Abstract
Objectives
During cardiopulmonary resuscitation (CPR), adequate coronary perfusion pressure (CPP) is essential for establishing return of spontaneous circulation. Current American Heart Association (AHA) guidelines recommend standardized interval administration of epinephrine for patients in cardiac arrest. The objective of this study was to compare short-term survival using a hemodynamic directed resuscitation strategy versus chest compression depth directed CPR in a porcine model of cardiac arrest.
Design
Randomized interventional study
Setting
Preclinical animal laboratory
Subjects
Twenty four female 3-month old swine
Interventions / Measurements
After 7 minutes of ventricular fibrillation, pigs were randomized to receive one of three resuscitation strategies: 1) Hemodynamic Directed Care (CPP-20): chest compressions (CCs) with depth titrated to a target systolic blood pressure of 100 mmHg and titration of vasopressors to maintain CPP > 20 mmHg; 2) Depth 33mm(D33): target CC depth of 33mm with standard AHA epinephrine dosing; or 3) Depth 51mm(D51): target CC depth of 51mm with standard AHA epinephrine dosing. All animals received manual CPR guided by audiovisual feedback for 10 minutes before first shock.
Main Results
45-minute survival was higher in the CPP-20 group (8/8) compared to D33 (1/8) or D51 (3/8) groups; p=0.002. Coronary perfusion pressures were higher in the CPP-20 group compared to D33 (p=0.004) and D51 (p=0.006), and in survivors compared to non-survivors (p<0.01). Total epinephrine dosing and defibrillation attempts were not different.
Conclusions
Hemodynamic directed resuscitation targeting CPPs > 20 mmHg during 10 minutes of CPR for VF cardiac arrest improves short-term survival, when compared to resuscitation with depth of compressions guided to 33mm or 51mm and standard AHA vasopressor dosing.
Keywords: cardiac arrest, cardiopulmonary resuscitation, coronary perfusion pressure, ventricular fibrillation, swine
Introduction
Successful cardiopulmonary resuscitation (CPR) is dependent on adequate myocardial blood flow [1-4]. Currently, guidelines for the treatment of cardiac arrest assume that all patients can be treated with a uniform chest compression (CC) depth despite a paucity of data indicating that a specific depth consistently provides adequate myocardial blood flow [5, 6]. A therapeutic strategy to titrate compression depth and vasopressor dosing to optimize physiological conditions for improved myocardial blood flow would presumably improve outcomes following cardiac arrest.
During CPR, coronary perfusion pressure (CPP), the aortic pressure minus the right atrial pressure during the relaxation (“diastolic”) phase of CPR, is the primary determinant of myocardial blood flow [1, 7, 8]. Both human and animal investigations have demonstrated a strong association between CPP and resuscitation outcomes [2, 3, 9-11]. Failure to generate a CPP of at least 15 – 20 mmHg during CPR is rarely associated with a successful resuscitation [1, 3, 10]. Importantly, many patients with in-hospital cardiac arrests are in intensive care units and have invasive hemodynamic monitoring [12, 13], so a hemodynamic directed CPR strategy targeted to attain an adequate CPP is feasible.
This randomized laboratory investigation compared short-term survival with a hemodynamic directed resuscitation strategy intended to attain CPPs > 20 mmHg (CPP-20) versus absolute depth-guided CPR in a porcine model of ventricular fibrillation (VF) cardiac arrest. We further subdivided the depth-guided CPR into two groups: one with CC depth targeted to previously documented “usual care” of 33 mm (D33) and one with CCs targeted to the American Heart Association AHA 2010 guideline recommended depth of 51 mm (D51). We hypothesized that the CPP-20 resuscitation strategy would improve short-term survival compared to either D33 or D51.
Materials and Methods
Animal Preparation
The experimental protocol was approved by The University of Pennsylvania Institutional Animal Care and Use Committee. Twenty-four healthy 3-month old female domestic swine were anesthetized and mechanically ventilated using a Datex Ohmeda anesthesia machine (Modulus SE) on a mixture of room air and titrated isoflurane (~1.0% to 2.5%) with a tidal volume of 12mL / kg, PEEP 6 cm H2O, rate of 12 breaths / minute, and titration of rate to maintain endtidal carbon dioxide (ETCO2) at 38 – 42 mmHg (NICO, Novametrix Medical Systems Inc.).
High fidelity, solid-state, micromanometer-tipped catheters (MPC-500, Millar Instruments) were advanced through the right femoral artery and external jugular vein into thoracic locations to measure continuous aortic and right atrial pressures respectively. A Swan-Ganz Thermodilution catheter (Edwards Lifesciences) was advanced into the pulmonary artery, and a bipolar pacing catheter (Edwards Lifesciences) was advanced into the right ventricle. All catheter placements were confirmed with fluoroscopy. Unfractionated heparin 200 U/kg was provided to prevent catheter clotting. Prior to obtaining any baseline measurements, all animals received 20 mL / kg of 0.9% normal saline intravenously to replace overnight fasting fluid deficits.
Measurements
Thermodilution cardiac outputs (ICU monitor: model HP66, Hewlett Packard) were obtained at baseline. Arterial blood gas specimens were obtained from the thoracic aorta at baseline (before VF), at 2 minutes, 4 minutes, and 6 1/2 minutes of VF, and then 2 1/2 minutes and 6 minutes after the initiation of CPR. Coronary perfusion pressure (CPP) was calculated by subtracting the mid-diastolic right atrial pressure from the mid-diastolic aortic pressure.
To guide and record manual CPR quality, the Philips Heart Start MRx defibrillator with QCPR option was deployed during the experimental protocol. Using force transducer / accelerometer technology, the defibrillator records CPR quality and provides audiovisual feedback to the chest compression (CC) provider for rate (CC/min), depth (mm), and incomplete chest wall recoil (residual leaning force (grams)) [14-17].
Experimental Protocol
Overview (Figure 1)
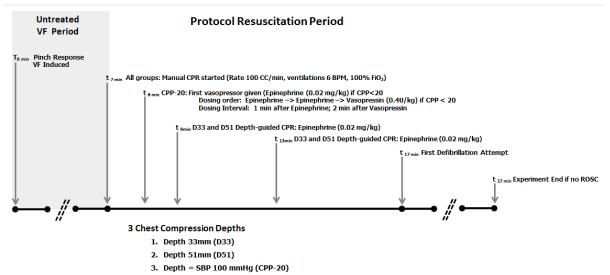
Figure 1.
Protocol design. During protocol resuscitation period, animals were randomized to receive one of three resuscitation strategies. SBP indicates systolic blood pressure. D33 and D51 refer to depth-directed CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg.
The protocol utilized in this experiment was designed to address CPR goals for VF cardiac arrest. VF was induced by electrical pacing. No changes in mechanical ventilation or oxygenation were made during the initial seven minutes of VF. Following seven minutes of untreated VF, animals were randomized to one of three different CPR and advanced life support strategies for a ten minute duration before attempts at defibrillation. A 10 minute interval of CPR was chosen before defibrillation as a practical approach, because this duration of CPR is necessary to adequately compare CPR techniques. Of note, most in-hospital CPR is at least 10 minutes in duration for both survivors and non-survivors [12]. The 7-minute untreated VF period was included so that the insult would be severe enough to allow discrimination of outcomes with the three protocols.
In all treatment arms, metronome-guided CCs were provided with a target rate of 100 CC/min and ventilations at 6 breaths per minute with 100% oxygen. Brief interruptions in CPR every two minutes mimicked pulse checks / rhythm analysis. Animals randomly received one of three resuscitation strategies: 1) Hemodynamic Directed Care (CPP-20): CCs with depth titrated to a target systolic blood pressure of 100 mmHg and titration of vasopressors to maintain CPP > 20 mmHg; 2) Depth 33mm (D33): target CC depth of 33mm [14-19] with standard AHA epinephrine dosing interval; or 3) Depth 51mm (D51): target CC depth of 51mm with standard AHA epinephrine dosing interval. Animals in the D33 and D51 groups received intravenous vasopressor (0.02 mg/kg epinephrine) every 4 minutes starting at minute 9 of the protocol (2 minutes after CPR was started). Animals in the CPP-20 group only received intravenous vasopressor if the CPP was < 20 mmHg, starting at minute 8 of the protocol. The order of drug administration in CPP-20 was Epinephrine (0.02 mg/kg) – Epinephrine (0.02 mg/kg) – Vasopressin (0.4 U/kg). The dosing interval was 1 minute between doses, and if vasopressin was given, 2 minutes elapsed before the cycle was restarted with another epinephrine dose. After 10 minutes of CPR (minute 17 of the protocol), the initial 200J biphasic waveform defibrillation attempt was provided. Resuscitation according to treatment strategy continued until sustained return of spontaneous circulation (ROSC) was attained or at minute 27 of the protocol (after an additional ten minutes of resuscitation post-initial defibrillation attempt). If ROSC was attained, the animals were supported for 45 minutes in a simulated intensive care setting. After ROSC, mechanical ventilation was provided with 100% oxygen and adjusted to obtain an ETCO2 of 38 to 42 mmHg. Isoflurane was administered as necessary. At 45 minutes, the animals were euthanized with pentobarbital and potassium chloride. All animals received a post-mortem examination for detection of visceral injuries.
Data Analysis / Outcomes
The primary outcome of the study was 45-minute ICU survival. Secondary outcomes included: 1) return of spontaneous circulation; 2) hemodynamic measures (specifically CPP); and 3) CPR quality variables. Statistical analysis was completed using the Stata-IC statistical package (Version 12.0, StataCorp, College Station, TX). Normality of continuous variables was assessed using the Skewness-Kurtosis test. Normally distributed continuous variables were described as mean ± SEM and compared by ANOVA. Continuous variables that were not normally distributed were described as median (25%, 75%) and evaluated by the Kruskal-Wallis test. Comparisons of dichotomous variables, such as 45-minute ICU survival and rate of return of spontaneous circulation were evaluated by Fisher’s exact test. Differences in CPPs over time and between treatment groups and between survivors / non-survivors were assessed using generalized estimating equations [20].
Results
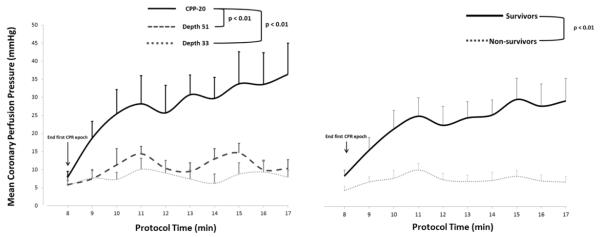
The primary outcome variable of 45-minute ICU survival and the secondary outcome variable of any ROSC were both significantly different across treatments (Table 1) with superior survival rates in the CPP-20 group. In a model using generalized estimating equations (GEE), coronary perfusion pressure (Figure 2) was significantly higher in the CPP-20 group compared to both D33 (p=0.01) and D51 (p=0.01), and higher in survivors compared to non-survivors irrespective of treatment group (p<0.01).
Table 1.
Rates of survival across treatment groups. Depth 33 and Depth 51 refer to depth-guided CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg. Rate of survival in CPP-20 higher compared to both Depth 33 (p=0.001) and Depth 51 (p=0.026).
| Depth 33 (n=8) |
Depth 51 (n=8) |
CPP-20 (n=8) |
p | |
|---|---|---|---|---|
| Survival [n (%)] | ||||
| Any ROSC | 1 (13) | 3 (38) | 8 (100) | 0.002 |
| 45 Minute ICU Survival | 1 (13) | 3 (38) | 8 (100) | 0.002 |
Figure 2.
Mean coronary perfusion pressure during each minute of CPR across treatment groups (left) and between survivors and non-survivors (right). Error bars represent SEM. D33 and D51 refer to depth-directed CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg.
Resuscitation Variables
Chest compression depth was significantly different among groups: D33 = 34 ± 0.8mm; D51 = 47 ± 0.5mm; CPP-20 = 44 ± 0.8mm (p<0.01). Other CPR quality variables were not different (rate = 100 ± 0.1 CC/min; no flow fraction = 3.0 ± 0.2%; no delivered compressions had leaning exceeding 2.5kg). Total number of vasopressors doses administered was not different (D33 = 5 (2, 5); D51 =5 (2, 5); CPP-20 = 3 (2.5, 6), p=0.57). Similarly, total number of epinephrine doses was not different (D33 = 5 (2, 5); D51 =5 (2, 5); CPP-20 = 2 (2, 4.5), p=0.16). By protocol design, CPP-20 received first vasopressor dose as early as 1 minute after initiation of CPR, compared to 2 minutes in the D33 and D55 groups. Overall number of defibrillation attempts was not different among groups (D33 = 4 (2, 5); D51 = 3 (1, 5); CPP-20 = 1 (1, 2), p=0.08). One surviving animal in the CPP-20 group required cardioversion for unstable narrow complex tachycardia during the intensive care unit period after ROSC.
Hemodynamics and Arterial Blood Gases
Hemodynamic variables were not different at pre-arrest baseline or at the end of untreated VF period (Table 2). During the last minute of the resuscitation period (minute 16 – 17), there were significant differences across treatment groups for aortic systolic and diastolic pressure and CPP but not right atrial diastolic pressure (Table 2). Higher CPP in the CPP-20 group compared to D33 and D55 was the result of higher aortic diastolic pressures in the CPP-20 group. There was a trend towards higher end tidal CO2 in the D51 and CPP-20 groups compared to D33 that did not reach statistical significance. There were no differences in arterial blood gases obtained at baseline, at the end of the untreated VF period, or after 6 minutes of CPR (Table 3).
Table 2.
| Depth 33 (n=7) |
Depth 51 (n=7) |
CPP-20 (n=7) |
p | |
|---|---|---|---|---|
| Baseline | ||||
| Weight (kg) | 32.3 (0.4) | 32.3 (0.5) | 31.3 (0.5) | 0.27 |
| CO (L/min) | 3.3 (0.3) | 3.0 (0.3) | 3.0 (0.3) | 0.78 |
| AoS | 110 (5) | 105 (6) | 104 (6) | 0.79 |
| AoD | 77 (6) | 78 (6) | 79 (6) | 0.98 |
| RAD | 14 (1) | 12 (1) | 13 (1) | 0.53 |
| CPP | 63 (6) | 68 (5) | 68 (7) | 0.78 |
| End of Untreated VF Period* | ||||
| AoS | 26 (1) | 25 (1) | 25 (2) | 0.99 |
| AoD | 20 (1) | 19 (1) | 21 (2) | 0.75 |
| RAD | 20 (1) | 19 (1) | 18 (1) | 0.24 |
| CPP | 2 (1) | 2 (1) | 3 (1) | 0.55 |
| End of Resuscitation Period† | ||||
| AoS | 62 (9) | 102 (15) | 109 (6) | 0.011‡,§ |
| AoD | 24 (3) | 26 (3) | 47 (6) | 0.0089∥,¶ |
| RAD | 17 (1) | 12 (3) | 13 (6) | 0.67 |
| CPP | 8 (2) | 10 (2) | 36 (9) | 0.0012**,†† |
| ETCO2 | 21 (2) | 28 (3) | 27 (1) | 0.10 |
Last epoch during untreated VF period (minute 6 – 7);
Last epoch during protocol resuscitation period CPR (minute 16 – 17). Pressures in mmHg. AoS indicates aortic systolic pressure; AoD, aortic diastolic pressure; RAD, right atrial diastolic pressure; CPP, coronary perfusion pressure; ETCO2, end tidal carbon dioxide. Depth 33 (D33) and Depth 51 (D51) refer to depth-guided CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg. Data presented as mean (SEM).
CPP-20 vs. D33: p=0.018;
p=0.006;
p=0.004.
D51 vs. D33: p=0.046.
CPP-20 vs. D51: p=0.012;
p=0.006.
Table 3.
Depth 33 and Depth 51 refer to depth-guided CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg.
| Depth 33 (n=7) |
Depth 51 (n=8) |
CPP-20 (n=8) |
p | |
|---|---|---|---|---|
| Baseline | ||||
| pH | 7.52 (0.01) | 7.51 (0.01) | 7.51 (0.01) | 0.49 |
| PCO2 (mmHg) | 43 (2) | 42 (2) | 44 (1) | 0.74 |
| PO2 (mmHg) | 134 (4) | 138 (11) | 144 (12) | 0.92 |
| End of Untreated VF* | ||||
| pH | 7.77 (0.01) | 7.78 (0.04) | 7.69 (0.05) | 0.25 |
| PCO2 (mmHg) | 16 (2) | 16 (3) | 23 (6) | 0.41 |
| PO2 (mmHg) | 92 (10) | 107 (9) | 100 (15) | 0.68 |
| After 6 Minutes of CPR† | ||||
| pH | 7.46 (0.04) | 7.41 (0.02) | 7.36 (0.03) | 0.07 |
| PCO2 (mmHg) | 35 (4) | 40 (3) | 52 (7) | 0.14 |
| PO2 (mmHg) | 367 (57) | 323 (55) | 193 (60) | 0.11 |
Sample drawn at 6min 30s during VF period and
at 6 minutes during protocol resuscitation period.
Discussion
In the present study, short-term survival from VF cardiac arrest was improved with hemodynamic directed CPR to maintain coronary perfusion pressure > 20 mmHg (CPP-20) compared to resuscitation with depth of compressions guided to 33mm or 51mm and standard AHA vasopressor dosing. Irrespective of treatment group, coronary perfusion pressures were higher in survivors compared to non-survivors consistent with previous investigations [1, 3, 10].
This animal model was intended to address CPR of 10 minutes duration for a VF associated cardiac arrest because this duration of CPR provided an opportunity to adequately compare CPR techniques. Of note, most in-hospital cardiac arrests have at least 10 minutes of CPR. Increasingly, in-hospital cardiac arrests are occurring in the intensive care setting, presumably in part due to the emergence of medical emergency teams [12, 13, 21-23]. Invasive hemodynamic monitoring is readily available for many of these patients, yet AHA recommendations for CPR focus on depth and rate of compressions and fixed interval vasopressor dosing rather than titrating compression depth and vasopressor dosing to invasive hemodynamics.
The relationship between CPP and myocardial blood flow, and in turn, resuscitation outcome is well established [1-4, 9-11]. However, to our knowledge, this is the first investigation to evaluate a treatment algorithm with manually provided CCs titrated to a hemodynamic goal (systolic blood pressure) and vasopressor administration targeted to CPPs in a VF large animal model. This new therapeutic strategy focuses on individualizing resuscitation to the appropriate hemodynamic goal rather than a standard “one-size-fits-all” strategy and could be applied during actual resuscitation attempts in the intensive care unit.
High quality quantitatively evaluated manual CPR (CC rate 100/min, full chest wall recoil, and a ventilation rate of 6/min) was provided to all three treatment groups. As planned, there were differences in CC depth across treatment groups. Previous investigations have shown that deeper compressions are associated with superior outcomes [18, 24, 25]. However, in our investigation, deeper compressions in the D51 group did not translate into improved survival despite excellent systolic blood pressures. Compared with the D51 group, the CPP-20 animals had similar systolic blood pressure and right atrial diastolic pressure but higher CPPs and were therefore more likely to survive. Thus, the primary difference was higher aortic diastolic pressure. The elevated aortic diastolic pressures and improved survival in in the CPP-20 animals compared to the D51 group is most likely attributable to the timing and titration of vasopressors in the CPP-20 group. As in previous studies, surviving animals, irrespective of treatment group, had substantially higher CPPs than non-surviving animals [2, 3, 10].
Quantitative noninvasive end-tidal carbon dioxide has been shown to correlate well with cardiac output and resuscitation outcomes [26-34]. Achieving ETCO2 levels > 10 – 15 mmHg during CPR has been associated with survival, and similarly, low ETCO2 (< 10 mmHg) is a strong predictor of unsuccessful CPR (death) [28, 33]. However, experimental data have established that ETCO2 correlates best with pulmonary blood flow and cardiac output rather than myocardial perfusion. In contrast, CPP correlates best with myocardial perfusion [8, 32, 34]. Current AHA guidelines recommend continuous ETCO2 monitoring during cardiac arrest resuscitation when available [5, 6]. In this laboratory investigation, ETCO2 levels in the last minute of CPR were as high or higher in the D51 group compared with the less deep compressions of the CPP-20 and D33 groups (Table 2) but did not result in an improved rate of ROSC or short-term survival. Across all treatment groups, CPP measurements were better predictors of 45 minute survival than the ETCO2 measurements, supporting the concept that invasive hemodynamic monitoring during CPR is superior to ETCO2 monitoring.
There are several limitations to our experimental design which must be considered when translating our results to the care of the cardiac arrest patient. First, our observation period after return of spontaneous circulation was only 45 minutes. The effect of targeting hemodynamic goals during resuscitation on long-term survival and neurological outcome has not been established. Nevertheless, the inability to achieve short-term survival in the depth-guided groups precludes the potential for long-term survival and good neurological outcomes. Importantly, we have now weaned two animals with this CPR protocol and CPP-20 goals from ventilator support after 45 minute survival, and both were survived for 24-hours with excellent Cerebral Performance Category scores of 1 after 24-hours [35, 36]. Second, the length of untreated VF (7 minutes) and the duration of CPR before first shock (10 minutes) may not realistically simulate resuscitation of the invasively monitored hospitalized patient. However, these intervals were necessary to achieve an insult severe enough to discriminate outcomes between the different resuscitation strategies, and to allow a clinically relevant 10 minute period of chest compressions without ROSC. Third, there were some differences in vasopressor administration between the groups. Vasopressors were often administered earlier in the CPP-20 group as per the CPP titration protocol. However, there were survivors in the CPP-20 group who received no more vasopressors than the median number of doses in the other two groups, and the total vasopressor doses did not differ among the groups. CPP-20 animals also received vasopressin when two doses of epinephrine could not maintain CPP >20 mmHg. Rather than continuing to administer a therapy (epinephrine) that was not achieving treatment goals (CPP > 20 mmHg), we chose to alter our vasopressor choice so as to evaluate a dynamic treatment algorithm that was targeted to subject hemodynamics. Improved outcomes from cardiac arrest with vasopressin administration in the clinical setting have not been observed [37-40]. However in these studies, vasopressin was not actively titrated to CPP, which may explain the superior survival outcomes in the CPP-20 group. Fourth, our study was not blinded. Those participating in the resuscitation attempts needed to be aware of the resuscitation strategy (depth guided vs. hemodynamically guided chest compressions). However, besides the statistical differences in chest compression depths, all other CPR quality variables were similar among the groups, minimizing the concern for bias. Finally, we evaluated only one model of in-hospital cardiac arrest, future studies should investigate whether a coronary perfusion pressure directed resuscitation strategy would also improve outcomes when other common etiologies of in-hospital arrests (e.g., respiratory failure/sepsis /shock) are evaluated.
Conclusions
In this model of ventricular fibrillation cardiac arrest, a resuscitation strategy titrated to a coronary perfusion pressure > 20 mmHg improved short-term survival when compared to resuscitation with depth of compressions guided to 33mm or 51mm and standard AHA vasopressor dosing. This resuscitation protocol individualizes therapy to the subject’s hemodynamic status in contrast to the usual “one-size-fits-all” strategy. As more in-hospital cardiac arrests are occurring in the intensive care setting, these findings may offer a promising new resuscitation strategy that could be applied in invasively monitored cardiac arrest patients.
Acknowledgments
This study was funded by the Laerdal Foundation for Acute Care Medicine, the National Institute of Neurological Disorders and Stroke (SHF K08), the National Institute of Child Health and Human Development (RMS K23), and The Russell Raphaely Endowed Chair Funds at The Children’s Hospital of Philadelphia
Dr. Becker has consulted for Philips Health Care, given expert testimony for various attorneys, received payment for lectures from Philips Medical Systems, received travel reimbursements from the American Heart Association, and has a patent from the University of Pennsylvania Technology Transfer. He has received grant support from Philips Medical Systems, Stavanger, Betheseda, Cardiac Science, BeneChill Inc, Zoll Medical Corp, PhysioControl, Medtronic Foundation, and Abbott Point of Care.
Footnotes
Financial Disclosure: The rest of the authors have not disclosed any potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halperin HR, Lee K, Zviman M, Illindala U, Lardo A, Kolandaivelu A, Paradis NA. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. The American journal of emergency medicine. 2010;28(2):195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16(4):241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 3.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA : the journal of the American Medical Association. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 4.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional blood flow and resuscitation in dogs. Annals of emergency medicine. 1984;13(2):79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 5.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S876–908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 7.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, Ewy GA. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 8.Kern KB, Lancaster L, Goldman S, Ewy GA. The effect of coronary artery lesions on the relationship between coronary perfusion pressure and myocardial blood flow during cardiopulmonary resuscitation in pigs. American heart journal. 1990;120(2):324–333. doi: 10.1016/0002-8703(90)90076-a. [DOI] [PubMed] [Google Scholar]
- 9.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Annals of emergency medicine. 1985;14(6):521–528. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehospital emergency care : official journal of the National Association of EMS Physicians and the National Association of State EMS Directors. 2010;14(1):78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders AB, Kern KB, Atlas M, Bragg S, Ewy GA. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. Journal of the American College of Cardiology. 1985;6(1):113–118. doi: 10.1016/s0735-1097(85)80261-8. [DOI] [PubMed] [Google Scholar]
- 12.Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Critical care medicine. 2010;38(1):101–108. doi: 10.1097/CCM.0b013e3181b43282. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA : the journal of the American Medical Association. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 14.Abella BS, Alvarado JP, Myklebust H, Edelson DP, Barry A, O’Hearn N, Vanden Hoek TL, Becker LB. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA : the journal of the American Medical Association. 2005;293(3):305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 15.Abella BS, Edelson DP, Kim S, Retzer E, Myklebust H, Barry AM, O’Hearn N, Hoek TL, Becker LB. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73(1):54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Edelson DP, Litzinger B, Arora V, Walsh D, Kim S, Lauderdale DS, Vanden Hoek TL, Becker LB, Abella BS. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Archives of internal medicine. 2008;168(10):1063–1069. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 17.Sutton RM, Niles D, Nysaether J, Abella BS, Arbogast KB, Nishisaki A, Maltese MR, Donoghue A, Bishnoi R, Helfaer MA, et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124(2):494–499. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 18.Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, Merchant RM, Hoek TL, Steen PA, Becker LB. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Nishisaki A, Nysaether J, Sutton R, Maltese M, Niles D, Donoghue A, Bishnoi R, Helfaer M, Perkins GD, Berg R, et al. Effect of mattress deflection on CPR quality assessment for older children and adolescents. Resuscitation. 2009;80(5):540–545. doi: 10.1016/j.resuscitation.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.Brilli RJ, Gibson R, Luria JW, Wheeler TA, Shaw J, Linam M, Kheir J, McLain P, Lingsch T, Hall-Haering A, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2007;8(3):236–246. doi: 10.1097/01.PCC.0000262947.72442.EA. quiz 247. [DOI] [PubMed] [Google Scholar]
- 22.Sharek PJ, Parast LM, Leong K, Coombs J, Earnest K, Sullivan J, Frankel LR, Roth SJ. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a Children’s Hospital. JAMA : the journal of the American Medical Association. 2007;298(19):2267–2274. doi: 10.1001/jama.298.19.2267. [DOI] [PubMed] [Google Scholar]
- 23.Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2009;10(3):306–312. doi: 10.1097/PCC.0b013e318198b02c. [DOI] [PubMed] [Google Scholar]
- 24.Kramer-Johansen J, Myklebust H, Wik L, Fellows B, Svensson L, Sorebo H, Steen PA. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71(3):283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Stiell IG, Brown SP, Christenson J, Cheskes S, Nichol G, Powell J, Bigham B, Morrison LJ, Larsen J, Hess E, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Critical care medicine. 2012;40(4):1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77(1):234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 27.Kalenda Z. The capnogram as a guide to the efficacy of cardiac massage. Resuscitation. 1978;6(4):259–263. doi: 10.1016/0300-9572(78)90006-0. [DOI] [PubMed] [Google Scholar]
- 28.Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. The New England journal of medicine. 1997;337(5):301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 29.Morley PT. Monitoring the quality of cardiopulmonary resuscitation. Current opinion in critical care. 2007;13(3):261–267. doi: 10.1097/MCC.0b013e32814b05bd. [DOI] [PubMed] [Google Scholar]
- 30.Ornato JP, Levine RL, Young DS, Racht EM, Garnett AR, Gonzalez ER. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Annals of emergency medicine. 1989;18(7):732–737. doi: 10.1016/s0196-0644(89)80005-8. [DOI] [PubMed] [Google Scholar]
- 31.Pokorna M, Necas E, Kratochvil J, Skripsky R, Andrlik M, Franek O. A sudden increase in partial pressure end-tidal carbon dioxide (P(ET)CO(2)) at the moment of return of spontaneous circulation. The Journal of emergency medicine. 2010;38(5):614–621. doi: 10.1016/j.jemermed.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 32.Sanders AB, Atlas M, Ewy GA, Kern KB, Bragg S. Expired PCO2 as an index of coronary perfusion pressure. The American journal of emergency medicine. 1985;3(2):147–149. doi: 10.1016/0735-6757(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 33.Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA : the journal of the American Medical Association. 1989;262(10):1347–1351. [PubMed] [Google Scholar]
- 34.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Critical care medicine. 1985;13(11):907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, Ewy GA. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Critical care medicine. 1994;22(2):282–290. doi: 10.1097/00003246-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Bircher N, Safar P. Cerebral preservation during cardiopulmonary resuscitation. Critical care medicine. 1985;13(3):185–190. doi: 10.1097/00003246-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Carroll TG, Dimas VV, Raymond TT. Vasopressin rescue for in-pediatric intensive care unit cardiopulmonary arrest refractory to initial epinephrine dosing: a prospective feasibility pilot trial. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13(3):265–272. doi: 10.1097/PCC.0b013e31822f1569. [DOI] [PubMed] [Google Scholar]
- 38.Duncan JM, Meaney P, Simpson P, Berg RA, Nadkarni V, Schexnayder S, National Registry of CPRI Vasopressin for in-hospital pediatric cardiac arrest: results from the American Heart Association National Registry of Cardiopulmonary Resuscitation. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2009;10(2):191–195. doi: 10.1097/PCC.0b013e31819a36f2. [DOI] [PubMed] [Google Scholar]
- 39.Stiell IG, Hebert PC, Wells GA, Vandemheen KL, Tang AS, Higginson LA, Dreyer JF, Clement C, Battram E, Watpool I, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358(9276):105–109. doi: 10.1016/S0140-6736(01)05328-4. [DOI] [PubMed] [Google Scholar]
- 40.Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH, European Resuscitation Council Vasopressor during Cardiopulmonary Resuscitation Study G A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. The New England journal of medicine. 2004;350(2):105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]