Summary
Cortisol's daytime rhythm is thought to be altered by aging and by exposure to chronic stress. However, measurement of an individual's usual cortisol rhythm is hampered by the effects of acute stressors, by differences between working days and weekends, by between-day variation in waking time and sleep duration, by variability in cortisol sampling times, and by possible variability in the timing of cortisol peak and nadir. Therefore, to determine differences in the usual daytime cortisol rhythm by age, socioeconomic status, and race/ethnicity, we measured salivary cortisol levels at four time-points, repeated over four days that included both weekdays and weekend days, in 1,693 men and women from a national sample, and used three alternate growth curve specifications for the underlying cortisol rhythm (linear spline, quadratic spline, piece-wise linear-cubic) in order to minimize the impact of sample timing and other methodological issues. Model-predicted mean values of (and demographic and socioeconomic differences in) cortisol peak, nadir, and area under the curve (AUC) were nearly identical across model specifications. Older age and male gender were independently associated with higher cortisol peak, nadir, and AUC. Low education and minority race/ethnicity status were independently associated with lower cortisol peak and higher nadir, but were not associated with AUC. We also found significant cortisol peak and AUC associations with waking time, sleep duration, and workday vs. weekend day status, suggesting the importance of measuring these confounders and of collecting cortisol measurements over multiple days in research studies. We conclude that daytime cortisol levels are higher in older age and in men compared to women, and that the daytime cortisol rhythm is flatter (more blunted) in less privileged segments of society. Flattening of daytime cortisol rhythms may represent one mechanism by which social stressors lead to poor health outcomes.
Keywords: Cortisol diurnal rhythm, socioeconomic status, age, gender, race, spline regression, cortisol peak, cortisol nadir, cortisol area under the curve
1. Introduction
Cortisol, a key regulator of inflammatory and major metabolic activity, and a primary agent of the neuroendocrine stress response, has an underlying circadian rhythm, with circulating levels peaking shortly after awakening, followed by a gradual decline during the waking day (Steptoe et al., 2003). Changes in this diurnal rhythm are seen with aging and also in individuals exposed to chronic or frequent life stresses. These changes include blunting or heightening of the morning peak and increases in the late evening nadir (Varadhan et al., 2008; Almeida et al, 2009). Such changes are thought to be part of the biological pathway by which chronic or frequent life stressors affect physical health (Lundberg, 2005; Cohen et al., 2007) and even mortality (Kumari et al, 2011). In addition to changes in the amplitude of the circadian oscillation, there has also been the suggestion that the timing of the nadir might be affected by age and possibly by chronic stress (Van Cauter, 1996).
However cortisol peak and nadir associations with socioeconomic disadvantage - a chronic stressor, have not been consistently found (Dowd et al., 2009). Inconsistencies in socioeconomic status (SES) associations with daytime cortisol levels may reflect diverging influences of ongoing and past stressors: While past exposure to frequent or severe stress is expected to raise resting cortisol levels (i.e., late evening nadir and overnight levels) and blunt morning peaks (Miller et al., 2007), ongoing daily stressors accentuate the morning rise and raise peak levels (Pruessner et al., 2003). Moreover, day-to-day variation in ongoing stressors, such as differences in work-related stress levels between weekdays and weekend days, can mask long-term changes in cortisol rhythms (Clow et al., 2004; Kunz-Ebrecht et al., 2004; Adam et al., 2006). Cortisol rhythms may also vary from one day to the next because of differences in the time of waking and/or duration of sleep (Edwards et al., 2001; Kumari et al., 2009). It has therefore been suggested that repeated measurement over multiple days is needed to assess underlying daytime cortisol rhythms (Hellhammer et al., 2007). Studies of daytime cortisol have also been plagued by between-person variation in the timing of the cortisol sample meant to capture the morning peak level; both early and late measurements underestimate the peak and blur between-person differences in cortisol trajectories (Kunz-Ebrecht et al., 2004).
Therefore, to address open questions about changes in the cortisol rhythm with age and SES, and to determine differences across demographic groups, while addressing these methodological concerns, we examined salivary cortisol measurements repeated over multiple days (including both weekdays and weekend days) in a large national sample, and used spline regressions with the first knot fixed at the expected time of the morning peak, to reduce the impact of sample timing on study findings (Ranjit et al., 2005). Our objective was to determine how the timing and magnitudes of the daytime cortisol peak and nadir, and the integrated cortisol exposure over the waking day (i.e., area under the curve) vary by demographic and socioeconomic characteristics, while adequately controlling for between-person and within-person (day-to-day) differences in waking time, length of bedtime during the preceding night, length of typical wake-day, and weekend vs. weekday status, and while allowing for possible variations (both between person and within person from day to day) in the timing of the cortisol peak and nadir.
2. Methods
Data came from the second wave of the Midlife in the United States Study (MIDUS), which included salivary cortisol collection on a sub-sample, as part of the National Study of Daily Experiences (NSDE). The MIDUS study, initiated in 1995, was designed to determine how social, psychological, and behavioral factors inter-relate to influence mental and physical health. The first wave collected sociodemographic and psychosocial data on 7,108 Americans, ages 25 to 74 years, from a sample of English-speaking, non-institutionalized adults residing in the contiguous 48 states, whose household included at least one telephone (recruited by random digit dialing), with oversampling of five metropolitan areas, twin pairs, and siblings (Brim et al., 2004). Of the original 7,108 MIDUS participants, 4,963 (70%) were successfully re-contacted and completed the MIDUS II 30-minute phone interview and two self-administered questionnaires 9-10 years later using the original protocol (Radler and Ryff, 2010). To increase the representation of urban African Americans in the sample, an additional 592 African Americans were recruited (to participate in MIDUS II) from Milwaukee, WI, with U.S. Census data used to obtain a diverse representation across household income, age, and gender (Slopen et al. 2010). In this second wave, as part of NSDE II, a random sub-sample (n= 2,022) of the MIDUS II sample also completed a daily telephone diary study about their experiences over eight consecutive days and collected four saliva samples per day (for cortisol assessments) on four consecutive days, starting from day 2 of the diary study (Almeida et al., 2009).
2.1. Study Sample
Of the 2,022 NSDE II participants, 1,733 provided at least one valid cortisol sample with sampling time, for a total of 6,883 days of cortisol data. We dropped data from 150 days when participants awoke before 0400h, an additional 115 days when the 3rd (pre-lunch) cortisol sample was 10 nmol or more higher than the 2nd (morning-peak) sample (as this likely reflects a time-recording error for one of the two saliva samples or cortisol measurement error after a meal), 64 days when respondents woke after 11am, and an additional 32 days for respondents who were awake more than 20 hours on a given day. This left us with a potential sample of 1,714 participants. Missing data on race/ethnicity, education, average wake-day length, or weekend day vs. workday status reduced the final analytic sample to 1,693 participants (from 1,409 families) with 6,318 days of cortisol collection and 24,388 salivary cortisol data points.
2.2. Measurements
Prior to their first NSDE II telephone interview, participants received an in-home saliva collection kit, which included a detailed instruction sheet, and sixteen numbered and color-coded salivettes (Sarstedt, Nümbrecht, Germany). Interviewers reviewed collection procedures with participants during the first interview, and saliva collection began the next day (day 2 of the eight-day study). On saliva collection days (days 2 through 5), participants were instructed to collect four saliva samples, one upon waking, one around 30 minutes after getting out of bed, one before lunch, and one at bed time. Data on the exact time of each saliva sample were obtained from nightly telephone interviews by study staff and on a paper-pencil log sent with the collection kit. In addition, a quarter of the respondents receive a “Smart Box” to store their salivettes, with a computer chip that recorded the time of box opening and closing. Correlations between self-reported times (paper-pencil log and nightly phone interviews) were above 0.9 for each of the four sampling points; correlations between self-reported times and times obtained from the “smart box” ranged from 0.75 to .95 across the four sampling points. Salivettes were frozen (at –60 °C) for storing and shipping. Cortisol concentrations were measured with a commercially available luminescence immunoassay (IBL, Hamburg, Germany).
Demographic and socioeconomic characteristics, age (in years), race/ethnicity, gender, highest level of educational attainment, and annual household income, were all self-reported in the MIDUS II questionnaire. We categorized age in three groups of similar size: <50 years old, 50-64 years old, and 65 years of age or older. We also collapsed race into two groups: Caucasian and non-Caucasian. The latter group was predominantly African American (67.5%), but also included Hispanics, Native Americans, and people of mixed race/ethnicity (n=77). Education was analyzed as a three-category variable indicating whether the respondent had completed high school or less; had some college education but not completed it; vs. had completed a college education. Annual household income was dichotomized at the median level ($60K) for purposes of analysis.
Additional variables that could potentially influence the daily cortisol trajectory were obtained for every day of the cortisol measurement from the participant during the nightly telephone interviews, and included the length of sleep the previous night, morning waking time, night bedtime, and whether or not it was a weekend day - a distinction that was made only for those who were employed. To capture the influence of too little sleep and too much sleep, we categorized the previous night's sleep time into three groups: <6 hours, 6-8 hours, and >8 hours. Waking time and bedtime were used to compute the length of the waking day, which was averaged over all eight diary days, to get average wake-day length for every participant.
A third set of variables that may mediate the associations between demographic/socioeconomic characteristics and daily cortisol trajectories were collected from participant responses to questionnaires, and included depression (yes/no), a count (range, 0 to 4) of major chronic health conditions (Piazza et al., 2012), body mass index (calculated from reported weight and height), current smoking status (yes/no), mean number of cigarettes smoked per day over the eight days of the diary study, mean physical activity (minutes per day) over the eight days, current use of oral steroid medications (yes/no), and current use of anti-anxiety or anti-depressant medications (yes/no).
2.3. Analysis
Since multiple previous studies have demonstrated that cortisol rhythms are driven by time elapsed since awakening and less by clock time (van Cauter, 1990; Steptoe et al., 2003; Clow et al., 2004; Fries et al., 2009; Kumari et al. 2010), we examined cortisol trajectories as a function of time since waking, after excluding measurements for days with extreme waking times (before 0400h and after 1100h) and extreme waking day lengths (longer than 20 hours).
Although participants had been instructed to take the second saliva sample of the day 30 minutes after waking, there was significant variability in actual time of sampling, with the second sample collected between 22.5 minutes and 37.5 minutes after waking in less than 65% of collection days. In 4% of collection days, the second sample was taken between 7.5 and 22.5 minutes after waking, in 20% of days between 37.5 and 60 minutes after waking, and in 11% of the days an hour or more after waking. There was even more variability in the timing of the 3rd (pre-lunch) and 4th (bedtime) saliva samples; this allowed us to examine cortisol values at different times during the day in the population (albeit across different individuals) to get a general idea of the shape of the mean daytime cortisol trajectory.
We first plotted salivary cortisol sample means over consecutive (non-overlapping) 15 minute intervals as a function of time since waking. Because of considerable right-sided skew in the distribution of cortisol, and because several bed-time cortisol measurements were smaller than 1 nmol/L, we also examined plots of the log of (cortisol + 1 nmol/L), also averaged over consecutive 15-minute intervals. A visual comparison of the two mean plots indicated that the log cortisol trajectory has less variability around straight-line segments than the raw cortisol trajectory (Taylor et al., 2011). A four-piece, piece-wise linear growth curve (with knots at 0.5 hours, 4.5 hours, and 15 hours after waking, as determined by an examination of the mean plots) fit the log cortisol data substantially better than it fit the raw cortisol data: R-square of 55.6% vs. 42.4%. We therefore chose to model the daytime trajectories of log cortisol rather than raw cortisol.
Next, to examine the consistency of the general shape of the daytime trajectory across the population, we plotted trajectories of log cortisol over different strata of the study sample: 1) age groups (<50, 50-64, and >64 years); 2) gender; 3) annual household income ($60K and less, more than $60K); 4) time of waking (before or after 0642h, the median waking time in the study); and 5) bedtime (before or after 2230h, the median bedtime in the study). The general form of the daytime log cortisol trajectories and the location of inflexion points were very consistent across these groups, with every group showing a morning peak 30 minutes after waking, then a steep decline from the peak for 4 hours, followed by a more gradual decline for 10.5 hours, and a final plateau or upturn late in the day (strata-specific plots not shown, but available on request).
Accordingly, we decided to model the daytime trajectories of log cortisol as spline functions of time since waking, with the first knot fixed at 0.5 hours (30 minutes). We experimented with three distinct specifications (parameterizations) of the trajectory of log-cortisol: 1) Linear spline: Piece-wise linear trajectory with four linear segments and three fixed knots at 0.5 hours, 4.5 hours, and 15 hours after waking, corresponding to the inflexion points noted in mean plots; 2) Piecewise linear-cubic: Two-piece growth curve, with an initial linear segment up to 0.5 hours after waking, followed by a cubic segment; 3) Quadratic spline with one knot at 0.5 hours after waking. Linear splines with fixed knots have been used previously to model diurnal cortisol rhythms (Ranjit et al. 2005; Dowd et al. 2009); here, we also examined alternate modeling approaches that impose greater degrees of smoothness in the cortisol trajectory and allow for the possibility that inflexion points vary from person to person. Compared to the linear spline model, the piecewise linear-cubic and quadratic spline models do not fix the second and third inflexion times across the population, and model a smoother decay after the peak. The quadratic spline model, in addition, also allows the timing of the peak to vary and models a smooth transition at the peak (Smith, 1979; Harrell et al., 1988).
We used multi-level (four hierarchical levels), linear mixed-effects regression to model the log-cortisol growth curves and to account for within-day, within-person, and within-family correlations in cortisol measurements. All growth curve parameters (intercept, slopes, quadratic, and cubic growth rates) were modeled to vary with individual-level primary demographic / socioeconomic predictors (age, gender, race, and education level) and the following covariates: average wake-day length (individual-level), waking time (day-level), and weekend vs. workday status (day-level). To account for correlation between members of the same family (twin pairs and siblings), we included a random intercept at the family level. To account for correlations between repeated measurements in the same individual, we included random effects at the individual level for all growth curve parameters. In addition, to capture correlations (in an individual) between repeated cortisol measurements in the same day, we included a random intercept at the day level and either a random initial decline slope (for the linear spline and the linear-cubic specifications) or a random quadratic growth rate (for the quadratic spline specification).
Model estimates of intercept, slopes, and quadratic and cubic growth rates were used to estimate the daily peak, the nightly nadir, and the integrated total log-cortisol exposure over 16 hours, or area under the log-cortisol curve (AUC), using formulas listed in the appendix. Robust error variance estimation was used to generate standard errors and confidence intervals for all trajectory parameters and effect sizes.
In supplementary analyses, we added eight potential mediators/confounders to the models (depression, count of health conditions, body mass index, smoking status, mean daily cigarette consumption, mean daily physical activity, oral corticosteroid medication use, and psychiatric medication use), and examined changes in the size of demographic/socioeconomic associations with cortisol trajectory parameters. Stata version 10 was used for all analyses.
3. Results
The study sample consisted of 1,693 individuals from 1,409 families. Forty one percent of the sample was between the ages of 50 and 64 years, 43% were male, and 14% were not Caucasian. Nearly forty percent of the sample had graduated from college, but 30% had high school or less education. Eight percent of the sample reported depression, 15% reported using anti-depressant or anti-anxiety medications, and 3% reported using oral corticosteroid medications. Mean number of major chronic health conditions (range, 0-4) was 0.9, and mean body mass index was 28 kg/m2 On three quarters of the sampling days, participants reported sleeping between six and eight hours the previous night, but on nearly 10% of the days, participants slept less than six hours the previous night. Less than one in eight sampling days (11.8%) were marked as non-work or weekend days by participants who reported being employed.
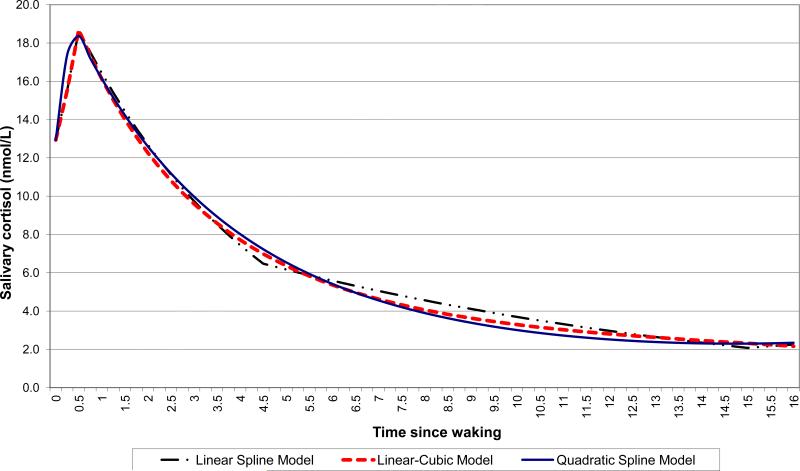
The model-predicted mean daytime cortisol trajectories from the three alternate growth-curve specifications (linear spline, linear-cubic, and quadratic spline) were very similar to each other (Figure 1), despite differences in mean slopes estimated by the three models (Table 1). The residual variance (not explained by the model) for the three alternate specifications was 0.178 for the linear spline model, 0.187 for the linear-cubic model, and 0.190 for the quadratic spline model, corresponding to model-explained percentage variance (pseudo R-squared) of 78.1%, 76.9%, and 76.6% respectively, for the linear spline, linear-cubic, and quadratic spline models.
Figure 1.
Model-predicted mean cortisol trajectory in the sample, based on three alternate growth curve specifications: linear spline, quadratic spline, and piecewise linear-cubic.
Table 1.
Parameters of the mean log-cortisol trajectory, as estimated by three alternate growth curve models
| Alternate Models* | Model Parameters ** | Derived from Model Parameters *** | ||||||
|---|---|---|---|---|---|---|---|---|
| Linear Spline Model: | Intercept (Waking Value) | Slope of morning rise | Slope of first rapid decline | Slope of afternoon decline | Slope of late evening rise | Peak Value | Nadir Value | AUC |
| Fixed effects | ||||||||
| Sample mean | 2.63 | + 0.69 | − 0.242 | − 0.084 | + 0.054 | 2.98 | 1.12 | 29.0 |
| Random effects | ||||||||
| Between days SD | 0.09 | 0.023 | ||||||
| Between persons SD | 0.31 | 0.29 | 0.069 | 0.041 | 0.099 | |||
| Between families SD | 0.23 | |||||||
| Residual SD | 0.42 | |||||||
| Linear-Cubic Model: | Intercept (Waking Value) | Slope of morning rise | Slope of initial decline | Quadratic decline rate | Cubic decline rate | Peak Value | Nadir Value | AUC |
| Fixed effects | ||||||||
| Sample mean | 2.63 | + 0.69 | − 0.289 | + 0.0175 | − 0.00042 | 2.98 | 1.20 | 28.6 |
| Random effects | ||||||||
| Between days SD | 0.08 | 0.012 | ||||||
| Between persons SD | 0.30 | 0.32 | 0.030 | 0.0000 | 0.00005 | |||
| Between families SD | 0.23 | |||||||
| Residual SD | 0.43 | |||||||
| Quadratic Spline Model: | Intercept (Waking Value) | Slope of initial rise | Quadratic growth rate | Quadratic decline rate | Peak Value | Nadir Value | AUC | |
| Fixed effects | ||||||||
| Sample mean | 2.63 | + 1.57 | − 1.8150 | + 1.8237 | 2.96 | 1.19 | 28.3 | |
| Random effects | ||||||||
| Between days SD | 0.07 | 0.0006 | ||||||
| Between persons SD | 0.30 | 0.03 | 0.0000 | 0.0008 | ||||
| Between families SD | 0.23 | |||||||
| Residual SD | 0.44 | |||||||
AUC = Area under the log-cortisol curve from waking to 16 hours after waking SD = standard deviation
All growth curve models indexed on time since waking Linear spline model = Piece-wise linear with knots at 0.5, 4.5, and 15 hours after waking Linear-cubic model = Two pieces; initial linear segment from waking to 0.5 hours after waking, followed by cubic segment that starts at 0.5 hours Quadratic spline model = Quadratic growth (with linear and quadratic terms) starting at waking plus a quadratic term that starts at 0.5 hours
Results of 4-level base models for log (cortisol + 1 nmol/L), adjusted only for individual's average length of waking day, his/her length of sleep the previous night, waking time on day of measurement, and weekend vs. workday status. Random effects included at day level for intercept (waking value) and rapid decline slope, at individual level for intercept (waking value) and all four slopes, and at family level for intercept (waking value) only. Units for cortisol: nmol/L. Thus, units for waking value: log(nmol/L) Units for time: hour. Thus units for slope: log(nmol/L) per hour, for quadratic rate: log(nmol/L) per hour2, for cubic rate: log(nmol/L) per hour3
Formulas for generating peak value, nadir value, and AUC from growth curve parameters are listed in the appendix. Units for peak and nadir: log(nmol/L). Units for AUC: log(nmol/L)-hour.
Consistent with the striking similarity between model-predicted mean trajectories (Figure 1), the model-predicted mean waking, peak, and nadir values and the AUC were also very similar across the three alternate specifications for the log-cortisol growth curve (Table 1). All growth curve parameters for the mean trajectory were statistically different from zero in each model; in addition, the four slopes in the linear spline model were statistically different from each other. There was significant clustering at each of the three higher levels (day, person, and family) as indicated by the random effect variances for the intercept and growth curve parameters in each model specification (Table 1). After back-transformation from log (cortisol + 1 nmol/L) to raw cortisol, model-predicted mean waking value was 12.9 nmol/L in all three specifications, predicted mean peak was 18.7 nmol/L in two of the three models and 18.4 nmol/L in the quadratic spline model, and predicted mean nadir was 2.1 nmol/L in the linear spline model and 2.3 nmol/L in the other two models. Back-transformation of mean slopes from the linear spline model yields the following mean growth and decay rates for the diurnal cortisol rhythm: Morning rise of 41% in 30 minutes, followed by 21.5% decline per hour for 4 hours, then 8.1% decline per hour for 10.5 hours, and a late evening rise (after 15 hours have passed since waking) of 5.6% per hour.
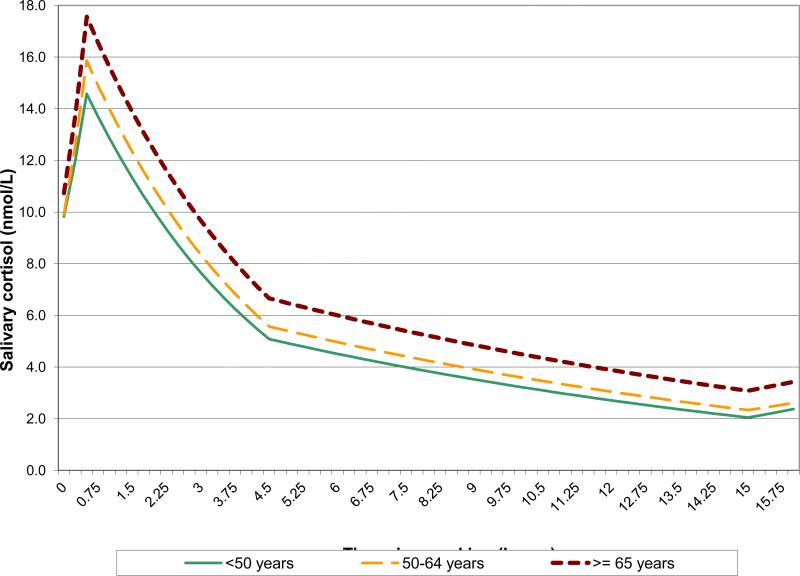
Demographic and socioeconomic differences in the daytime cortisol trajectory were consistent across models. In the interest of brevity, we present results from the full models for two of the three growth curve specifications: linear spline model and linear-cubic model (Table 2), and visually illustrate the demographic differences using the results from only the full linear spline model (Figures 2-4). Older age and male gender were associated with higher cortisol values during the entire day. Older individuals, compared to younger participants, had significantly higher average waking values, peaks, nadirs, and AUC (Table 2, Figure 2). There was a clear ‘dose-response’ with the oldest (65 years and older) group having significantly higher average peak, nadir, and AUC than the middle-age (50-64 years) group, and the middle age group having higher values than the youngest (<50 years) group. Men, compared to women, also had higher average waking value, nadir, and AUC (Table 2). The average male-female differences in waking value, nadir, and AUC were as large as the average differences between the middle-age and youngest-age groups.
Table 2.
Adjusted Associations (and 95% confidence intervals)* with Log Cortisol Trajectory Parameters
| A. Linear Spline Model** | ||||||||
|---|---|---|---|---|---|---|---|---|
| Waking Value | Slope of morning rise | Slope of first rapid decline | Slope of afternoon decline | Slope of late evening rise | Peak Value | Nadir Value | AUC | |
| Age (ref: <50 y) | ||||||||
| 50-64 y | + 0.01 [−0.04, +0.06] | + 0.14 [+0.06, 0.22] | + 0.000 [−.014, +.013] | + 0.001 [−.006, +.009] | − 0.02 [−0.06, +0.01] | + 0.08 [+0.03, +0.13] | + 0.09 [+0.01, +0.17] | + 1.29 [+0.37, +2.22] |
| 65-74 y | + 0.08 [+0.02, +0.14] | + 0.19 [+0.10, 0.28] | + 0.014 [−.001, +.029] | + 0.006 [−.002, +.014] | − 0.02 [−0.06, +0.02] | + 0.17 [+0.11, +0.23] | + 0.29 [+0.20, +0.39] | + 3.90 [+2.87, +4.93] |
| Sex: Male | + 0.11 [+0.07, +0.15] | − 0.19 [−0.26, −0.12] | + 0.034 [+.023, +.046] | − 0.006 [−.012, +.000] | − 0.03 [−0.06, +0.01] | + 0.01 [−0.03, +0.06] | + 0.09 [+0.02, +0.16] | + 1.72 [+0.93, +2.50] |
| Race: Non-white | − 0.29 [−0.35, −0.23] | + 0.11 [+0.01, +0.22] | + 0.040 [+.023, +.056] | + 0.025 [+.016, +.034] | + 0.02 [−0.02, +0.06] | − 0.23 [−0.30, −0.17] | + 0.19 [+0.08, +0.29] | + 0.05 [−1.07, +1.17] |
| Education (ref: College grad) | ||||||||
| Some college | − 0.08 [−0.13, −0.03] | − 0.01 [−0.09, +0.07] | + 0.019 [+.006, +.033] | + 0.001 [−.006, +.008] | + 0.01 [−0.03, +0.05] | − 0.08 [−0.13, −0.03] | + 0.01 [−0.08, +0.09] | − 0.19 [−1.13, +0.75] |
| <= High school | − 0.14 [−0.19, −0.09] | + 0.08 [−0.00, +0.16] | + 0.015 [+.001, +.028] | + 0.010 [+.003, +.018] | − 0.02 [−0.06, +0.02] | − 0.10 [−0.16, −0.05] | + 0.06 [−0.02, +0.15] | − 0.21 [−1.16, +0.74] |
| Average wake-day length (per hour) | − 0.01 [−0.02, +0.01] | + 0.08 [+0.05, +0.11] | − 0.001 [−.006, +.004] | − 0.005 [−.008, −.001] | + 0.02 [+0.00, +0.04] | + 0.03 [+0.01, +0.05] | − 0.02 [−0.05, +0.02] | + 0.21 [−0.13, +0.55] |
| Weekend day | − 0.09 [−0.13, −0.05] | − 0.07 [−0.18, +0.03] | + 0.016 [+.000, +.031] | + 0.007 [−.001, +.014] | + 0.00 [−0.04, +0.05] | − 0.13 [−0.17, −0.09] | + 0.00 [−0.05, +0.06] | − 0.79 [−1.28, −0.29] |
| Waking before 6:40am | + 0.00 [−0.03, +0.03] | + 0.12 [+0.05, +0.19] | + 0.012 [+.001, +.023] | − 0.011 [−.017, −.005] | − 0.02 [−0.05, +0.02] | + 0.06 [+0.03, +0.09] | − 0.00 [−0.05, +0.05] | + 0.91 [+0.49, +1.33] |
| Sleep duration (ref: 6-8 hours) | ||||||||
| < 6 hours | − 0.07 [−0.11, −0.03] | + 0.03 [−0.08, 0.14] | + 0.019 [−.001, +.036] | + 0.004 [−.005, +.014] | − 0.04 [−0.08, −0.00] | − 0.06 [−0.10, −0.01] | + 0.06 [−0.01, +0.14] | + 0.38 [−0.20, +0.96] |
| > 8 hours | − 0.01 [−0.04, +0.03] | − 0.04 [−0.13, 0.06] | − 0.005 [−.019, +.009] | + 0.002 [−.004, +.008] | + 0.07 [+0.01, +0.13] | − 0.02 [−0.06, +0.02] | − 0.02 [−0.07, +0.03] | − 0.48 [−0.94, −0.02] |
| B. Linear-Cubic Model** | ||||||||
|---|---|---|---|---|---|---|---|---|
| Waking Value | Slope of morning rise | Slope of initial decline | Quadratic decline rate | Cubic decline rate | Peak Value | Nadir Value | AUC | |
| Age (ref: <50 y) | ||||||||
| 50-64 y | + 0.01 [−0.04, +0.06] | + 0.15 [+0.07, 0.23] | − 0.010 [−.031, +.011] | + 0.0024 [−.0014, +.0061] | − 0.00012 [−0.00028, +0.00005] | + 0.08 [+0.03, +0.14] | + 0.08 [+0.00, +0.15] | + 1.36 [+0.47, +2.24] |
| 65-74 y | + 0.08 [+0.03, +0.14] | + 0.19 [+0.10, 0.20] | − 0.004 [−.020, +.028] | + 0.0016 [−.0028, +.0060] | − 0.00010 [−0.00030, +0.00009] | + 0.18 [+0.12, +0.24] | + 0.27 [+0.19, +0.36] | + 3.89 [+2.90, +4.88] |
| Sex: Male | + 0.11 [+0.07, +0.15] | − 0.19 [−0.26, −0.12] | + 0.038 [+.020, +.057] | − 0.0028 [−.0061, +.0005] | + 0.00003 [−0.00011, +0.00018] | + 0.02 [−0.03, +0.06] | + 0.08 [+0.02, +0.15] | + 1.88 [+1.13, +2.63] |
| Race: Non-white | − 0.29 [−0.35, −0.23] | + 0.12 [+0.02, +0.23] | + 0.048 [+.021, +.075] | − 0.0029 [−.0076, +.0017] | + 0.00011 [−0.00009, +0.00031] | − 0.23 [−0.29, −0.16] | + 0.18 [+0.09, +0.27] | + 0.01 [−1.05, +1.09] |
| Education (ref: College grad) | ||||||||
| Some college | − 0.08 [−0.12, −0.03] | − 0.01 [−0.09, +0.08] | + 0.026 [+.004, +.048] | − 0.0028 [−.0067, +.0011] | + 0.00010 [−0.00007, +0.00027] | − 0.08 [−0.13, −0.03] | + 0.01 [−0.07, +0.08] | − 0.22 [−1.12, +0.68] |
| <= High school | − 0.14 [−0.19, −0.09] | + 0.08 [−0.00, +0.17] | + 0.007 [−.015, +.029] | + 0.0015 [−.0024, +.0055] | − 0.00009 [−0.00027, +0.00008] | − 0.10 [−0.16, −0.05] | + 0.04 [−0.03, +0.12] | − 0.19 [−1.10, +0.73] |
| Average wake-day length (per hour) | − 0.00 [−0.02, +0.01] | + 0.07 [+0.04, +0.11] | + 0.014 [+.005, +.023] | − 0.0036 [−.0051, −.0021] | + 0.00018 [+0.00011, +0.00024] | + 0.03 [+0.01, +0.05] | + 0.02 [−0.01, +0.04] | + 0.24 [−0.09, +0.57] |
| Weekend day | − 0.09 [−0.13, −0.05] | − 0.08 [−0.18, +0.03] | + 0.021 [−.006, +.048] | − 0.0019 [+.0065, +.0027] | + 0.00006 [−0.00013, +0.00026] | − 0.13 [−0.17, −0.09] | − 0.01 [−0.06, +0.03] | − 0.88 [−1.41, −0.35] |
| Waking before 6:40 am | + 0.00 [−0.02, +0.03] | + 0.12 [+0.04, +0.19] | + 0.038 [+.018, +.058] | − 0.0053 [−.0089, −.0016] | + 0.00017 [+0.00001, +0.00033] | + 0.06 [+0.03, +0.09] | + 0.03 [−0.01, +0.07] | + 1.52 [+1.08, +1.96] |
| Sleep duration (ref: 6-8 hours) | ||||||||
| < 6 hours | − 0.08 [−0.12, −0.03] | + 0.03 [−0.08, 0.15] | + 0.017 [−.012, +.046] | + 0.0007 [−.0042, +.0057] | − 0.00009 [−0.00037, +0.00011] | − 0.06 [−0.11, −0.01] | + 0.05 [−0.01, +0.11] | + 0.61 [+0.02, +1.21] |
| > 8 hours | − 0.00 [−0.04, +0.03] | − 0.04 [−0.14, 0.05] | + 0.009 [−.017, +.035] | − 0.0037 [−.0084, +.0012] | + 0.00021 [−0.00001, +0.00043] | − 0.03 [−0.07, +0.01] | − 0.02 [−0.06, +0.03] | − 0.77 [−1.28, −0.26] |
Statistically significant associations (p value < 0.05) are in bold font, and marginally significant associations (0.05<= p < 0.075) are in italics.
See footnotes to Table 1 for model descriptions and units for effect sizes
AUC = Area under the log-cortisol curve from waking to 16 hours after waking
Figure 2.
Mean cortisol trajectory by age group, as estimated by the linear spline model
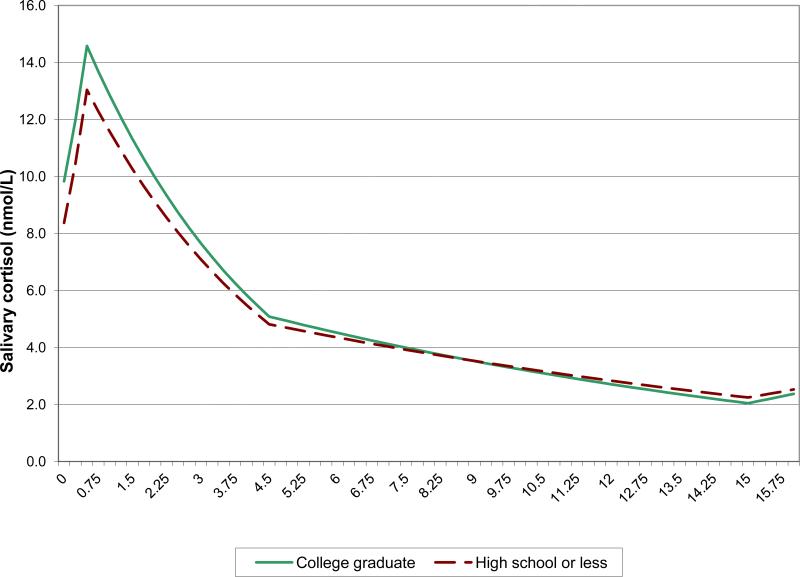
Figure 4.
Mean cortisol trajectory in the highest and lowest education groups, as estimated by the linear spline model
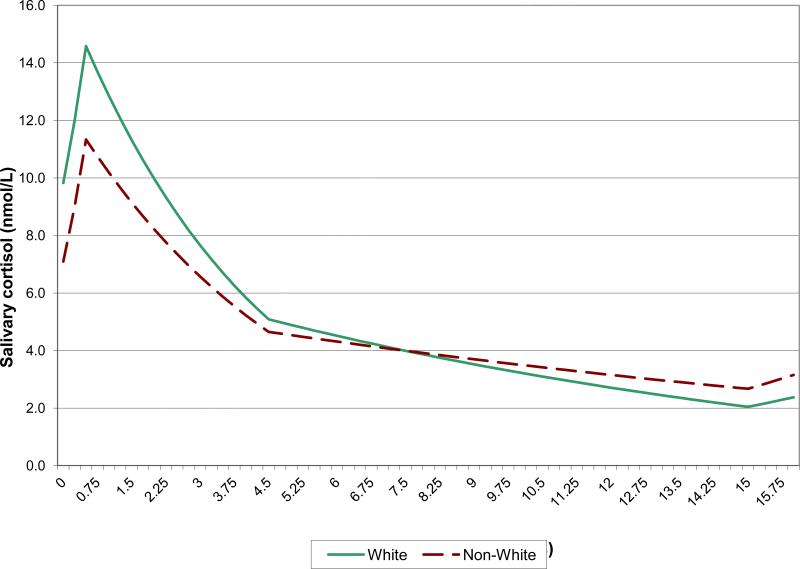
In contrast to the effects of old age and male gender, which were to raise the entire cortisol trajectory, race and SES affected peak and nadir in different directions. Thus, non-Caucasians had lower average waking and peak values but higher average nadirs (i.e., flatter cortisol rhythms, on average) compared to Caucasians, and their AUCs were no different (Table 2 and Figure 3). Similarly, less educated individuals had lower average waking and peak values than more educated individuals, but their nadirs and AUCs were comparable (Table 2). Although those with less education achieved smaller cortisol peaks than those more educated, their cortisol levels declined more gradually from the peak, so that there was no difference in nadir by level of education (Figure 4). Both minority race status and low education status were associated with blunting of the morning cortisol peak, and minority race status was associated additionally with higher resting levels, so that the dynamic range in minority race participants was limited from both above and below (Fig 3). Race differences in average waking and peak values were more than twice the corresponding differences between the lowest and highest education groups.
Figure 3.
Mean cortisol trajectory by race, as estimated by the linear spline model
In addition to these demographic differences, there were also substantial between-person and day-to-day differences in cortisol rhythms by waking time, length of the average wake-day, duration of sleep the previous night, and weekend vs. working-weekday status (Table 2). People who stayed awake longer on average (as determined by the 8-day average of wake-day lengths) had faster morning rises and higher peaks, but comparable waking values, nadirs, and AUCs. Peak values and AUCs were also higher on days that participants awoke early (before 0640h). In contrast, employed individuals had lower waking values, peaks, and AUCs on weekend days compared to weekdays. Similarly sleeping more than eight hours the previous night was associated with lower AUC (Table 2).
In supplementary analyses that included controls for potential mediators (health conditions, health behaviors, body mass index, and medications), demographic and socioeconomic differences in daily cortisol trajectories were minimally changed (data not shown).
4. Discussion
This national study documented striking socioeconomic and demographic differences in daytime cortisol trajectories. Disadvantaged individuals in our society, both those with less education and those from minority race/ethnicity communities, had flatter cortisol rhythms, with lower morning peaks and higher late-evening nadirs. Consistent with a dose response, mean cortisol peak was highest in college graduates, significantly lower in those with some college-level education, and lowest in those with no more than high school education. The differences in mean peak and nadir levels by race (between white and non-white individuals) were larger in magnitude than differences by education (between college graduates and those with at-most high school education). Low education and minority race/ethnicity status were associated with lower peaks and higher nadirs, but the accumulated daytime exposure to cortisol, (as measured by the log-cortisol AUC) was not different by either race/ethnicity or level of education.
In contrast, age and gender associations with cortisol peak levels were in the same direction as their associations with cortisol nadir, leading to significant differences in accumulated daytime exposure to cortisol. Thus, the entire daytime cortisol trajectory (including waking, peak, and nadir values and AUC) was higher in older than in younger participants and in men than in women. There was a clear dose response with age, with the oldest group having significantly higher mean levels (and AUC) than the middle group (ages 50-64 years), and the latter having significantly higher mean levels (and AUC) than the youngest group. The male-female differences in mean nadir and AUC were comparable to corresponding differences between the mid-age and youngest-age groups.
The education and race/ethnicity differences in cortisol daytime trajectories seen here are consistent with the chronic stress hypothesis, wherein chronic stress leads to both a blunting of the cortisol awakening response due to burnout and a diminished ability to recover from daily challenges, so that cortisol resting nadirs are elevated (Pruessner et al., 1999; Morgan et al., 2002; Kudielka et al., 2006; Miller et al. 2007). Our findings confirm and extend findings from previous studies that, at least partly, suggest flattening of daytime cortisol rhythms in less privileged groups. Lower salivary cortisol levels in the morning have been documented in African Americans compared to Caucasians (Bennett et al., 2004; Cohen et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Skinner et al., 2011) and in less educated compared to more educated individuals (Bennett et al., 2004; Hajat et al., 2010; Dowd et al., 2011). Some of the same studies have also found higher evening levels of salivary cortisol in African Americans (Cohen et al., 2006; DeSantis et al., 2007; Skinner et al., 2011) and the less educated (Cohen et al., 2006), but no differences in overall exposure to cortisol, as measured by the daytime AUC, by either race or education (Cohen et al., 2006; Hajat et al., 2010). Since flatter cortisol trajectories over the waking day have been associated, at least cross-sectionally, with lower cognitive functioning (Fiocco et al., 2006; Stawski et al., 2011) and with frailty (Varadhan et al., 2008), such flattening of daytime cortisol rhythms may represent an important biological pathway from chronic stress to poor health and functioning.
In contrast to the blunting of dynamic range (lower peaks and higher nadirs) seen in association with both low SES and minority race/ethnicity status, older age and male gender were independently associated with increase in both peaks and nadirs, as seen previously by van Cauter et al. (1996). Similar age effects have been reported by others (Deuschle et al., 1997; Touitou and Haus, 2000; Larsson et al., 2009), but gender differences have not been observed as consistently. The higher nadirs seen in the less educated and in non-whites, and the higher nadir and AUCs seen in the elderly and in men, are all consistent with the generally poorer health observed in these demographic groups. It suggests that such changes in the diurnal cortisol rhythm may represent one potential biological pathway to poorer health in these groups.
Our study also found that relative to waking time, the timing of peaks and nadirs is broadly consistent across demographic groups and is not affected by non-extreme differences in waking time, justifying the use of splines with fixed knots to model daytime cortisol trajectories, as first suggested by Ranjit et al. (2005). These findings are consistent with previous studies that have found no effect of gender or age on the timing of the morning cortisol peak (van Cauter et al., 1996). Touitou and Haus (2000) concluded that the cortisol circadian rhythm is inherently robust and is not affected by day-to-day changes in waking time and even by jet lag.
In contrast to the stability of peak and nadir timing, our study did find that cortisol levels and slopes vary with time of waking, duration of sleep the previous night, length of the average wake-day, and weekend vs. weekday status. These findings are consistent with previous studies showing that waking time and sleep duration independently affect cortisol levels and slopes (Edwards et al., 2001; Kumari et al., 2009), and that the awakening response is greater on working weekdays than on weekend days (Kunz-Ebrecht et al., 2004). Taken together, these findings highlight the importance of collecting cortisol data over multiple days including both working weekdays and weekend days, and of controlling for variations in waking time on the day of collection, sleep duration the previous night, typical wake-day length, and weekend vs. weekday status. This is especially important because sleep duration and waking times vary significantly across demographic groups and by SES (van Cauter and Spiegel, 1999; Lichstein et al., 2004).
Other methodology-related findings from this study were that a) piece-wise linear trajectories are a better fit to log-transformed cortisol than to raw cortisol, consistent with first-order decay in cortisol from its peak, as first documented in serum and saliva by Umeda et al. (1981) and confirmed in later studies (Purnell et al., 2004); b) linear spline modeling does as good a job of estimating mean trajectories and group differences in cortisol trajectories as higher-order models (that do not fix inflexion times across the population and fit smoother transitions between phases of the trajectory). Estimated mean waking values, peaks, nadirs, and AUCs, as well as group differences in these parameters were remarkably consistent across alternate growth curve specifications. In comparison, slope estimates varied from model to model. This is primarily because slopes from the linear spline model represent average slopes over each segment, while slopes from higher-order models represent instantaneous initial slope in a segment. The observed difference between initial and average recovery slope for instance, suggests that slope estimates from the linear spline model would be sensitive to where the second knot is placed. Unfortunately, the placement of the second knot has been different in different research studies (e.g., Ranjit et al., 2005, Hajat et al., 2010; Dowd et al., 2011), limiting recovery slope comparisons across studies. Inflexion points are more likely to be detected where data density is high (Dowd et al., 2011); thus empiric knot placement is dependent on the collection strategy (specifically, timing of saliva collection) employed by the study. However, since estimates of cortisol levels (waking, peak, and nadir values) and AUC and their associations with predictors were shown to be robust to model specification, they should be less affected by collection strategy and knot placement.
The deleterious downstream effects of cortisol on health are thought to be from sustained increases in cortisol levels and accumulated exposure (Porter and Landfeld, 1998; Purnell et al., 2004; Agnostis et al., 2009); thus nadir levels and AUC are likely to be more directly relevant to health, than are slopes.
Lastly, our study also found that there is significant correlation (clustering) within collection days and within individuals, as apparent from the variances of random effects at both the day level and person level, demonstrating the importance of using multi-level modeling or related approaches to account for this clustering when estimating standard errors and p values.
Limitations of our study include its cross-sectional design, which limit causal inference; however, the demographic and socioeconomic predictors examined (chronological age, gender, race/ethnicity, and educational attainment) are stable, and could not have been affected by diurnal cortisol rhythms. Moreover, the dose responses seen for both education and age are very suggestive of causal roles for them in daily cortisol trajectories, although a common causal precedent (such as genetics or family environment) for low educational attainment and blunted cortisol trajectories cannot be ruled out. A second major limitation of our study is between-participant variability in cortisol sampling time (relative to time of waking) which affects measured cortisol values. Our use of smart boxes that record the clock time that the box containing the salivette was opened, combined with our use of spline models with fixed knots reduces, but does not completely eliminate, the impact of sampling time on cortisol trajectory parameters, since waking times were self-reported and not objectively measured.
The study also has several major methodological strengths, which include a large national sample, measurement of cortisol over multiple days including at least one weekend day, objective recording of cortisol sampling time using smart boxes, use of splines with a knot fixed at the expected peak time to allow estimation of the cortisol peak value even when the second sample is not timed exactly at the peak, use of multi-level models to account for within-day, within-person and within-family correlations, and inclusion of controls for differences in waking time and sleep duration. To our knowledge, this is also the first study that did a head-to-head comparison of linear and higher-order piecewise models for cortisol daytime trajectories.
In conclusion, this study demonstrates the ability of linear spline modeling of log-transformed cortisol data to robustly estimate cortisol peak, nadir, and AUC, and their associations with hypothesized predictors. We found that daytime trajectories were flatter (with lower peaks and higher nadirs) in less privileged segments of our society, and that cortisol levels over the day were higher in older ages and in men compared to women, which is consistent with worse overall health on average in socially disadvantaged compared to more advantaged individuals, in old compared to the young, and in men compared to women. Such alterations in the diurnal cortisol rhythm in disadvantaged individuals may represent one mechanism by which social stressors lead to poor health outcomes.
Acknowledgements
This work was supported by the National Institute on Aging under grants P01-AG020166, R01-AG019239, R01-AG033067, R01-AG020166, and P30-AG028748, and by the National Institutes of Health's General Clinical Research Centers Program under grant M01-RR00865. The first wave of MIDUS was supported by the John D. and Catherine T. MacArthur Foundation. The Robert Wood Johnson Foundation Health & Society Scholars program and the National Science Foundation's UCLA Interdisciplinary Relationship Science Program also partly supported this work.
Abbreviations
- MIDUS
Midlife in the United States
- NSDE
National Study of Daily Experiences
- SES
Socioeconomic Status
Appendix. Peak, Nadir, and Area Under the Curve Formulas
Formulas for calculating the log-cortisol morning peak (at 0.5 hours after waking), evening nadir (at 15 hours after waking), and the integrated total log-cortisol exposure (area under the curve, AUC) over 16 hours, from growth curve parameters, are provided below. Units for peak and nadir are log(nmol/L) and for AUC log(nmol/L)-hour. Peak and nadir timings could have varied from person to person (and even from day to day), and two of the growth curve model specifications allowed for such variation; however, the mean trajectory in the sample peaked at 0.5 hours and nadired at 15 hours in all three model specifications (Figure 2).
Growth Curve Specification 1: Linear Spline Model
Growth Curve Parameters: Intercept, Slope1, Slope2, Slope3, and Slope4. The intercept is the waking value (in log(nmol/L) and the slopes (in units of log(nmol/L) per hour) for each of the four linear segments: Time since waking 0 to 0.5 hours, 0.5 to 4.5 hours, 4.5 to 15 hours, and more than 15 hours.
Peak = Intercept + 0.5 *slope1
Nadir = Peak + 4 *slope2 + 10.5 *slope3
AUC = 16 *Intercept + 7.875 *slope1 + 54 *slope2 + 65.625 *slope3 + 0.5 *slope4
Growth Curve Specification 2: Linear-Cubic Model
Growth Curve Parameters: Intercept, Slope1, Slope2, Quadratic2, and Cubic2. The intercept is the waking value (in log(nmol/L), slope1 is the morning rise slope (in units of log(nmol/L) per hour), and slope2, quadratic2, and cubic2 are the parameters of the cubic recovery segment that starts 0.5 hours after waking.
Peak = Intercept + 0.5 *slope1
Nadir = Peak + 14.5 *slope2 + 14.52 *quadratic2 + 14.53 *cubic2
AUC = 16 *Intercept + 7.875 *slope1 + 15.52 *0.5*slope2 + 15.53 *0.333*quadratic2 + 15.54 *0.25*cubic2
Growth Curve Specification 3: Quadratic Spline Model
Growth Curve Parameters: Intercept, Slope1, Quadratic1, and Quadratic2. The intercept is the waking value (in log(nmol/L), slope1 is the initial slope immediately after waking (in units of log(nmol/L) per hour), quadratic1 is the quadratic growth rate, and quadratic2 is the rate of the additional quadratic growth that starts 0.5 hours after waking.
Peak = Intercept + 0.5 *slope1 + 0.52 *quadratic1
Nadir = Intercept + 15 *slope1 + 152 *quadratic1+ 14.52 *quadratic2
AUC = 16 *Intercept + 162 *0.5*slope1 + 163 *0.333*quadratic1 + 15.53 *0.333*quadratic2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Arun Karlamangla and Dave Almeida designed the study and wrote the protocol. Drs. Esther Friedman and Arun Karlamangla undertook the statistical analyses. Drs. Robert Stawksi, Teresa Seeman, and Arun Karlamangla interpreted the findings. Dr. Karlamangla also managed the literature searches and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. PNAS. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnostis P, Athyros VG, Tziomalos K, et al. The pathogenetic role of cortisol in the metabolic syndrome. J Clin Endocrinol Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Inter-individual differences and intra-individual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24:819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: A preliminary investigation. Ethnicity & Health. 2004;9(4):337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. How Healthy are We? : A National Study of Well-being at Midlife. University of Chicago Press; Chicago: 2004. [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The Awakening Cortisol Response: Methodological Issues and Significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and distress. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/Ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socioeconomic status, cortisol, and allostatic load: A review. Intl J Epidemiol. 2009:1–13. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Ranjit N, Do DP, Young EA, House JS, Kaplan GA. Education and levels of salivary cortisol over the day in US adults. Ann Behav Med. 2011;41:13–20. doi: 10.1007/s12160-010-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: Relation to cognitive functioning. Stress. 2006;9(3):143–152. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The Cortisol Awakening Response (CAR), Facts and Future Directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez Roux A, Franklin TG, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FK, Jr., Lee KL, Pollock BG. Regression models in clinical studies: Determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, et al. Several daily measurements are necessary to assess the cortisol rise after awakening: State and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Bellingrath S, Hellhammer DH. Cortisol in burnout and vital exhaustion: An overview. G Ital Med Lav Ergon. 2006;28(1 Suppl 1):34–42. [PubMed] [Google Scholar]
- Kumari M, Badrick E, Ferrie J, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II Study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, et al. Measures of social position and cortisol secretion in an aging population: Findings from the Whitehall II Study. Psychosom Med. 2010;72:27–34. doi: 10.1097/PSY.0b013e3181c85712. [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-Cause and cardiovascular mortality: Findings from the Whitehall II Study. J Clin Endocrinol Metab. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–28. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ. Epidemiology of Sleep: Age, Gender, and Ethnicity. Lawrence Erlbaum Associates; Mahwah: 2004. [Google Scholar]
- Lundberg U. Stress hormones in health and illness. Psychoneuroendocrinology. 2005;30(10):1017–1021. doi: 10.1016/j.psyneuen.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Marin TJ, Martin TM, Blackwell E, Stetler C, Miller GE. Differnetiating the impact of episodic and chronic stressors on hypothalamic-pituitary-adrenal axis regulation in young women. Health Psychol. 2007;26(4):447–455. doi: 10.1037/0278-6133.26.4.447. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must It come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Cho T, Hazlett G, Coric V, Morgan J. The impact of burnout on human physiology and on operational performance: A prospective study of soldiers enrolled in the Combat Diver Qualification Course. Yale J Biol Med. 2002;75:199–205. [PMC free article] [PubMed] [Google Scholar]
- Piazza JR, Charles ST, Sliwinski M, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Annals of Behavioral Medicine. Advance online publication. 2012 doi: 10.1007/s12160-012-9423-0. doi: 10.1007/s12160-012-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter NM, Landfeld PW. Stress hormones and brain aging: Adding injury to insult? Nature Neuroscience. 1998;1(1):3–4. doi: 10.1038/196. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien S. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Brandon DD, isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–287. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff C. Who Participates? Accounting for Longitudinal Retention in the MIDUS National Study of Health and Well-Being. J Aging Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30:615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux A, Sanchez B, Seeman T, Shea S, Shrager S, Watson K. Association of Salivary Cortisol Circadian Pattern With Cynical Hostility: Multi-Ethnic Study of Atherosclerosis. Psychosom Med. 2009;71:748–755. doi: 10.1097/PSY.0b013e3181ad23e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Develop Psychopath. 2011;23:1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL. Splines as a useful and convenient statistical tool. The American Statistician. 1979;33(2):57–62. [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: Timing is everything. J Gerontol B Psychol Sci Soc Sci. 2011;66B(suppl 1):i71–i81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot M. Socioeconomic Status and Stress-Related Biological Responses Over the Working Day. Psychosom Med. 2003;65:461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Karlamangla AS, Friedman EM, Seeman TE. Early environment affects neuroendocrine regulation in adulthood. Soc Cogn Affect Neurosci. 2011;6(2):244–251. doi: 10.1093/scan/nsq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou Y, Haus E. Alterations with aging of the endocrine and neuroendocrine circadian systems in humans. Chronobiol Intl. 2000;17(3):369–390. doi: 10.1081/cbi-100101052. [DOI] [PubMed] [Google Scholar]
- Umeda T, Hiramatsu R, Iwaoka T, Shimada T, Miura F, Sato T. Use of saliva for monitoring unbound free cortisol levels in serum. Clinica Chimico Acta. 1981;110:245–253. doi: 10.1016/0009-8981(81)90353-3. [DOI] [PubMed] [Google Scholar]
- Varadhan R, Walston J, Cappola AR, et al. Higher levels and blunted diurnal variation of cortisol in frail older women. J Geron Med Sci. 2008;63A(2):190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- van Cauter E. Diurnal and Ultradian Rhythms in Human Endocrine Function: A Mini Review. Hormone Research. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- van Cauter E, Spiegel K. Sleep as a mediator of the relationship between socioeconomic status and health: A hypothesis. Annals New York Academ Sci. 1999;896:254–261. doi: 10.1111/j.1749-6632.1999.tb08120.x. [DOI] [PubMed] [Google Scholar]