Abstract
Intestinal stem cells (ISCs) are responsible for renewal of the epithelium both during normal homeostasis and following injury. As such they have significant therapeutic potential. However, it is unknown whether ISCs can survive tissue storage. We hypothesized that, although the majority of epithelial cells may die, ISCs would remain viable for at least 24 h at 4°C. To explore this hypothesis, jejuni of C57Bl6/J or Lgr5-LacZ mice were removed and either processed immediately or placed in phosphate buffered saline (PBS) at 4°C. Delayed isolations of epithelia were performed after 24, 30, or 48 h storage. At the light microscope level, despite extensive apoptosis of villus epithelial cells, small intestinal crypts remained morphologically intact through 30 h and ISCs were identifiable via Lgr5-LacZ positivity. Electron microscopy showed that ISCs retain high integrity through 24 h. When assessed by flow cytometry, ISCs were more resistant to degeneration than the rest of the epithelium, including neighboring Paneth cells, with higher viability across all time points. Culture of isolated crypts showed no loss of capacity to form complex enteroids after 24 h tissue storage, with efficiencies after 7 days of culture remaining above 80%. By 30 h storage, efficiencies declined but budding capability was retained. We conclude that, with delay in isolation, ISCs remain viable and retain their proliferative capacity. In contrast, the remainder of the epithelium, including the Paneth cells, exhibits degeneration and programmed cell death. If these findings are recapitulated with human tissue, storage at 4°C may offer a valuable temporal window for harvest of crypts or ISCs for therapeutic application.
Keywords: Intestinal stem cells, light and electron microscopy, viability, resistance to storage, enteroid culture
Introduction
The intestinal epithelium is the most rapidly proliferating tissue in the mammalian body, with complete turnover of the majority of cells occurring every 3–4 days. Pioneering work of Cheng and Leblond in the early 1970s suggested that this turnover arises from stem cells located deep in the intestinal crypts (Cheng and Leblond, 1974). The general concept that these stem cells give rise to all differentiated lineages within the epithelium has stood the test of time and has now been confirmed by numerous groups (see reviews by Garrison, et al., 2009, King and Dekaney, 2013, Montgomery and Breault, 2008, Shaker and Rubin, 2010, Wong, 2004). Additionally, as more sophisticated tools have become available, accumulated evidence indicates that there are in fact at least two relatively distinct populations of intestinal stem cells (ISCs). The most extensively studied population is one that is marked by the G-protein-coupled receptor Lgr5. This population is actively cycling and is normally responsible for the day-to-day homeostatic turnover of the epithelium (Barker, et al., 2012). In contrast, there is another population, marked by Bmi1, m-Tert, Hopx, Lrig1 and Dclk1, which is relatively quiescent and appears to be responsible for epithelial regeneration after injury (Carlone and Breault, 2012, Lund, 2012, Potten, et al., 2009, Scoville, et al., 2008).
The use of preparations which maintain ISCs in their niche, such as isolated crypts or organoids (i.e. crypts with adherent myofibroblasts), has long been recognized as having valuable therapeutic applications for situations where a critical mass of the intestine is damaged. Several labs have reported functional engraftment of tissue-engineered small intestine grown from organoid units(Avansino, et al., 2005, Bitar and Raghavan, 2012, Levin and Grikscheit, 2012). Initially, a critical limiting factor in such applications was the inability to expand intestinal organoids in vitro (Gupta, et al., 2006). Recently however, culture conditions have been reported which allow net expansion of murine crypts (Fuller, et al., 2012, Sato, et al., 2009), thus bringing therapeutic transplantation significantly closer to reality. To date, however, all studies of ISC/crypt expansion and transplantation have utilized freshly isolated, healthy tissue (Lahar, et al., 2011, Sato, et al., 2011, Yui, et al., 2012). While such an approach is easy in the laboratory, in the clinic that may rarely be the case. Therapeutic sources would typically include tissue collected either postmortem or at surgery. In either case the ability to store tissue prior to crypt or ISC isolation would constitute a significant advantage.
Data from other organ systems suggest that therapeutically useful cells are present postmortem. Hematopoeitic stem cells have long been known to survive with delayed bone marrow harvest (Liu, et al., 1978, Mugishima, et al., 1985, Yesus, et al., 1981). More recently, hepatocytes were found viable and capable of engraftment after harvest up to 27 h postmortem (Erker, et al., 2010). On the basis of these findings, we hypothesized that following storage of intestinal tissue at 4°C, ISCs would remain viable even if other components of the epithelium degenerate. To investigate this hypothesis, viability was assessed by light and electron microscopy, flow cytometry, and crypt culture. The results indicated, that just as in other tissues, stem cells of the small intestine are remarkably resistant to apoptosis and necrosis during storage up to 30 h postmortem.
Materials and methods
Animals and Reagents
All animal procedures were approved by the Institutional Animal Care and Use Committee at the respective two institutions. Male, C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Heterozygote breeder pairs of Lgr5-LacZ mice (Barker, et al., 2007) were generously provided by Lexicon Pharmaceuticals (The Woodlands, TX). All mice were housed 4–5 per cage on a 12 h light dark cycle in American Association for Accreditation of Laboratory Animal Care-approved facilities and were 6–8 weeks of age at the time of usage. Mice were killed by overdose of isoflurane (until breathing stopped) followed by cervical dislocation, then the jejunum was removed and used as described in Experimental Design below.
Reagents used for histology were: anti-cleaved caspase-3 [Asp 175] (Cell signaling #9661, Danvers, MA), and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside [X-gal] (SIGMA #7240-90-6). Antibodies used for flow cytometric analysis included: Alexa Fluor 647 (Alexa 647) conjugated anti-CD31 (Biolegend #102415, San Diego, CA), Alexa 647 conjugated anti-CD45 (Biolegend #103124), Pacific Blue conjugated anti-CD24 (Biolegend #101820), Fluorescein isothiocyanate (FITC) conjugated bovine anti-rabbit IgG (Santa Cruz Biotechnology #SC-2365, Santa Cruz, CA), and FITC conjugated lectin from Ulex europaeus [UEA] (SIGMA #L9006, St. Louis, MO).
Experimental Design
In the first experiment, viability of the entire jejunal epithelium was assessed following immediate dissociation, as compared with 24 h storage under various conditions: a) storage in phosphate buffered saline (PBS) at 4°C after initial flushing of the intestine; b) storage in PBS at 4°C without flushing of the intestinal contents; and c) placing the entire mouse at 4°C. Jejunal epithelial cells were isolated via the EDTA/dispase method as previously described, except that dithiothreitol was omitted (Formeister, et al., 2009). Each epithelial preparation was stained with trypan blue, then trypan-positive (dead) and trypan-negative (alive) cells were counted in a hemocytometer. Viability was expressed as the number of alive cells as a percentage of total (alive plus dead) cells. While this approach gives useful information regarding the overall pattern of epithelial viability, it should be recognized that absolute values for percent viable may be an over estimation if some proportion of the dead cells (i.e. part of the denominator) become degraded to the point of being unrecognizable as cells assessed for overall viability by trypan blue.
For all subsequent experiments, jejunum of C57BL6/J or Lgr5-LacZ mice was dissected, flushed with ice cold PBS and split into four aliquots for immediate processing (0 h) and delayed processing (24, 30, and 48 h). For delayed processing, flushed jejunum was kept in a 15mL conical of PBS at 4°C for 24, 30, or 48 h. When splitting the intestine of each mouse jejunum, sections were taken either with diagonal sectioning or small transverse sections, which were systematically split between time points, to prevent regional differences between time points.
Histology/ Microscopy
Tissue was pinned flat and fixed with 4% paraformaldehyde (PFA) for 3 h at room temperature (RT) or processed for whole-mount β-galactosidase (X-gal) staining. After whole-mount staining and/or fixation, tissue was immersed in 70% ethanol for at least 24 h and then paraffin embedded into a single block per time point. Longitudinal sections of 6 m were utilized for morphology and immunohistochemistry.
Slides were evaluated using an Axio Imager A1 microscope and pictures taken with an AxioCam MRC 5 high-resolution camera (Carl Zeiss, Microimaging, Thornwood, NY). Overall morphology was examined with hematoxylin and eosin (H&E). Apoptosis was assessed with cleaved caspase-3. For caspase-3, heat induced epitope retrieval was performed in a decloaking chamber at 120°C for 30 s followed by 90°C for 10 s (DAKO #S1699, Glostrup, Denmark). Sections were treated with 3% H2O2 for 10 min at RT, then incubated for 1 h in blocking buffer (DAKO X0909, Glostrup, Denmark) or 10% normal goat serum at RT. Subsequently, sections were incubated with primary antibody at 1:800 (cleaved caspase-3) in antibody diluent (Thermo #TA125-ADQ, Waltham, MA) at 4°C overnight. Sections were rinsed in Tris buffer between all applications of antibodies, reagents and endogenous peroxidase blocking step. Secondary antibody, biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, #111-065-144, West Grove, PA), was applied at 1:500 in antibody diluent (Thermo #TA125-ADQ, Waltham, MA) for 1 h at RT. Vectastain Elite avidin-biotin horseradish peroxidase complex in Tris, was applied at 1:50 (Vector Labs, #PK6100, Burlingame, CA) for 30 min at RT. Quatro chromogen, 3,3′ diaminobenzidine (Thermo #TA125-QHDX, Waltham, MA) was then applied for 1 min. Finally, sections were counterstained with either nuclear fast red, or hematoxylin, dehydrated, cleared in xylene and DPX coverslipped.
β-galactosidase staining
To determine whether the Lgr5 positive crypt base stem cells remain over time, whole mount staining was performed with intestinal tissue from male heterozygote Lgr5-LacZ mice as per published protocol with slight modifications (Takeda, et al., 2011). All segments were pinned flat and flushed with 3mM dithiothreitol to remove mucus. Briefly, jejunum was fixed with glutaraldehyde and PFA, incubated with X-gal solution for 16 h overnight at RT, and then further fixed with 4% PFA. Tissue was then transferred to cassettes in 70% ethanol and embedded in paraffin.
Electron microscopy (EM)
For assessment of the structural integrity of the intestine at 0 and 24 h time points, 5mm pieces of jejunum were immersion-fixed in 2% PFA/2.5% glutaraldehyde in 0.15M sodium phosphate buffer, pH 7.4, and stored at 4°C for several days before processing. Following several washes in 0.15M sodium phosphate buffer, the samples were post-fixed in 1% osmium tetroxide/1.25% potassium ferrocyanide in 0.15M sodium phosphate buffer, pH 7.4, for 1 h (Russel and Burguet, 1977). Samples were dehydrated in a graded series of ethanols, followed by propylene oxide, and infiltrated and embedded in Polybed 812 resin (Polysciences, Inc., Warrington, PA). 1μm semithin transverse sections were cut with a glass knife, mounted on slides, stained with 1% toluidine blue and viewed using a light microscope to select the region of interest. Ultrathin sections (70nm) were cut using a diamond knife, mounted on 200 mesh copper grids and stained with 4% aqueous uranyl acetate and Reynold’s lead citrate (Reynolds, 1963). Samples were observed using a LEO EM910 transmission electron microscope operating at 80kV (Carl Zeiss Microscopy, Peabody, MA) and digital images were acquired using a Gatan Orius SC1000 CCD Digital Camera with Digital Micrograph 3.11.0 (Gatan, Inc., Pleasanton, CA). The integrity of cells at the crypt base was assessed by a pathologist who was blinded to the experimental conditions.
Flow Cytometry
Jejunal epithelial cells were isolated as described under Experimental Design and 1 million cells were then incubated in 100 μL of PBS for 45 min on ice with either: no antibody, isotype-FITC Bovine IgG (0.5 μg); CD31-Alexa674 (0.5 μg) and CD24-PB (0.25 μg); or CD45-Alexa647 (0.5 μg), CD31-Alexa647, CD24-PB, and UEA-FITC (1:250). Samples were then rinsed and resuspended in 500 μL of PBS with addition of 1 μg of propidium iodide (PI). Cells were analyzed using a Beckman-Coulter (Dako) CyAn ADP. Debris was first excluded based on size via bivariate plot of forward scatter (FSC) versus side scatter. Doublets were excluded using successive gating on both a bivariate plot of pulse-width versus FSC and a bivariate plot of FSC area versus FSC linear. CD45+ and CD31+ events, representing leukocytes and endothelial cells respectively (von Furstenberg, et al., 2011), were then excluded based on positivity for Alexa-647. To determine which crypt base cell populations survive over the course of time, the remaining cells were analyzed for PI, CD24 and UEA, to assess viability of ISC and Paneth cell fractions as previously described (von Furstenberg, et al., 2011, Wong, et al., 2012). A separate aliquot was also analyzed for overall viability by trypan blue.
Tissue Harvest for Intestinal Crypt Culture
Crypts were isolated, cultured, and quantified as previously published. (Fuller, et al., 2012) Proximal jejunum of male C57Bl6/J mice was removed and processed (0 h) or placed in PBS at 4°C for 24, 30, or 48 h (n= 6, 8, 8, 4 respectively). Tissue was manually shaken for crypt removal after incubation with 3mM EDTA. Pelleted crypts were resuspended in growth factor reduced Matrigel (BD Bioscience) supplemented with EGF (50ng/mL), Noggin (100ng/mL), R-spondin (500ng/mL) and wnt3a (5ng/mL). All concentrations are for total well volume. With the exception of wnt3a, these conditions are as described by Sato et al. (Sato, et al., 2009) Crypts in 10μL of Matrigel were then plated in 6 experimental replicates per time point on 48 well plates. Each was then overlaid with Advanced DMEM/F12 (GIBCO, Carlsbad, CA) supplemented with Hepes (10mM), N2 (1:100), B27 (1:50), L-glutamine (1:100), and penicillin/streptomycin (1:100) (Invitrogen). The final number of crypts plated was counted per well and followed over the course of 7 days for enterosphere and enteroid formation. Definition of these structures was as per recent nomenclature recommendations (Stelzner, et al., 2012). Enteroid efficiencies were defined as a percentage of enteroids formed divided by the number of crypts initially plated. Among formed enteroids, lateral crypt buds were counted across a minimum of two wells and ten enteroids per well per mouse.
Statistics and Quantification
To assess the effect of storage conditions on epithelial viability, 1-way analysis of variance with Bonferroni post-test corrections for multiple comparisons was performed in GraphPad Prism 5 (LaJolla, CA). For analysis of viability over time by cell type (ISC v Paneth) a 2-way analysis of variance was performed in GraphPad Prism 5. To assess the maintenance of culture efficiency and crypt budding over time of tissue storage at 4°C, 1-way analysis of variance with Bonferroni post-test corrections for multiple comparisons was performed in GraphPad Prism 5. P-values less than 0.05 were considered statistically significant.
Results
Viable intestinal cells are present at least up to 24 hours post mortem
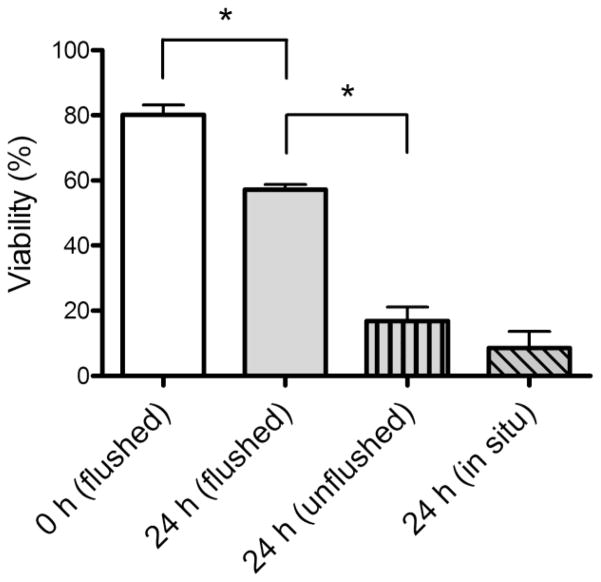
Based on the report that viable hepatocytes can be harvested from postmortem mice that have been stored as intact animals at 4°C (Erker et al. 2010), we initially assessed whether viable epithelial cells could be isolated from jejunal tissue stored for 24 h at 4°C under three different conditions: a) flushed intestine; b) removal of intestine with no flushing; and c) an entire carcass with intestine left in situ. As can be seen in Fig. 1, viability of the entire epithelium (as assessed by trypan blue staining after isolation) remained above 50% after 24 h when the intestine was flushed prior to storage, but decreased significantly when the tissue was left unflushed or in situ. Therefore, for all further experiments the jejunum was flushed prior to storage in PBS at 4°C.
Fig. 1.
Viability of jejunal epithelial cells following storage of tissue at 4°C. Cells were isolated either immediately (0 h flushed) or after 24 h storage of jejuni either: flushed with PBS (flushed); removed from carcass but not flushed (unflushed); or left within the whole mouse (in situ). Viability was measured by trypan blue staining. Data are shown as means ± SEM (n=3) *P < 0.05
Morphology of the small intestinal crypts remains intact through 30 hours at 4°c
Tissue architecture, was initially assessed by hematoxylin and eosin (H&E) staining performed after 0, 24, 30, and 48 h after storage. The results (Fig. 2 a–e) demonstrate overall maintenance of crypt architecture through 30 h postmortem. Meanwhile, by 30 h the villi begin to slough from their laminal cores and are nearly completely absent by 48 h. At 48 h some tissue sections exhibit conservation of crypts (Fig. 2 d). However, in other areas there are crypts detaching from the submucosa with demonstrable cellular shrinkage (Fig. 2 e).
Fig. 2.
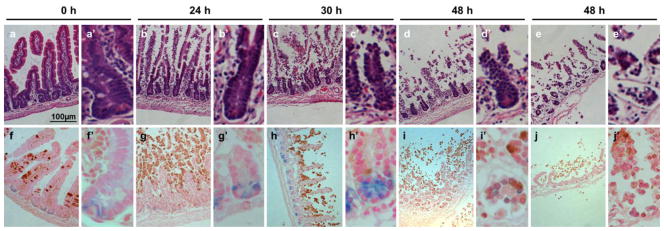
Architecture of flushed jejunal tissue stored for 4°C for 0, 24, 30, and 48 h. (a–e) H&E for demonstration of morphology. (f–j) cleaved caspase-3 staining (brown) for apoptosis and co-staining for Lgr5-LacZ (blue). Each time point is shown at low power (a–j) and high power (a′–j′) respectively. For the 48 h time point two different regions are shown to illustrate the variability observed. Because the tissue is very fragile at this stage it was very difficult to get good sections. Nevertheless: panels “d” and “i” show a region where the mucosa is nearly denuded, but the muscle layers have remained intact and negative for cleaved caspase-3; whereas panels “e” and “j” show a region where the muscle layers are also destroyed. Scale bar shown is 100μm (a).
To further assess the viability of the crypt compartments, we used tissue from Lgr5-LacZ mice to simultaneously identify apoptotic cells by staining with antibodies to cleaved caspase-3 and Lgr5 positive ISCs by LacZ staining (Fig. 2 f–j). Consistent with the H&E staining of wild-type mice, the crypts of Lgr5-LacZ mice retain good architecture through 30 h but show signs of degeneration by 48 h. Moreover, through 24 h crypts show no signs of apoptosis, despite extensive activated caspase-3 staining on the villi. By 48 h postmortem, there is near complete loss of the apoptotic villi, and at this time, the crypts begin to display caspase positivity (Fig. 2 i and j). As the ISCs reside within the crypt base and are responsible for maintenance of the small intestinal epithelium, we further characterized our tissue sections for the presence of crypt base columnar ISCs marked by Lgr5. Lgr5-LacZ expressing stem cells (Barker, et al., 2007) are clearly evident through 30 h but cannot be identified by 48 h delayed isolation (Fig. 2 f–j).
Small intestinal stem cells are resistant to degeneration
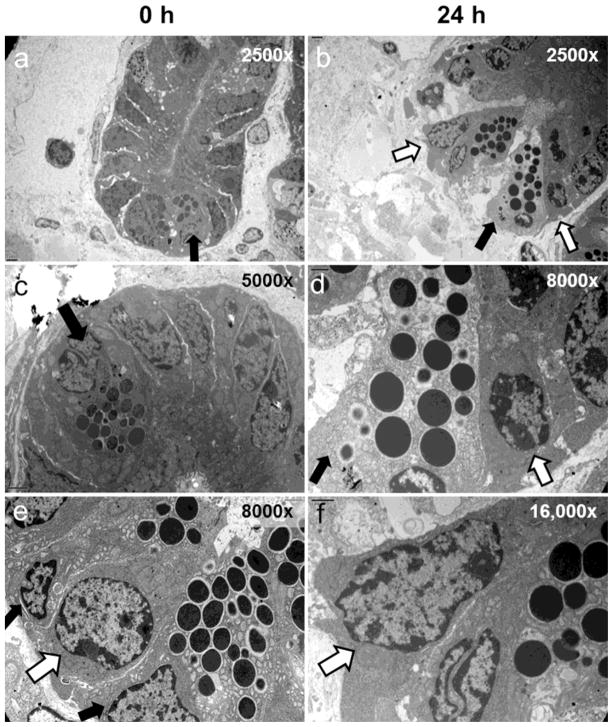
The EM images in Fig. 3 show that at the ultrastructural level the ISCs of the crypt base appear to retain high integrity after 24 h storage of tissue. In contrast, some of the adjacent Paneth cells have become necrotic at this time, although others still appear to be relatively healthy with intact electron dense secretory vesicles.
Fig. 3.
EM images of jejunal tissue stored for 0 or 24 h at 4°C. Each vertical panel shows crypts of increasing magnification: left panel 0 h; right panel 24 h. Open arrows point to crypt base columnar ISCs compared to closed arrows which indicate Paneth cells (with electron dense granules).
Based on the report that the CD24loUEA− population represents a relatively pure fraction of ISC by flow cytometry (Wong, et al., 2012), we examined viability of that fraction using PI-positivity to identify dead cells. In the same epithelial preparations, the viability of Paneth cells was assessed utilizing the CD24loUEA+ fraction. As can be seen in Fig. 4a, there was a decline in the percent of viable stem cells and Paneth cells over time postmortem (p<0.001). However, ISCs are significantly more viable (Fig. 4c) than their neighboring Paneth cells (p<0.001) (Fig. 4d). This difference is most pronounced by 30h, (Fig. 4a).
Fig. 4.
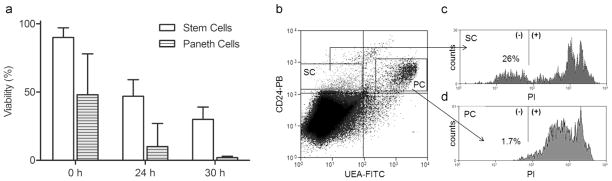
Viability of ISCs and Paneth cells isolated from jejunal tissue stored at 4°C for 0, 24, and 30 h. (a) ISC viability was computed as live (PI−) CD24loUEA− cells as a % of total CD24loUEA− cells and Paneth cell viability as live (PI−) CD24loUEA+ cells as % of total CD24loUEA+ cells. Data displayed as means ± SEM (n=4). (b) Example of gating strategy as illustrated by a 30 h sample, with gates termed (SC) for stem cell and (PC) for Paneth cell. The CD24loUEA− (SC) and CD24loUEA+ (PC) subpopulations are gated forward onto single parameter histograms (panels “c” and “d” respectively) to determine the % of cells negative for PI (live).
Small intestinal stem cells retain proliferative capacity after storage at 4°c
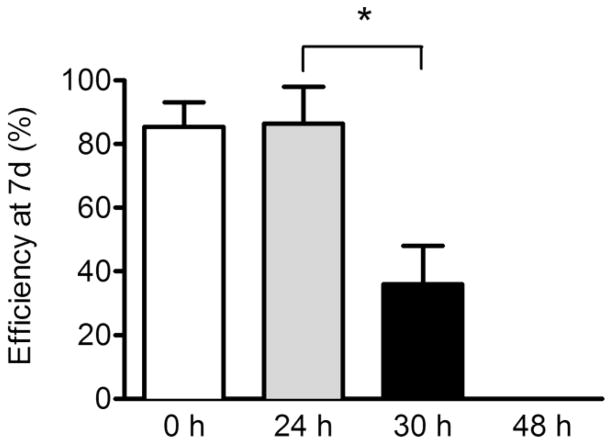
As ISCs appeared viable postmortem, we further assessed their functionality via in vitro crypt culture. Freshly isolated crypts showed culture efficiencies over 80% and remarkably there was no decline in culture efficiency after a 24 h delay in isolation (Fig. 5). Efficiency dropped to 36% when crypts were isolated from tissue that had been stored for 30 h and no crypts formed enteroids when storage was extended to 48 h. However, if enteroids form, they retain their proliferative capacity as evidenced by no statistically significant differences in their ability to form buds (Fig. 6a, p > 0.3). Multilobulated structures that equally progressed from enterospheres to enteroids were observed in tissue that had been stored for 0, 24, and 30 h. Moreover, the observation of approximately 6 buds per enteroid from tissue that had been stored for 30 h (Fig. 6b) is not only within the range of that observed for fresh tissue in the current study, but is also consistent with the extent of budding reported for crypts isolated from fresh tissue in a previous study (Fuller, et al., 2012).
Fig. 5.
Survival and growth of murine crypts isolated from tissue stored at 4°C for 0, 24, 30, and 48 h. Data are shown as enteroid forming efficiency after 7 d of crypt culture, with means ± SEM (n= 7, 7, 7, 4) *P < 0.05.
Fig. 6.
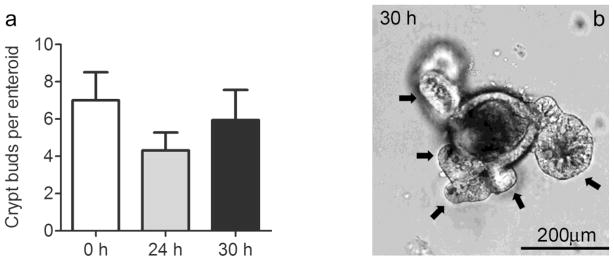
Proliferative capacity of murine ISC after storage at 4°C as measured by enteroid budding after 7d of crypt culture. (a) Composite data for 0, 24, 30 h displayed as means ± SEM (n= 6,6,5) P > 0.3. (b) Representative enteroid from 30 h condition with black arrows marking crypt buds, scale bar of 200μm shown.
Discussion
Current advances in tissue engineering and cellular transplantation models require analysis of potential tissue sources, both autologous and heterologous. Some tissue types have proven viable after delayed isolation and useful for therapy (Erker, et al., 2010, Mugishima, et al., 1985). For the intestine, numerous studies have shown that this tissue is remarkably rejuvenative after various types of in vivo damage, due to ISC subpopulations being relatively resistant to cell death (Barker, et al., 2012, Potten and Hendry, 1975, Wright, 2000). However, survival of intestinal tissue postmortem has not been systematically investigated. In this study, we sought to determine if viable and therapeutically useful ISCs were present and functional after delayed isolation.
Our initial experiment explored various storage conditions with the rationale that depending on the nature of the harvest (e.g. heterologous tissue postmortem versus autologous tissue via surgery) there may be limited opportunity to prepare the tissue for storage. In this experiment our assessment was based on viability of the entire epithelium. The data (Fig. 1) made a convincing case, that to ensure reasonable viability after 24 h storage at 4°C, the intestinal tissue needs to not only be removed from the body, but also flushed prior to storage. Whether loss of viability in the tissue stored in situ or without flushing could have reflected slower cooling or adverse effects of intestinal contents remains to be investigated. Likewise, the possibility that the ISC fraction may be resistant even under adverse storage conditions remains open to question. Nevertheless on the basis on these initial observations we chose to use flushed tissue for all subsequent experiments.
Our images of tissue architecture after storage for various times revealed dramatic differences between villi and crypts. While the villi showed extensive apoptosis at 24 h and almost complete disintegration by 48 h, the crypts remained essentially intact. Moreover healthy ISCs at the crypt bases were observed both at the light microscopic (by Lgr5-LacZ) and the EM level. Similar morphology of apoptotic villus loss with crypt preservation has also been noted in models of intestinal ischemia and reperfusion (Itoh, et al., 2002, Park and Haglund, 1992, Udassin, et al., 1994). Although no studies have looked specifically at the ISCs following ischemia and reperfusion, thymidine incorporation was noted at the base of the crypt in one study at the time of regeneration (Park and Haglund, 1992). Given the common findings of villus degeneration and crypt preservation, the same protective mechanisms may be in place both ex vivo following storage at 4°C and in vivo following ischemia at body temperature. As many of the pathologic diseases resulting in intestinal failure are secondary to ischemia, our data may suggest that ISCs remain viable in these disorders.
Beyond the morphologic evidence of ISC viability, we found they are capable of supporting crypt growth in vitro after delayed isolation up to 30 h. Furthermore, once growth is established in vitro, cells maintain their proliferative capacity as evidenced by the lack of significant difference in the number of buds observed in enteroids derived from 30 h tissue as compared with fresh tissue. Successful expansion of crypts in vitro is consistent with our finding that the ISCs are more resistant to degeneration than their neighboring Paneth cells over time as demonstrated via flow cytometry and EM. However, we cannot say if these dying Paneth cells still play a role in protecting their neighboring stem cells as has been demonstrated in vivo for live Paneth cells (Parry, et al., 2012). Protection by compromised Paneth cells would be consistent with a study which explored bone marrow transplant of both apoptotic support cells and live hematopoietic stem cells and found improved engraftment and declined rates and severity of graft v host disease (Pessach, et al., 2012). Interestingly, despite lack of nutrients the majority of cell death appears to occur via apoptosis in a controlled fashion.
As viable crypts are present and expandable, they can provide a tissue source for cellular transplantation or tissue engineering small intestine. Furthermore, as culture techniques improve, single ISCs may provide therapeutic benefits. Proof of principle for cellular transplantation was recently reported by Yui, et al. who successfully transplanted colonoids into a mouse model of colitis. The aim of their study was to replace damaged mucosal surface area via colonoid delivery per anus; engraftment of colonoids and restoration of colonic architecture was achieved (Yui, et al., 2012). Although, as noted in a recent commentary (Shaker and Rubin, 2012), much work still needs to be done to prove functionality, this cellular approach may prove beneficial for some patients. But, for other patients a tissue engineering approach to provide additional absorptive surface area may be necessary. Tissue engineered small intestines are now well characterized and have shown abrogation of nutritional deficits in a small animal models and feasibility in a large animal model (Avansino, et al., 2005, Grikscheit, et al., 2004, Sala, et al., 2009). While transplantation may provide one therapy for short bowel syndrome and intestinal failure, the finding of viable ISCs after prolonged storage may also help us understand in vivo regeneration of bowel following damage.
An exciting area for future investigation would be to determine which cell is responsible for our observed renewal in vitro. Current evidence supports the existence of actively cycling ‘workhorse’ ISCs and slowly cycling ‘quiescent’ ISCs (Carlone and Breault, 2012, Li and Clevers, 2010, Potten, et al., 2009, Scoville, et al., 2008). Additionally, under stress these cells may interconvert to repopulate the crypt (Montgomery, et al., 2011, Takeda, et al., 2011, Tian, et al., 2011, Yan, et al., 2012). In other states of damage and adaptation like resection, doxorubicin, radiation, or development a marked expansion is noted within the stem cell compartment (Dehmer, et al., 2011, Dekaney, et al., 2007, Dekaney, et al., 2009, Van Landeghem, et al., 2012). We hypothesize that the same mechanisms may be in play as cells survive delayed isolation and then are able to proliferate in vitro. From the irradiation model, an expansion is noted in both the active and quiescent stem pools, but it is unclear which cell population initiates the proliferative response to damage (Van Landeghem, et al., 2012). Likewise, although we detect ISCs marked by Lgr5 after 30 h delayed isolation, we cannot say definitively that these cells are responsible for initiating growth in vitro. More research is needed to define the interrelationships among crypt cells during damage and repair. Nevertheless, without knowing which stem cell compartment is responsible - we do know that isolation in a delayed fashion provides viable ISCs which are capable of growth and proliferation in vitro, and may be therapeutically beneficial.
Acknowledgments
Funding: This work was supported an AGA Student Research Fellowship Award (KMS) and by the National Institutes of Health Grants R01-DK083325 (MAH), P30 DK034987 (SJH, MAH), and U01-DK085547 (SJH, MAH). The latter grant is part of the Intestinal Stem Cell Consortium, a collaborative research project funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases. Additionally, this work received support from the UNC Department of Surgery (MKF), and the UNC Flow Cytometry Core Facility is supported in part by NCI Center Core Support Grant (P30CA06086) to the UNC Lineberger Comprehensive Cancer Center.
The authors wish to thank: Dr. Joe Galanko in the UNC Center for Gastrointestinal Biology and Disease for assistance with statistical analysis, Ashley Ezzel in the Cell and Molecular Physiology Core at UNC for assistance with histology; Victoria Madden, Steven Ray, and Dr. Robert Bagnell of the UNC School of Medicine Microscopy Services Lab for sample preparation and useful input on electron microscopy; Drs. Christopher Dekaney, Scott Magness, and P. Kay Lund for useful discussions.
Contributor Information
Megan K. Fuller, Department of Surgery, University of North Carolina at Chapel Hill
Denver M. Faulk, Department of Surgery, Cincinnati Children’s Hospital Medical Center
Nambirajan Sundaram, Department of Surgery, Cincinnati Children’s Hospital Medical Center.
Maxime M. Mahe, Department of Surgery, Cincinnati Children’s Hospital Medical Center
Kara M. Stout, Department of Medicine and Cell Biology & Physiology, University of North Carolina at Chapel Hill
Richard J. von Furstenberg, Department of Medicine and Cell Biology & Physiology, University of North Carolina at Chapel Hill
Brian J. Smith, Department of Medicine and Cell Biology & Physiology, University of North Carolina at Chapel Hill
Kirk K. McNaughton, Department of Medicine and Cell Biology & Physiology, University of North Carolina at Chapel Hill
Noah F. Shroyer, Department of Pediatrics: Division of Gastroenterology, Hepatology & Nutrition Cincinnati Children’s Hospital Medical Center
Michael A. Helmrath, Department of Surgery, Cincinnati Children’s Hospital Medical Center
Susan J. Henning, University of North Carolina at Chapel Hill, 4341 Medical Biomolecular Research Building (MBRB), CB# 7555, 111 Mason Farm Rd, Chapel Hill, NC 27599-7555
References
- Avansino JR, Chen DC, Hoagland VD, Woolman JD, Haigh WG, Stelzner M. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg. 2005;201:710–720. doi: 10.1016/j.jamcollsurg.2005.06.270. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den BM, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: Strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Bitar KN, Raghavan S. Intestinal tissue engineering: Current concepts and future vision of regenerative medicine in the gut. Neurogastroenterol Motil. 2012;24:7–19. doi: 10.1111/j.1365-2982.2011.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlone DL, Breault DT. Tales from the crypt: The expanding role of slow cycling intestinal stem cells. Cell Stem Cell. 2012;10:2–4. doi: 10.1016/j.stem.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. American Journal of Anatomy. 1974;141:537–562. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Dehmer JJ, Garrison AP, Speck KE, Dekaney CM, Van Landeghem L, Sun X, Henning SJ, Helmrath MA. Expansion of intestinal epithelial stem cells during murine development. PLoS One. 2011;6:e27070. doi: 10.1371/journal.pone.0027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2007;293:G1013–G1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;297:G461–G470. doi: 10.1152/ajpgi.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erker L, Azuma H, Lee AY, Guo C, Orloff S, Eaton L, Benedetti E, Jensen B, Finegold M, Willenbring H, Grompe M. Therapeutic liver reconstitution with murine cells isolated long after death. Gastroenterology. 2010;139:1019–1029. doi: 10.1053/j.gastro.2010.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct sox9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2009;296:G1108–G1118. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MK, Faulk DM, Sundaram N, Shroyer NF, Henning SJ, Helmrath MA. Intestinal crypts reproducibly expand in culture. J Surg Res. 2012;178:48–54. doi: 10.1016/j.jss.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison AP, Helmrath MA, Dekaney CM. Intestinal stem cells. J Pediatr Gastroenterol Nutr. 2009;49:2–7. doi: 10.1097/MPG.0b013e3181ad3021. [DOI] [PubMed] [Google Scholar]
- Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, Vacanti JP. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240:748–754. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestine--current status. Biomacromolecules. 2006;7:2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- Itoh H, Yagi M, Hasebe K, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K. Regeneration of small intestinal mucosa after acute ischemia-reperfusion injury. Dig Dis Sci. 2002;47:2704–2710. doi: 10.1023/a:1021049004188. [DOI] [PubMed] [Google Scholar]
- King SL, Dekaney CM. Small intestinal stem cells. Curr Opin Gastroenterol. 2013;29:140–145. doi: 10.1097/MOG.0b013e32835cf253. [DOI] [PubMed] [Google Scholar]
- Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martin MG, Dunn JC. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One. 2011;6:e26898. doi: 10.1371/journal.pone.0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Grikscheit TC. Tissue-engineering of the gastrointestinal tract. Curr Opin Pediatr. 2012;24:365–370. doi: 10.1097/MOP.0b013e328352ec19. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PI, Ogawa M, Crook L, Ochia R, Upshur JK. Proliferative function of cadaveric bone marrow cells. Am J Hematol. 1978;5:145–150. doi: 10.1002/ajh.2830050210. [DOI] [PubMed] [Google Scholar]
- Lund PK. Fixing the breaks in intestinal stem cells after radiation: A matter of DNA damage and death or DNA repair and regeneration. Gastroenterology. 2012;143:1144–1147. doi: 10.1053/j.gastro.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Breault DT. Small intestinal stem cell markers. Journal of Anatomy. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk MEG, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mtert) expression marks slowly cycling intestinal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugishima H, Terasaki P, Sueyoshi A. Bone marrow from cadaver donors for transplantation. Blood. 1985;65:392–396. [PubMed] [Google Scholar]
- Park PO, Haglund U. Regeneration of small bowel mucosa after intestinal ischemia. Crit Care Med. 1992;20:135–139. doi: 10.1097/00003246-199201000-00026. [DOI] [PubMed] [Google Scholar]
- Parry L, Young M, El Marjou F, Clarke AR. Evidence for a crucial role of paneth cells in mediating the intestinal response to injury. Stem Cells. 2012;31:776–785. doi: 10.1002/stem.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessach I, Shimoni A, Nagler A. Apoptotic cells in allogeneic hematopoietic stem cell transplantations: “Turning trash into gold”. Leuk Lymphoma. 2012;53:2130–2135. doi: 10.3109/10428194.2012.690099. [DOI] [PubMed] [Google Scholar]
- Potten CS, Gandara R, Mahida YR, Loeffler M, Wright NA. The stem cells of small intestinal crypts: Where are they? Cell Proliferation. 2009;42:731–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Hendry JH. Differential regeneration of intestinal proliferative cells and cryptogenic cells after irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1975;27:413–424. doi: 10.1080/09553007514550411. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high ph as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel L, Burguet S. Ultrastructure of leydig cells as revealed by secondary tissue treatment with a ferrocyanide-osmium mixture. Tissue Cell. 1977;9:751–766. doi: 10.1016/0040-8166(77)90040-4. [DOI] [PubMed] [Google Scholar]
- Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205–212. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de WM, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: Intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. TranslRes. 2010;156:180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker A, Rubin DC. Stem cells: One step closer to gut repair. Nature. 2012;485:181–182. doi: 10.1038/485181a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzner M, Helmrath M, Dunn JCY, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, Wong MH, Yu J. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302:1359–1363. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang QH, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udassin R, Vromen A, Haskel Y. The time sequence of injury and recovery following transient reversible intestinal ischemia. J Surg Res. 1994;56:221–225. doi: 10.1006/jsre.1994.1035. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct sox9-egfp-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:1111–1132. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Furstenberg RJ, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST, Henning SJ. Sorting mouse jejunal epithelial cells with cd24 yields a population with characteristics of intestinal stem cells. AmJPhysiol GastrointestLiver Physiol. 2011;300:G409–G417. doi: 10.1152/ajpgi.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MH. Regulation of intestinal stem cells. JInvestigDermatolSympProc. 2004;9:224–228. doi: 10.1111/j.1087-0024.2004.09304.x. [DOI] [PubMed] [Google Scholar]
- Wong VWY, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MWB, Watt FM, Winton DJ, Clevers H, Jensen KB. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of erbb signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NA. Epithelial stem cell repertoire in the gut: Clues to the origin of cell lineages, proliferative units and cancer. IntJ ExpPathol. 2000;81:117–143. doi: 10.1046/j.1365-2613.2000.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers bmi1 and lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesus YW, Kenneally C, Taylor HM. Preservation of hematopoietic stem cells in cadaveric marrow. Am J Clin Pathol. 1981;76:205–207. doi: 10.1093/ajcp/76.2.205. [DOI] [PubMed] [Google Scholar]
- Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult lgr5(+) stem cell. Nature Medicine. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]