Abstract
Objective
To determine whether 1 of 2 vaccines based on dendritic cells (DCs) and poxvectors encoding CEA (carcinoembryonic antigen) and MUC1 (PANVAC) would lengthen survival in patients with resected metastases of colorectal cancer (CRC).
Background
Recurrences after complete resections of metastatic CRC remain frequent. Immune responses to CRC are associated with fewer recurrences, suggesting a role for cancer vaccines as adjuvant therapy. Both DCs and poxvectors are potent stimulators of immune responses against cancer antigens.
Methods
Patients, disease-free after CRC metastasectomy and perioperative chemotherapy (n = 74), were randomized to injections of autologous DCs modified with PANVAC (DC/PANVAC) or PANVAC with per injection GM-CSF (granulocyte-macrophage colony-stimulating factor). Endpoints were recurrence-free survival overall survival, and rate of CEA-specific immune responses. Clinical outcome was compared with that of an unvaccinated, contemporary group of patients who had undergone CRC metastasectomy, received similar perioperative therapy, and would have otherwise been eligible for the study.
Results
Recurrence-free survival at 2 years was similar (47% and 55% for DC/PANVAC and PANVAC/GM-CSF, respectively) (χ2 P = 0.48). At a median follow-up of 35.7 months, there were 2 of 37 deaths in the DC/PANVAC arm and 5 of 37 deaths in the PANVAC/GM-CSF arm. The rate and magnitude of T-cell responses against CEA was statistically similar between study arms. As a group, vaccinated patients had superior survival compared with the contemporary unvaccinated group.
Conclusions
Both DC and poxvector vaccines have similar activity. Survival was longer for vaccinated patients than for a contemporary unvaccinated group, suggesting that a randomized trial of poxvector vaccinations compared with standard follow-up after metastasectomy is warranted. (NCT00103142)
Keywords: cancer vaccine, CEA, poxvectors, T-cell responses, antibody responses
Although unresectable metastatic colorectal cancer (CRC) is usually incurable, 5-year survivals of 10% to 50% have been reported after complete resection of hepatic or pulmonary metastases.1–4 Recurrences are reduced by adjuvant chemotherapy5–7 but remain frequent, suggesting the need for new therapies. Because the host immune response against CRCs is associated with prolonged survival,8 active immunotherapy in which anticancer immune responses are augmented by vaccines has been of considerable interest. The potential utility of immunotherapy for CRC has been suggested by studies in which better clinical outcome was observed in patients who developed immune responses against antigens included in their vaccines.9,10 Prior studies of vaccination after CRC metastasectomy have suggested clinical benefits in subgroup analyses.11–14
Among the antigens targeted by vaccines, carcinoembryonic antigen (CEA) and MUC1 are attractive because they are overexpressed in many CRCs, yet minimally expressed in normal cells,15,16 and they contain epitopes recognizable by cytolytic T cells and antibodies.17–20 Vaccines incorporating CEA or MUC1 have induced immune responses against tumors expressing these antigens.21,22
A variety of platforms have been developed to serve as cancer vaccines, but the most immunogenic strategies include viral vectors encoding tumor antigens23 and dendritic cells (DCs)24 modified with proteins, peptides, mRNA, and viral vectors. Poxviruses have been extensively tested because they possess innate immunostimulatory properties.25 Diversified prime-boost immunizations with poxvectors encoding CEA and MUC1 along with the TRIad of COstimulatory Molecules [CD80 (B7.1), CD54 (ICAM-1), and CD58 (LFA-1)], designated TRICOM26,27 (called PANVAC-VF), have demonstrated clinical and immunologic activities.28 We have extensively tested a series of DC vaccines loaded with CEA peptide29 or mRNA,30 and more recently, observed that autologous DC loaded with CEA-expressing poxvectors could induce the greatest magnitude of CEA-specific T-cell and antibody responses in cancer patients.31 Nonetheless, autologous DC generation is logistically complex, requiring production of a unique product for each patient.
We wished to develop and test a vaccine strategy for use in the adjuvant therapy of resected metastatic CRC. Before performing a randomized study of vaccination compared with standard care, we designed a prospective, randomized phase II clinical trial to address whether prime-boost immunization with DCs modified ex vivo with PANVAC or PANVAC alone would reduce the rate of tumor recurrence, induce more potent tumor antigen–specific immune responses, and prolong survival in the setting of minimal residual disease after liver or lung metastasectomy and after completion of standard chemotherapy. In concert with CTEP (Cancer Therapy Evaluation Program of the National Cancer Institute), a decision was made to first perform a randomized trial to choose a preferred vaccine strategy before undertaking a much larger, pivotal, randomized trial of vaccination compared with no vaccination.
METHODS
Patients
Participants in this 7-site study provided signed informed consent approved by the local governing institutional review board. The major study requirements were histologically confirmed hepatic or pulmonary metastases of colorectal adenocarcinoma that had been completely resected and receipt of a minimum of 2 months of perioperative systemic chemotherapy determined by the treating physician. We chose a minimum of 2 months, based on treatment patterns among those having metastasectomies at the participating institutions and based on EORTC Intergroup trial 40983, which studied perioperative chemotherapy for patients with resected hepatic metastases and reported a range of 1 to 6 doses of chemotherapy before and after surgery.6 Recovery from surgical wound complications, Karnofsky performance status 70% or more, adequate organ function, and no evidence of immune compromise or history of HIV infection were also required.
Study Drugs
PANVAC-V and PANVAC-F were manufactured by Therion Biologics Corporation as part of a Collaborative Research and Development Agreement between Therion and the National Cancer Institute. PANVAC-V is a recombinant vaccinia virus based on TBC-Wy, a parental vaccinia virus derived from the Wyeth vaccine strain that contains the genes for CEA, MUC1, and the 3 costimulatory molecules B7.1, ICAM-1, and LFA-3. The encoded CEA and MUC1 proteins each contain a modification in a single antigenic epitope [for CEA, CAP1(6D) (YLSGADLNL) and for MUC1, MUC1(L93) (ALWGQDVTSV)]. PANVAC-F contains the same 5 recombinant genes inserted into the genome of a parental fowlpox virus derived from the POXVAC-TC vaccine strain. GM-CSF (Leukine) was purchased from the manufacturer.
For patients who received the DC vaccines, a 4-hour leukapheresis product was shipped to the central cell processing facility at Duke University Medical Center. The peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation over Ficoll, and the fraction adherent to plastic flasks after 2 hours incubation were then cultured with GM-CSF and IL-4 for 7 days to enrich for DCs as previously described.31 The DC/PANVAC-V product consisted of 1 × 107 DCs mixed with PANVAC-V [2 × 108 plaque-forming units (pfu)] and the DC/PANVAC-F product consisted of 1 × 107 DCs mixed with PANVAC-F (1 × 109 pfu). These are collectively referred to as DC/PANVAC.
Randomization and Masking
Patients were randomized with equal probability to arm A (DC/PANVAC) or arm B (PANVAC/GM-CSF), with no masking of treatment arm.
Study Treatment
Arm A patients received the DC/PANVAC-V vaccine subcutaneously and intradermally (approximately 900 μL subcutaneously and 100 μL intradermally) into the thigh. Beginning 28 ± 7 days after the first injection and then every 28 ± 7 days thereafter for a total of 3 additional visits, the DC/PANVAC-F vaccine was injected subcutaneously and intradermally (approximately 900 μL subcutaneously and 100 μL intradermally) into the same thigh as previous injections. Arm B patients received PANVAC-V (2 × 108 pfu) for 1 dose, followed by PANVAC-F (1 × 109 pfu) every 28 days for 3 doses subcutaneously into the thigh. GM-CSF (100 μg) was given subcutaneously for 4 consecutive days at the injection sites after each administration of PANVAC-V and PANVAC-F.
Analysis of T-cell Response by IFNγ ELISpot Assay
Cryopreserved PBMCs collected prevaccination (week 0) and 1 week after the completion of all 4 vaccinations (week 13) were analyzed for antigen-specific reactivity by ELISpot assay as previously described.31
Clinical Outcome and Adverse Events
Patients were followed for recurrence with computed tomographic scans and/or magnetic resonance images of the chest, abdomen, and pelvis every 3 months for 2 years, then at the discretion of their physician, and finally for survival. RECIST 1.0 criteria were used to determine relapse. Clinical data were recorded utilizing caBIG metadata standards and software including the Cancer Central Clinical Database (C3D) and the Cancer Central Clinical Participant Registry (C3PR).
In a preplanned analysis, a contemporaneous group of 123 patients with liver metastases and 38 patients with lung metastases who had undergone hepatic or pulmonary metastasectomy at Duke University Medical Center between January 1995 and February 2007 (Duke contemporary group) was also compared with the vaccinated patients taken as a group. Contemporary patients were included only if they would have met the major inclusion criteria of the randomized study (ie, having had complete resections with negative margins and multiagent chemotherapy with irinotecan and/or oxaliplatin, and biologic agents) and were younger than 77 years (the oldest age for a vaccinated patient), had not received immunotherapy, and had not experienced a recurrence within 6 months (182.5 days) of their metastasectomy (to account for the fact that vaccinated patients could not have relapsed before initiation of the vaccinations).
Statistical Analysis
The preplanned clinical endpoints were 2-year recurrence-free survival (RFS) and overall survival (OS). The RFS was measured from the date of metastasectomy, with relapse defined as documented disease recurrence at any site. Within each treatment, a regimen was considered efficacious if the 90% exact lower confidence bound of the 2-year RFS estimate was 0.32 or more, the 2-year RFS calculated from data available during study design from patients undergoing metastasectomy. Follow-up beyond 2 years from the date of last injection was not required in this study; however, all sites were queried for updated recurrence and survival data as of September 2010. The OS was measured from metastasectomy until death from any cause, and patients were censored on the date of last known follow-up. Survival curves, median survival times, and 95% confidence intervals for RFS and OS for each treatment arm and the Duke historical controls were estimated using the method of Kaplan and Meier. Survival curves were compared using the log rank or Wilcoxon tests.
Secondary endpoints included induction of CEA and specific immune responses. A positive immune response by ELISpot was de-fined as described at the 2002 Society of Biologic Therapy Workshop on “Immunologic Monitoring of Cancer Vaccine Therapy”: a T-cell response was considered positive if the mean number of spots in 6 wells with experimental antigen (ie, PANVAC-F) exceeded the mean number of spots in 6 control wells (ie, rF-TRICOM) by 10 and the difference between the 6 wells containing the experimental antigen and the 6 control wells is statistically significant at a level of P ≤ 0.05 using the Student t test.32 We compared the proportion of immune responses between treatment arms using the χ2 test.
RESULTS
Patient Demographics
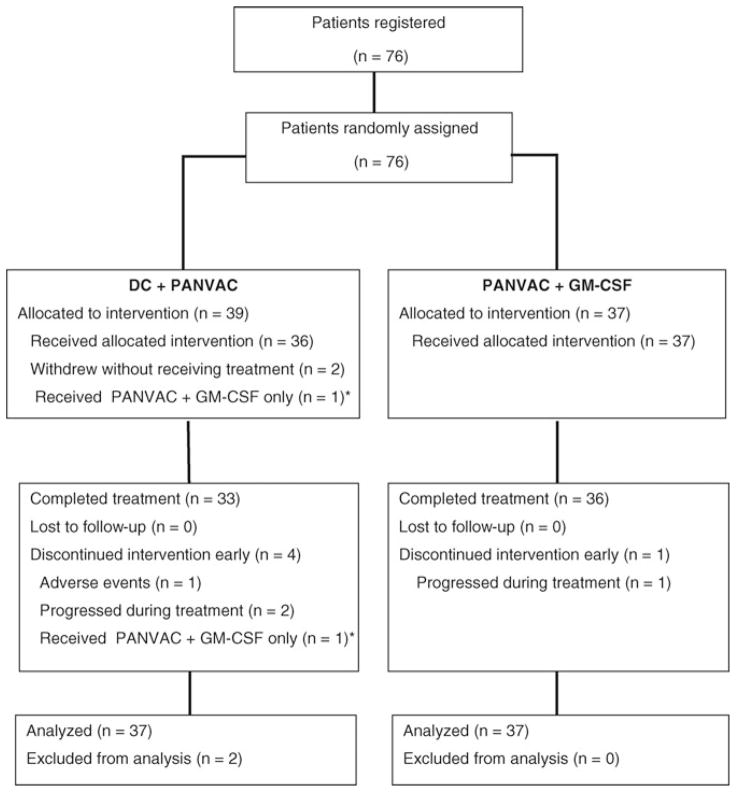
Between January 2005 and September 2009, a total of 76 patients from 7 sites were enrolled and randomized to the 2 arms of the study (38 in each arm) (see the CONSORT diagram in Fig. 1). Two patients on the DC/PANVAC arm withdrew consent before treatment, and no data were captured for these patients. Demographic and baseline disease characteristics are given in Table 1 for the 74 patients who received any vaccinations and for the contemporary unvaccinated patients. Among those with complete data (n = 25), a median of 12 doses (range, 4–14) of multiagent chemotherapy (FOLFOX or FOLFIRI or XELOX) was administered perioperatively and 80% of patients received bevacizumab with their perioperative therapy. Overall, the arms of the study were well balanced, but there were more patients with multiple hepatic lesions in the DC/PANVAC arm (a negative prognostic factor) than with the PANVAC vector arm.
FIGURE 1.
CONSORT diagram. Patient disposition in this clinical trial is described. *Analyzed with DC/PANVAC arm by intention to treat analysis.
TABLE 1.
Patient Demographics
| Characteristic | No. (%)
|
P (PANVAC Trial Total vs Contemp.) | |||
|---|---|---|---|---|---|
| PANVAC + DCs (N = 37) | PANVAC (N = 37) | Total (N = 74) | Duke Contemp. (N = 161) | ||
| Age at resection, median (range), yr | 53.6 (25–77) | 52.0 (33–74) | 53.5 (25–77) | 60.1 (34–77) | 0.001 |
| Sex | 0.13 | ||||
| Male | 16 (43.2) | 19 (51.3) | 35 (47) | 93 (57.7) | |
| Female | 21 (56.8) | 18 (48.7) | 39 (53) | 68 (42.3) | |
| Race | 0.08 | ||||
| White | 33 (89) | 32 (86.4) | 65 (88) | 135 (83.8) | |
| Nonwhite | 3 (8) | 4 (10.8) | 7 (9) | 23 (14.3) | |
| Unknown | 1 (3) | 1 (2.7) | 2 (3) | 3 (1.9) | |
| Years to diagnosis of metastases | 0.07 | ||||
| ≤2 | 24 (64.9) | 28 (75.6) | 52 (70) | 119 (73.9) | |
| >2 | 6 (16.2) | 4 (10.8) | 10 (14) | 38 (23.6) | |
| Unknown | 7 (18.9) | 5 (13.6) | 12 (16) | 4 (2.4) | |
| Site of metastasis | 0.9 | ||||
| Liver | 26 (70.3) | 31 (83.8) | 57 (77) | 123 (76.4) | |
| Lung | 11 (29.7) | 6 (16.2) | 17 (23) | 38 (23.6) | |
| No. nodules | <0.001 | ||||
| 1 | 11 (29.7) | 17 (46.0) | 28 (38) | 96 (59.6) | |
| 2–4 | 16 (43.3) | 12 (32.4) | 28 (38) | 61 (37.9) | |
| >4 | 4 (10.8) | 5 (13.5) | 9 (12) | 1 (0.6) | |
| Unknown | 6 (16.2) | 3 (8.1) | 9 (12) | 3 (1.9) | |
| CEA, mean (range) | 2.0 (0.6–13.0), N = 18 | 1.8 (0.5–15.0), N = 23 | 1.9 (0.5–15), N = 41 | Not available | — |
Contemp. indicates contemporary.
Treatment and Toxicity
Seventy patients received all immunizations, whereas 4 patients discontinued immunizations early (3 on the DC/PANVAC arm and 1 on the PANVAC arm): 1 because of grade 3 urticaria on the DC/PANVAC arm and 3 because of progressive disease. The majority of the toxicities on the trial were injection site reactions (63% grade 1, 2 for the DC/PANVAC arm and 64% grade 1, 2 for the PANVAC arm), low-grade fevers (17% grade 1, 2 for the DC/PANVAC arm and 31% grade 1, 2 for the PANVAC arm), muscle pain (11% grade 1 for the DC/PANVAC arm and 11% grade 1 for the PANVAC arm), and fatigue (26% grade 1, 2 for the DC/PANVAC arm and 34% grade 1, 2 for the PANVAC arm).
Recurrence-Free and Overall Survival
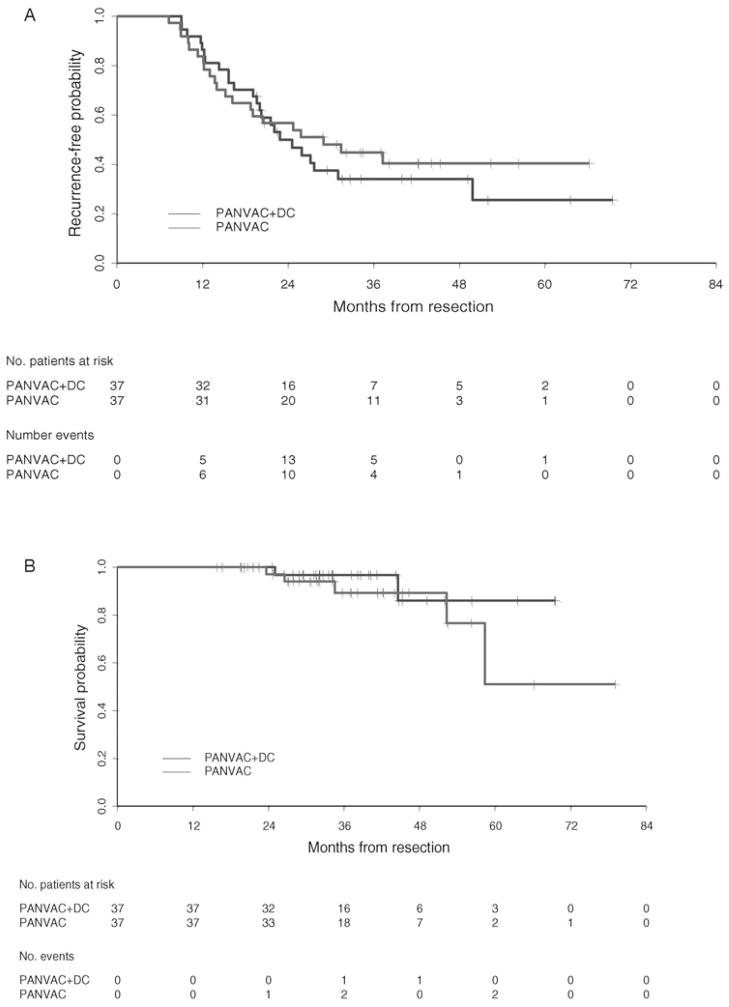
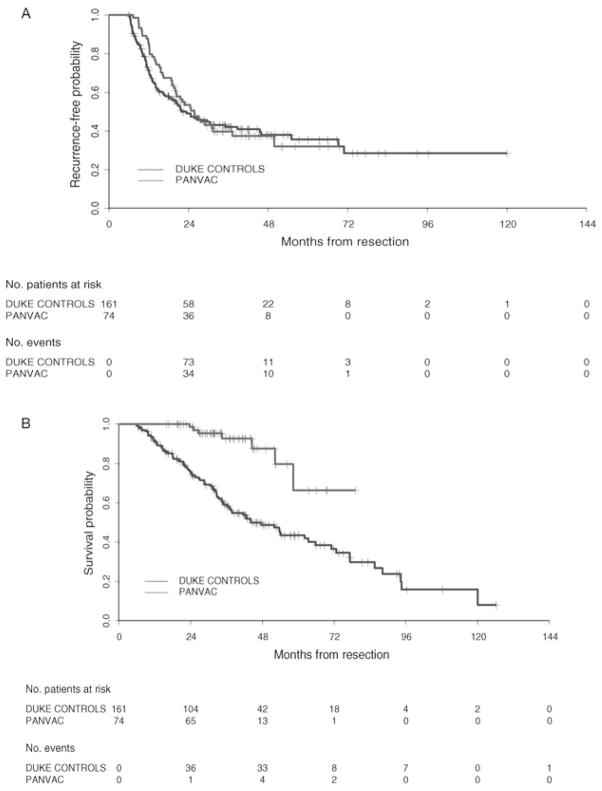
Thirty-four of 37 and 35 of 37 subjects on the DC/PANVAC and PANVAC arms, respectively, met criteria for inclusion in this analysis (ie, minimum follow-up ≥2 years or recurrence before 2 years). Median follow-up time among randomized patients was 35.7 months. The proportions of randomized patients who did not experience recurrence within 24 months of resections were 16 of 34 (47%) and 20 of 36 (55%) for the DC/PANVAC arm and the PANVAC arm, respectively. The 90% lower confidence bounds for the proportion of patients who were recurrence free at 2 years were 0.35 for the DC/PANVAC arm and 0.43 for the PANVAC arm. There were 2 of 37 deaths in the DC/PANVAC arm and 5 of 37 deaths in the poxvector arm. Survival curve estimates for RFS and OS by treatment arm are illustrated in Figures 2A and 2B, respectively.
FIGURE 2.
A, Recurrence-free survival for randomized patients receiving DCs loaded with PANVAC or PANVAC plus GM-CSF measured from the date of metastasectomy, with relapse defined as documented disease recurrence at any site. Median RFS (95% CI) was 22.9 (19.6–49.8) and 28.9 (16.2, not reached), months, respectively. B, OS for randomized patients receiving DCs loaded with PANVAC or PANVAC plus GM-CSF measured from metastasectomy until death from any cause. Patients were censored on the date of last known follow-up. 95% CI indicates 95% confidence interval.
Immune Response Data
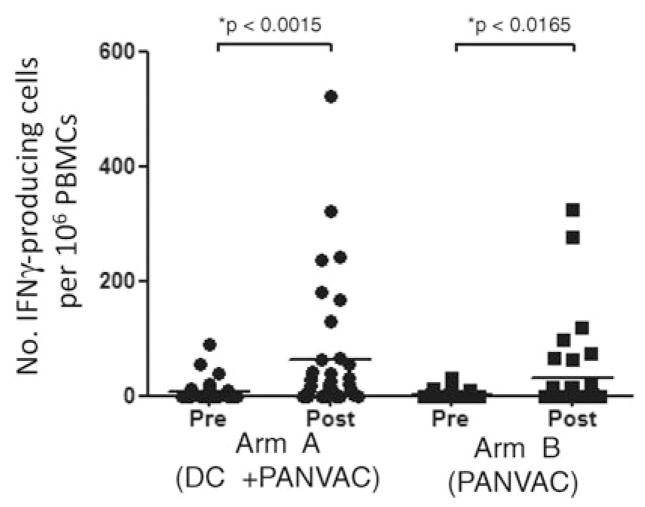
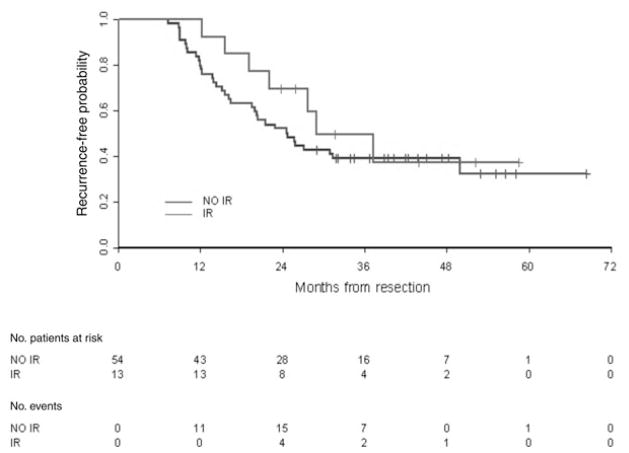
ELISpot assays were performed on PBMCs collected before initiating the immunizations and after all the immunizations were completed (Fig. 3). The majority of the patients had undetectable levels of CEA-specific T-cell responses before immunization. After the immunizations, although there were numerically more T-cell responders to the DC/PANVAC, it was not statistically different from the percentage of T-cell responders to the PANVAC alone (Table 2). For those with a T-cell response, the magnitude of the induced CEA-specific T cells was similar between the 2 arms of the study (Fig. 3). We also determined whether clinical outcome was associated with immune responses. Early after immunization, a trend existed for a longer RFS among all enrolled patients who developed a tumor antigen-specific T-cell response than those with no T-cell response (Fig. 4); however, over time, the RFS curves became inseparable and there was no difference in OS by T-cell response.
FIGURE 3.
ELISpot analysis after vaccinations. Patient PBMCs were analyzed pre- (week 0) and post- (week 13) vaccinations for arm A (+DC) and arm B (no DC) by IFNγ ELISpot. Patient PBMCs were stimulated 20 to 24 hours with PANVAC-F (moi 10) and fowlpox-TRICOM (moi 10) in a standard ELISpot assay. The number of IFNγ-producing cells per 106 PBMCs for each subject analyzed is represented for the response to PANVAC-F minus the fowlpox-TRICOM response (solid black circles for arm A; solid black squares for arm B). The mean response is depicted as a solid black bar pre- and postimmunization for each study arm. The P value reported is based on the Wilcoxon signed rank test calculated by Graph-Pad Prism. moi indicates multiplicity of infection.
TABLE 2.
CEA-Specific Immune Responders by ELISpot by Arm of the Study
| T-cell Response | PANVAC + DCs | PANVAC | Total |
|---|---|---|---|
| No | 26 | 28 | 54 |
| Yes | 9 (25.7%) | 4 (12.5%) | 13 |
| Total | 35 | 32 | 67 |
Fisher exact test P = 0.22.
FIGURE 4.
Recurrence-free survival by ELISpot immune response for patients enrolled and treated on the PANVAC trial (Wilcoxon P = 0.08). IR indicates immune responders; NR, no immune response.
Comparison of Outcome With Contemporary Controls
Although the primary purpose of this study was to compare the 2 vaccine strategies, we planned to compare the clinical outcome for the vaccinated patients with a contemporary group of patients who had undergone CRC metastasectomy but had not been vaccinated (Duke contemporary controls). As shown in Table 1, the characteristics of this group were similar to those of the vaccinated patients except that the contemporary group had slightly better prognostic features such as fewer metastatic nodules. All had received multiagent chemotherapy (FOLFOX, FOLFIRI, or XELOX) in the perioperative period or after relapse, and bevacizumab was given concurrently in 67%. Median follow-up time was 50.1 months among the Duke contemporary group. Survival plots for RFS and OS for the 2 study arms combined versus the Duke contemporary group are provided in Figures 5A and 5B, respectively. The estimate of 2-year RFS in the contemporary group was 0.44 with 90% lower confidence bounds of 0.38. This proportion was similar to that observed in the vaccine groups. There was a significant OS advantage among the patients treated with the vaccines taken as a group versus the contemporary group (log rank P < 0.0001).
FIGURE 5.
A, Recurrence-free survival for vaccinated patients combined versus contemporary, unvaccinated controls. RFS was measured from the date of metastasectomy until documented disease recurrence at any site. Median RFS (95% CI) was 21.9 (16.9–38.8) and 25.7 (20.0–37.2), months, respectively. B, OS for vaccinated patients combined compared with contemporary, unvaccinated controls. OS was measured from metastasectomy until death from any cause. Patients were censored on the date of last known follow-up. Median OS (95% CI) was not reached and 44.1 (36.2–63.4), months, respectively. 95% CI indicates 95% confidence interval.
DISCUSSION
Because vaccines based on autologous antigen-presenting cells33 and poxvectors34 have established clinical benefit, we wished to compare vaccination with an autologous cell therapy versus recombinant viral vectors in patients with no evidence of disease but a high risk of recurrence after surgical resection of CRC metastases. We intended to choose the superior strategy for a future phase III study, based on which yielded better clinical outcomes in time-dependent endpoints such as RFS or OS, with consideration to be given to the vaccination strategies that enhanced immune response. This strategy was justified, as previous studies have suggested longer survival in patients with a positive immune response to vaccination as determined by the ELISpot assay.14 There was no difference in clinical outcome (progression-free survival [PFS] or OS) between the 2 vaccine strategies. Although there were numerically more T-cell responders against CEA after DC/PANVAC, the results were not significant; therefore, we could not choose one or the other arm as superior.
In addition to the comparison of the 2 vaccination strategies, we planned a comparison between vaccinated patients and a well-defined, nonrandomized, contemporary group with similar characteristics [ie, had complete resections with negative margins and had received at least 2 months of perioperative chemotherapy, were of similar age, had a similar proportion of lung and liver metastases, and had received multiagent chemotherapy (fluoropyrimidines, irinotecan, oxaliplatin, and biologic agents)]. The contemporary group had slightly better prognostic features such as fewer metastatic nodules. Vaccinated participants had a 2-year RFS similar to that of contemporary, unvaccinated patients; however, survival for vaccinated subjects exceeded that of the contemporary, unvaccinated group. Although this contemporary group was not randomized, its outcomes (RFS = 25.7 months and OS = 44.1 months) are similar to those reported for patients who had undergone CRC metastasectomy in other authors’ experiences. Andres et al35 calculated 3- and 5-year survival rates of 69% and 46%, respectively, for CRC patients undergoing liver metastases resections after 1996. The 3- and 5-year disease-free survival rates for the best prognosis group were 45% and 38%, respectively. Choti et al36 reported actuarial OS was 57% at 3 years and 40% at 5 years, with a median survival of 46 months (not reached in the more recent time period. Disease-free survival was 63% at 1 year, 28% at 3 years, and 20% at 5 years, with a median recurrence-free survival of 16 months (19 months in the more recent period.) In the Arru et al study,37 median survival was 36 months, regardless of time period of the metastasectomy. The actuarial survival was 90.6% after 1 year, 51.0% after 3 years, and 27.5% after 5 years. The RFS was not reported. Pawlik et al38 reported the 3- and 5-year actuarial survival rates were 74% and 58%, respectively, with median survival 74 months and a median time to recurrence of 10.5 months. This suggests that our contemporary unvaccinated group was an appropriate comparator because it mirrored the outcome of patients who had undergone metastasectomy across several studies. Nonetheless, we recognize the limitations of utilizing historical controls in assessing the efficacy of new interventions and caution against interpreting these data as proof of clinical benefit. These hypothesis-generating results support the pursuit of a rigorous randomized trial to provide evidence of clinical benefit.
This report adds to growing list of immunotherapy studies in which there is no difference in PFS or RFS but an improvement in overall survival. Because some chemotherapeutic agents can induce immunogenic cancer cell death39,40 and because all patients in this study had received chemotherapy before study enrollment and the majority are assumed to have done so if they relapsed, it is possible that the immune response may not prevent recurrences by itself but may act in synergy with other therapies to lengthen survival. Other immune effects that evolve over time, such as epitope or antigenic spreading of the immune response, or the involvement of other immune effectors not specifically measured in this study, such as NK (natural killer) cells, could also impact survival. Chemotherapy may also alter the effector and immunomodulatory cell content of tumors or the host.41 In addition, microscopic tumor metastases may initially progress and then subsequently regress as has been observed in studies with immunotherapies such as ipilimumab and which has led to the development of new response criteria for immunotherapies.42,43 Finally, it is possible that other mechanisms of action for the immune effectors, such as altering the invasive behavior of persistent tumor cells, could result in improved clinical outcome but no reduction in tumor burden.
Our study is one of the few that has compared a DC-based vaccine with another strategy in patients with malignancy. Ex vivo loading of DCs is hypothesized to provide greater efficiency of antigen loading than would occur with injection of the vectors into the skin, but it is not clear whether this translates into better immunologic or clinical benefit. DC vaccination did not prolong PFS or OS compared with DTIC (dacarbazine) in patients with melanoma.44 In this study, matured DCs were pulsed with melanoma peptides and it is possible that the peptides were not adequately presented. Nonetheless, in certain subgroups of patients such as those with HLA-A2+ immune type and 100% performance status, survival did seem longer with DCs.
Another important outcome of this study was the observation of CEA-specific T-cell responses to vaccination despite recent systemic chemotherapy. There was no obvious correlation between type or extent of chemotherapy and T-cell response, although most subjects received a comparable number of cycles of similar multiagent chemotherapy with concurrent bevacizumab and thus correlations would be difficult to draw. We attempted to analyze the MUC1-specific T-cell response, using a MUC1 HLA-A2–restricted peptide and did not see a response in any of the patients. Unfortunately, we were unable to further analyze T-cell responses against MUC1 because we did not have available a peptide cocktail that would cover all HLA types, nor did we have a vector containing exclusively MUC1.
CONCLUSIONS
The DC-based vaccine was not superior to the viral vector vaccine alone. In addition, although there was not a randomized untreated control group and thus survival comparisons are hypothesis generating, we observed, as have others, that OS should be a key clinical endpoint in immunotherapy studies. We believe that the data support a phase III study of vaccination compared with standard follow-up care after CRC metastasectomy and perioperative chemotherapy. Because the viral vector can be administered to patients without the need for an apheresis and an ex vivo manufacturing process to generate autologous DCs, it is technically much simpler to administer and we propose that it is currently the most feasible vaccine platform to test in a future pivotal trial. Ongoing preclinical studies continue to explore the potential utility and superiority of cellular based vaccines.
Acknowledgments
Supported by NIH 2P01 CA078673-07 (PI: H. Kim Lyerly; Project PI: Michael Morse, MD) and by Golfers Against Cancer for aspects of the analysis. This trial is registered with ClinicalTrials.gov (NCT00103142).
We thank Delila Serra, Amanda Summers, Wiguins Etienne, Karrie Comatas, and Manar Ghanayem for technical assistance in the manufacture of the dendritic cell vaccines and immunologic assays; Sharon Peplinski for assistance with flow cytometry; and Liz Anderson, RN, and Cyndy Simonson, ANP, for help with patient accrual and management. We also thank the clinical trial coordinators at Duke University Medical Center, Lombardi Comprehensive Cancer Center, MD Anderson Cancer Center, Moffitt Cancer Center, Providence Portland Medical Center, Wake Forest University Baptist Medical Center, and Medical University of South Carolina where patients were recruited and treated. We also appreciate the expertise, provided by Drs Jeffrey Schlom and James Gulley, National Cancer Institute, Thomas Shuetz, formerly of Therion, and Dennis Panicali, formerly of Therion, in the use and analysis of the poxvector vaccines and for helpful suggestions with the manuscript. We thank Mr W. Gordon Cole for serving as a patient advocate.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- 1.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Association Francaise de Chirurgie. Br J Surg. 1997;84:977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitry E, Guiu B, Cosconea S, et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59:1383–1388. doi: 10.1136/gut.2010.211557. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Nagai K, Kobayashi A, et al. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg. 2009;96:1058–1065. doi: 10.1002/bjs.6682. [DOI] [PubMed] [Google Scholar]
- 5.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 6.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Vermorken JB, Claessen AM, van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomized trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 10.Ockert D, Schirrmacher V, Beck N, et al. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res. 1996;2:21–28. [PubMed] [Google Scholar]
- 11.Schlag P, Manasterski M, Gerneth T, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer: first evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother. 1992;35:325–330. doi: 10.1007/BF01741145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze T, Kemmner W, Weitz J, et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58:61–69. doi: 10.1007/s00262-008-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posner MC, Niedzwiecki D, Venook AP, et al. A phase II prospective multi-institutional trial of adjuvant active specific immunotherapy following curative resection of colorectal cancer hepatic metastases: Cancer and Leukemia Group B Study 89903. Ann Surg Oncol. 2008;15:58–64. doi: 10.1245/s10434-007-9654-7. [DOI] [PubMed] [Google Scholar]
- 14.Barth RJ, Jr, Fisher DA, Wallace PK, et al. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res. 2010;16:5548–5556. doi: 10.1158/1078-0432.CCR-10-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berinstein NL. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol. 2002;20:2197–2207. doi: 10.1200/JCO.2002.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 17.Tsang K, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 18.Barnd DL, Lan MS, Metzgar RS, et al. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotera Y, Fontenot JD, Pecher G, et al. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–2860. [PubMed] [Google Scholar]
- 20.Ras E, van der Burg SH, Zegveld ST, et al. Identification of potential HLA-A *0201 restricted CTL epitopes derived from the epithelial cell adhesion molecule (Ep-CAM) and the carcinoembryonic antigen (CEA) Hum Immunol. 1997;53:81–89. doi: 10.1016/S0198-8859(97)00032-3. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J. Carcinoembryonic antigen-based vaccines. Semin Oncol. 2003;30:30–36. doi: 10.1016/s0093-7754(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 22.Tang CK, Katsara M, Apostolopoulos V. Strategies used for MUC1 immunotherapy: human clinical studies. Expert Rev Vaccines. 2008;7:963–975. doi: 10.1586/14760584.7.7.963. [DOI] [PubMed] [Google Scholar]
- 23.Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17:359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palucka K, Ueno H, Zurawski G, et al. Building on dendritic cell subsets to improve cancer vaccines. Curr Opin Immunol. 2010;22:258–263. doi: 10.1016/j.coi.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arlen PM, Kaufman HL, DiPaola RS. Pox viral vaccine approaches. Semin Oncol. 2005;32:549–555. doi: 10.1053/j.seminoncol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Marshall J, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3974. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 28.Mohebtash M, Tsang KY, Madan RA, et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011;17:7164–7173. doi: 10.1158/1078-0432.CCR-11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse MA, Deng Y, Hull S, et al. A phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res. 1999;5:1331–1338. [PubMed] [Google Scholar]
- 30.Morse MA, Nair SK, Mosca PJ, et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest. 2003;21:341–349. doi: 10.1081/cnv-120018224. [DOI] [PubMed] [Google Scholar]
- 31.Morse MA, Clay TM, Hobeika AC, et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 32.Keilholz U, Weber J, Finke JH, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 34.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andres A, Majno PE, Morel P, et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15:134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]
- 36.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arru M, Aldrighetti L, Castoldi R, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 38.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;24:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesniere A, Apetoh L, Ghiringhelli F, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 43.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]