Abstract
The gastric pathogen Helicobacter pylori causes peptic ulcer disease and gastric cancer. We have reported that in H. pylori-activated macrophages, nitric oxide (NO) derived from inducible NO synthase (iNOS) can kill the bacterium, iNOS protein expression is dependent on uptake of its substrate L-arginine (L-Arg), the polyamine spermine can inhibit iNOS translation by inhibiting L-Arg uptake, and inhibition of polyamine synthesis enhances NO-mediated bacterial killing. Because spermine oxidase (SMO), which back-converts spermine to spermidine, is induced in macrophages by H. pylori, we determined its role in iNOS-dependent host defense. SMO shRNA knockdown in RAW 264.7 murine macrophages resulted in a marked decrease in H. pylori-stimulated iNOS protein, but not mRNA expression, and a 90% reduction in NO levels; NO production was also inhibited in primary murine peritoneal macrophages with SMO knockdown. There was an increase in spermine levels after H. pylori stimulation that rapidly decreased, while SMO knockdown caused a greater increase in spermine that was sustained. With SMO knockdown, L-Arg uptake and killing of H. pylori by macrophages was prevented. Overexpression of SMO by transfection of an expression plasmid prevented the H. pylori-stimulated increase in spermine levels, and led to increased L-Arg uptake, iNOS protein expression and NO production, and H. pylori killing. In two human monocytic cell lines, U937 and THP-1, overexpression of SMO caused a significant enhancement of NO production with H. pylori stimulation. By depleting spermine, SMO can abrogate the inhibitory effect of polyamines on innate immune responses to H. pylori by enhancing antimicrobial NO production.
Keywords: Macrophages, Gastric cancer, Spermine oxidase, Host defense, Innate immune response, Polyamines
Introduction
H. pylori is a microaerophilic Gram-negative bacteria that selectively colonizes the human stomach and causes chronic gastritis, peptic ulcers, and gastric cancer (Marshall and Warren 1984; Correa 1992). H. pylori infection is the strongest known risk factor for gastric cancer (Parkin, et al. 2001; Parsonnet, et al. 1991). While only a fraction of infected individuals develop symptomatic clinical disorders, the prevalence of H. pylori infection worldwide makes these diseases a significant public health burden (Polk and Peek 2010). The stomach is the third most common anatomical site for digestive system cancer, and the second leading cause of cancer death worldwide (Parkin, et al. 2001). The most recent available data indicate that in the year 2004 in the United States, where prevalence of infection was 42%, H. pylori-associated diseases cost approximately $10 billion per year, including the direct costs for health care and drugs and the indirect costs due to loss of work (Everhart 2008).
Although H. pylori infection results in an innate and adaptive immune response in the host, the bacterium persists for the life of the host (Wilson and Crabtree 2007). H. pylori has evolved numerous strategies to evade the immune response including induction of apoptosis in macrophages (Chaturvedi, et al. 2004; Gobert, et al. 2002a) and T cells (Wang, et al. 2001; Gebert, et al. 2003; Ganten, et al. 2007), limiting the bactericidal effects of macrophages (Bussiere, et al. 2005; Chaturvedi, et al. 2007; Lewis, et al. 2010), varying the antigenic repertoire of surface-exposed proteins (Aras, et al. 2003) and actively suppressing the host adaptive immune response (Wang, et al. 2001). Macrophages are coordinators of the immune response to pathogens and act as a first line of defense against any pathogenic bacteria (Wilson and Crabtree 2007). The exposure of macrophages to pathogenic bacteria or bacterial antigens results in induction of inducible nitric oxide (NO) synthase (iNOS) and production of NO, a free radical species that mediates cytotoxic and cytostatic effects against pathogenic microbes (Schneemann, et al. 1993; Huang, et al. 2002). We have demonstrated that H. pylori induces iNOS expression and NO production in macrophages in vitro in a contact-independent manner (Wilson, et al. 1996; Gobert, et al. 2002b). Moreover, to maximize the production of NO and its bactericidal effect, macrophages require high levels of the iNOS substrate, L-arginine (L-Arg), in culture medium (Chaturvedi, et al. 2007). We have also shown that H. pylori-activated macrophages require L-Arg uptake into cells via cationic amino acid transporter 2 for the expression of iNOS protein and production of NO (Chaturvedi, et al. 2010). Additionally, H. pylori infection in mice results in an increase in iNOS mRNA expression in gastric macrophages, but a relatively modest increase in the levels of iNOS protein and NO in these cells (Chaturvedi, et al. 2010).
Upon uptake into the cell, L-Arg can be metabolized by either iNOS, or arginase I (Arg1) or arginase II (Arg2), to NO plus L-citrulline, or L-ornithine, respectively (Satriano 2004; Morgan 1994). Further, ornithine decarboxylase (ODC) metabolizes L-ornithine to the polyamine putrescine (Pegg and Casero 2011; Pegg and McCann 1982). Spermidine synthase and spermine synthase convert putrescine into the higher polyamines spermidine and spermine, respectively (Pegg and Casero 2011; Pegg and McCann 1982). Spermine can be back-converted to spermidine by spermine oxidase (SMO) or by a two-step process in which spermidine/spermine N1-acetyltransferase (SSAT) acetylates spermine into acetylspermine and this metabolite is subsequently converted to spermidine by acetyl polyamine oxidase (Pegg 2008; Pegg and Casero 2011).
Polyamines are biogenic polycations, and have been implicated in the regulation of various biological functions (Pegg and McCann 1982), including inhibition of NO production in activated macrophages (Chaturvedi, et al. 2012). We have shown that H. pylori infection increases ODC expression in macrophages in vitro and in vivo (Gobert, et al. 2002a; Chaturvedi, et al. 2004; Chaturvedi, et al. 2010), and also the levels of polyamines, specifically spermine (Chaturvedi, et al. 2010; Chaturvedi, et al. 2004). Inhibition of ODC by siRNA in vitro increases L-Arg uptake into macrophages and results in an increase in the levels of iNOS protein expression and NO production in H. pylori-activated macrophages (Chaturvedi, et al. 2010). Pharmacological inhibition of ODC by DFMO in H. pylori-infected mice also increases L-Arg uptake in gastric macrophages and the levels of iNOS protein and NO production in these cells (Chaturvedi, et al. 2010). Since H. pylori infection induces SMO in macrophages, in this study we sought to determine if this facilitates L-Arg uptake and iNOS-dependent NO production by decreasing spermine in macrophages.
Materials and Methods
Materials
All reagents used for cell culture, RNA extraction, and reverse transcription (RT)-PCR were obtained from Invitrogen. All other chemicals were purchased from Sigma (St. Louis, MO). For knockdown experiments siRNA were designed and utilized as described (Chaturvedi, et al. 2004; Chaturvedi, et al. 2010) and transfection reagents were purchased from Invitrogen (Grand Island, NY).
Bacteria, cells, and culture conditions
H. pylori SS1 was grown and used as described previously (Wilson, et al. 1996; Gobert, et al. 2002a; Gobert, et al. 2002b). Macrophages were activated with H. pylori lysate (HPL) prepared with a French press, and multiplicity of infection (MOI) was determined in lysates as described (Wilson, et al. 1996; Gobert, et al. 2002a; Gobert, et al. 2002b). For H. pylori killing studies, live H. pylori was separated from macrophages by filter supports (pore size, 0.4 μm; Transwell; Corning Inc., Corning, NY) (Gobert, et al. 2001; Bussiere, et al. 2005; Chaturvedi, et al. 2007). The murine macrophage cell line RAW 264.7 was maintained in complete Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. U937 and THP-1 human monocytic cell lines were maintained in complete Roswell Park Memorial Institute (RPMI) medium containing 10% fetal calf serum, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. Murine peritoneal macrophages were isolated from C57BL/6 mice and were cultured in complete DMEM as described (Bussiere, et al. 2005; Chaturvedi, et al. 2010).
Generation of stable murine macrophage cell lines
The murine macrophage cell line RAW 267.4 was transfected with Lipofectamine (Invitrogen, Grand Island, NY) and 4 μg of targeting plasmid (SMO shRNA) or scrambled non-targeting control plasmid (Scr shRNA). The targeting plasmid contained the following oligonucleotides (Invitrogen, Grand Island, NY) in the pSilencer 2.1-U6 neo expression vector (Ambion, Austin, TX): SMO forward, 5′-GATCCGCACTTCTTGAGCAGGGTTTTCAAGAGAAACCCTGCTCAAGAAGTGCTTTT TTGGAAA-3′ and SMO reverse, 5′-AGCTTTTCCAAAAAAGCACTTCTTGAGCAGGGTTTC TCT TGAAAACCCTGCTCAAGAAGTGCG-3′. After transfection, cells were grown in DMEM with 500 μg/ml G148. Cells were tested for knockdown of SMO mRNA expression by RT-PCR and the clone with maximum knockdown of SMO was used for experiments.
RT-PCR
Levels of SMO and iNOS mRNA expression were assessed by RT-PCR as described previously (Chaturvedi, et al. 2004; Chaturvedi, et al. 2007). After activation with HPL, total RNA was isolated from cells using TRIzol reagent. Subsequently, RNA from each sample was reverse transcribed and PCR was conducted using 2 μl of cDNA and 1 unit of Taq DNA polymerase. For SMO and iNOS, 15 pmol each of sense and antisense primers were used, with 3 pmol each of the β-actin primers in a multiplex reaction, as described previously (Chaturvedi, et al. 2004; Chaturvedi, et al. 2007). One PCR cycle consisted of the following: 94 °C for 1 min, 60 °C for 1.5 min, and 72 °C for 1.8 min. The total numbers of cycles were 30 for SMO and 32 for iNOS. A final elongation step of 10 min at 72 °C was used for each reaction. PCR products were run on 2% agarose gels with 0.4 μg/ml ethidium bromide. Stained bands were visualized under UV light and photographed with a digital gel documentation system (EDAS 290 and 1D software, Eastman Kodak Co.).
Real-time PCR
cDNA was prepared from each samples using 2 μg of RNA. PCRs were performed using an Opticon 2 thermal cycler (MJ Research, Cambridge, MA) and SYBR Green Master Mix (Molecular Probes, Eugene, OR) with 5.4 nM primers for murine SMO, and β-actin (Chaturvedi, et al. 2004; Chaturvedi, et al. 2007), or human ODC, SMO, and β-actin as described (Xu, et al. 2004; Chaturvedi, et al. 2010; Chaturvedi, et al. 2011). Thermal cycling conditions included an initial denaturation step (94 °C for 2 min) and 40 cycles (94°C for 30 s, 60°C for 30 s, and 72°C for 30 s). Relative expression was calculated from threshold values (CT) of the target and reference genes.
Polyamine measurement
Spermine levels were determined as described previously (Chaturvedi, et al. 2004; Chaturvedi, et al. 2010). After stimulation, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer. Samples were then dansylated and reverse phase high performance liquid chromatography was performed for detection of polyamines as reported previously (Chaturvedi, et al. 2004; Chaturvedi, et al. 2010). Protein concentrations were determined by the method of Bradford (Chaturvedi, et al. 2010).
L-Arg uptake
L-Arg uptake transport into cells was measured as described previously (Chaturvedi, et al. 2010). Cells were activated with HPL for 6, 12, 18, or 24 h, followed by washing with L-Arg-free medium. The uptake studies were initiated by adding 10 μl of [14C]-L-Arg (specific activity 308 μCi/mmol) for 1 min in L-Arg-free medium. After this incubation time, cells were washed with cold L-Arg-free medium supplemented with excess L-Arg (10 mM). Cells were lysed with RIPA buffer and supernatants were collected. Cell lysates were mixed with scintillation fluid and the [14C] content was determined in a scintillation counter. Protein was measured in cell lysates as described (Chaturvedi, et al. 2010). L-Arg transport values were expressed as nmol [14C]-L-Arg/min/mg protein.
Immunoblot analysis
Cells were treated with HPL, and were lysed and Western blotting for iNOS and β-actin was performed as described previously (Bussiere, et al. 2005; Chaturvedi, et al. 2007). iNOS (130 kDa) and β-actin (42 kDa) proteins were detected with a rabbit polyclonal iNOS antibody (1/1000; BD Biosciences, San Jose, CA ) and a mouse polyclonal β-actin antibody (1/5000; Sigma, St. Louis, MO), respectively. Chemiluminescent detection was performed as described (Bussiere, et al. 2005; Chaturvedi, et al. 2007).
Measurement of NO
The concentration of the oxidized metabolite of NO, nitrite (NO2−), was assessed by the Griess reaction, as described previously (Bussiere, et al. 2005; Chaturvedi, et al. 2007).
Transient transfection of SMO siRNA in peritoneal macrophages
siRNA duplexes that targeted mouse SMO nucleotides 467– 487, numbered from the start codon (sense, 5′-GGACGUGGUUGAGGAAUUC-3′; antisense, 5′-CCUGCACCAACUCCUUAAG-3′) were utilized as described previously (Chaturvedi, et al. 2004). Scrambled control siRNA (Scr siRNA) that has no sequence homology to any known genes was used as the control. Briefly, peritoneal macrophages were transfected with 20 μl of the 20 μM stock duplex SMO siRNA or control Scr siRNA and 100 μl of optiMEM medium (Invitrogen, Grand Island, NY). This mixture was combined with 5 μl of LipofectAMINE 2000 (Invitrogen) in 100 μl of optiMEM. Peritoneal macrophages were transfected with this mixture for 24 h. Cells were treated with HPL for 72 h.
Transient transfection of SMO
Murine RAW 267.4 and human THP-1 and U937 cells were transfected with 200 ng of pcDNA3.1-SMO using LipofectAMINE Plus and optiMEM medium. The cell culture medium was changed 6 h after transfection to complete DMEM for RAW 264.7, and complete RPMI medium for THP-1 and U937 cells.
H. pylori killing assay
Live H. pylori placed above Transwell filter supports were incubated with or without 1 × 106 macrophages in complete DMEM for 24 h at an MOI of 200. Colony forming units were determined after 24 h of co-culture by serial dilution and culture as described previously (Gobert, et al. 2001; Bussiere, et al. 2005; Chaturvedi, et al. 2007).
Determination of SMO activity
RAW 264.7 cells transfected with the SMO plasmid or vector alone were stimulated with HPL at an MOI of 100. SMO enzyme activity was assayed in cell lysates as described previously (Chaturvedi, et al. 2004; Xu, et al. 2004). In brief, cells were homogenized in 80 μM borate buffer, pH 9.0, and 100 μl of lysate from each sample was added to 5 nmol luminol with 570 milliunits of horseradish peroxidase and 250 μM spermine. Luminol-dependent chemiluminescence was determined using a Monolight 3010 luminometer with two reagent injectors. The SMO activities were expressed as nmol of H2O2/mg protein/min.
Results
Knockdown of SMO results in accumulation of spermine and attenuation of L-Arg uptake in macrophages
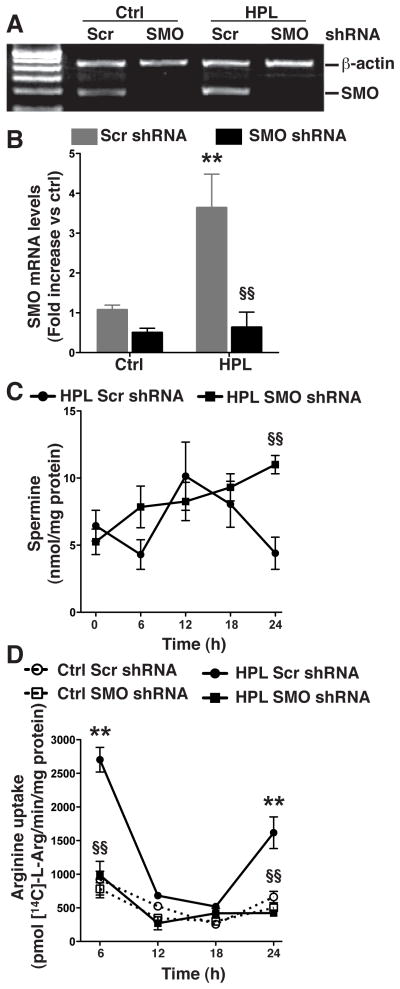
We have previously shown that activation of macrophages with H. pylori increases the levels of ODC, spermine, and SMO, (Chaturvedi, et al. 2004) while exogenous spermine acts to inhibit L-Arg uptake and iNOS-derived NO production (Bussiere, et al. 2005; Chaturvedi, et al. 2010). We also reported that inhibition of ODC activity by siRNA knockdown decreases spermine levels (Bussiere, et al. 2005) and increases L-Arg uptake in H. pylori-activated macrophages (Chaturvedi, et al. 2010). In the current study we wished to assess the effects of SMO on the levels of spermine and L-Arg uptake. To this end, we stably transfected RAW 264.7 cells with SMO shRNA that resulted in complete knockdown of SMO mRNA expression under basal conditions in the unstimulated control cells, as well as after activation with H. pylori (Fig. 1A and 1B). Levels of SMO mRNA expression were measured by RT-PCR and as well as by real-time qPCR, to ensure high sensitivity, and both techniques showed a substantial knockdown of SMO mRNA expression. While levels of spermine in cells transfected with control scrambled (Scr) shRNA increased between 6–12 h following activation with H. pylori lysates (HPL) and then decreased over the subsequent 12 h, in cells with stable knockdown of SMO, spermine levels increased continuously in a time-dependent manner (Fig. 1C) and were significantly elevated 24 h post activation (6.66 ± 1.11 vs. 8.33 ± 0.94 nmol/mg of protein; p<0.01). As shown in Fig. 1D, in cells transfected with Scr shRNA, HPL stimulation resulted in an induction of L-Arg uptake at 6 h after activation, with a loss of uptake at 12 h and 18 h when spermine levels in Fig. 1C were increased, followed by a subsequent increase in uptake at 24 h, when spermine levels were again decreased. In sharp contrast, in cells with knockdown of SMO there was a complete attenuation of HPL-stimulated L-Arg uptake at all time points from 6–24 h after activation (Fig. 1D), consistent with the increased spermine levels in these cells. Together, these observations suggest that SMO induction in H. pylori-activated macrophages prevents the accumulation of spermine that would otherwise prevent LArg uptake, and has a net effect of facilitating sustained L-Arg uptake into activated macrophages.
Figure 1.
Effect of stable knockdown of SMO on the levels of spermine and L-Arg uptake. RAW 267.4 cells were transfected with an expression vector containing scrambled (Scr) shRNA or SMO shRNA, and cells were selected with G148. Selected clones were activated with H. pylori lysate (HPL) at a multiplicity of infection (MOI) of 100. (A) Levels of SMO mRNA expression, by semi-quantitative RT-PCR, and (B) real-time PCR. (C) Levels of spermine at the times indicated. (D) L-Arg transport assessed as uptake of 14C-L-Arg into cells at the times indicated. In A–D, data are from 3 experiments performed in duplicate. **p < 0.01 vs control cells transfected with Scr shRNA; and §§p < 0.01 vs HPL-stimulated cells transfected with Scr shRNA.
SMO attenuation reduces iNOS protein expression and NO levels in macrophages
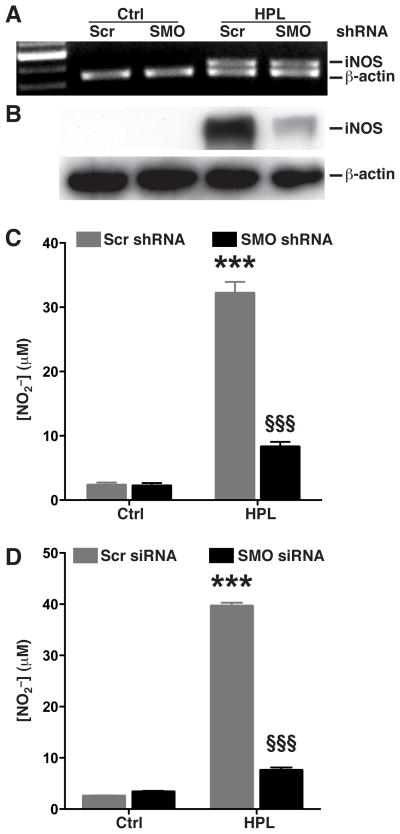
Our earlier reports indicated that addition of spermine or inhibition of L-Arg uptake with L-lysine (L-Lys) attenuates the H. pylori-stimulated increase of iNOS protein levels and NO production in macrophages (Bussiere, et al. 2005; Chaturvedi, et al. 2010). In the current studies, when we assessed iNOS mRNA levels by RT-PCR, we observed equal levels in macrophages transfected with either Scr or SMO shRNA upon activation with HPL (Fig. 2A). However, Western blotting demonstrated markedly attenuated induction of iNOS protein levels in cells with SMO knockdown (Fig. 2B). Similarly, NO production in HPL-activated cells was significantly reduced in macrophages transfected with SMO shRNA (from 31.27 ± 1.73 to 8.12 ± 0.88 μM; p<0.001; Fig. 2C). We confirmed these effects in primary cells using murine peritoneal macrophages transfected with either Scr or SMO siRNA; macrophages with Scr siRNA produced significant amounts of NO upon activation with HPL while macrophages transfected with SMO siRNA did not (Fig. 2D). Our results indicate that H. pylori-induced iNOS protein and NO production in macrophages is dependent on simultaneous induction of SMO, such that functional SMO acts to enhance iNOS and NO levels.
Figure 2.
Effect of stable knockdown of SMO on H. pylori-stimulated iNOS expression and NO production. (A–C) RAW 264.7 cells stably transfected with Scr shRNA or SMO shRNA were activated with HPL at an MOI of 100 for 24 h. (A) mRNA expression assessed by RT-PCR at 6 h after stimulation with HPL. (B) Western blot analysis for iNOS (130 kDa) and β-actin (42 kDa) 24 h after stimulation. In A and B, data are representative of 3 separate experiments. (C) NO2− levels, measured by Griess assay. (D) Peritoneal macrophages were transiently transfected with either Scr siRNA or SMO siRNA, and NO2− levels were measured 72 h after activation with HPL. For C and D, ***p < 0.001 vs unstimulated control cells transfected with Scr shRNA or siRNA, and §§§p < 0.001 vs HPL-stimulated cells transfected with Scr shRNA or siRNA. Data are from 3 experiments.
Knockdown of SMO prevents killing of H. pylori by macrophages
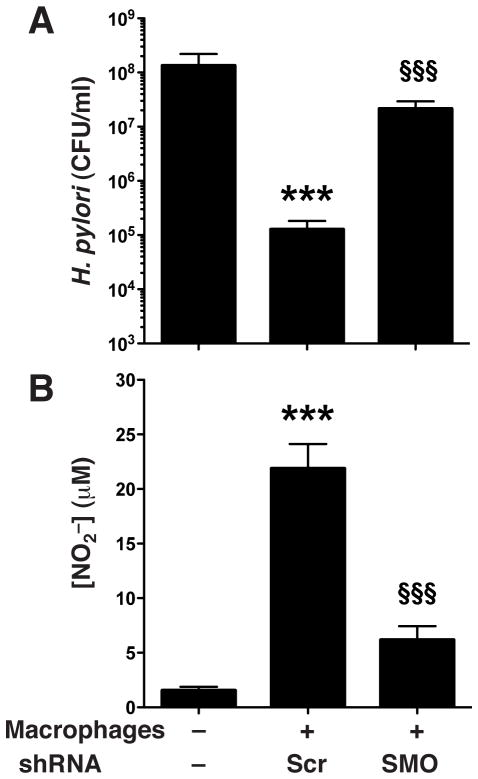
Since our previous study noted that exogenous spermine blocks the ability of macrophages to kill H. pylori in vitro (Bussiere, et al. 2005) via inhibition of bactericidal NO production, and our data in the present study indicate that knocking down SMO causes accumulation of spermine and reduction in NO production in HPL-activated macrophages, we next sought to determine the effect of SMO knockdown on H. pylori killing. Macrophages transfected with control Scr shRNA and cultured with live H. pylori separated by a Transwell membrane support caused a substantial, three-log-order reduction in levels of bacteria (Fig. 3A). In contrast, macrophages stably transfected with SMO shRNA failed to kill H. pylori (Fig. 3A). When NO in the supernatant was measured, macrophages with SMO knockdown produced significantly lower levels (21.88 ± 2.23 vs. 6.16 ± 1.26; p<0.001; Fig. 3B).
Figure 3.
Killing of H. pylori by SMO shRNA-transfected RAW 264.7 murine macrophages. Live H. pylori placed above Transwell filter supports was incubated for 24 h at an MOI of 100 with RAW 264.7 cells below the filters that were transfected with Scr shRNA or SMO shRNA. CFU were determined after 24 h of coculture. (A) H. pylori survival. (B) NO2− levels in supernatants. ***p < 0.001 vs no macrophages; and §§§p < 0.001 vs macrophages transfected with Scr shRNA. Data are from 2 separate experiments performed in duplicate. Scr, scrambled.
Ectopic overexpression of SMO in macrophages leads to spermine depletion and enhanced L-Arg uptake upon activation with H. pylori
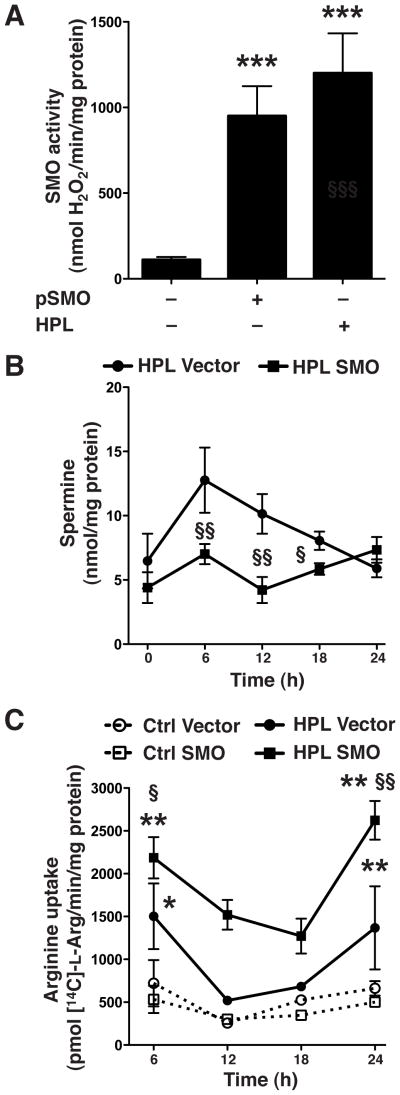
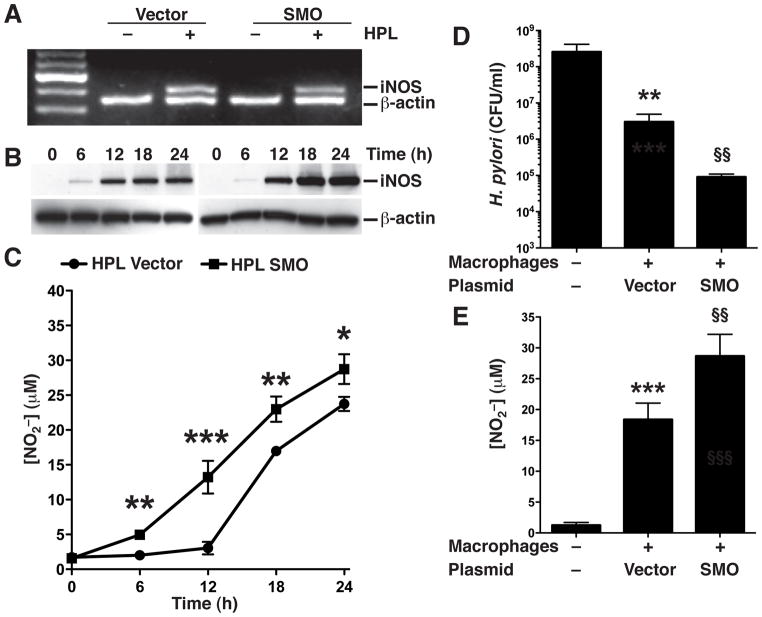
To further ascertain the biological role of SMO in regulation of the levels of spermine, iNOS protein, and NO production, we transfected RAW 264.7 macrophages with a plasmid expressing full length SMO cDNA. Measurement of SMO enzyme activity indicated that expression of SMO in transfected macrophages without HPL activation achieved a similar level as in HPL-activated cells transfected with the empty vector (950.63 ± 173.72 vs. 1200.23 ± 233.12 nmol/mg protein; Fig. 4A). HPL activation of empty vector-transfected macrophages resulted in elevated levels of spermine from 6 to 18 h compared to that seen in cells transfected with the SMO plasmid (Fig. 4B). Concomitantly, there was increased L-Arg uptake at all time points post-activation with HPL in macrophages transfected with the SMO expression plasmid (Fig. 4C) compared to macrophages transfected with vector alone. Taken together, these results indicate that overexpression of SMO reduces H. pylori-induced spermine accumulation and causes a sustained increase in L-Arg uptake.
Figure 4.
Effects of SMO overexpression on levels of spermine and L-Arg uptake. RAW 267.4 cells were transiently transfected with vector alone or vector containing SMO cDNA. Transfected cells were activated with HPL at an MOI of 100. (A) SMO activity. (B) Levels of spermine at the times indicated. (C) L-Arg uptake, assessed as transport of 14C-L-Arg into cells at the times indicated. In A–C, data are from 3 experiments performed in duplicate. *p < 0.05; **p < 0.01, ***p < 0.001 vs unstimulated control cells transfected with empty vector; and §p < 0.05; §§p < 0.01 vs HPL-stimulated cells transfected with the empty vector.
Ectopic expression of SMO in macrophages increases levels of iNOS protein and NO production
Since we had observed that H. pylori stimulation of macrophages overexpressing SMO had decreased levels of spermine and sustained L-Arg uptake, we next sought to determine the effect of SMO overexpression on levels of iNOS and NO production. HPL activation resulted in equally increased levels of iNOS mRNA in macrophages transfected with either empty vector or the SMO expression plasmid (Fig. 5A). When we assayed iNOS protein expression by Western blotting, we found that HPL activation of macrophages transfected with vector alone induced increased iNOS levels that were further increased in macrophages with ectopic overexpression of SMO (Fig. 5B). We also observed that macrophages transfected with vector alone did not increase the production of NO until 18 h post stimulation with HPL, whereas stimulated macrophages transfected with the SMO plasmid produced significantly increased amounts of NO beginning at 6 h (p<0.01; Fig. 5C) that increased in a time-dependent manner for the subsequent 18 h. These increases in NO production corresponded to the observed increase in the levels of iNOS protein indicating that expression of SMO can abrogate the inhibitory effect of spermine on iNOS protein expression and production of NO.
Figure 5.
Effect of SMO overexpression on H. pylori-stimulated iNOS expression, NO production, and bacterial killing. RAW 267.4 cells were transiently transfected with vector alone or vector containing SMO cDNA. Transfected cells were activated with HPL at an MOI of 100 for the times indicated. (A) mRNA expression assessed by RT-PCR at 6 h after stimulation with HPL. (B) Western blot analysis for iNOS (130 kDa) and β-actin (42 kDa). In A and B, data are representative of 3 separate experiments. (C) NO2− levels, measured by Griess assay. *p <0.05; **p < 0.01; ***p < 0.001 vs cells transfected with empty vector. Data are from 4 experiments. In D and E, live H. pylori placed above Transwell filter supports was incubated for 24 h at an MOI of 100 with macrophages transfected with vector alone or vector containing SMO cDNA. CFU were determined after 24 h of coculture. (D) H. pylori survival. (E) NO2− levels in culture supernatants. **p < 0.01, ***p < 0.001 vs no macrophages; and §§p < 0.01 vs empty vector-transfected macrophages. Data are from 2 separate experiments performed in duplicate.
Macrophages with ectopic expression of SMO exhibit enhanced killing of H. pylori
We previously reported that spermine blocks the ability of macrophages to kill H. pylori by blocking iNOS-associated NO production (Bussiere, et al. 2005). In the present study we observed that ectopic expression of SMO results in inhibition of spermine accumulation and more NO production in HPL-activated macrophages. We therefore measured the effect of SMO overexpression on macrophage killing of H. pylori. Macrophages transfected with control vector and cultured with H. pylori separated by a Transwell membrane caused a two-log-order reduction in the level of bacteria compared with samples cultured in the absence of macrophages, while macrophages transfected with the SMO expression plasmid reduced the number of live H. pylori by an additional 90% (Fig. 5D). When NO was measured in the macrophage supernatants, the SMO-overexpressing macrophages produced significantly higher levels (18.37 ± 2.69 vs. 28.66 ± 2.53 μM; p<0.01) after co-culture with H. pylori (Fig. 5E). These data indicate that SMO expression in macrophages facilitates NO production and associated bacterial killing during H. pylori infection.
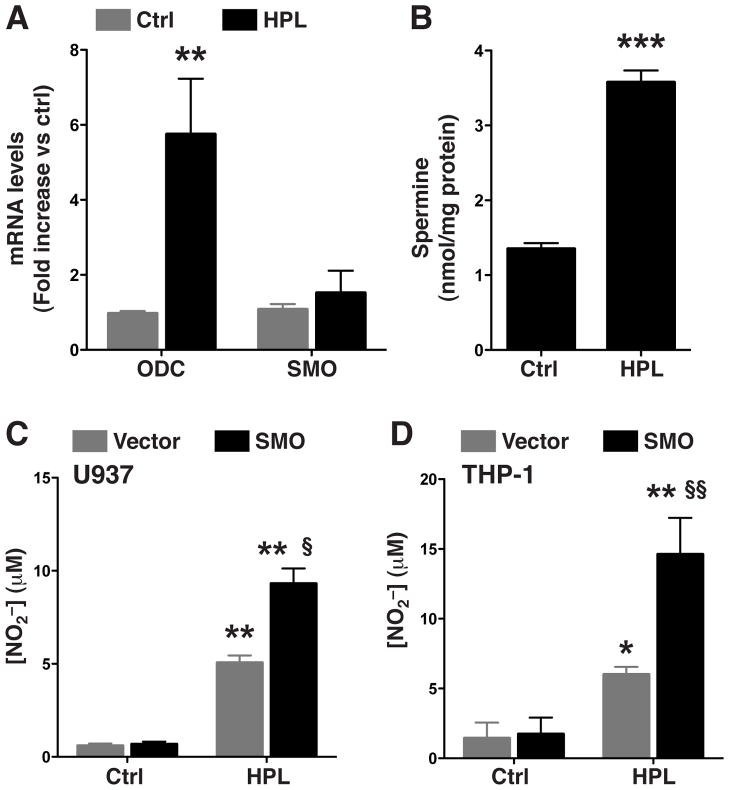
H. pylori increases levels of ODC mRNA and spermine but not SMO mRNA
To assess the ability of HPL to increase the levels of ODC, SMO, and spermine in human monocytes, we activated THP-1 cells with HPL. The HPL activation of THP-1 cells resulted in an increase in the level of ODC mRNA (0.97 ± 0.15 vs. 5.74 ± 1.48 fold increase; p<0.01) but not that of SMO mRNA (Fig 6A). Consistent with these findings, the level of spermine after HPL activation was also elevated in THP-1 cells (1.35 ± 0.07 vs. 3.57 ± 0.15 nmol/mg protein; p<0.001; Fig 6B).
Figure 6.
Levels of ODC, SMO mRNA and spermine in HPL-activated human monocytic cell lines and effect of SMO overexpression on H. pylori-stimulated NO. (A and B) THP-1 cells were activated with HPL activated with HPL at an MOI of 100 for the times indicated. (A) mRNA expression assessed by real-time PCR at 6 h after stimulation with HPL. (B) Levels of spermine at 24 h after stimulation with HPL. (C and D) U937 and THP-1 cells were transiently transfected with vector alone or with SMO plasmid vector and activated with HPL at an MOI of 100 for 72 h. NO2− levels, measured by Griess assay for (C) U937 cells and (D) THP-1 cells. *p < 0.05, **p < 0.01; vs unstimulated control cells transfected with empty vector; §p < 0.05, §§p < 0.01 vs HPL-stimulated cells transfected with empty vector. Data are from 3 experiments.
Overexpression of SMO increases NO production in human monocytes
To expand our findings to human immune cells, we transfected THP-1 and U937 monocytic cell lines with either vector alone or the SMO expression plasmid. In both cell lines, activation with HPL in cells transfected with vector alone produced modest amounts of NO, while stimulated SMO-transfected cells generated significantly higher NO levels (Fig. 6C and 6D). Thus, the human cell line data also indicates that the host innate immune response to H. pylori infection can be enhanced by SMO expression.
Discussion
In the current study we demonstrated that induction of iNOS by H. pylori is tightly regulated by metabolism of the polyamine spermine. We have previously reported that H. pylori increases polyamine synthesis in macrophages in an ODC-dependent manner (Chaturvedi, et al. 2004; Bussiere, et al. 2005). The time kinetics for the levels of spermine in macrophages after H. pylori stimulation indicated a maximum level of spermine after 12 h, which rapidly decreased to control levels at 18 h and further significantly decreased below the levels of the control at 24 h (Chaturvedi, et al. 2004). The observed decrease in spermine levels after 12 h could be due to catabolism of spermine by SMO or SSAT, or efflux of spermine. Spermine efflux requires acetylation of spermine by SSAT (Pegg 2008). We have published that H. pylori induces SMO activity in a time-dependent manner in macrophages that peaked at 18 h post-infection (Chaturvedi, et al. 2004), whereas SSAT activity did not significantly increase until the 18 h time point (Chaturvedi, et al. 2004). Moreover, the levels of SSAT activity were also very low compared to SMO activity (Chaturvedi, et al. 2004). In the present study we observed that knockdown of SMO increased the levels of spermine in a timedependent manner and prevented the decrease in spermine levels at 18 h postactivation. Conversely, in macrophages transfected with the SMO expression plasmid, activation with H. pylori did not cause an increase in the levels of spermine. These results indicate that SMO is an important regulator of spermine levels in H. pyloriactivated macrophages.
L-Arg transport is essential for expression of iNOS protein and NO production in H. pylori-activated macrophages (Kakuda, et al. 1999; Nicholson, et al. 2001; Chaturvedi, et al. 2010). In the present study, macrophages transfected with the SMO shRNA vector did not exhibit an increase in L-Arg uptake upon activation with H. pylori, whereas ectopic expression of SMO in macrophages resulted in a sustained level of LArg transport in stimulated cells. We have shown that L-Arg uptake is primarily mediated by CAT2 in H. pylori-activated macrophages, and that spermine inhibits L-Arg transport activity without altering the mRNA or protein levels of CAT2 (Chaturvedi, et al. 2010). It should be noted that spermine also inhibited L-Arg uptake in LPS-activated rat alveolar macrophages (Mossner, et al. 2001). Our results indicate that SMO knockdown caused loss of catabolism of spermine and thus accumulation of spermine, which inhibited L-Arg uptake into H. pylori-activated macrophages. In contrast, overexpression SMO increased the catabolism of spermine in H. pylori-activated cells, preventing accumulation of spermine and thus facilitating uptake of L-Arg into macrophages. Similarly, we have demonstrated previously that knockdown of ODC, the rate-limiting enzyme for polyamine synthesis, resulted in attenuation of the H. pylori-induced increase in the levels of spermine (Bussiere, et al. 2005), facilitating L-Arg uptake in macrophages (Chaturvedi, et al. 2010).
Spermine has been shown to inhibit iNOS expression and NO production in activated macrophages (Chaturvedi, et al. 2010; Satriano 2004). We have shown that spermine inhibits NO production by blocking translation of iNOS protein in H. pylori-activated macrophages (Bussiere, et al. 2005). In the present study, SMO knockdown led to accumulation of spermine and this inhibited iNOS protein expression and NO production in H. pylori-activated macrophages. Consistent with the role of SMO in regulating spermine levels in activated macrophages, overexpression of SMO depleted levels of spermine and allowed for increased levels of H. pylori-induced iNOS protein expression and NO production. These results confirm our previous findings that not only exogenous spermine addition, but also stimulated endogenous polyamine synthesis due to H. pylori infection can restrict the levels of iNOS and NO. Overexpression of SMO did not affect the levels of iNOS mRNA, but enhanced the levels of iNOS protein at 12–24 h post activation, indicating that spermine depletion enhances iNOS translation, consistent with our previous studies showing that iNOS translation is tightly regulated by L-Arg availability (Chaturvedi, et al. 2007). In the present study we observed an increase in NO production at 6 h post-activation in cells with SMO overexpression, although iNOS protein was not different from mock-transfected cells at this time point. A likely explanation is that the increased levels of L-Arg uptake that we found with SMO overexpression at 6 h can provide a sufficient increase in the substrate for iNOS to allow enhanced production of NO. It should be noted that spermine itself has been reported to have oxyradical scavenging activity (Ha, et al. 1998), so intracellular spermine levels could also possibly directly reduce the levels of free radical species, including nitrogen radicals such as NO.
It should be noted that bactericidal activity of iNOS requires generation of copious amount of NO. We observed in this study that knockdown of SMO resulted in complete attenuation of L-Arg uptake and NO production in macrophages that led to enhanced bacterial survival. In contrast, overexpression of SMO led to increase NO production that resulted in increased bacterial killing. Together, these findings suggest that upregulation of SMO in macrophages represents a key facilitator of innate immunity. Since substantial SMO expression leads to apoptosis of activated macrophages due to generation of H2O2 (Chaturvedi, et al. 2004), there is clearly a difficult balancing act for the cell as it faces the exposure to H. pylori itself and its released soluble factors that can readily activate these cells. Therefore, the macrophages that do not undergo apoptosis may have submaximal SMO expression and thus suboptimal depletion of spermine, leading to limited antimicrobial NO production and contributing to survival of the pathogen in the gastric niche.
Human monocytes have attenuated NO production in response to bacterial stimulation (Schneemann, et al. 1993). In the present study, we found that activation of human THP-1 cells with H. pylori resulted in increased levels of ODC and spermine, but unlike murine macrophages, there was no induction of SMO. These results suggest that in human monocytes with H. pylori infection, deficiency in catabolism of spermine due to a lack of induction of SMO leads to increased levels of spermine and limited NO production. Indeed, facilitation of spermine depletion by SMO overexpression resulted in increased production of NO in the monocytic cell lines.
Our study establishes SMO as a critical facilitator of NO production and bacterial killing. Further, our data in human cells provide a possible explanation for poor NO responses to cytokines or bacterial stimuli in human monocytes. We have shown that inhibition of ODC by DFMO in H. pylori-infected mice resulted in increased levels of LArg uptake, iNOS protein and NO production in gastric macrophages (Chaturvedi, et al. 2010). This DFMO treatment resulted in a decrease in H. pylori colonization (Chaturvedi, et al. 2010) an effect attributable to enhanced NO production. In total our data suggest that spermine contributes to the immunopathogenesis of H. pylori infection. Therefore, in humans, consumption of increased levels of dietary polyamines, especially spermine, could contribute to increased intracellular spermine, and thus may impair the host innate immune response; dietary restriction of spermine could perhaps prove useful in chemoprevention of gastric cancer. In summary, SMO can be a key enhancer of NO production in macrophages, and targeted strategies such as blocking the synthesis of polyamines or modulating the diet to deplete spermine could be useful as an adjuvant therapy in H. pylori infection to enhance the antimicrobial activity of innate immune response cells.
Acknowledgments
This work was supported by National Institutes of Health grants R01DK053620, R01AT004821, P01CA028842, and P01CA116087 (to K.T.W.), K01AT007324 (to R.C.), R01CA051085 and R01CA098454 (to R.A.C.), the Vanderbilt University Digestive Disease Research Center grant (P30DK058404), and a Merit Review Grant (1I01BX001453) from the Office of Medical Research, Department of Veterans Affairs (to K.T.W.)
Abbreviations
- H. pylori
Helicobacter pylori
- NO
nitric oxide
- iNOS
inducible NO synthase
- L-Arg
L-arginine
- Arg1
arginase I
- Arg2
arginase II
- ODC
ornithine decarboxylase
- SMO
spermine oxidase
- H2O2
hydrogen peroxide
- siRNA
small interfering RNA
- shRNA
small hairpin RNA
- DFMO
α-difluoromethylornithine
- SSAT
spermidine/spermine N1-acetyltransferase
References
- Aras RA, Fischer W, Perez-Perez GI, Crosatti M, Ando T, Haas R, Blaser MJ. Plasticity of repetitive DNA sequences within a bacterial (Type IV) secretion system component. J Exp Med. 2003;198 (9):1349–1360. doi: 10.1084/jem.20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere FI, Chaturvedi R, Cheng Y, Gobert AP, Asim M, Blumberg DR, Xu H, Kim PY, Hacker A, Casero RA, Jr, Wilson KT. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem. 2005;280 (4):2409–2412. doi: 10.1074/jbc.C400498200. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, de Sablet T, Piazuelo MB, Sarvaria AR, Cheng Y, Closs EI, Casero RA, Jr, Gobert AP, Wilson KT. Polyamines Impair Immunity to Helicobacter pylori by Inhibiting L-Arginine Uptake Required for Nitric Oxide Production. Gastroenterology. 2010;139(5):1686–1698. 1698 e1681–1686. doi: 10.1053/j.gastro.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Lewis ND, Algood HM, Cover TL, Kim PY, Wilson KT. L-arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect Immun. 2007;75 (9):4305–4315. doi: 10.1128/IAI.00578-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA, Jr, Correa P, Gobert AP, Polk DB, Peek RM, Jr, Wilson KT. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori. CagA Gastroenterology. 2011;141(5):1696–1708. e1691–1692. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279 (38):40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, de Sablet T, Coburn LA, Gobert AP, Wilson KT. Arginine and polyamines in Helicobacter pylori-induced immune dysregulation and gastric carcinogenesis. Amino Acids. 2012;42 (2–3):627–640. doi: 10.1007/s00726-011-1038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52 (24):6735–6740. [PubMed] [Google Scholar]
- Ganten TM, Aravena E, Sykora J, Koschny R, Mohr J, Rudi J, Stremmel W, Walczak H. Helicobacter pylori-induced apoptosis in T cells is mediated by the mitochondrial pathway independent of death receptors. Eur J Clin Invest. 2007;37 (2):117–125. doi: 10.1111/j.1365-2362.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301 (5636):1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- Gobert AP, Cheng Y, Wang JY, Boucher JL, Iyer RK, Cederbaum SD, Casero RA, Jr, Newton JC, Wilson KT. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002a;168 (9):4692–4700. doi: 10.4049/jimmunol.168.9.4692. [DOI] [PubMed] [Google Scholar]
- Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci U S A. 2001;98 (24):13844–13849. doi: 10.1073/pnas.241443798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert AP, Mersey BD, Cheng Y, Blumberg DR, Newton JC, Wilson KT. Cutting edge: urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J Immunol. 2002b;168 (12):6002–6006. doi: 10.4049/jimmunol.168.12.6002. [DOI] [PubMed] [Google Scholar]
- Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. 1998;95 (19):11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, DeGraves FJ, Lenz SD, Gao D, Feng P, Li D, Schlapp T, Kaltenboeck B. The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease. Proc Natl Acad Sci U S A. 2002;99 (6):3914–3919. doi: 10.1073/pnas.062578399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda DK, Sweet MJ, Mac Leod CL, Hume DA, Markovich D. CAT2-mediated L-arginine transport and nitric oxide production in activated macrophages. Biochem J. 1999;340 ( Pt 2):549–553. [PMC free article] [PubMed] [Google Scholar]
- Lewis ND, Asim M, Barry DP, Singh K, de Sablet T, Boucher JL, Gobert AP, Chaturvedi R, Wilson KT. Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages. J Immunol. 2010;184 (5):2572–2582. doi: 10.4049/jimmunol.0902436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1 (8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Morgan DM. Polyamines, arginine and nitric oxide. Biochem Soc Trans. 1994;22 (4):879–883. doi: 10.1042/bst0220879. [DOI] [PubMed] [Google Scholar]
- Mossner J, Hammermann R, Racke K. Concomitant down-regulation of L-arginine transport and nitric oxide (NO) synthesis in rat alveolar macrophages by the polyamine spermine. Pulm Pharmacol Ther. 2001;14 (4):297–305. doi: 10.1006/pupt.2001.0297. [DOI] [PubMed] [Google Scholar]
- Nicholson B, Manner CK, Kleeman J, MacLeod CL. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem. 2001;276 (19):15881–15885. doi: 10.1074/jbc.M010030200. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325 (16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294 (6):E995–1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- Pegg AE, Casero RA., Jr Current status of the polyamine research field. Methods Mol Biol. 2011;720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;243 (5):C212–221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10 (6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriano J. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids. 2004;26 (4):321–329. doi: 10.1007/s00726-004-0078-4. [DOI] [PubMed] [Google Scholar]
- Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167 (6):1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- Wang J, Brooks EG, Bamford KB, Denning TL, Pappo J, Ernst PB. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J Immunol. 2001;167 (2):926–934. doi: 10.4049/jimmunol.167.2.926. [DOI] [PubMed] [Google Scholar]
- Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133 (1):288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Wilson KT, Ramanujam KS, Mobley HL, Musselman RF, James SP, Meltzer SJ. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996;111 (6):1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]
- Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Jr, Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64 (23):8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]