Abstract
Advancing age results in altered cognitive and neuroimaging‐derived markers of neural integrity. Whether cognitive changes are the result of variations in brain measures remains unclear and relating the two across the lifespan poses a unique set of problems. It must be determined whether statistical associations between cognitive and brain measures truly exist and are not epiphenomenal due solely to their shared relationships with age. The purpose of this study was to determine whether cerebral blood flow (CBF) and gray matter volume (GMV) measures make unique and better predictions of cognition than age alone. Multivariate analyses identified brain‐wide covariance patterns from 35 healthy young and 23 healthy older adults using MRI‐derived measures of CBF and GMV related to three cognitive composite scores (i.e., memory, fluid ability, and speed/attention). These brain‐cognitive relationships were consistent across the age range, and not the result of epiphenomenal associations with age and each imaging modality provided its own unique information. The CBF and GMV patterns each accounted for unique aspects of cognition and accounted for nearly all the age‐related variance in the cognitive composite scores. The findings suggest that measures derived from multiple imaging modalities explain larger amounts of variance in cognition providing a more complete understanding of the aging brain. Hum Brain Mapp 34:3267–3279, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: aging, multiple modality imaging, cognitive decline, cerebral blood flow, gray matter volume, multivariate analysis
INTRODUCTION
Advancing age is associated with concurrent changes in cognition, cerebral blood flow (CBF) and gray matter volume (GMV), but causal relationships among the three have not been fully established. Age‐related differences in cognition include the domains of memory, fluid ability, and processing speed [Salthouse, 2011, 1996]. Age‐related cognitive changes accompany age‐related alterations of CBF, as measured regionally and globally by positron emission tomograhy (PET) and magnetic resonance imaging (MRI) [Buijs et al., 1998; Chen et al., 2011; Duara et al., 1984; Hagstadius and Risberg, 1989; Marchal et al., 1992; Martin et al., 1991; Pantano et al., 1984; Parkes et al., 2004], but there has been limited investigation of direct relationships between age‐related CBF and cognitive changes in healthy individuals [Bertsch et al., 2009; Duara et al., 1984; Heo et al., 2010; Rabbitt et al., 2006]. The recent work by Bertsch et al. 2009 demonstrated negative associations between CBF and task performance among both young and older adults.
The relationship between age and GMV, globally and regionally, has received much broader investigation (for reviews see Raz and Rodrigue 1962; Sowell et al., 2002). For instance, age‐related GMV changes are associated with cognitive performance across multiple domains [Brickman et al., 2007; Gong et al., 2005; Kaup et al., 2011; Reuben et al., 2011; Salthouse, 2011; Schretlen et al., 2000; Taki et al., 2011; Zimmerman et al., 2006]. A recent review of the structural correlates of cognition across that lifespan demonstrated that the direction of these changes is in dispute [Kaup et al., 2011]. Most studies demonstrated positive relationships between GMV and cognitive performance: larger brain volume associated with better cognitive performance. However, there were also findings of negative relationships and null results. Furthermore, this review found that relationships between structure and cognition remained stable across the lifespan, supporting the concept of neural reserve [Stern, 2009; Stern et al., 2005].
The purpose of this study was to examine the degree to which cognitive changes across the lifespan are explained by age and brain‐wide covariance patterns of CBF and GMV. Before addressing this aim, it is important to establish that any identified brain‐cognitive relationship truly exists and does not reflect the alternative possibility that it is the result of an epiphenomenal association between the measures, that is, a secondary result, due to their shared relationships with age [Salthouse and Ferrer‐Caja, 2003]. One question is whether relationships between neural (CBF and GMV) and cognitive measures are age‐dependent or age‐independent? In addition, do the brain measures have unique associations with cognition? These questions were addressed using guidelines and criteria put forth by Kraemer et al. 2011 and Salthouse 1996.
One approach of finding brain correlates of cognition uses multivariate techniques to identify brain‐wide neural‐cognitive patterns [Alexander et al., 1999]. From this class of techniques, we used the multivariate covariance approach called scaled subprofile modeling (SSM) [Moeller et al., 1987]. This method successfully derived structural and functional patterns related to aging [Alexander et al., 2006; Bergfield et al., 2010; Brickman et al., 2007; Moeller et al., 1996] and to performance on cognitive measures across the lifespan [Alexander et al., 1999]. Focusing on brain‐wide MRI measures of CBF and GMV, we identified separate covariance patterns associated with three cognitive domains: memory, fluid ability, and speed/attention across healthy young and older adults. Identification of covariance patterns, as opposed to identification of individual regions provides a potential brain‐wide reproducible metric [Brickman et al., 2008] that may assist in dissociating normal age‐related cognitive changes from pathological aging [Bergfield et al., 2010; Spetsieris and Eidelberg, 2011]. This approach provides one summary measure per covariance pattern, per individual, for use in subsequent statistical analyses.
We hypothesized that CBF and GMV pattern expressions have age‐dependent and age‐independent relationships with cognition and that these relationships are not epiphenomenal due to strong, but independent, relationships of the brain and cognitive measures with age group. Furthermore, although there is evidence for a causal relationship between CBF and GMV [Fierstra et al., 2010], there is also evidence of dissociations between age‐related changes in CBF and GMV [Chen et al., 2011]. We therefore hypothesized that CBF and GMV measures will each have their own unique predictive relationships with the cognitive measures. We used commonality analyses to summarize results by parsing the unique and common variance in cognitive measures associated with age, CBF, and GMV [McPhee and Seibold, 1979; Zientek and Thompson, 2006]. These analyses determined whether CBF, GMV, or their combination accounted for the most amount of variance in cognition and whether they are stronger correlates of cognition than age alone.
MATERIALS AND METHODS
Study Sample
Data came from ongoing neuroimaging studies of normal aging conducted within the Cognitive Neuroscience Division of the Taub Institute at Columbia University. Participants were recruited through random market mailings to individuals living within 10 miles of the medical center campus in northern Manhattan. All MRI acquisition and cognitive testing were performed during a single visit. Initial participant groups included 35 healthy young and 26 healthy older adults, but data from three older subjects were not used due to scan artifact in their arterial spin labeled (ASL)/CBF images. Thus, the final sample comprises 35 young adults (mean age ± S.D. = 24.34 ± 3.19; 14M/21F) and 23 older adults (mean age ± S.D. = 66.39 ± 4.11; 9M/14F) who were similar in education (Young mean ± S.D. = 15.74 ± 1.63; Older mean ± S.D. = 16.17 ± 1.82; P > 0.05). Participants were screened with medical, neurological, psychiatric, and neuropsychological evaluations to ensure that they had no neurological or psychiatric disease or cognitive impairment. The screening procedure included a detailed interview that excluded individuals with a self‐reported history of major‐or unstable medical illness, hypertension, significant neurological history (e.g., epilepsy, brain tumor, and stroke), history of brain trauma with a loss of consciousness for greater than 5 min, history of diagnosis of an Axis I psychiatric disorder [American Psychiatric Association, 1994]. Individuals taking psychotropic medications were also excluded. Older participants were evaluated for dementia with the Mattis Dementia Rating Scale [Mattis, 1988] and those scoring below 135 were excluded. Performance on subsequent neuropsychological testing was further used to rule out dementia or mild cognitive impairment. Individuals with well‐managed diabetes were eligible for participation. Written informed consent approved by the local ethics committee of Columbia University was obtained from all participants.
Cognitive Measures
Based on previous work from our laboratory, three cognitive composite scores were created for memory, speed/attention, and fluid ability as derived from confirmatory factor analyses of multiple cognitive tests per cognitive domain [Siedlecki et al., 2009].
Memory
Memory was defined as the latent construct score of three subscores of the selective reminding task (SRT—total, delayed recall, and delayed recognition; [Buschke and Fuld, 1974]). For this task, participants were read a list of 12 words and were asked to recall the words after each of six trials. After each recall attempt, participants were reminded of the words they failed to recall. SRT‐total is the total number of recalled words for all trials and has a maximum score of 72. SRT‐delayed recall refers to the number of correctly recalled words after a 15‐min delay. SRT‐delayed recognition refers to the number of correctly recognized words when each of the 12 words is presented with three distracters.
Speed/attention
Speed/attention was defined as a construct of the Wechsler adult intelligence scale‐revised (WAIS‐R; [Wechsler, 1981]) digit symbol subtest and the trail making test [Reitan and Wolfson, 1993]. The digit symbol test involves writing the symbol corresponding to each single‐digit in a list of numbers using a key at the top of the test form as quickly as possible. The time to complete the Trails A (numbers only) from the trail making test was used.
Fluid ability
Fluid ability generally refers to novel problem solving and tests of abstract reasoning. The Raven's matrix reasoning tests tend to have the highest loadings on this construct, while a number of studies have found that fluid ability has strong relationships to WCST [Salthouse, 2005] and to working memory, including the letter number sequencing [Salthouse, 2005; Salthouse and Pink, 2008]. Fluid ability was defined as a construct comprising the WAIS‐III [Wechsler, 1997] letter number sequencing subtest and the matrix reasoning test [Raven, 1962]. The letter number sequencing test involves participants repeating verbally presented lists of intermixed letters and numbers in alphabetical and numerical order. The list lengths increase with each subsequent trial. The matrix reasoning subtest requires participants to determine which pattern in a set of eight possible patterns best completes a missing cell of a matrix.
MRI Data Acquisition
All scanning took place on a 1.5 Tesla Philips Intera MRI scanner. CBF was calculated from an arterial spin labeling MRI sequence and GMV from a T 1‐weighted sequence. Spin‐echo continuous ASL (SE‐CASL) was acquired while subjects were instructed to rest quietly with eyes open. The specific parameters included: labeling duration = 2,000 ms, postlabeling delay (PLD) = 800 ms, echo time/repetition time = 35 ms/5,000 ms, flip angle = 90°; 64 × 58 acquisition matrix; in‐plane resolution = 3.4 mm × 3.4 mm; slice thickness = 7.4 mm; gap = 1.5 mm; 15 transaxial slices per volume. Slices were acquired in ascending mode (inferior to superior) with a slice acquisition time of 64 ms resulting in an effective PLD range of 800–1,760 ms. The total CBF image pairs were 30 per participant.
Labeling induced the flow‐driven adiabatic inversion of the water spins with a block‐shaped radio‐frequency pulse 2,000 ms long and 3.5 μT amplitude applied in the presence of a z‐gradient 2.5 mT/m [Alsop and Detre, 1998]. Off‐resonance effects were corrected for with an amplitude modulated (sinusoidal, 250 Hz) radio‐frequency pulse of the same power and gradient as applied prior for collection of the control image [Alsop and Detre, 1998]. The labeling plane was positioned 40 mm inferior to the lower edge of the imaging volume.
T 1‐weighted MP‐RAGE images were acquired (echo time/repetition time = 3 ms/25 ms; flip angle = 45°; 107 slices; 256 × 256 grid; FOV = 230 mm × 230 mm × 186 mm).
Image Processing
Study specific normalization template
Structural images from a randomly selected subsample of 26 young adults and all 26 elders with high quality structural images were used in template creation. The subsample of young adults ensured that the template was not biased toward either age group. Although the final analyses used 23 of the older adults, the template derivation used structural data from 26, the maximal number of older participants. The T 1‐weighted images were segmented into four probability classes: gray matter, white matter, cerebrospinal fluid (CSF), and other tissues using the unified segmentation routines in SPM5 (Wellcome Department of Cognitive Neurology) [Ashburner and Friston, 2000, 2005; Good et al., 2001]. Initially, the segmented images remained in native space, and then a 12 parameter linear affine transformation was calculated to transform all the original native images to standard space defined by the Montreal Neurological Institute. This transformation was applied to the gray matter, white matter, and CSF probability images. A mean image within each tissue class and across both age groups was calculated to create probability maps for each of the three tissue classes. These three prior probability maps constitute the study specific normalization template.
Gray matter volume
The T 1‐weighted anatomical images were first segmented into three tissue types: gray matter, white matter, and CSF, using the unified segmentation routines in SPM5 (Wellcome Department of Cognitive Neurology) [Ashburner and Friston, 2000, 2005; Good et al., 2001] and the study‐specific normalization template described above. This procedure uses a single generative model to correct for image intensity nonuniformity (bias), registration with tissue class priors, and tissue classification. The result is a classification for each voxel based on the probability that it belongs to each tissue type. Each image segment therefore contains measures of tissue densities in each voxel location. The images were spatially normalized to the study specific normalization template using 12 degrees of freedom affine transforms and nonlinear warping. Once warped, the images were modulated using the Jacobian determinant, which converts the density images into measures of absolute volume at each voxel location [Good et al., 2001]. The resultant modulated, spatially normalized gray matter probability maps were spatially smoothed with an isometric Gaussian smoothing kernel of 8 mm3 at its full‐width at half‐maximum (FWHM) to result in GMV maps.
Cerebral blood flow
ASL images were processed using the “ASLtbx” toolbox from the laboratory of Dr. John Detre [Wang et al., 2008]. Using the recommended preprocessing analysis stream, the following steps were performed: (2005) image pairs (label and control) were separately realigned to the mean of the control images, (1999) the mean of the control images was used to calculate the coregistration parameters required to bring the CBF images into the same space as each participant's high‐resolution anatomical image, (2006) masked to exclude all locations outside the brain using a mask derived from the high‐resolution anatomical image using the brain extraction tool [Smith, 2002], (1996) spatially smoothed using a Gaussian kernel of 8 mm3 at its FWHM, (1998) CBF images for each label/control pair were calculated using the simplified two‐compartment model [Alsop and Detre, 1996; Wang et al., 2002] using simple subtraction between the image pairs [Aguirre et al., 2005], (1994) image pairs were investigated to eliminate global spikes which may result from spatial location offset due to head motion as defined by the ASLtbx, (2000) mean CBF across all image pairs not excluded in the previous step was calculated, and (2005) all CBF images were spatially normalized to the study specific template.
Statistical Analyses
Scaled subprofile modeling
Multivariate analyses were performed to identify brain patterns of CBF and GMV related to each cognitive measure using the SSM approach implemented in the principal components analysis (PCA) toolbox (http://groups.google.com/group/gcva) [Habeck et al., 2005; Habeck and Stern, 2007]. The analyses were conducted separately for each of the two brain measures and each of the three cognitive measures. All images were first masked using a group level gray matter mask including only voxels with a greater than 20% probability of being gray matter. The resultant masked images from all participants in each image modality were subjected to SSM analyses.
Briefly, a PCA is performed on the data after the subject means were subtracted from each voxel without further transforms, producing a series of principal component images and their respective subject scaling factors (SSF). These scaling factors reflect the degree to which each individual expressed each component image. The covariance patterns relating CBF and GMV to the cognitive data for all three domains were calculated by regressing the optimal collection of SSF's taken from the first 12 principle components, using the Akaike information criterion criteria [Burnham and Anderson, 2002] against the cognitive scores in separate multiple regression models. The stability of the voxels within the resultant covariance patterns were tested using 1,000 bootstrap resamples. Voxels with bootstrap estimates of |Z| > 1.96, P (two‐tailed) < .025, were considered significant network nodes. This analysis provided localization of the key “nodes” of the covariance pattern [Habeck et al., 2005]. For display purposes only, a cluster extent threshold of 50 voxels was used.
Criterion models
Are brain measures truly related to cognition or are the brain and cognitive variables related to each other due to their strong correlations with age? A series of regression models was calculated with each cognitive composite score serving as a separate dependent variable. The independent variables were the individual expressions of the brain patterns entered first and age group entered in a second step.
Are relationships between the neural measures (CBF and GMV) and the cognitive measures age dependent or age independent? An ANOVA model for each cognitive measure and each brain measure was calculated with the cognitive composite score serving as the dependent variable. The independent variables were age group, expression of a brain pattern, and their interaction. A significant interaction term indicates that expression of the brain pattern significantly differs between the age groups suggesting that it is not representative of both age groups.
Do CBF and GMV each account for unique aspects of cognition? To test whether CBF and GMV capture independent aspects of cognition, three ANOVA models were used using each of the cognitive composite scores as dependent measures and age group, CBF and GMV as the independent measures. Support for expression of both CBF and GMV patterns accounting for unique aspects of cognition is demonstrated by both brain measures being significant in the models.
Commonality analysis
Commonality analysis is a procedure that decomposes variance to determine the unique and nonunique relationships between independent and dependent variables [McPhee and Seibold, 1979; Seibold and McPhee, 1978; Zientek and Thompson, 2006]. The variables of interest include the cognitive variables (C) as the dependent measures and age (A), individual expression of the CBF pattern related to the cognitive measure (CBF), individual expression of the GMV pattern related to the cognitive measure (GMV) as independent variables. The calculations are as follows:
The variables in parentheses correspond to the independent variables used to predict the dependent variable; U i represents unique variance of the dependent variable accounted for by variable i; Ci,j is the variance in the dependent variable that is common to both variables i and j; R 2(X,Y,Z) represents the total variance accounted for in the dependent variable by the three independent measures (X,Y,Z) using multiple regression; r 2(X) represents the squared correlation between the dependent variable and the independent variable: X. These measures are summarized in the Venn diagram shown in Figure 1A.
Figure 1.
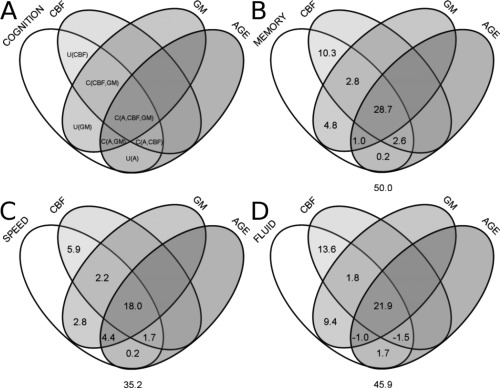
Venn diagrams summarizing commonality results. (A) General four variable Venn diagram where labels correspond to the (U)nique and (C)ommon variance components of the cognitive variable as calculated in the text. Results for the (B) memory composite score, (C) speed/attention composite score, and (D) the fluid ability composite score. Values under each Venn diagram correspond to the total variance accounted for in the respective cognitive measure.
RESULTS
Cognitive Scores
The correlation coefficients between the cognitive composite scores and each variable comprising the scores are shown in Table 1.
Table 1.
Means (S.D.) of cognitive variables and the correlations between the components and the three factors
| Young mean (S.D.) | Elder mean (S.D.) | Correlation Coefficients | |||
|---|---|---|---|---|---|
| Memory | Fluid | Speed/Attn | |||
| Memory | |||||
| SRT total recall | 60.60 (5.87) | 53.35 (8.58) | 0.878 | 0.440 | 0.431 |
| SRT delayed recall | 10.94 (1.35) | 8.61 (2.35) | 0.914 | 0.483 | 0.505 |
| SRT delayed recognition | 12.00 (0.00) | 11.78 (0.60) | 0.674 | 0.484 | 0.401 |
| Fluid ability | |||||
| WAIS‐3 letter‐number | 13.86 (2.86) | 11.83 (3.21) | 0.480 | 0.897 | 0.479 |
| WAIS‐3 Matrix | 20.86 (3.93) | 15.57 (5.84) | 0.525 | 0.897 | 0.475 |
| Speed | |||||
| WAIS‐R digit symbol | 67.51 (10.07) | 55.04 (13.02) | 0.591 | 0.595 | 0.781 |
| Trail making Test A | 26.94 (13.85) | 34.00 (9.44) | −0.250T | −0.236T | −0.781 |
| Cognitive factors | |||||
| Memory | 15.68 (0.53) | 14.61 (1.20) | — | ||
| Fluid ability | 4.60 (0.75) | 3.70 (1.10) | 0.560 | — | |
| Speed | 2.03 (0.92) | 1.05 (0.81) | 0.538 | 0.532 | — |
| Covariance patterns | |||||
| CBF (Mem) | 15.66 (0.34) | 14.64 (0.56) | 0.667 | 0.542 | 0.524 |
| GMV (Mem) | 15.61 (0.35) | 14.71 (0.51) | 0.607 | 0.475 | 0.526 |
| CBF (fluid) | 4.56 (0.34) | 3.76 (0.59) | 0.593 | 0.599 | 0.490 |
| GMV (fluid) | 4.57 (0.36) | 3.74 (0.43) | 0.467 | 0.567 | 0.358 |
| CBF (speed) | 1.90 (0.31) | 1.24 (0.47) | 0.532 | 0.437 | 0.498 |
| GMV (speed) | 1.95 (0.34) | 1.17 (0.47) | 0.581 | 0.416 | 0.553 |
| Age group | −0.524 | −0.447 | −0.484 | ||
Note. All group mean differences were significant at P< 0.05. All correlation coefficients are significant at P < 0.001, except for the two marked with (x)T. Numbers in bold are within cognitive‐construct values.
SSM Analyses
The SSM analyses identified a pattern of CBF and GMV associated with each cognitive composite score. Expression of the identified brain patterns was significantly related to the cognitive composite scores. In all cases, the brain patterns accounted for more variance in cognitive composite scores than age group, see Table 2. Scatter plots of the cognitive scores and the predicted scores by both CBF and GMV covariance patterns are shown in Figure 2. It is important to emphasize that covariance patterns are derived using all voxels within the brain and Figure 3 highlights brain areas that most reliably contribute to the patterns. The similarity of the covariance patterns was measured using spatial correlation, Supporting Information Table SI.
Table 2.
Analysis of variance results for the memory score
| Memory | R 2 | F(d.f.) | η2 | P |
|---|---|---|---|---|
| Model I | 0.44 | |||
| CBF | 44.76 (1,56) | 0.66 | 0.000 | |
| Model II | 0.45 | |||
| CBF | 16.96 (1,55) | 0.49 | 0.000 | |
| Age Group | 0.138 (1,55) | 0.04 | 0.712 | |
| Model III | 0.47 | |||
| CBF | 13.075 (1,54) | 0.39 | 0.001 | |
| Age Group | 2.068 (1,54) | 0.19 | 0.156 | |
| CBF × age group | 2.026 (1,54) | 0.19 | 0.160 | |
| Model I | 0.37 | |||
| GMV | 32.75 (1,56) | 0.61 | 0.000 | |
| Model II | 0.38 | |||
| GMV | 9.67 (1,55) | 0.39 | 0.003 | |
| Age Group | 1.26 (1,55) | 0.15 | 0.266 | |
| Model III | 0.40 | |||
| GMV | 8.43 (1,54) | 0.37 | 0.005 | |
| Age group | 1.31(1,54) | 0.15 | 0.257 | |
| GMV × age group | 1.21(1,54) | 0.15 | 0.276 |
Figure 2.
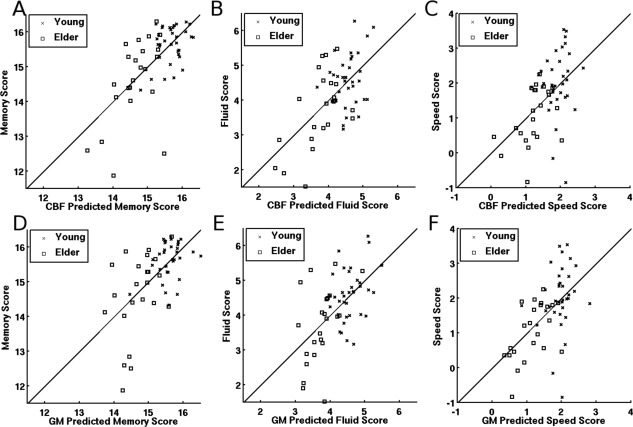
Scatter plots of the three cognitive scores and their predicted values using the individual expressions of the CBF (top row) and GMV (bottom row) covariance patterns. The lines in each plot represent the no‐change reference, the point of perfect prediction. Scatterplots (A) and (D) show that four older adults appear to have outlying memory scores. Excluding these four individuals did not alter any of the significant findings described below; therefore, all results include the entire study sample.
Figure 3.
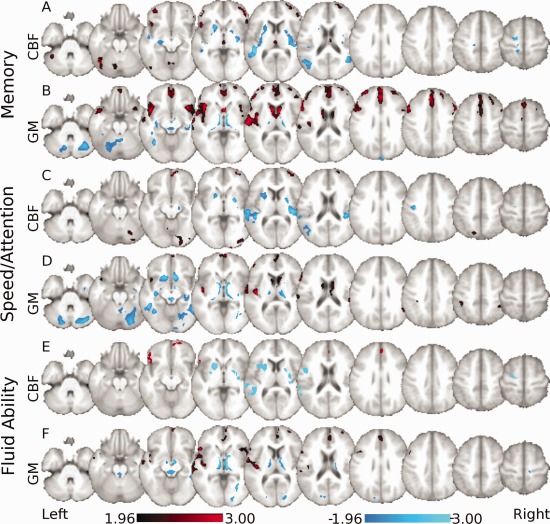
Neural covariance patterns of the cognitive composite scores as derived across all participants. Voxels labeled red represent regions that strongly co‐vary and have relatively greater values within the respective network. Voxels labeled blue represent regions that strongly co‐vary and have relatively lower values within the respective network. (A) CBF pattern related to the memory composite score is characterized by relative increased CBF in the cerebellum and middle orbital frontal lobe associated with relative decreased blood flow in the hippocampus, temporal cortex, and postcentral gyrus. (B) GMV pattern related to the memory composite score includes relative increased GMV in the temporal cortex, caudate, and medial prefrontal cortex and is associated with relative decreased volume within the thalamus, cerebellum, and fusiform regions. (C) CBF pattern related to the speed/attention composite score is characterized by relative increased CBF in middle and superior frontal regions, calcarine sulcus, parietal, and cerebellar regions, with relative decreased CBF in the putamen, temporal gyri, and occipital cortex. (D) Gray matter pattern related to the speed/attention composite score includes relative increased GMV in temporal regions, prefrontal, and parietal regions and caudate with relative decreased volume within the cerebellum, fusiform, and hippocampal regions. (E) CBF pattern related to the fluid ability composite score is characterized by relative increased CBF within prefrontal cortical areas and the anterior cingulate with relative decreased blood flow within the temporal gyri putamen and precentral regions. (F) Gray matter pattern related to the fluid ability composite score includes relative increased GMV within temporal gyri and prefrontal cortical regions with relative decreased volume within the thalamus, temporal, cuneus, and occipital regions.
Memory Covariance Pattern
The CBF and GMV brain patterns of memory are displayed in Figure 3A, B and the voxel locations of the key nodes of the networks are in Supporting Information Tables SII and SIII. Expression of the CBF pattern accounted for 44.4% of the variance in the memory scores, expression of the GMV pattern accounted for 36.9%, and age accounted for 32.1%.
Speed/Attention Covariance Pattern
The CBF and GMV brain patterns of speed/attention are displayed in Figure 3C, D, and the voxel locations of the key nodes of the networks are in Supporting Information Tables SIV and SV. Expression of the CBF pattern accounted for 24.7% of the variance in the speed/attention scores, expression of the GMV pattern accounted for 30.5% and age accounted for 24.3%.
Fluid Ability Covariance Pattern
The CBF and GMV brain patterns of fluid ability are displayed in Figure 3E, F, and the voxel locations of the key nodes of the networks are in Supporting Information Tables SVI and SVII. Expression of the CBF pattern accounted for 35.8% of the variance in the fluid ability scores, expression of the GMV pattern accounted for 32.1% and age accounted for 21.1%.
Criterion Models Results
Criterion 1: Brain patterns significantly related to the cognitive composite scores were identified. Testing a series of regression models indicated that the relationships between expression of the brain patterns and the respective cognitive composite scores remained significant after age group was entered into the models, Tables 2, 3, 4. This finding provides support that the relationship between expression of the brain patterns and the cognitive composite scores is not an artifactual result due to strong correlations with age group.
Table 3.
Analysis of variance results for the fluid ability score
| Fluid | R 2 | F(d.f.) | η | P |
|---|---|---|---|---|
| Model I | 0.36 | |||
| CBF | 31.26 (1,56) | 0.60 | 0.000 | |
| Model II | 0.36 | |||
| CBF | 14.15 (1,55) | 0.45 | 0.000 | |
| Age group | 0.48 (1,55) | 0.09 | 0.489 | |
| Model III | 0.38 | |||
| CBF | 10.45 (1,54) | 0.40 | 0.002 | |
| Age group | 1.21 (1,54) | 0.15 | 0.276 | |
| CBF × age group | 1.03 (1,54) | 0.14 | 0.314 | |
| Model I | 0.32 | |||
| GMV | 26.47 (1,56) | 0.56 | 0.000 | |
| Model II | 0.32 | |||
| GMV | 10.01 (1,55) | 0.39 | 0.003 | |
| Age group | 0.19 (1,55) | 0.06 | 0.662 | |
| Model III | 0.33 | |||
| GMV | 10.22 (1,54) | 0.40 | 0.002 | |
| Age group | 0.94 (1,54) | 0.13 | 0.339 | |
| GMV × age group | 0.84 (1,54) | 0.122 | 0.363 |
Table 4.
Analysis of variance results for the speed/attention score
| Speed | R 2 | F(d.f.) | η | P |
|---|---|---|---|---|
| Model I | 0.25 | |||
| CBF | 18.44 (1,56) | 0.50 | 0.000 | |
| Model II | 0.29 | |||
| CBF | 4.33 (1,55) | 0.27 | 0.042 | |
| Age Group | 3.31 (1,55) | 0.24 | 0.074 | |
| Model III | 0.30 | |||
| CBF | 3.64 (1,54) | 0.25 | 0.062 | |
| Age Group | 1.66 (1,54) | 0.17 | 0.202 | |
| CBF × age group | 0.637 (1,54) | 0.11 | 0.428 | |
| Model I | 0.31 | |||
| GMV | 24.73 (1,56) | 0.56 | 0.000 | |
| Model II | 0.32 | |||
| GMV | 7.27 (1,55) | 0.35 | 0.009 | |
| Age Group | 1.43 (1,55) | 0.16 | 0.237 | |
| Model III | 0.33 | |||
| GMV | 6.59 (1,54) | 0.33 | 0.013 | |
| Age Group | 1.43 (1,54) | 0.16 | 0.237 | |
| GMV × age group | 0.73 (1,54) | 0.11 | 0.396 |
Criterion 2: The relationships between the neural (CBF and GMV) covariance patterns and the cognitive measures were age independent as demonstrated by nonsignificant interaction terms in all ANOVA models, Tables 2.
Criterion 3: The two brain measures each accounted for unique aspects of cognition. Expression of the CBF and GMV covariance patterns each accounted for large, unique aspects of the memory and fluid ability. For the speed/attention score only the GMV brain measure was significant, Table 5. Tests of the interactions between age group and the neural measures were nonsignificant for all six combinations of neural and cognitive measures, Supporting Information Table SVIII.
Table 5.
ANOVA models testing whether the brain patterns are overlapping proxies for cognition
| R 2 | F(d.f.) | η | P | |
|---|---|---|---|---|
| Memory | 0.51 | |||
| Age group | 0.81 (1,54) | 0.12 | 0.372 | |
| CBF | 13.38 (1,54) | 0.45 | 0.001 | |
| GMV | 6.55 (1,54) | 0.33 | 0.013 | |
| Fluid | 0.46 | |||
| Age group | 1.46 (1,54) | 0.16 | 0.232 | |
| CBF | 13.29 (1,54) | 0.45 | 0.001 | |
| GMV | 9.26 (1,54) | 0.39 | 0.004 | |
| Speed | 0.35 | |||
| Age group | 0.18 (1,54) | 0.05 | 0.669 | |
| CBF | 2.43 (1,54) | 0.21 | 0.125 | |
| GMV | 5.22 (1,54) | 0.30 | 0.026 |
Commonality Analysis
Commonality analyses used the percent variance accounted for (R 2) from the analyses presented in Tables 2, 3, and 5 to summarize and extend the above results. In addition, the calculations required the R 2 values for regression models of memory, fluid ability, and speed/attention including the respective CBF and GMV predictors (R 2(CBF,GMV)), these values were 0.498, 0.442 and 0.350, respectively.
For the memory composite score, see Figure 1B, the CBF, GMV, and age group variables accounted for 50.0% of the total variance and age group accounted for only 0.2% unique variance. For the speed/attention composite score, see Figure 1C, the CBF GMV and age group variables accounted for 35.2% of the total variance and age group accounted for only 0.2% unique variance. For the fluid ability composite score, see Figure 1D, the CBF, GMV, and age group variables accounted for 45.9% of the total variance and age group accounted for only 1.7% unique variance. Notably, for fluid ability, there are negative variance values suggesting that one of the variables acted as a “suppressor” variable, as discussed below. An alternate presentation of the commonality results using bar graphs of relative variance accounted for is included in Supporting Information materials, Supporting Information Figures S1–S3.
DISCUSSION
We identified CBF and GMV covariance patterns related to (associated with) performance on three cognitive composite scores. These covariance patterns accounted for age group‐dependent and independent variance in the cognitive scores. We found that the relationship between the covariance patterns and cognition was similar across the age groups. Finally, the CBF and GMV brain patterns each accounted for their own significantly large, unique aspects of the memory and fluid ability cognitive scores.
This work identified patterns of CBF and GMV whose relationships to cognitive scores were invariant across the age groups. Advancing age moves individuals along this continuum without significantly altering the relationship as previously shown in the literature [Kaup et al., 2011]. Our findings are similar to demonstrations that cognitive tests scores steadily decline across the lifespan with configural and metric invariance [Siedlecki et al., 2008].Therefore, relationships between the different neuropsychological test scores and between the test scores and age do not change [Salthouse, 2004]. Brain measures show similar results across the lifespan. In healthy individuals, global GMV slowly decreases across a large age range [Fotenos et al., 2005], with no age‐related increase in this rate of change. The same gradual decline across the lifespan is shown with measures of CBF [Lu et al., 2011].
Interpretation of the individual brain areas comprising the CBF and GMV covariance patterns must be tempered by the consideration that it is the entire spatial pattern that is used to calculate the relationships between the brain measures and the cognitive scores. The directionality of the voxel‐wise loadings within the patterns, represents the deviation of that region from the combined young and elder mean (see Spetsieris and Eidelberg 2004 for detailed discussions of these topics). In positively labeled regions, increased relative blood flow or volume is related to higher cognitive scores while negatively labeled regions, decreased relative blood flow or volume, are associated with higher cognitive score. Although it is difficult to compare the covariance patterns to results from univariate analyses, aspects of our identified neural‐cognitive relationships have been previously identified in the literature.
In one study, examining the association between CBF and cognition in aging resting CBF measures within all brain lobes was related to selective attention in young adults and tonic alertness in the older adults [Bertsch et al., 2009]. Our finding of a relationship between CBF in the putamen and speed is similar to that of Berentand colleagues, who used the digit symbol task, which is one measure comprising our speed factor [Berent et al., 1988]. Our finding of CBF within the hippocampus related to the memory factor is also in line with work by Bangen et al. 2009. Global measures of CBF and brain volume have also been linked to age‐related cognitive changes [Rabbitt et al., 2006]. Rabbit and colleagues found that global CBF was related to cognitive speed but neither global CBF nor global brain volume was related to fluid ability. The authors postulated that their use of global measures masked regional relationships [Rabbitt et al., 2006]. This thesis is supported by our findings of CBF and GMV brain patterns associated with fluid ability.
Recent work by Taki et al. 2005 identified cognitive measures related to GMV within the bilateral temporal gyri and the hippocampus. In a study by Gong et al. 2005 fluid ability was related to GMV within the medial PFC, similar to this study. Meanwhile fluid ability, but not memory, was related to hippocampal GMV in another study from our group [Reuben et al., 2011]. Although the hippocampus was not a strong node in our GMV‐fluid ability covariance pattern, the hippocampus was a strong node in the GMV‐memory pattern. This discrepancy could result from the differences in sample sizes between these two studies (76 vs. 58 for our study) or more likely result from the fact that SSM methods collectively identify regions related to the cognitive scores. Therefore, while the GMV of the hippocampus may have an independent relationship with fluid ability [Reuben et al., 2011] its relationship with memory is in conjunction with many other brain regions. Although there are many other studies relating cognition and GMV, a comprehensive review by Raz and Rodrigue discuss that the overall findings in the literature are weak. Furthermore, questions about whether age‐related differences in GMV precede changes in CBF, or the reverse, are in need of further study [Raz and Rodrigue, 2006].
A limitation of this work is the lack of external validation of the patterns through forward application to new study samples. It will be important to replicate these findings in an independent sample by forward applying the derived brain patterns [Bergfield et al., 2010; Brickman et al., 2008; Spetsieris and Eidelberg, 2011]. Forward application can rank an individual based on where they fall on each of the brain‐cognitive relationships. Such rankings can then be included as additional biomarkers in assessing someone's aging process.
The benefit of using an established set of criteria [Kraemer et al., 2001] before making any conclusions about neural‐cognitive relations is that it is systematic and provides clear understanding of the relationships among age, markers of neural functioning, and cognition. These criteria, when combined with recommendations suggested by Van Petten 2007 related to aging and neuropsychology hypotheses, may help shed light on disparate neural‐memory findings in the literature.
Visual inspection of the covariance patterns suggests limited spatial overlap between the CBF and GMV patterns (see Fig. 3). Despite this, expression of the CBF and GMV brain patterns shared large amounts of variance with each other for the memory, speed/attention, and fluid ability cognitive composite scores (31.5%, 20.2%, and 23.7%, respectively). Furthermore, expression of the brain patterns correlated with all three cognitive domains, not only the domain they were derived from (see Table 1). This lack of complete specificity of the brain patterns is not surprising for two main reasons. The patterns were derived with the effect of age group in the cognitive scores and the cognitive scores themselves were intercorrelated. Therefore, the derived brain patterns all capture the age effect and the intercorrelations resulting in some commonality of the brain patterns.
The use of two brain measures proved advantageous over either measure alone. The results indicate that the multiple modality neuroimaging approach provides a more complete picture of the aging brain and its impact on cognition. Furthermore, the two brain measures account for nearly all the age group‐related variance in the cognitive scores, and for large amount of additional variance: 18% for memory, 24.8% for fluid ability, and 10.9% for speed/attention as calculated by summing the independent and combined effects of CBF and GMV in Figure 1. It is important to point out however that this work used discrete age groups and not a continuum across the adult life. This leaves open the possibility that the actual neural‐cognitive relationships are nonlinear through adulthood [Heo et al., 2010] as opposed to linear, as tested in this work. Future work will overcome this limitation by including a more complete sampling of ages and test for forward applicability of these covariance patterns [Bergfield et al., 2010; Brickman et al., 2008; Spetsieris and Eidelberg, 2011].
In addition, this study used a rather small sample. This relatively small sample may be the reason for the nonsignificant P‐value when testing the relation between speed and CBF in the presence of age group and the interaction effect see Model III, Table 4. The moderate effect size (η = 0.251) suggests that this analysis is underpowered and requires a sample size of 127 to reach significance of α = 0.05 and β = 0.80 (calculated using G*Power3 [Faul et al., 2007]). An additional limitation of this work is the exclusive focus on gray matter. Large bodies of work exist focusing on age‐related alterations in white matter pathology [Brickman et al., 2006, 2011; Gunning‐Dixon and Raz, 2003; Rabbitt et al., 2007] and integrity [Charlton et al., 2008; Gold et al., 2008; Madden et al., 2009; Zahr et al., 2009] and their impact on cognition. It is possible that white matter measures could explain additional variance in our cognitive measures. Future work will incorporate similar measures for investigation of white matter effects on cognition within the context of joint investigation of CBF and GMV.
It is important to point out that we did not investigate the neural mediation of the effects of aging on cognition nor mediation between the brain measures [Vaidya et al., 2007]. Such approaches test whether age‐related cognitive variability is the causal result of the brain measures. Rather, we identified patterns of neural CBF and GMV related to cognitive performance that are not proxies of age group and not the result of epiphenomenal associations due to shared relationships with age group. Although mediation analyses would use brain measures as mechanistic proxies for aging, we identified neural correlates of specific cognitive measures. Traditional mediation analyses [Baron and Kenny, 1986; Shrout and Bolger, 2002] would not be appropriate in the current analyses based on statistical circularity. Our neural correlates of cognition were defined using the cognitive measures; therefore, our procedure was to determine if there existed a neural‐cognitive relationship. Use of the identified neural‐cognitive relationship in subsequent mediation analyses, within the same data set, would be circular [Kriegeskorte et al., 2009; Vul et al., 2009]. The data driven nature and lack of a priori regional assumptions however make the results from this work prime targets for selection of ROIs in future work and formal tests of neural mediation.
Although the commonality analyses display the strength of the relationships between variables, they can also potentially identify the presence of negative amounts of variance accounted for, as was the case for the fluid ability measure. Negative values represent suppression effects between the regressors in the model that result when an independent variable (in this case age group) correlates more highly with other independent variables in the model (in this case CBF and GMV) than the dependent variable (fluid ability) [Conger and Jackson, 1972; McPhee and Seibold, 1979]. Such findings point to the need for careful testing and interpretation of neural‐cognitive relationships as influenced by the complex processes underlying advancing age.
We identified covariance patterns of CBF and GMV that predicted composite cognitive scores from three domains. These brain patterns accounted for nearly all the age group related variance in the cognitive measures. Additionally, expression of the CBF and GMV brain patterns each accounted for additional age group‐independent variance in the cognitive scores. The identified neural‐cognitive relationships were invariant across the age groups and the combination of the GMV and CBF patterns made better predictors of cognition than age group or either measure alone. Finally, this carefully designed analytic framework ensures that the identified neural‐cognitive relationships truly exist and are not the result of epiphenomenal associations with age group.
Supporting information
Supporting Information
REFERENCES
- Aguirre GK, Detre JA, Wang J (2005): Perfusion fMRI for functional neuroimaging. Int Rev Neurobiol 66:213–236. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Mentis MJ, Van Horn JD, Grady CL, Berman KF, Furey ML, Pietrini P, Rapoport SI, Schapiro MB, Moeller JR (1999): Individual differences in PET activation of object perception and attention systems predict face matching accuracy. Neuroreport 10:1965–1971. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre‐Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, Hampel H, Rapoport SI, Moeller JR (2006): Regional network of magnetic resonance imaging gray matter volume in healthy aging. Neuroreport 17:951–956. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA (1996): Reduced transit‐time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 16:1236–1249. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA (1998): Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 208:410–416. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994):Diagnostic and Statistical Manual of Mental Disorders.Washington, DC:American Psychiatric Press. [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11( 6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Jak AJ, Wierenga CE, Salmon DP, Bondi MW (2009): Differential age effects on cerebral blood flow and BOLD response to encoding: Associations with cognition and stroke risk. Neurobiol Aging 30:1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA (1986): The moderator‐mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Berent S, Giordani B, Lehtinen S, Markel D, Penney JB, Buchtel HA, Starosta‐Rubinstein S, Hichwa R, Young AB (1988): Positron emission tomographic scan investigations of Huntington's disease: Cerebral metabolic correlates of cognitive function. Ann Neurol 23:541–546. [DOI] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE. (2010): Age‐related networks of regional covariance in MRI gray matter: Reproducible multivariate patterns in healthy aging. NeuroImage 49:1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E (2009): Resting cerebral blood flow, attention, and aging. Brain Res 1267:77–88. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E (2006): Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry 60:444–453. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y (2007): Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging 28:284–295. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y (2008): A forward application of age associated gray and white matter networks. Hum Brain Mapp 29:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, Brown TR, DeCarli C, Stern Y (2011): White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging 32:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs PC, Krabbe‐Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, Mali WP (1998): Effect of age on cerebral blood flow: Measurement with ungated two‐dimensional phase‐contrast MR angiography in 250 adults. Radiology 209:667–674. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR 2002Model Selection and Multimodel Inference: A Practical Information‐Theoretic Approach.New York, NY:Springer. [Google Scholar]
- Buschke H, Fuld PA (1974): Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24:1019–1025. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG (2008): A structural equation modeling investigation of age‐related variance in executive function and DTI measured white matter damage. Neurobiol Aging 29:1547–1555. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH (2011): Age‐associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 55:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger AJ, Jackson DN (1972): Suppressor variables, prediction, and the interpretation of psychological relationships. Educ Psychol Meas 32:579–599. [Google Scholar]
- Duara R, Grady C, Haxby J, Ingvar D, Sokoloff L, Margolin RA, Manning RG, Cutler NR, Rapoport SI (1984): Human brain glucose utilization and cognitive function in relation to age. Ann Neurol 16:703–713. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007): G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. [DOI] [PubMed] [Google Scholar]
- Fierstra J, Poublanc J, Han JS, Silver F, Tymianski M, Crawley AP, Fisher JA, Mikulis DJ (2010): Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatry 81:290–293. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL (2005): Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology 64:1032–1039. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD (2008): Age‐related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging 31:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong QY, Sluming V, Mayes A, Keller S, Barrick T, Cezayirli E, Roberts N (2005): Voxel‐based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage 25:1175–1186. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14( 1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Raz N (2003): Neuroanatomical correlates of selected executive functions in middle‐aged and older adults: A prospective MRI study. Neuropsychologia 41:1929–1941. [DOI] [PubMed] [Google Scholar]
- Habeck C, Stern Y (2007): Neural network approaches and their reproducibility in the study of verbal working memory and Alzheimer's disease. Clin Neurosci Res 6:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR (2005): A new approach to spatial covariance modeling of functional brain imaging data: Ordinal trend analysis. Neural Comput 17:1602–1645. [DOI] [PubMed] [Google Scholar]
- Hagstadius S, Risberg J (1989): Regional cerebral blood flow characteristics and variations with age in resting normal subjects. Brain Cogn 10:28–43. [DOI] [PubMed] [Google Scholar]
- Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF (2010): Resting hippocampal blood flow, spatial memory and aging. Brain Res 1315:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT (2011): A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci 23:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D (2001): How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry 158:848–856. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI (2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC (2011): Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral Cortex 21:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA (2009): Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Petit‐Taboue MC, Sette G, Travere JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC (1992): Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 49:1013–1020. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS (1991): Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689. [DOI] [PubMed] [Google Scholar]
- Mattis S (1988):Dementia Rating Scale.Odessa:Psychological assessment resources. [Google Scholar]
- McPhee RD, Seibold DR (1979): Rationale, procedures, and applications for decomposition of explained variance in multiple regression analyses. Commun Res 6:345–384. [Google Scholar]
- Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA (1987): Scaled subprofile model: A statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab 7:649–658. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D (1996): The metabolic topography of normal aging. J Cereb Blood Flow Metab 16:385–398. [DOI] [PubMed] [Google Scholar]
- Pantano P, Baron JC, Lebrun‐Grandie P, Duquesnoy N, Bousser MG, Comar D (1984): Regional cerebral blood flow and oxygen consumption in human aging. Stroke 15:635–641. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS (2004): Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn Reson Med 51:736–743. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, Pendleton N (2006): Losses in gross brain volume and cerebral blood flow account for age‐related differences in speed but not in fluid intelligence. Neuropsychology 20:549–557. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Lunn M, Thacker N, Lowe C, Pendleton N, Horan M, Jackson A (2007): White matter lesions account for all age‐related declines in speed but not in intelligence. Neuropsychology 21:363–370. [DOI] [PubMed] [Google Scholar]
- Raven J (1962).Advanced Progressive Matrices, Set II.London:H.K. Lewis. [Google Scholar]
- Raz N, Rodrigue KM (2006): Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D 1993The Halstead‐Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation.Tuscon, AZ:Neuropsychology Press. [Google Scholar]
- Reuben A, Brickman AM, Muraskin J, Steffener J, Stern Y (2011): Hippocampal atrophy relates to fluid intelligence decline in the elderly. J Int Neuropsychol Soc 17:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2004): What and when of cognitive aging. Curr Directions Psychol Sci 13:140–144. [Google Scholar]
- Salthouse TA (2005): Relations between cognitive abilities and measures of executive functioning. Neuropsychology 19:532–545. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996): The processing‐speed theory of adult age differences in cognition. Psychol Rev 103:403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2011): Neuroanatomical substrates of age‐related cognitive decline. Psychol Bull 137:753–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer‐Caja E (2003): What needs to be explained to account for age‐related effects on multiple cognitive variables? Psychol Aging 18:91–110. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Pink JE (2008): Why is working memory related to fluid intelligence? Psychon Bull Rev 15:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P (2000): Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age‐related differences in fluid intelligence. J Int Neuropsychol Soc 6:52–61. [DOI] [PubMed] [Google Scholar]
- Seibold DR, McPhee RD (1978): Commonality analyses: A method for decomposing explaine dvariance in multiple regression alayses. Hum Commun Res 5:355–365. [Google Scholar]
- Shrout PE, Bolger N (2002): Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods 7:422–445. [PubMed] [Google Scholar]
- Siedlecki KL, Honig LS, Stern Y (2008): Exploring the structure of a neuropsychological battery across healthy elders and those with questionable dementia and Alzheimer's disease. Neuropsychology 22:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MSV, Wright CB (2009): Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan study. J Int Neuropsychol Soc 15:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW (2004): Mapping changes in the human cortex throughout the span of life. Neuroscientist 10:372–392. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Eidelberg D (2011): Scaled subprofile modeling of resting state imaging data in Parkinson's disease: Methodological issues. Neuroimage 54:2899–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2009): Cognitive reserve. Neuropsychologia 47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R (2005): Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 15:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Wu K, Kawashima R, Fukuda H (2011): Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn 75:170–176. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Paradiso S, Boles Ponto LL, McCormick LM, Robinson RG (2007): Aging, grey matter, and blood flow in the anterior cingulate cortex. Neuroimage 37:1346–1353. [DOI] [PubMed] [Google Scholar]
- Van Petten C (2004): Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta‐analysis. Neuropsychologia 42:1394–1413. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H (2009): Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 4:274–290. [DOI] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Li L, Listerud J, Gonzalez‐At JB, Schnall MD, Detre JA (2002): Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med 48:242–254. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández‐Seara MA, Childress AR, Detre JA (2008): Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imag 26:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981).Wechsler Adult Intelligence Scale Revised.New York:The Psychological Corporation. [Google Scholar]
- Wechsler D (1997).Wechsler Memory Scale—III San Antonio, TX:Psychological Coorporation. [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV (2009): Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. Neuroimage 44:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientek LR, Thompson B (2006): Commonality analysis: Partitioning variance to facilitate better understanding of data. J Early Intervention 28:299–307. [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E (2006): The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry 14:823–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


