Summary
Schedules of intermittent food delivery induce excessive fluid intake, termed schedule-induced polydipsia (SIP), and hypothalamic-pituitary-adrenal (HPA) axis activation is important for the expression and maintenance of this adjunctive behavior. Previous work has focused of examining the relationship between water intake and plasma corticosterone (CORT) in rats at a single or a limited range of fixed time (FT) intervals. However, little remains known regarding SIP and the corresponding stress response 1) across the bitonic function that epitomizes adjunctive behavior, 2) when ethanol is the available fluid, and 3) when a species other than rat or multiple strains are studied. Here we report the findings from ethanol-preferring C57BL/6J (B6) and non-preferring DBA/2J (D2) mice serially exposed to progressively larger FT intervals (0 → 60 min) and given access to either water or a 5% v/v ethanol solution. Following 2 weeks of experience with each schedule, blood samples were collected at the conclusion of the last 60-min session to evaluate CORT and the blood ethanol concentration (BEC) achieved. While both strains exhibited a bitonic function of ethanol intake and BEC that peaked at or near a 5-min interval, only D2 mice showed a similar response with water. In contrast, CORT levels rose monotonically with incremental increases in the FT interval regardless of the strain examined or fluid type offered, indicating that glucocorticoid release likely reflects the aversive aspects of increasing intervals between reinforcement rather than engagement in adjunctive behavior. These findings also caution against the use of a single intensity stressor to evaluate the relationship between stress and ethanol intake, as the magnitude of stress appears to affect ethanol consumption in a non-linear fashion.
Keywords: schedule-induced polydipsia, adjunctive behavior, excessive alcohol consumption, blood ethanol concentration, drinking patterns, stress, HPA axis, corticosteroid, C57BL/6 mice, DBA/2 mice
1. Introduction
Schedule-induced (adjunctive) drinking occurs when fluid is available and small quantities of food are delivered intermittently at fixed time (FT) intervals that constitute sub-optimal reinforcement magnitude for the animal (Falk, 1961, 1984). The term polydipsia refers to the excessive nature of adjunctive drinking; consumption of 2-fold or greater volume under scheduled conditions versus baseline intake (Falk, 1971). One characteristic of adjunctive behavior is a bitonic function between the amount of behavior generated and the interval length between scheduled food pellet deliveries. If the rate of food presentation is frequent or rare, then adjunctive behavior is not generated. In contrast, intervals falling between these extremes (termed the ‘effective range’) generate excessive levels of behavior (Falk, 1971). Regarding schedule-induced polydipsia (SIP), earlier work in rats demonstrated incremental increases in water intake during schedule exposure with intervals between 5 sec and 3 min in duration, followed by a progressive decline in consumption with larger intervals up to 8 min (Falk, 1966; Falk, 1969; Flory, 1971). More contemporary applications of SIP with ethanol or sweetener (sucrose or saccharin) solutions in rats and mice have largely adopted the practice of investigating only a single interval schedule, usually an interval between 30 sec and 2 min (as this approximates the maximal polydipsic response historically observed with water). However, similar parametric analyses of the bitonic function with fluids other than water and within a species other than rat have yet to be conducted. Given that ethanol can serve as a reinforcer, exhibits an anxiolytic profile, and results in intoxication when consumed in excessive quantities, it is likely that important differences in the polydipsic response given ethanol to drink versus water exist.
The effective range of pellet delivery for SIP has been related to an increase in hypothalamic-pituitary-adrenal (HPA) axis activity. For example, in rats it is well established that the schedule conditions that induce adjunctive behaviors also activate the HPA axis (Brett and Levine, 1979, 1981; Dantzer et al., 1988; Dantzer and Mormede, 1981; Lopez-Grancha, et al., 2006; Mittleman et al., 1988; Tazi et al., 1986). The increase in HPA activity is thought to reflect an enhanced arousal and vigilance as well as “conflict” in motivational forces upon behavior that are associated with obtaining intermittent reinforcement (Dantzer and Mormede, 1981; Falk, 1971, 1977). Specifically, under conditions that induce adjunctive behavior, there are heightened circulating levels of adrenal hormones such as corticosterone (CORT), the catecholamines epinephrine and norepinephrine (Brett and Levine, 1979, 1981; Dantzer et al., 1988; Lopez-Grancha, et al., 2006; Mittleman, 1988; Tazi et al., 1986), and the pituitary hormones prolactin (Dantzer et al., 1988) and adrenocorticotropic hormone (ACTH; Helms et al., 2012). In general, the elevations in these hormones persist as long as the schedule is in effect and the animal is not given the opportunity to escape (Dantzer et al., 1988, Tazi et al., 1986, Lopez-Grancha et al., 2006). Acquisition of water SIP over successive sessions was reduced in rats following either adrenalectomy or administration of the CORT synthesis inhibitor metyrapone, but was restored in adrenalectomized rats that received CORT replacement (Levine and Levine, 1989; Mittleman et al., 1992). Further, elevations in plasma CORT over baseline (massed-feeding) conditions were observed in conjunction with an interval schedule associated with the generation of water SIP in rats, but not following exposure to an ineffective schedule (Lopez-Grancha et al., 2006). Collectively, these observations suggest that stress-response mediators like CORT are integral to the development and maintenance of excessive drinking in SIP procedures. However, it is not known if CORT is correlated with adjunctive drinking across the entire bitonic function of interval schedules.
While experimental variables (intermittent schedule, level of food deprivation, etc.) play prominent roles in the generation of adjunctive behavior, it is clear that genetic background also exerts influence. First, earlier examinations of SIP in outbred rat lines yielded large individual differences in responsiveness, with only some subjects demonstrating excessive water intake when under a fixed interval schedule (Dantzer et al., 1988; Lopez-Grancha et al., 2008; Mittleman et al., 1988; Moreno and Flores, 2012). Second, comparisons between strains (DeCarolis et al., 2003; Mittleman et al., 2003) and selected lines (Gilpin et al., 2008; Moreno et al., 2010) of rodents have identified significant divergence in the expression of water and ethanol polydipsia when tested at either a single interval or a limited range of intervals. However, the presence or absence of strain differences in SIP responsiveness may be reliant upon the interval schedule chosen for investigation. For instance, one study found no strain difference between C57BL/6 (B6) and DBA/2 (D2) mice in the expression of water polydipsia under a 1 min interval schedule (Mittleman et al., 2003) whereas a second study reported a pronounced difference when a 2.5 min interval was implemented (Symons & Sprott, 1976). Again, a more complete examination of the entire bitonic function in these inbred mouse strains may help reconcile the disparity between these earlier observations. Further, concomitant measurement of plasma CORT would help determine whether differences in stress axis responsiveness to interval schedules are associated with between-strain variability in expression of adjunctive drinking.
Thus, the goals of the current work were to identify the FT interval associated with the greatest amount of drinking, assess whether this peak FT is specific to the fluid type offered (5% v/v ethanol versus water) or the mouse strain examined (ethanol-preferring B6 versus ethanol-avoiding D2 mice), and evaluate the relationship between plasma CORT and the pattern of drinking that occurs across the bitonic function.
2. Methods
2.1. Animals
Male mice (B6 and D2; n = 12/strain) were acquired from Jackson Laboratories (Sacramento, CA, USA), single-housed in standard shoebox caging, provided ad libitum access to rodent chow and water, and placed under a modified 12/12-hr light/dark schedule with lights off at 1200 hrs (noon). Throughout a 1-week acclimation period, mice were weighed daily and home cage water intake was measured to the nearest 0.1 g at 0900 hrs. During week 2, food restriction was initiated, and mice were adjusted to 90% of free-feeding body weight. In addition to the food pellets acquired during daily drinking sessions, mice were supplemented with a daily ration of rodent chow to maintain the targeted body weight restriction. Ad libitum water access was always provided in the home cage. The local Institutional Animal Care and Use Committee reviewed and approved all procedures in compliance with the guidelines set forth in the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research and the U.S. National Institutes of Health.
2.2. Apparatus
Eight operant conditioning chambers (14 × 13 × 16 cm; Med-Associates Inc., St Albans, VT) were outfitted with a stainless steel rod floor, a pellet dispenser, a trough-style pellet receptacle, a mounting block for a stationary drinking spout, a lickometer controller, a quick disconnect harness, and a house light. The left wall of each chamber contained a centrally positioned pellet receptacle, connected externally via polypropylene tubing to a pellet dispenser that delivered 20 mg sugar pellets (dustless precision pellets; Bio-Serv, Frenchtown, NJ). On the same wall a mounting block was positioned immediately to the left of the food receptacle to minimize the proximity between food delivery and fluid access. A 50-ml fluid reservoir attached to a double-ball bearing drinking spout (Ancare, Bellmore, NY) was mounted to the block. An identical drinking tube was supplied to mice in the home cage for ad libitum water consumption. The mounting block also contained a connection point for an input wire that relayed cumulative lick information to the lickometer controller. The quick disconnect harness was attached to the steel rod floor and provided the electrical ground required for lick detection. The house light (100 mA) was positioned on the top center of the right-side wall. Each chamber was set within a sound-attenuating cabinet (56 × 38 × 36 cm), and an exhaust fan provided ventilation and masked external noise. All chamber inputs and outputs were controlled by a modular data acquisition system interfaced with LabVIEW software (National Instruments Corporation, Austin, TX). Custom programming was composed to manage chamber function and data management throughout the drinking sessions.
2.3. Schedule-induced polydipsia (SIP) and experimental design
Three cohorts of mice were run 5–6 days/week between 0900 and 1200 hrs. Sugar pellets (20 mg) were delivered automatically into the food receptacle at FT intervals, and no response was required from the animals. Mice were serially exposed to the following FT interval schedules (expressed in minutes): FT-0, FT-0.5, FT-1, FT-2, FT-3, FT-5, FT-7, FT-10, FT-20, and FT-∞ (infinity). All sessions were 60-min in duration and began with the delivery of a sugar pellet (except the FT-0 schedule). FT-0 was a massed-feeding condition in which 120 pellets (the maximum number of pellets presented during the schedules examined; i.e., FT-0.5) were placed in the food receptacle prior to session start. During the FT-∞ schedule no additional pellets were delivered following the inaugural pellet drop at session start. FT schedules were run in 2-week increments. At the conclusion of the final session at each FT interval, a blood sample was collected from each mouse for evaluation of plasma CORT levels. When ethanol was the available fluid (see below), an additional aliquot of blood was obtained to measure blood ethanol concentration (BEC) at each interval. Two weeks of exposure to each interval was chosen based on earlier work that suggested CORT levels and drinking behavior tend to stabilize after 10 or more SIP sessions on a given schedule (Lopez-Grancha et al., 2006).
Half of the mice from each strain (n = 6) received tap water as the sole fluid available whereas the other half was presented with a 5% v/v ethanol (5E) solution during the 60-min sessions. The ethanol concentration was selected based on previous work demonstrating that 5E resulted in the largest intake volume and highest BECs in rats (Falk et al., 1972; Roehrs & Samson, 1980) and mice (Mittleman et al., 2003). Dependent measures recorded during each drinking session included fluid volume, g/kg ethanol intake, total licks on drinking spout, number of sugar pellets consumed, and body weight. The g/kg intake values were derived from the volume of 5E consumed and the respective body weight.
2.4. Measurement of plasma CORT and BEC
Mice were gently restrained by hand and the medial saphenous vein (located at inner thigh) was punctured with the tip of a 20-gauge needle. Pooled blood was then collected into either a heparinized hematocrit tube (Fisher Scientific, Pittsburgh, PA) for CORT measurement (~40-μl) or a 20-μl micro-capillary pipet (Kimble Glass Inc., Vineland, NJ) for BEC analysis. Sampling for CORT was always conducted first to minimize the duration of experimenter-handling prior to assessment of this stress-axis mediator. Gauze was then applied to the collection site until bleeding ceased. For CORT assessment, blood samples were centrifuged for 20-min at 914 x g to isolate plasma, and then stored at −80°C until assayed. Steroid concentrations were measured using a commercially available 125I radioimmunoassay kit (ImmuChem Double Antibody Corticosterone for rodents; MP Biomedicals, Orangeburg, NY). The manufacturer supplied protocol was implemented, with minor modifications. In brief, CORT concentration in plasma samples was single-determined via interpolation from a standard curve derived from six calibrators (ranging from 25–1000 ng/ml). Based on previous experience with this assay, the plasma volume tested was 5-μl. The intra- and inter-assay variability was 1.5 ±0.1% and 2.5±0.3%, respectively.
For BEC measurement, whole blood was treated as previously described (Finn et al., 2007). Briefly, the blood samples were diluted into 500 μl of 4 mM n-propanol matrix constituted with deionized water. Crimp-top vials (Agilent Technologies, Santa Clara, CA) containing the blood sample in matrix were capped, thoroughly mixed, and then evaluated via ambient headspace sampling gas chromatography using an Agilent 6890N machine with DB-ALC1 column. Six pairs of ethanol standards (0.1–3.0 mg/ml), which included n-propanol as an internal standard, were run in parallel to permit interpolation of unknown sample concentrations.
2.5. Statistical analyses
The dependent variables evaluated were fluid volume (ml), total licks, ethanol intake (g/kg), BEC (mg/ml), and plasma CORT (ng/ml). Drinking patterns throughout the session were also monitored via lickometer circuits that were interfaced to each drinking spout. First, this allowed for analysis of the lick distributions within the various FT intervals. Second, it permitted the evaluation of bout micro-architecture, including variables such as 1st bout size (licks), % total licks in the 1st bout, and the rate of drinking onset (licks/min during initial 20-min of session). Based on previous experience, a drinking bout was defined as the number of licks that occur with < 60-sec pause between successive licks (Ford et al., 2005a, 2005b, 2008). For each dependent variable, 2-way repeated measures analysis of variance (RM ANOVA) was conducted with factors strain and FT interval. Separate analyses were carried out for each fluid type offered (5E or water). In the event that a significant factorial interaction was detected, pair-wise comparisons were subsequently evaluated. For repeated measure comparisons across FT intervals a Dunnett’s test was performed, with the FT-0 baseline values serving as ‘controls’. Correlations between drinking variables, BEC, and plasma CORT were evaluated with the Pearson Product Moment test. All analyses were performed by SigmaStat for Windows version 3.5 (Systat Software Inc., San Jose, CA), with the threshold for statistical significance set at P < 0.05. All figures were derived from Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA).
One 5E-drinking B6 mouse died unexpectedly overnight in its home cage early in the experimental time course, and was excluded from all statistical analyses. On occasion, it was not possible to collect the sufficient blood volume required to assay plasma CORT, BEC, or both. In these cases, data points were treated as missing values.
3. Results
Baseline body weight (BW) for all mice prior to food restriction was 24.1 ± 0.3 grams. The average BW of mice throughout the FT interval manipulations was 21.7 ± 0.2 grams, and this reflected a restriction to 90.3 ± 0.2% of free-feeding BW.
3.1. SIP: ethanol
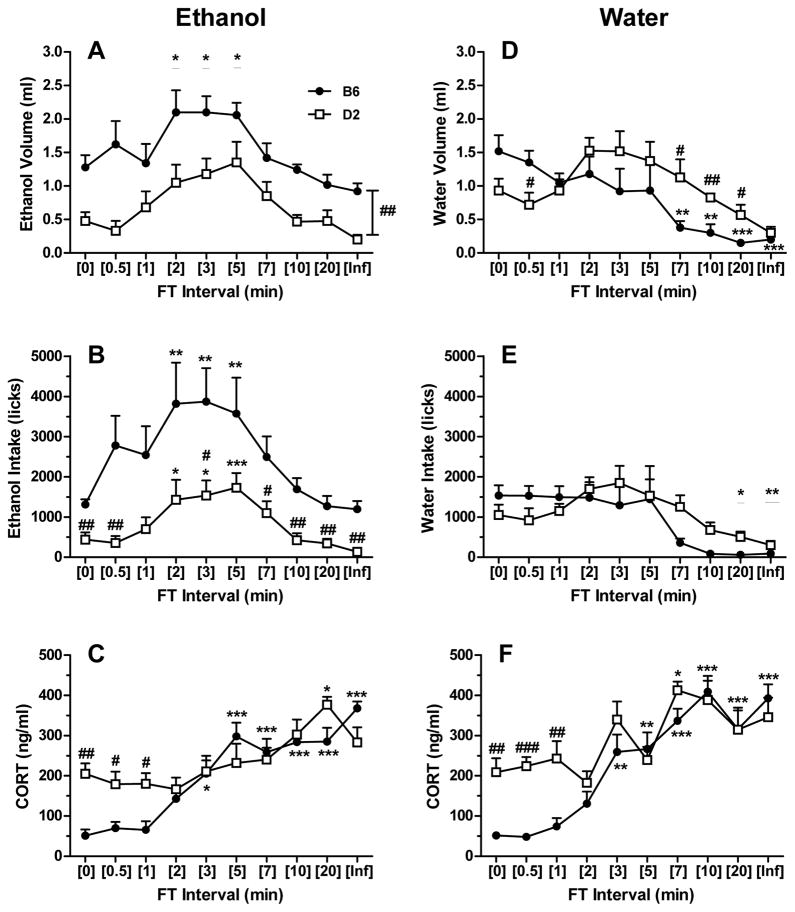
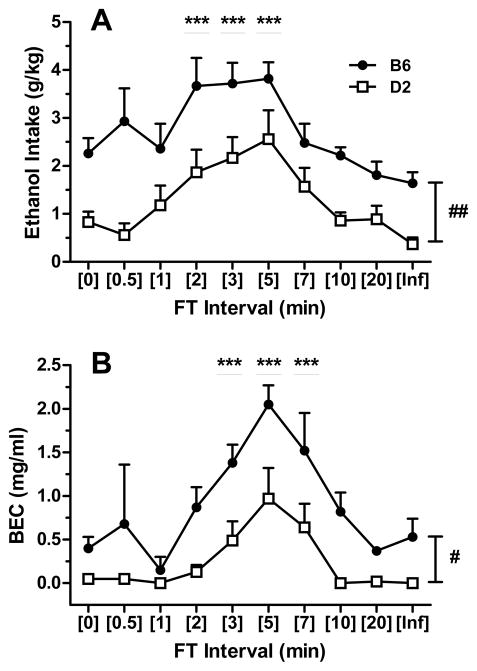
Ethanol intakes followed a bitonic function across FT intervals in both B6 and D2 mouse strains, with peak drinking levels occurring at or near the 5-min FT interval (i.e., FT-5; Fig. 1A-B and Fig. 2A). The relationship between FT interval length and ethanol intake was similar, regardless of whether intake was reported as volume, licks or g/kg values. This was consistent with the strong positive correlations between these intake measures in B6 and D2 mice (each P < 0.001; Table 1). In general, licks represented a more finite measurement of ethanol intake, as low volume intakes sometimes approached the limit of detection (0.1 ml). There were main effects of strain [F(1,9) = 13.03; P < 0.01] and interval [F(9,81) = 13.33; P < 0.001] for ethanol volume, effects of strain [F(1,9) = 9.82; P < 0.05], interval [F(9,81) = 14.39; P < 0.001] and factorial interaction [F(9,81) = 2.12; P < 0.05] for ethanol licks, and effects of strain [F(1,9) = 12.39; P < 0.01] and interval [F(9,81) = 13.17; P < 0.001] for g/kg ethanol intake. Ethanol intakes (volume and g/kg) during FT-2, FT-3, and FT-5 schedules were significantly greater when compared to respective baseline (i.e., FT-0; massed-feeding condition) values (Ps < 0.05 for volumes; Ps < 0.001 for g/kg), independent of strain (Fig. 1A and Fig. 2A, respectively). Similar findings were noted for ethanol licks in the B6 (Ps < 0.01) and D2 (Ps < 0.05) strains at the same intervals (Fig. 1B). Further, the ethanol-preferring B6 mice exhibited a significantly greater amount of ethanol licks under the FT-0 schedule than the D2 mice (P < 0.01; Fig. 1B), and their intakes (regardless of the metric) were larger than those of D2 mice at each FT interval examined (Fig. 1A-B and Fig. 2A). Analysis of BEC revealed significant main effects of strain [F(1,9) = 11.24; P < 0.01] and interval [F(9,66) = 17.14; P < 0.001], but no factorial interaction. Overall, BECs were significantly elevated during FT-3, FT-5, and FT-7 schedules when compared to FT-0 (all Ps < 0.001; Fig. 2B) and the B6 mice exhibited significantly higher BECs than D2 mice (P < 0.05). Interestingly, the steep ascending limb of the BEC curves (FT-2 thru FT-5) corresponded to g/kg intakes that were only modestly different across these intervals within each strain, suggesting that factors other than total session ethanol intake contributed to the BECs observed (see drinking pattern analyses below). Despite this, significant correlations between BEC and g/kg, volume, or licks of ethanol intake were observed in both strains (all Ps < 0.001; Table 1).
Figure 1.
Schedule-induced intake of ethanol (panels A–B) or water (panels D–E) and respective CORT concentrations (panels C and F) across FT intervals. Each data point depicts the mean ± SEM of B6 (n=5–6) or D2 (n=6) mice. *p < 0.05, **p < 0.01 and ***p < 0.001 versus within-strain baseline (FT-0, massed-feeding) value by Dunnett test. #p < 0.05, ##p < 0.01 and ###p < 0.001 versus B6 strain within respective FT interval by Tukey test. Underlined or bracketed levels of significance refer to main effects of either FT interval or strain in the absence of strain x interval interactions.
Figure 2.
Comparison of g/kg ethanol intake (panel A) and BEC (panel B) across FT intervals. Each data point depicts the mean ± SEM of n=5–6 mice/strain. ***p < 0.001 versus within-strain baseline (FT-0, massed-feeding) value by Dunnett test. #p < 0.05 and ##p < 0.01versus B6 strain within respective FT interval by Tukey test. Underlined or bracketed levels of significance refer to main effects of either FT interval or strain in the absence of strain x interval interactions.
Table 1.
Correlations between ethanol intake variables, BEC and CORT. Analyses encompass all FT intervals examined. The correlation coefficient (r), level of significance (P) and sample size (n) are reported for each relationship. Significant correlations are bolded
| 5E ml | 5E licks | BEC | CORT | |
|---|---|---|---|---|
| B6 mice | ||||
| 5E g/kg |
r = 1.00; P < 0.001 n = 50 |
r = 0.85; P < 0.001 n = 50 |
r = 0.77; P < 0.001 n = 36 |
r = 0.00; P = 1.00 n = 47 |
| 5E ml | --- |
r = 0.87; P < 0.001 n = 50 |
r = 0.75; P < 0.001 n = 36 |
r = −0.02; P = 0.90 n = 47 |
| 5E licks | --- | --- |
r = 0.70; P < 0.001 n = 36 |
r = 0.00; P = 0.98 n = 47 |
| BEC | --- | --- | --- | r = 0.31; P = 0.07 n = 35 |
|
| ||||
| D2 mice | ||||
| 5E g/kg |
r = 1.00; P < 0.001 n = 60 |
r = 0.89; P < 0.001 n = 60 |
r = 0.77; P < 0.001 n = 59 |
r = 0.04; P = 0.76 n = 59 |
| 5E ml | --- |
r = 0.91; P < 0.001 n = 60 |
r = 0.75; P < 0.001 n = 59 |
r = 0.01; P = 0.97 n = 59 |
| 5E licks | --- | --- |
r = 0.69; P < 0.001 n = 59 |
r = −0.17; P = 0.19 n = 59 |
| BEC | --- | --- | --- | r = 0.14; P = 0.30 n = 58 |
There was a main effect of interval [F(9,77) = 13.17; P < 0.001] as well as a strain x interval interaction [F(9,77) = 3.01; P < 0.01] for CORT levels during SIP sessions with ethanol. Baseline CORT levels were 4-fold greater in D2 versus B6 mice (Fig. 1C; P < 0.01), and significant strain differences were also observed at FT-0.5 and FT-1 schedules (each P < 0.05). CORT concentrations in B6 mice increased significantly starting at FT-3 (P < 0.05), reached a plateau at FT-5 (P < 0.001), and remained elevated with schedules FT-7 thru FT-∞ (all Ps < 0.001) when compared to within-subject baseline levels. In contrast, CORT levels in D2 mice rose more modestly with incremental lengthening of the FT interval from 2 to 20 min, with only the CORT levels at FT-20 reaching statistical significance versus respective baseline values (P < 0.05). When taking into account all FT interval schedules, correlations between CORT and ethanol intake or BEC did not meet statistical significance in either strain (Table 1).
3.2. SIP: water
While water intakes followed a bitonic function across FT intervals in D2 mice, a steady decline in the water intakes of B6 mice occurred as the FT interval was progressively lengthened (Fig. 1D-E). As with ethanol self-administration, strong positive correlations between water volume and lick values were observed for both strains (each P < 0.001; Table 2). There was a main effect of interval [F(9,90) = 7.74; P < 0.001] and a strain x interval interaction [F(9,90) = 3.03; P < 0.01] for water volume consumed. In B6 mice water intakes were significantly reduced during the FT-7 and FT-10 schedules (each P < 0.01) as well as the FT-20 and FT-∞ schedules (each P < 0.001) when compared to baseline volumes associated with FT-0 (Fig. 1D). Although water volume consumed by D2 mice across FT intervals did not differ versus within-subject baseline values, between strain differences were apparent during both the ascending (FT-0.5; P < 0.05) and descending (FT-7 thru FT-20; all Ps < 0.05) limbs of the bitonic function. Similar profiles of water intake were observed with the dependent variable of licks (Fig. 1E), but a two-way RM ANOVA determined only a main effect of interval [F(9,90) = 7.45; P < 0.001], with significantly less water licks occurring overall during FT-20 (P < 0.05) and FT-∞ (P < 0.01) schedules versus baseline levels.
Table 2.
Correlations between water intake variables and CORT. See Table 1 for additional details
| Water licks | CORT | |
|---|---|---|
| B6 mice | ||
| Water ml |
r = 0.93; P < 0.001 n = 60 |
r = −0.50; P < 0.001 n = 58 |
| Water licks | --- |
r = −0.48; P < 0.001 n = 58 |
|
| ||
| D2 mice | ||
| Water ml |
r = 0.95; P < 0.001 n = 60 |
r = −0.12; P = 0.38 n = 57 |
| Water licks | --- | r = −0.16; P = 0.25 n = 57 |
Main effects of strain [F(1,10) = 15.62; P < 0.01] and interval [F(9,85) = 16.31; P < 0.001] as well as a strain x interval interaction [F(9,85) = 2.40; P < 0.05] were detected for CORT levels during water SIP (Fig. 1F). As with ethanol SIP, baseline CORT measures (at FT-0) were approximately 4-fold greater in D2 versus B6 mice (P < 0.01) with access to water, and this strain difference in CORT concentrations was also apparent at the FT-0.5 (P < 0.001) and FT-1 (P < 0.01) schedules. B6 mice exhibited significantly elevated CORT levels over their respective baseline measures during FT-3 and FT-5 schedules (each P < 0.01) as well as FT-7 thru FT-∞ schedules (all Ps < 0.001). As with ethanol, water-consuming D2 mice demonstrated more modest elevations in plasma CORT across FT intervals (Fig. 1F), with levels of this hormone rising significantly over baseline during exposure to the FT-7 schedule (P < 0.05). Unlike ethanol SIP, CORT levels during water SIP in B6 mice were found to be significantly and negatively correlated to both water volume and licks (each P < 0.001; Table 2). No relationship between CORT concentrations and water intake was determined for D2 mice.
3.3. Comparison of CORT response in ethanol versus water SIP
One of our a priori hypotheses was that the anxiolytic profile of ethanol would be manifested as a rightward or downward shift in CORT concentrations across FT intervals when compared to water SIP. To determine potential differences in CORT response as a function of the fluid type offered, separate analyses were conducted for each strain. In B6 mice, neither a main effect of fluid type nor a fluid type x interval interaction was detected. However, a main effect of fluid type [F(1,10) = 10.78; P < 0.01], but no factorial interaction, was realized for CORT levels in D2 mice. Ethanol SIP in D2 mice was associated with an overall lower level of CORT than water SIP in this same strain (P < 0.01; comparing open squares in panels C and F of Fig. 1).
3.4. Drinking patterns across FT intervals
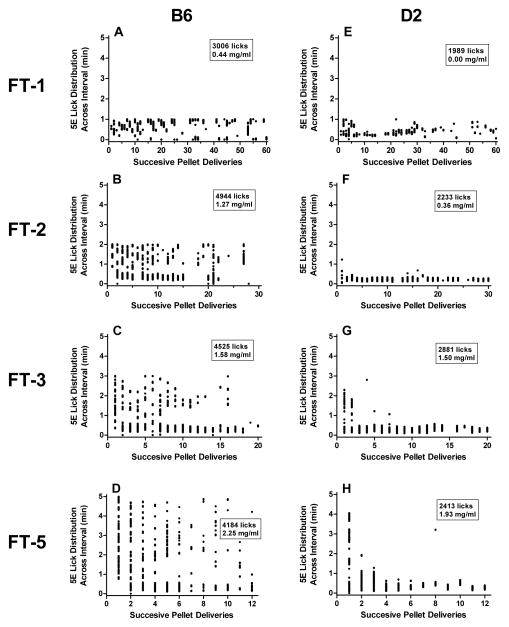
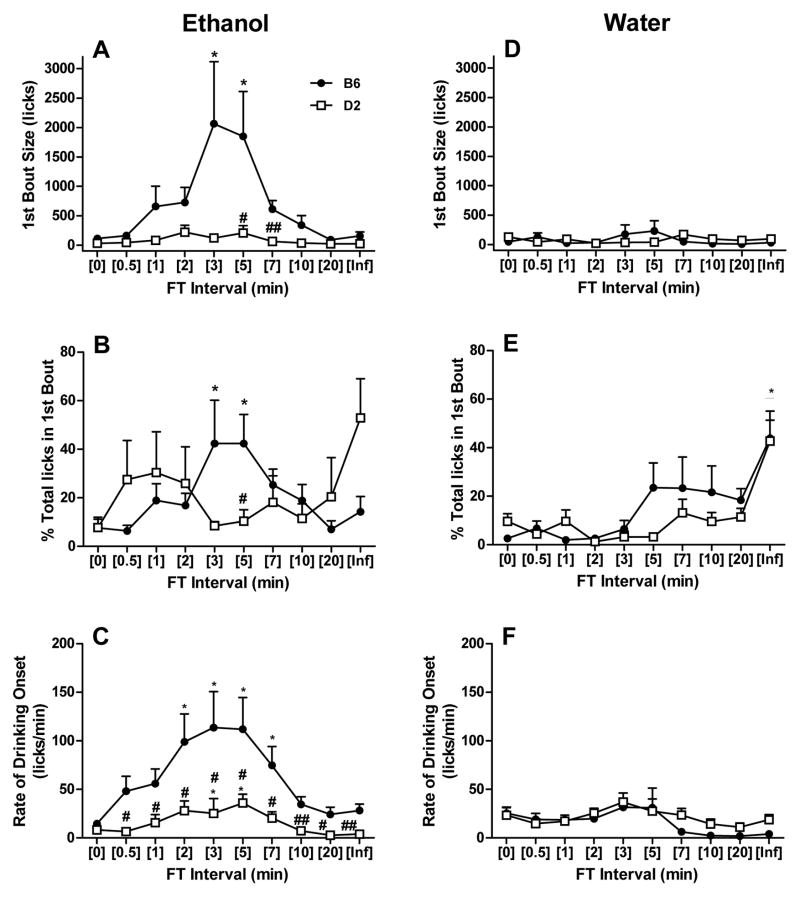
Shifts in ethanol lick patterns between pellet deliveries occurred across FT interval schedules in representative B6 and D2 mice (Fig. 3). While an increase in post-session BEC between FT-1 and FT-2 schedules can be partially attributed to increases in total licks, further incremental elevations in BEC within each strain between FT-2 and FT-5 were not always accompanied by a greater number of licks, and in the case of the representative B6 mouse shown, a modest decline in total licks was observed (Fig. 3, panels B–D). In general, B6 and D2 mice became more efficient at concentrating their consumption immediately after each pellet delivery (i.e., within the first 60-sec) as the FT interval lengthened. B6 mice also tended to exhibit a heightened onset by drinking persistently throughout the initial third of the 1-hr sessions. To further test this supposition we analyzed the 1st bout size, percentage of total licks in the 1st bout and the rate of drinking onset in ethanol-drinking mice (Fig. 4A–C). There were main effects of strain [F(1,9) = 6.14; P < 0.05] and interval [F(9,81) = 5.02; P < 0.001], as well as a strain x interval interaction [F(9,81) = 3.74; P < 0.001] for the size of the 1st ethanol bout. B6 mice significantly augmented their first bout size by 18- and 16-fold, respectively, during FT-3 and FT-5 schedules versus FT-0 schedule exposure (Ps < 0.05; Fig. 4A). In contrast, the 1st bout sizes in D2 mice were not significantly different across FT intervals. Assessment of the percentage of total licks in the 1st bout similarly yielded a strain x interval interaction [F(9,77) = 2.39; P < 0.05], and ethanol-drinking B6 mice increased this measure from 8% to 42% as the FT interval was lengthened from 0 to 5 min (Fig. 4B). Significant main effects of strain [F(1,9) = 9.08; P < 0.05] and interval [F(9,81) = 11.86; P < 0.001] and a strain x interval interaction [F(9,81) = 3.22; P < 0.01] were also detected for the rate of ethanol drinking onset during the initial 20-min of the session. Although no strain difference was observed during baseline (FT-0), B6 mice exhibited significantly greater rates of ethanol drinking onset at all FT interval schedules when compared to D2 mice (all Ps < 0.05), and B6 rates during FT-2 thru FT-7 schedules (P < 0.001) were significantly elevated over within-strain FT-0 values (all Ps < 0.05; Fig. 4C). More subtle, yet significant within-strain increases in rates also occurred at the FT-3 and FT-5 intervals in D2 mice (each P < 0.05). In general, comparative analyses in water drinking mice revealed that B6 and D2 strains exhibited similar drinking patterns, and that water bout dynamics remained largely unaltered by incremental lengthening of the FT interval from 0 to 60 (i.e., FT-∞) minutes (Fig. 4D–F). Notably, main effects on interval were found for the % total licks in the first water bout [F(9,90) = 8.67; P < 0.001] and rate of drinking onset [F(9,90) = 2.66; P < 0.01].
Figure 3.
Comparison of 5% v/v ethanol (5E) lick distributions within FT intervals throughout 60-min sessions. Licks from a representative B6 (left panels, A–D) or D2 (right panels, E–H) mouse are shown at four different FT intervals (1, 2, 3 and 5-min) that correspond to the ascending limb of the BEC curves illustrated in Figure 2B. The total number of 5E licks and the resultant BEC post-session is reported in each panel.
Figure 4.
Comparison of ethanol and water bout patterns across FT intervals. The 1st bout size (panels A and D), % total licks in first bout (panels B and E), and rate of drinking onset (panels C and F) are shown for both strains when provided access to ethanol (left) or water (right), respectively. Each bar depicts the mean ± SEM of n=5–6 mice/strain. *p < 0.05versus within-strain baseline (FT-0, massed-feeding) value by Dunnett test. #p < 0.05 and ##p < 0.01 versus B6 strain within respective FT interval by Tukey test. Underlined levels of significance refer to a main effect of FT interval in the absence of a strain x interval interaction.
No relationship between BEC and any of the three bout parameters was noted in ethanol-drinking mice of either strain (data not shown). However, ‘% total licks in the first bout’ was significantly correlated with CORT values during ethanol SIP in B6 mice (r = 0.33; P < 0.05; n = 47) and water SIP in both B6 (r = 0.48; P < 0.001; n = 58) and D2 (r = 0.28; P < 0.05; n = 57) strains. A similar trend towards significance was observed for these two variables in ethanol-drinking D2 mice (r = 0.24; P = 0.08; n = 55). CORT values were also negatively associated with the ‘rate of drinking onset’, but only in water-drinking B6 mice (r = −0.32; P < 0.05; n = 58).
4. Discussion
Here we present the first demonstration of a bitonic function for adjunctive drinking of ethanol, and further establish that a similar function operates for schedule-induced ethanol polydipsia in mice as that initially characterized for water in rats (Falk, 1966; Falk, 1969; Flory, 1971). While both ethanol-preferring B6 and non-preferring D2 mice exhibited adjunctive drinking when ethanol is presented, only D2 mice engaged in water SIP. Unexpectedly, B6 mice linearly reduced water intake as the FT interval increased, indicating that water intake is prandial in nature for B6 mice, and not adjunctive. This realization may explain why previously reported strain differences in water drinking were inconsistently observed between B6 and D2 mice as the interval schedule was varied (Mittleman et al., 2003; Symons & Sprott, 1976). The schedules of reinforcement (FT-3 and FT-5) that generated peak amounts of excessive behavior are identified here, and it is suggested that earlier examinations using a single FT interval of shorter duration (i.e., 0.5-min or 1-min) likely employed sub-optimal schedules for the study of adjunctive drinking.
Associations between CORT and multiple ethanol intake measures across FT intervals did not meet the threshold for statistical significance (Table 1). The strongest relationship detected was that between CORT and BEC in B6 mice, for which only a trend towards a significant correlation was observed. This effect was largely driven by the parallel rises in CORT (Fig. 1C) and BEC (Fig. 2B) that occurred along the ascending (FT-0 thru FT-5), but not the descending, limb of the bitonic function. Two factors potentially contributed to plasma CORT during ethanol SIP in both strains. First, ethanol exposure can acutely augment plasma CORT concentrations in rodents under certain conditions whereas chronic exposure is associated with a neuroendocrine tolerance characterized by a blunted HPA axis response (i.e., CORT) to ethanol (Lee and Rivier, 1997; Richardson et al., 2008). We have shown that male B6 mice have modest increases in plasma CORT following a 2-hr limited access session to 10% ethanol and water when compared to a water only control group (Finn et al., 2004), and it is possible that the prolonged exposure to ethanol in the present study led to a blunted CORT response as the intervals increased. This possibility would be consistent with earlier work demonstrating a progressive blunting of the HPA response in rats as the magnitude of self-administered ethanol increased (Richardson et al., 2008). Second, apart from the effects of ethanol, FT schedules of intermittent food pellet delivery are also known to affect plasma CORT (Dantzer et al., 1988; Lopez-Grancha et al., 2006; Mittleman et al., 1988; Tazi et al., 1986). Indeed, CORT is important for the acquisition and expression of adjunctive water drinking during schedule exposure (Levine and Levine, 1989; Mittleman et al., 1992). In the current work, it is likely that the FT schedule condition was the predominant factor influencing the CORT response because the escalation of CORT concentrations was found across the same FT intervals when either ethanol or water was the available fluid. Additionally, the decline in ethanol intake during the descending limb of the bitonic function for adjunctive behavior was not accompanied by a decrease in plasma CORT (which followed a monotonic function), suggesting that ethanol’s ability to alter HPA activity is insufficient to explain the CORT response. Lastly, the most significant rise in plasma CORT occurred between FT-1 and FT-5 (especially in B6 mice), and this marked the transition from mice consuming 75–80% of food pellets presented during the session (under FT-1) to consuming 100% under FT intervals of 2-min and greater (data not shown). A related behavioral process that cannot be discounted in relation to the current findings and may have contributed to the CORT profile across intervals includes gnawing on the pellets. Gnawing is a stereotypic (or competing adjunctive) behavior that mice express in the face of adverse environmental conditions, and when engaged in leads to a dampening of HPA axis activation (Dantzer, 1991). Thus, CORT levels across the bitonic function may best reflect the reality that reinforcement (and the accompanying ability to gnaw on pellets) becomes increasingly scarce as the FT interval lengthened across the 60-min sessions.
Two important points can be taken from this conclusion. One, self-administration of ethanol at levels associated with binge drinking (i.e., BEC > 0.8 mg/ml) does not augment CORT within a SIP procedure, which would not be apparent if one were to examine a single FT interval or select range of intervals falling exclusively within the ascending limb of the bitonic function. However, this interpretation is congruent with earlier findings indicating no correlation between endogenous CORT levels and ethanol intakes (Fahlke et al., 1994). Second, mice with extensive opportunities to associate drinking with intoxicating BEC levels (throughout ascending limb and at peak FT) do not drink to alleviate the stress associated with schedule conditions presented during the descending limb of the bitonic function, indicating that the lower frequency of food reinforcement is the prevailing factor in the decline of adjunctive drinking and independent of continually elevated CORT levels.
While parallel rises in BEC and CORT were apparent in both mouse strains during the ascending limb of the bitonic function (FT-0 thru FT-5), the absence of a similar relationship between these variables during the descending limb of the function suggests that opponent processes are at play. Falk and others hypothesized that the bitonic function of adjunctive behavior is a product of two competing influences (drives) on behavior (Falk, 1981; Grant & Johanson, 1989). Specifically, there is an ‘engage’ drive, where behavior is maintained by the positively-reinforcing effects of the delivered stimulus (e.g., food) and an opposing ‘escape’ drive where behavior is governed by the delay of reinforcement within interval schedules. In general, as the FT interval between food reinforcement increases, behavior directed at obtaining the reinforcer declines (Herrnstein, 1970) and behavior directed at escaping the schedule escalates (Lydersen et al., 1980). Hypothetically, the interval at which these two oppositional forces on behavior are equal (i.e., intersect) is the interval of greatest ‘conflict’ in which the animal must reconcile the equal and opposing drives of whether to escape or to remain engaged. With this theoretical framework in mind, it was anticipated that the FT interval that generates the peak amount of adjunctive drinking should also be the interval that generates the most conflict (stress), and thus the largest activation of the HPA axis. Although adjunctive ethanol drinking peaked at or near the FT-5 schedule in both strains (Fig. 1A) it was clear that the CORT levels tended to escalate further (particularly in D2 mice) with exposure to longer FT intervals (Fig. 1C). This apparent lack of correspondence between peak volume consumed and maximal HPA axis response may be attributable to the hormonal measure chosen, as earlier work by Dantzer and colleagues (1988) found that elevated water intake was associated with a decline in plasma CORT values in rats, but other stress indicators like plasma catecholamines remained high. In the present study with mice, CORT levels may reflect the aversive aspects of increasing intervals between reinforcement, thus a monotonic increase with interval length, rather than a reflection of engaging in an adjunctive behavior (Mittleman et al., 1988, 1992). One potential confound with this interpretation is that serial testing of FT intervals from shortest to longest was conducted versus a counterbalanced design. This raises the possibility that cumulative experience with the schedule-induced polydipsia procedure or chronic exposure to large ethanol doses may have influenced the results obtained with the longer FT intervals that were examined towards the latter end of the experimental time course.
Maximal water intake for both strains approximated 1.5 ml during the 60-min sessions. Although this peak water intake occurred during different schedules for B6 (FT-0) and D2 (FT-3) mice, it demonstrated that the two strains were capable of ingesting similar volumes of water in the allotted session time. In contrast, B6 mice consumed up to 3-fold more ethanol than D2 mice at each FT interval examined (1.0 – 2.1 ml and 0.3 – 1.4 ml, respectively), even though peak intakes were observed during the FT-5 schedule in both strains. These observations are consistent with the recent demonstration of higher schedule-induced ethanol intake in B6 versus D2 mice on a FT-1 schedule (Mittleman et al., 2003) and with previous characterization of ethanol-preferring and ethanol-avoiding drinking phenotypes for B6 and D2 mice, respectively, within 2-bottle choice drinking (Belknap et al., 1993; Yoneyama et al., 2008) and operant-based reinforcement (Risinger et al., 1998) procedures. In the current work, extensive exposure to the FT-5 interval (2-weeks) culminated in BECs that have been associated with a pattern of binge drinking (i.e., > 0.8 mg/ml BEC; see DHHS-NIH, 2004) for both strains. However, the approximately 2-fold greater BEC achieved in B6 versus D2 mice (2 mg/ml versus 1 mg/ml) was likely attributable to an overall higher ethanol intake (by 35%) as well as a 8-fold greater 1st bout size, 4-fold greater percentage of total intake within the first bout, and 2-fold greater rate of drinking onset in B6 versus D2 mice. Taken in the context of findings from earlier strain comparison studies, pre-absorptive factors such as aversion to the orosensory properties of ethanol likely limited the BEC achieved by D2 mice under the induction schedules examined, as D2 mice consume ethanol in bouts only under conditions that bypass pre-absorptive factors associated with the oral route of self-administration (Fidler et al., 2011; Risinger et al., 1998). It is also worth mentioning that the use of sugar pellets as reinforcement in the current work could conceivably have reduced the absorption of ethanol and the resultant BEC in both strains, although this a controversial issue in rodents self-administering ethanol (for review, see the Gauvin, 1999) and is unlikely to explain the strain differences noted above.
Notably, the ethanol self-administration patterns observed in B6 mice under a FT-5 schedule resemble a drinking topography previously identified in cynomolgus monkeys that was predictive of chronic heavy drinking (Grant et al., 2008). In this non-human primate model, approximately 40% of individual subjects became heavy drinkers (> 3 g/kg/day) following an induction procedure that involved intermittent food pellet delivery under a FT-5 schedule. A drinking topography associated with rapid ethanol intake in the fewest number of bouts was most predictive of a future excessive drinking phenotype, with the size of the largest bout and the % of ethanol consumed in a single bout carrying particular significance (Grant et al., 2008). In the current work with mice we analyzed similar indices of 1st bout size, % of total licks in the first bout, and rate of drinking onset (lick/min in initial 20-min of the session) and discovered that ethanol-drinking B6 mice under a FT-5 schedule uniquely expressed this topography of rapid drinking when compared to ethanol-drinking D2 mice, to both strains of water-drinking mice, and to the same ethanol-drinking B6 mice under sub-optimal FT schedules (Fig. 4). In contrast, ethanol-drinking D2 mice tended to titrate their consumption across multiple, small samplings (Fig. 3E–H and Fig. 4A), findings that was consistent with monkey subjects that went on to become non-heavy drinkers. Also consistent between mice and monkeys was the negative acceleration of drinking that occurred within the FT interval immediately following the pellet delivery, an indication that drinking was under strong schedule control and that it was characteristically ‘adjunctive’ in nature (Falk, 1971). Importantly, this pattern was observed regardless of the fluid type presented, the mouse strain evaluated (Fig. 3), and whether or not an individual monkey became a chronic heavy drinker (Grant et al., 2008). Comparable findings in rats confirm this fundamental pattern of drinking within an FT interval (Lopez-Crespo et al., 2004; Mittleman et al., 1992). Collectively, the current observations identify an excessive drinking phenotype in B6 mice associated with the peak FT interval (FT-5) that is consistent with earlier work in cynomolgus monkeys, and suggest that a SIP model may be useful in exploring the biological bases of hazardous consumption patterns in humans.
In conclusion, a multitude of studies have addressed the relationship between stress and ethanol self-administration, but have yielded equivocal results. It was surmised by Becker and colleagues (2011) that a systematic assessment along the quantitative dimension of stress within a given procedure (with all other variables held constant) would be helpful in teasing apart the modulatory role of stress on ethanol drinking. Here we systematically lengthened the FT interval between food pellet deliveries while keeping all other experimental variables constant, and uncovered a complex relationship between plasma CORT and ethanol intake that may help resolve the mixed results stemming from a vast literature exploring the link between stress and excessive ethanol consumption. Additional investigation is clearly needed before firm conclusions can be drawn about this tenuous link, with future studies preferably addressing manipulations of the stress axis as an experimental rather than a correlation variable. Importantly, the common practice of applying a psychogenic or physical stressor of single intensity is likely to be insufficient for dissecting this relational link, as the level of stress generated (and CORT or other physiological response elicited) may influence the drinking outcome in a non-linear fashion, or not at all. These variable outcomes would be consistent with the notion that only a sub-group of drinkers are likely to develop their drinking repertoires in response to ‘stress-relief by ethanol’ as the primary motivating factor (Sillaber and Henniger, 2004).
Acknowledgments
This research was supported by the NIH, including a pilot project grant from the Integrative Neuroscience Initiative on Alcoholism: Stress and Anxiety of Alcohol Abuse (AA13641), a Mentored Research Scientist Development Award (AA16849), the Oregon National Primate Research Center (OD 011092), as well as funds and resources from the Department of Veterans Affairs. The authors would also like to thank Christopher Snelling and Michelle Tanchuck for their technical support in the analyses of blood ethanol and plasma corticosterone concentrations, and Steven Gonzales for composing program files that were essential for the running of operant chamber equipment.
The authors would also like to thank Christopher Snelling and Michelle Tanchuck for their technical support in the analyses of blood ethanol and plasma corticosterone concentrations, and Steven Gonzales for composing program files that were essential for the running of operant chamber equipment.
Footnotes
Conflict of interest
The authors declare no conflict on interest.
Contributors
Ford designed the study and wrote the protocol. Ford, Grant and Finn managed the literature searches and analyses. Steele and McCracken collected the data and conducted an initial phase of analysis, whereas Ford conducted the final analyses. Ford wrote the first draft of the manuscript. This manuscript has been read and approved by all authors, and we have exercised due care in ensuring the integrity of the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Brett LP, Levine S. Schedule-induced polydipsia suppresses pituitary-adrenal activity in rats. J Comp Physiol Psychol. 1979;93:946–956. doi: 10.1037/h0077619. [DOI] [PubMed] [Google Scholar]
- Brett LP, Levine S. The pituitary-adrenal response to “minimized” schedule-induced drinking. Physiol Behav. 1981;26:153–158. doi: 10.1016/0031-9384(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Stress, stereotypies and welfare. Behavioural Processes. 1991;25:95–l02. doi: 10.1016/0376-6357(91)90012-O. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Mormède P. Pituitary-adrenal consequences of adjunctive activities in pigs. Horm Behav. 1981;15:386–395. doi: 10.1016/0018-506x(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Terlouw C, Mormède P, Le Moal M. Schedule-induced polydipsia experience decreases plasma corticosterone levels but increases plasma prolactin levels. Physiol Behav. 1988;43:275–279. doi: 10.1016/0031-9384(88)90187-4. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Myracle A, Erbach J, Glowa J, Flores P, Riley AL. Strain-dependent differences in schedule-induced polydipsia: an assessment in Lewis and Fischer rats. Pharmacol Biochem Behav. 2003;74:755–763. doi: 10.1016/s0091-3057(02)01071-7. [DOI] [PubMed] [Google Scholar]
- DHHS-NIH. NIAAA Newsletter. Vol. 3. DHHS-NIH; Bethesda: 2004. Winter. NIAAA Council Approves Definition of Binge Drinking; p. 3. [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, Hård E, Söderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;11:195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Falk JL. Production of polydipsia in normal rats by an intermittent food schedule. Science. 1961;133:195–196. doi: 10.1126/science.133.3447.195. [DOI] [PubMed] [Google Scholar]
- Falk JL. Schedule-induced polydipsia as a function of fixed interval length. J Exp Anal Behav. 1966;9:37–39. doi: 10.1901/jeab.1966.9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Conditions producing psychogenic polydipsia in animals. Ann N Y Acad Sci. 1969;157:569–593. doi: 10.1111/j.1749-6632.1969.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Falk JL. The nature and determinants of adjunctive behavior. Physiol Behav. 1971;6:577–588. doi: 10.1016/0031-9384(71)90209-5. [DOI] [PubMed] [Google Scholar]
- Falk JL. The origin and functions of adjunctive behavior. Anim Learn Behav. 1977;5:325–335. [Google Scholar]
- Falk JL. The environmental generation of excessive behavior. In: Mule SJ, editor. Behavior in excess: An examination of the volitional disorders. New York: Free Press; 1981. pp. 313–337. [Google Scholar]
- Falk JL. Excessive behavior and drug-taking: Environmental generation and self-control. In: Levison PK, editor. Substance Abuse, Habitual Behavior, and Self-Control. Westview Press; Boulder: 1984. pp. 81–122. [Google Scholar]
- Falk JL, Samson HH, Winger G. Behavioral maintenance of high concentrations of blood ethanol and physical dependence in the rat. Science. 1972;177:811–813. doi: 10.1126/science.177.4051.811. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Flory RK. The control of schedule-induced polydipsia: Frequency and magnitude of reinforcement. Learning and Motivation. 1971;2:215–227. [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005a;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005b;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, Finn DA. Inhibition of 5α-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1408–1416. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV. Blood alcohol concentrations in rats drinking or intubated with ethanol. Alcohol Clin Exp Res. 1999;23:1945–1947. doi: 10.1111/j.1530-0277.1999.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Badia-Elder NE, Elder RL, Stewart RB. Schedule-induced polydipsia in lines of rats selectively bred for high and low ethanol preference. Behav Genet. 2008;38:515–524. doi: 10.1007/s10519-008-9224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Johanson CE. The generation of adjunctive behavior under conditions of drug self-administration. Behav Pharmacol. 1989;1:221–234. [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Jeng S, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol Clin Exp Res. 2012;36:995–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. J Exp Anal Behav. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci. 1997;17:8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R, Levine S. Role of the pituitary-adrenal hormones in the acquisition of schedule-induced polydipsia. Behav Neurosci. 1989;103:621–637. doi: 10.1037//0735-7044.103.3.621. [DOI] [PubMed] [Google Scholar]
- López-Crespo G, Rodríguez M, Pellón R, Flores P. Acquisition of schedule-induced polydipsia by rats in proximity to upcoming food delivery. Learn Behav. 2004;32:491–499. doi: 10.3758/bf03196044. [DOI] [PubMed] [Google Scholar]
- López-Grancha M, López-Crespo G, Venero C, Cañadas F, Sánchez-Santed F, Sandi C, Flores P. Differences in corticosterone level due to inter-food interval length: implications for schedule-induced polydipsia. Horm Behav. 2006;49:166–172. doi: 10.1016/j.yhbeh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- López-Grancha M, Lopez-Crespo G, Sanchez-Amate MC, Flores P. Individual differences in schedule-induced polydipsia and the role of gabaergic and dopaminergic systems. Psychopharmacology. 2008;197:487–498. doi: 10.1007/s00213-007-1059-6. [DOI] [PubMed] [Google Scholar]
- Lydersen T, Perkins D, Thome S, Lowman E. Choice of timeout during response-independent food schedules. J Exp Anal Behav. 1980;33:59–76. doi: 10.1901/jeab.1980.33-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Jones GH, Robbins TW. The relationship between schedule-induced polydipsia and pituitary-adrenal activity: pharmacological and behavioral manipulations. Behav Brain Res. 1988;28:315–324. doi: 10.1016/0166-4328(88)90134-9. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Blaha CD, Phillips AG. Pituitary-adrenal and dopaminergic modulation of schedule-induced polydipsia: behavioral and neurochemical evidence. Behav Neurosci. 1992;106:408–420. doi: 10.1037//0735-7044.106.2.408. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res. 2003;27:918–925. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Moreno M, Cardona D, Gómez MJ, Sánchez-Santed F, Tobeña A, Fernández-Teruel A, Campa L, Suñol C, Escarabajal MD, Torres C, Flores P. Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology. 2010;35:1198–1208. doi: 10.1038/npp.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Flores P. Schedule-induced polydipsia as a model of compulsive behavior: neuropharmacological and neuroendocrine bases. Psychopharmacology. 2012;219:647–659. doi: 10.1007/s00213-011-2570-3. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Roehrs TA, Samson HH. Schedule-induced ethanol polydipsia: function of ethanol concentration. Pharmacol Biochem Behav. 1980;13:291–294. doi: 10.1016/0091-3057(80)90086-6. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Ann Med. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Symons JP, Sprott RL. Genetic analysis of schedule induced polydipsia. Physiol Behav. 1976;17:837–839. doi: 10.1016/0031-9384(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Mormede P, Le Moal M. Pituitary-adrenal correlates of schedule-induced polydipsia and wheel running in rats. Behav Brain Res. 1986;19:249–256. doi: 10.1016/0166-4328(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]