Abstract
Differentiation methods often rely exclusively on growth factors to direct mouse embryonic stem cell (ESC) fate, but the niche also contains fibrillar extracellular matrix (ECM) proteins, including fibronectin (FN) and laminin, which could also direct cell fate. Soluble differentiation factors are known to increase ECM expression, yet ECM's ability to direct ESC fate is not well understood. To address the extent to which these proteins regulate differentiation when assembled into a matrix, we examined mouse ESC embryoid bodies and found that their ability to maintain pluripotency marker expression was impaired by soluble serum FN. Embryoid bodies also showed a spatiotemporal correlation between expression of FN and GATA4, a marker of definitive endoderm (DE), and an inverse correlation between FN and Nanog, a pluripotency marker. Maintenance of mouse ESC pluripotency prevented fibrillar matrix production, but induction medium created lineage-specific ECM containing varying amounts of FN and laminin. Mouse ESC-derived matrix was unlike conventional fibroblast-derived matrix, which did not contain laminin. Naïve mouse ESCs plated onto ESC- and fibroblast-derived matrix exhibited composition-specific differentiation. With exogenously added laminin, fibroblast-derived matrix is more similar in composition to mouse ESC-derived matrix and lacks residual growth factors that mouse ESC matrix may contain. Naïve mouse ESCs in DE induction medium exhibited dose-dependent DE differentiation as a function of the amount of exogenous laminin in the matrix in an α3 integrin-dependent mechanism. These data imply that fibrillar FN is necessary for loss of pluripotency and that laminin within a FN matrix improves DE differentiation.
Keywords: mouse embryonic stem cells, extracellular matrix, integrin signaling, endoderm development
Introduction
Embryonic stem cells (ESCs) employ a wide variety of external cues to regulate their balance between self-renewal and differentiation. During development, these cues are expressed with precise temporal and spatial control to result in appropriate cellular arrangements [1]. Complex sets of soluble growth factors, intended to recapitulate the early developmental regulation of a specific lineage, have been used in vitro to differentiate ESCs [2]; for example, addition of Activin A, a TGF-β family protein, and Wnt3a induces initial endoderm marker expression [3, 4]. However, differentiation protocols can also inadvertently include or stimulate the production of ECM proteins. For example, induction of mouse ESCs to definitive endoderm (DE) by Activin A involved changing the substrate on which the cells were plated from a collagen- to a FN-based substrate [5]. Many growth factors have also been linked to extracellular matrix (ECM) upregulation, e.g., TGF-β stimulates fibronectin (FN) production [6] and can sequester and regulate the presentation of soluble cues [7]. Though growth factors are clearly an important differentiation regulator, these observations motivate the examination of whether combinations of ECM proteins, e.g. FN and laminin, and the integrin-mediated signaling which they induce play a role in directing ESC fate in general [8].
FN is expressed and localized to mouse endoderm marker-expressing cells in vivo [9] as well as many endoderm-derived tissues [10–12]. Laminin is also upregulated during endoderm specification in vivo, where it forms a basement membrane between the primitive ectoderm and endoderm [13]. In fact, β1-integrin, which binds FN, laminin and other matrix proteins, has been shown to be required for endoderm differentiation in mouse embryoid bodies [14]. When secreted, FN and laminin bind to integrin receptors and are assembled into a 3-dimensional fibrillar scaffold alongside other matrix proteins to support cells [15]. Much like the signaling cascades initiated by growth factors, integrin activation via ECM binding initiates intracellular signaling pathways in mouse embryonic stem cells (e.g. MAPK/ERK and Rho-ROCK [16]) that mirror key aspects of embryonic development [1], including the regulation of pluripotency [17, 18], proliferation [19], and differentiation [20, 21]. Yet these studies typically utilize a defined ECM without necessarily defining the endogenous ECM that is produced by differentiating ESCs. This ESC matrix is likely to be as complex as that found in mature tissues [10] and while many reductionist studies have examined the effects of matrix components on properties of mouse embryonic stem cells [22], examining how a combination of matrix-based cues or ECM proteins influence stem cells has been limited to high throughput 2-dimensional assays [20]. Therefore, we employed multicellular embryoid bodies (EBs), ESC-derived matrix, and fibrillar matrix designed with specific protein composition to investigate how the presence of FN and laminin in a 3-dimensional matrix regulate mouse ESC differentiation, especially into DE. Together these data implicate endogenous ECM as important co-regulators of mouse ESC fate along with the growth factors that induce their production.
Materials and Methods
Cell Culture
All cells were cultured at 37°C in a humidified incubator containing 5% carbon dioxide. Mouse fibroblast (NIH-3T3) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum (Thermo Scientific), 4mM L-glutamine, 1mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B for 4 days prior to passaging. Murine ESCs (cell line CCE, Stem Cell Technologies, [23]; cell line R1, American Type Culture Collection, [24]) were maintained in DMEM containing 2mM L-glutamine, 1mM sodium pyruvate, 50 U/mL penicillin, 50 μg/mL streptomycin, 1mM non-essential amino acids, 15% fetal bovine serum screened for mouse ESCs (Thermo Scientific), 100 μM 1-thioglycerol and 103 U/mL leukemia inhibitory factor. ESCs were grown on 0.1% gelatin-coated plates and passaged 1:10 as a single-cell suspension once the plate reached 80% confluence, about every 2 days, to maintain their undifferentiated state. CCE cells that stably express GFP under the control of the Nanog promoter were kindly provided by Dr. Ihor Lemischka (Mt. Sinai Medical School) and are termed Nanog-GFP ESCs [25].
To differentiate ESCs as a monolayer, cells were seeded as a single cell suspension on the indicated substrate at a density of 104 cells/cm2 in a differentiation medium composed of 1:1 Iscove's modified Dulbecco's medium and Ham's F-12 nutrient mixture, 2mM L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 0.1% bovine serum albumin, 450 μM 1-thioglycerol and the indicated concentration of fetal clone II serum (Thermo Scientific). Differentiation medium was supplemented with 10% serum [26], 10% serum and 20 ng/mL BMP-4 (R&D Systems) [27], or 1% serum, 100 ng/mL Activin A and 10 ng/mL Wnt3a [3] to induce neural progenitor, mesoderm, and definitive endoderm (DE) lineage specification, respectively (Supplemental Table 1). 10 μg/mL of GoH3 α6-integrin function blocking antibody (Beckman Coulter), 5 μg/mL of Ralph 3.1 α3-integrin function blocking antibody (Developmental Hybridoma Bank, Iowa City, IA), or 50 μg/mL of rabbit IgG control antibody were selectively added to differentiation cultures to disrupt integrin binding. In addition to including blocking antibodies in differentiation medium during culture (which was replenished every 2 days), ESCs in suspension were also incubated in differentiation medium containing the antibody at 37°C for 1 hour prior to seeding.
To differentiate ESCs in embryoid bodies (EBs), the multi-cell aggregates were formed by culturing 105 ESCs/mL in differentiation medium containing 15% serum in non-adherent 6 cm petri dishes for the indicated time. 100 μg/mL of soluble heparin or 6 μg/mL of BIIG2 α5-integrin function blocking antibodies (Developmental Hybridoma Bank, Iowa City, IA) was selectively added to EB cultures in the same manner as described above to competitively bind FN dimerization sites [28] or block α5-integrin function, respectively [29]. As noted above, medium during culture was replenished every 2 days to continuously block the interaction. Unless otherwise noted, cell culture products purchased were from Invitrogen and other reagents purchased from Sigma-Aldrich.
Preparation of Extracellular Matrix and Gelatin Culture Substrates
To prepare gelatin-coated substrates, tissue culture plates were incubated with 0.1% gelatin for 30 minutes. Fibrillar extracellular matrix was prepared from the indicated cell type using an established protocol [15]. For fibroblasts, mouse laminin-111 (Southern Biotech) was added exogenously to the culture medium when indicated. Briefly, 104 cells/cm2 were grown in growth medium for fibroblasts or induction medium for mouse ESCs for 7 days. Cells were then washed with PBS, wash buffer I (2 mM magnesium chloride, 2 mM EGTA, 100 mM sodium phosphate, pH 9.6), incubated at 37°C for 15 minutes in lysis buffer (8 mM sodium phosphate, 1% NP-40, pH 9.6) and replaced with fresh lysis buffer for an additional 60 minutes before final washes with wash buffer II (10 mM sodium phosphate, 300 mM potassium chloride, pH 7.5), PBS, and sterile water. Matrices were sterilized under ultraviolet radiation for 15 minutes prior to seeding.
Metabolic FN Labeling
ESCs and EBs were cultured for the indicated periods of time in the absence or presence of serum FN, and subsequently labeled with 100 μCi/mL [35S] methionine (MP Biomedicals; Solon, OH) for 24 hours before collection of the medium and lysis of cells in mRIPA buffer. FN was isolated from culture medium and lysates using gelatin-Sepharose binding. Isolated FN was then reduced by addition of 0.1 M dithiothreitol and separated by electrophoresis. Gels were dried, placed on a phosphor storage screen, and bands detected and quantified using a Storm 860 system (GE Healthcare Life Sciences). Data plotted represents total FN produced in both lysates and media. Though cells were plated at similar densities, to normalize gel loading to account for any proliferation differences, protein concentration was determined from a β-N-acetylglucosaminidase activity assay performed on cell lysates.
Deoxycholate-Solubility Assay
A deoxycholate (DOC)-solubility assay was used to separate cellular components from DOC-insoluble cell-derived extracellular matrix for western blot analysis, as previously described [30]. Briefly, cultured cells were washed with PBS and lysed with DOC lysis buffer (2% sodium deoxycholate, 20 mM Tris·Cl, 2 mM EDTA, 2mM PMSF, pH 8.8). The cell lysate was collected and passed through a 27-G needle five times to shear DNA and reduce viscosity. The lysates were micro-centrifuged at 18,400 g for 20 minutes to pellet the DOC-insoluble fraction (DOC-insoluble ECM). The supernatant (cell lysis component) was removed and the DOC-insoluble fraction was washed once with fresh DOC lysis buffer and then resuspended in SDS-solubilization buffer (4% SDS, 20 mM Tris·Cl, 2 mM EDTA, 2mM PMSF, pH 8.8).
Western Blotting
Cells were lysed using mRIPA buffer [31] or DOC lysis buffer [30], as indicated. If a DOC-lysis buffer was used, the DOC-insoluble fraction was solubilized in a SDS-solubilization buffer [30]. Samples were separated by electrophoresis under reducing and denaturing conditions, transferred to a nitrocellulose membrane and immunoblotted using ab8245 mouse anti-GAPDH monoclonal antibody (1:104; Abcam), ab18976 rabbit anti-Oct4 polyclonal antibody (1:500; Abcam), PAX6 mouse-anti Pax6 monoclonal antibody (1:200; Developmental Studies Hybridoma Bank), ab20680 goat anti-Brachyury polyclonal antibody (1:200; Santa Cruz Biotechnology, Inc.), sc-9053 rabbit anti-GATA4 polyclonal antibody (1:200; Santa Cruz Biotechnology, Inc.), AF1924 goat anti-SOX17 polyclonal antibody (1:200; R&D Systems), R457 rabbit anti-fibronectin polyclonal antiserum (1:2000; [32]), ab11575 rabbit anti-laminin-111 polyclonal antibody (1:1000; raised against laminin from EHS basement membrane; Abcam) or ab34710 rabbit anti-collagen type I polyclonal antibody (1:2000; Abcam) and the appropriate horseradish peroxidase-conjugated goat or donkey IgG (1:104). Western blots were developed with ECL substrate (Pierce) and the integrated densities of bands within the linear range of the film were analyzed using Image J. GAPDH was used to normalize all data, which was plotted as a fold change from EBs grown with complete serum (Figure 1) or from ESCs grown in maintenance medium (Figure 4).
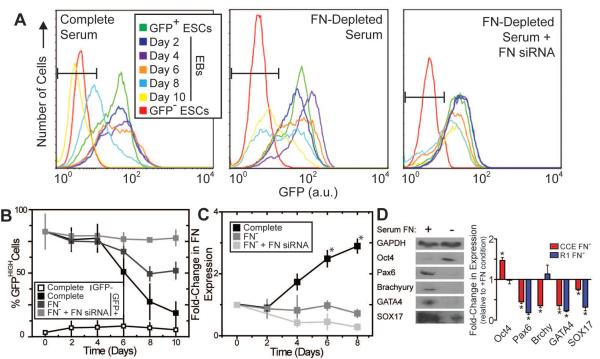
Figure 1. Mouse ESC self-renewal and differentiation is affected by FN.
Mouse ESCs (CCE cell line), expressing GFP under the control of a Nanog promoter, were cultured as embryoid bodies (EBs). (A) EBs, grown in conditions with complete serum or FN-depleted serum ± siRNA for FN, were dissociated into single cells and analyzed by flow cytometry. Mouse ESCs lacking Nanog-GFP were labeled GFP− ESCs (red lines) and used to set a GFPHIGH threshold in B. The starting populations of undifferentiated Nanog-GFP cells were labeled GFP+ ESCs (green lines). (B) The GFPHIGH ESC fraction, determined from the gate in A, as defined by the GFP− ESCs, decreases with time depending on the specific culture conditions. (C) EBs grown in conditions with complete serum or FN-depleted serum (labeled as FN−) ± siRNA for FN were metabolically labeled with [35S] methionine, and the labeled FN produced during labeling was analyzed by SDS-PAGE and phosphorimaging (images not shown). Data is normalized to the FN produced by undifferentiated ESCs at day 0. (D) Mouse ESCs (CCE and R1) were cultured as EBs for six days in medium with complete (+) or FN-depleted serum (−). Cells were lysed and equal amounts of protein were separated by SDS-PAGE and blotted with Oct4 (self-renewal), Pax6 (neural progenitor), Brachyury (mesoderm), GATA4 (definitive endoderm), SOX17 (definitive endoderm) and GAPDH (loading control) antibodies (left). The fold-change in protein expression of the FN− culture relative to the FN+ culture was quantified (mean ± standard error from 3 independent samples) (right). (A–C) * p < 0.05 as determined by ANOVA. (D) * p<0.05 as determined by a one-sample t-test comparing the sample to a theoretical mean of 1.0.
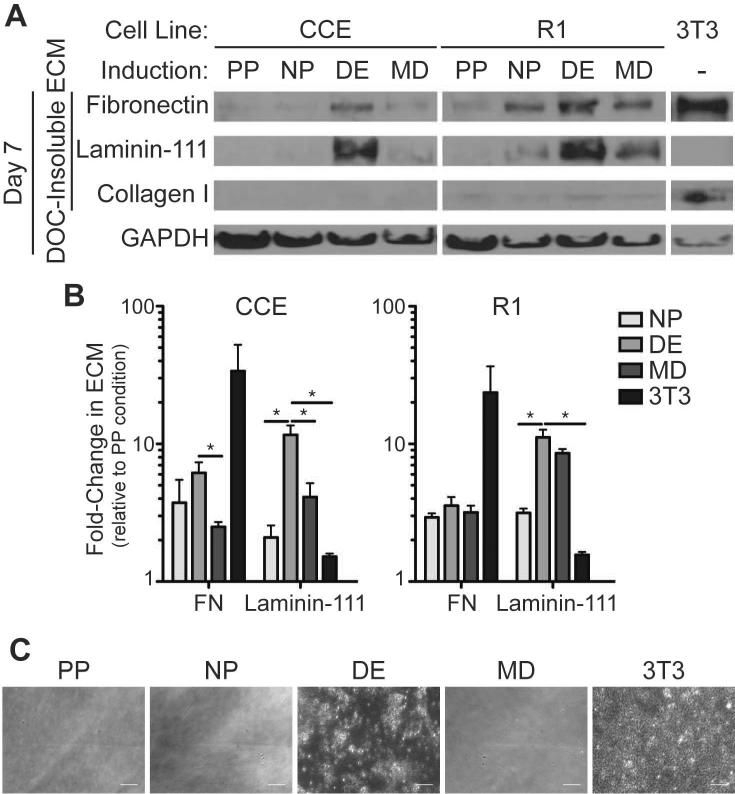
Figure 4. Mouse ESC-derived ECM is distinct from 3T3 fibroblast-derived ECM and is affected by induction medium.
Mouse ESCs (CCE and R1) and 3T3 fibroblasts were cultured for 7 days in pluripotency (PP), neural progenitor (NP), definitive endoderm (DE), mesoderm (MD), or 3T3 fibroblast medium. (A) A DOC-solubility assay was used to isolate the DOC-soluble (cell lysis) fraction from the DOC-insoluble extracellular matrix. Both fractions were separated by SDS-PAGE and blotted for GAPDH and extracellular matrix proteins, respectively (top). (B) The fold-change in expression of the indicated extracellular matrix protein was quantified relative to the pluripotency (PP) condition and the GAPDH content in the DOC-soluble fraction (right) (mean ± standard error from 3 independent samples). (C) The cell-derived extracellular matrices from each culture condition were decellularized and imaged by phase-contrast microscopy. Scale bar is 50 μm. * p < 0.05 as determined by ANOVA.
Fluorescence-Activated Cell Sorting Analysis
The indicated ESCs maintained in self-renewing conditions or grown as EBs in either complete fetal clone II serum (Thermo Scientific) or FN-depleted fetal clone II serum (FN removed by gelatin-Sepharose affinity chromatography [15]) were analyzed by flow cytometry for their expression of GFP under the control of a Nanog promoter. After washing in PBS, EBs were dissociated using a 5 minute treatment of 0.05% trypsin under light agitation. Cells were centrifuged before being resuspended for analysis in a FACScan cytometer (Becton Dickinson). Fluorescence was measured at 488 nm and data were gated to measure single cells.
Immunofluorescence Staining
Cell cultures to be immunofluorescently stained were fixed with 3.7% formaldehyde for 20 minutes at room temperature. When staining for intracellular proteins, samples were subsequently permeabilized with 0.5% Triton X-100 for 5 minutes at 37°C. Samples were blocked for 1 hour with 2% ovalbumin at 37°C and stained using R457 rabbit anti-fibronectin polyclonal antiserum (1:500; [32]), sc-9053 anti-GATA4 polyclonal antibody (1:200; Santa Cruz Biotechnology, Inc.), ab3280 mouse anti-actin polyclonal antibody (1:1000; Abcam), rhodamine-phalloidin (1:500; Invitrogen), ab11575 rabbit anti-laminin-111 polyclonal antibody (1:100; raised against laminin from EHS basement membrane; Abcam), or AF1924 goat anti-SOX17 polyclonal antibody (1:20; R&D Systems) and the appropriate Alexa dye- conjugated goat or donkey IgG (1:500). Cells were additionally labeled with Hoechst (1:2000) stain as indicated. Samples were examined by either a CARV or CARV II confocal microscope (BD Biosciences) mounted on a Nikon Eclipse TE2000-U microscope with IP Lab software or Nikon Ti-S microscope with Metamorph 7.6 software. Image J software with a custom analysis macro was used to determine line profiles (Figure 2). Briefly, a line was drawn from the center to edge of the EB at its widest confocal cross-section (Figure 2A). This line was averaged over all 360 degrees and then averaged across a population of EBs in the same culture condition, resulting in line plots from the center to the edge of EBs (Figure 2B–D; Supplemental Figure 1).
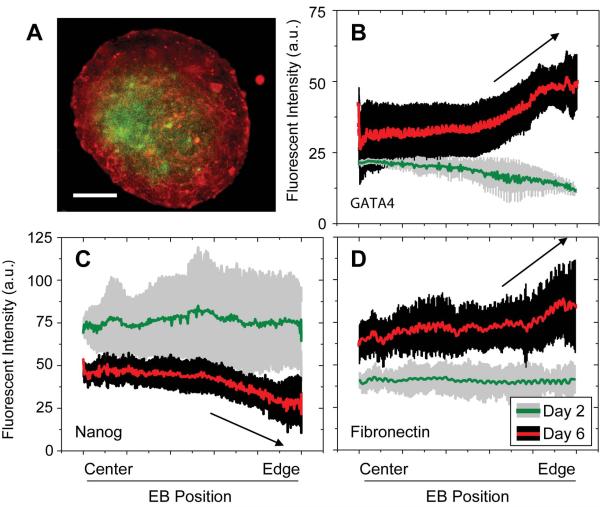
Figure 2. Fibronectin expression correlates with endoderm but not self-renewal markers in embryoid bodies.
(A) Immunofluorescent images of Nanog-controlled GFP (green) and fibronectin (red) were made at the widest confocal cross-section of EBs (CCE cell line) cultured in complete media. Custom image analysis software was used to determine the average fluorescent intensity from the EB center to the edge, summed together for all 360 degrees. Scale bar is 100 μm. (B–D) Protein distributions are shown for GATA4 (definitive endoderm), Fibronectin, and Nanog-controlled GFP at days 2 and 6 of EB culture (>50 EBs/group from 5 independent samples).
Quantitative Polymerase Chain Reaction (qPCR)
RNA was isolated from adherent ESCs or cultured EBs at the indicated time points using Trizol according to the manufacturer's instructions and cDNA was prepared from 2 μg of RNA as described elsewhere [28]. qPCR was performed (40 cycles, 95°C for 15 seconds followed by 60°C for 1 minute) for differentiation markers using an ABI Prism 7900 HT, the primer sets indicated in Supplemental Table 2 as designed via PrimerQuest software (Integrated DNA Technologies), and the iQ SYBR Green Supermix. Data were analyzed using SDS 2.3 software (Applied Biosystems), which calculated expression based on a standard curve generated by a FN plasmid [33]. GAPDH was used to normalize all data, which was plotted as a fold change from undifferentiated mouse ESC control samples. Note that the variance in threshold cycle for GAPDH across all samples from Figures 5 and 6 was less than 0.03 and 0.08 cycles, respectively.
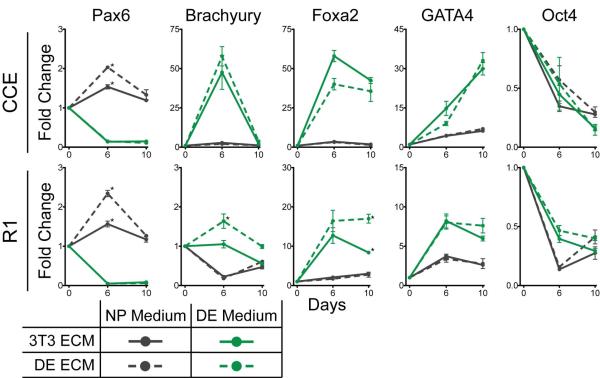
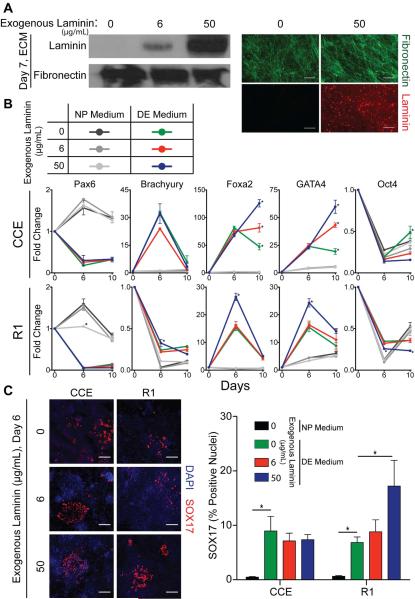
Figure 5. Mouse ESC differentiation is affected by composition of ECM substrate.
Pluripotent mouse ESCs (CCE and R1) were cultured for up to 10 days on the indicated substrate in either neural progenitor (NP) or definitive endoderm (DE) differentiation medium. The fold-change in expression of neural progenitor (Pax6), mesoderm (Brachyury), definitive endoderm (Foxa2 and GATA4), and pluripotency (Oct4) markers were determined by quantitative PCR, relative to an initial population of pluripotent mouse ESCs at day 0 (mean ± standard error from 3 independent samples). * p < 0.05 as determined by ANOVA and is a comparison of gene expression on the culture substrates in the same induction medium.
Figure 6. Laminin containing ECM promotes DE induction.
3T3 fibroblasts were grown for 7 days in fibroblast culture medium supplemented with up to 50 μg/mL mouse laminin-111 (Southern Biotech). (A) The DOC-insoluble extracellular matrix was separated by SDS-PAGE and blotted for laminin and fibronectin (left). Maximum intensity z-stack projections were created from confocal cross-sections of the immunostained decellularized ECM (fibronectin (green), laminin (red)) (right). Scale bar is 20 μm. (B) 3T3 fibroblast-derived ECMs, grown with the indicated concentration of exogenous mouse laminin-111, were decellularized and reseeded for up to 10 days with pluripotent mouse ESCs (CCE and R1) in either neural progenitor (NP) or definitive endoderm (DE) differentiation medium. The fold-change in expression of neural progenitor (Pax6), mesoderm (Brachyury), definitive endoderm (Foxa2 and GATA4), and pluripotency (Oct4) markers were determined by quantitative PCR, relative to an initial population of pluripotent mouse ESCs at day 0 (mean ± standard error from 3 independent samples). (C) Immunofluorescent images of SOX17 (red) and DAPI (blue) stained nuclei were taken after CCEs and R1s has been differentiated on decellularized fibroblast-derived ECM for 6 days (left). Scale bar is 100 μm. The percentage of SOX17 positive nuclei was quantified (>103 nuclei/group) (mean ± standard error) (right). (B) * p < 0.05 as determined by ANOVA and is a comparison of gene expression on the culture substrates in the same induction medium. (C) * p < 0.05 as determined by ANOVA.
Statistical Analysis
All statistical analyses were performed using Prism 5 (GraphPad Software, Inc.). Differences among three or more groups were assessed by ANOVA with Tukey's post hoc analysis to identify statistical differences when the p-value is less than 0.05. Unpaired t-tests were used when comparing two groups as indicated. All data are presented as mean ± standard error of the mean. Experimental data are shown for experiments performed in triplicate.
Results
Fibronectin is Necessary for Loss of Pluripotency and Correlates with Endoderm Differentiation in Embryoid Bodies
Embryos lacking FN have early developmental defects [34] and fail to complete gastrulation [35], suggesting that FN may be required for initial fate specification. To examine the influence that FN may have on differentiation, mouse CCE ESCs that express GFP under the control of a Nanog promoter [25] were cultured as multicellular embryoid bodies (EBs) to induce their differentiation while monitoring GFP expression and endogenous FN production. Between days 4 and 8 in culture, a decrease in GFP expression occurred as assessed by flow cytometry (Figure 1A; left), resulting in a 3-fold reduction of the GFPHIGH population (Figure 1B), and thus indicating lower Nanog promoter activity. Metabolic labeling showed that FN production preceded the change in GFP expression, resulting in a 3-fold increase in FN production by day 8 when normalized to undifferentiated ESCs at day 0 (Figure 1C). To determine whether changes in FN levels affected Nanog promoter activity, EBs were grown in FN-depleted serum with or without small interfering RNA (siRNA) targeting FN (see Supplemental Table 3 for sequence). Unlike complete serum, a roughly equal number of cells remained GFPHIGH as lost fluorescence when cultured in FN-depleted serum (Figure 1A; center and 1B), and the dramatic increase in FN production was no longer detected by metabolic labeling (Figure 1C). EBs grown in FN-depleted serum with FN siRNA contained many GFPHIGH cells for the duration of the culture period (Figure 1A; right and 1B) and exhibited decreased FN production versus untreated cells as a confirmation of the FN siRNA's effect (Figure 1C).
Though these data indicate a sequential relationship between FN and the loss of self-renewal, they do not show whether the absence of FN would also delay the expression of differentiation markers. To address this, EBs from both CCE and R1 mouse ESCs cultured in complete serum and in FN-depleted serum for 6 days were analyzed by western blotting to assess expression of germ layer markers. When compared to complete serum, CCE and R1 EBs cultured in FN-depleted serum exhibited increased expression of the self-renewal marker Oct4 and reduced expression of the ectoderm marker Pax6, the endoderm markers SOX17 and GATA4, and the mesoderm marker Brachyury, although R1 cells saw a slight but not statistically significant increase in Brachyury expression and no change in Oct4 expression (Figure 1D).
Since FN is localized to endoderm cells in vivo [9] and is abundant in many endoderm-derived tissues [10–12] the spatial and temporal localization of endoderm-markers and fibronectin in EBs were compared. Nanog, GATA4, and FN images of the widest confocal cross-sections of CCE EBs (Figure 2A) grown with medium containing complete serum were taken at days 2 and 6, and subsequent analyses produced line plots of the average staining intensity from EB center to edge. Both GATA4 (Figure 2B) and FN (Figure 2D) showed increased staining as a function of culture time. Levels of GATA4 and FN also increased with distance from the EB center at day 6 (arrows) but not at day 2. To quantitatively measure the correlation between protein expression and position for FN and GATA4, Pearson's Product-Moment Coefficient, a measure of positively (+1) or negatively (−1) correlated variables, was calculated. This coefficient increased from 0.11 on day 2 to 0.70 on day 6, showing that as EBs develop, FN and GATA4 exhibit similar distributions. In contrast, Nanog-controlled GFP expression (Figure 2C) decreased with both time and position towards the EB edge, and thus its correlation coefficient with both GATA4 and FN was negative at day 6: −0.58 and −0.85, respectively. FN and GATA4 expression in EBs treated with heparin, which interferes with FN matrix assembly but not production [28], did not increase with position towards the EB edge but did increase over time (Supplemental Figure 1C–E). Similarly, treatment with the antibody BIIG2, which blocks α5-integrin binding to FN [29], prevented the increase in FN and GATA4 at the EB periphery (Supplemental Figure 1F–H). It is important to note that mouse ESCs do not express many other fibronectin-binding alpha integrins [19]. It should also be noted that these confocal microscopy data are not likely affected by antibody diffusion as actin antibody and phalloidin staining produce line profiles with a correlation coefficient of 0.78 in EBs made from CCE ESCs (Supplemental Figure 1A, B). Together these data suggest that spatial and temporal changes in FN assembly may be coupled with mouse ESC fate.
Endoderm-Inducing Growth Factors cause Assembly of a Fibrillar ECM with Composition that is Distinct from other Lineages
Unlike EBs, monolayer culture can be highly controlled to induce mouse ESC differentiation down a specific lineage. To further establish a link between extracellular matrix proteins that are expressed as a result of the growth factors present in ESC cultures and endoderm induction, mouse ESCs were plated onto gelatin-coated substrates and cultured for 7 days in medium that either maintained pluripotency (PP) [36] or induced differentiation to mesoderm (MD) [27], definitive endoderm (DE) [3], or neural progenitors (NP) [26] (Supplemental Table 1). Fibrillar FN matrix was observed in the DE induction conditions, compared to pluripotent mouse ESCs and the other differentiation conditions of CCE cells where FN was mostly punctate (Figure 3). Quantitative comparison of assembled matrix using a DOC-solubility assay [30] indicated differences in both composition and amount of DOC-insoluble matrix produced in each condition (Figure 4A); for example, CCE and R1 ESCs in differentiating conditions produced 2-to 6-fold more FN matrix than when cultured in PP medium (Figure 4B). These assembly differences are not likely due to different serum FN concentrations as PP medium has the highest serum concentration (Supplemental Table 1). DE medium also induced 30–550% more laminin-111 expression than ESCs in NP or MD media (Figure 4A, B). In contrast, no matrix from either ESC line produced substantial type I collagen (Figure 4A). These cells, regardless of culture conditions, also produced matrix with different composition from the conventional matrices of 3T3 fibroblasts [15] which are enriched in fibronectin and also produce some type I collagen (Figure 4A). These data show that more matrix is assembled in DE medium than in other differentiation media, which is supported by decellularization studies. After 7 days in culture, CCE ESC and 3T3 fibroblast cultures were decellularized, removing cellular but preserving matrix components [37]. Only matrices derived from 3T3 fibroblasts and ESCs in DE medium remained on the substrates following decellularization (Figure 4C).
Figure 3. Differentiating mouse ESCs assemble a fibrillar fibronectin matrix.
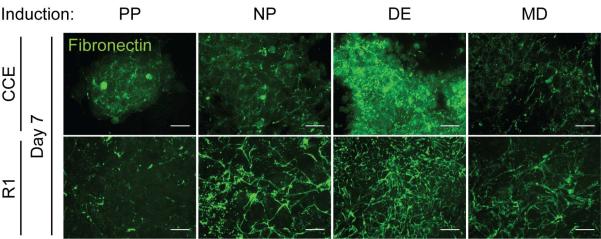
Mouse ESCs (CCE and R1) were cultured for 7 days in pluripotency (PP), neural progenitor (NP), definitive endoderm (DE), or mesoderm (MD) medium (Supplemental Table 1). Maximum intensity z-stack projections were created from confocal cross-sections of the immunostained fibronectin matrix (green). Scale bar is 20 μm.
Laminin-111 Incorporated into Fibroblast-Derived ECM Dose-Dependently Improves Endoderm Differentiation
To examine whether differentiation depends on or is affected by ECM components in the presence of induction medium, pluripotent ESCs were cultured on decellularized matrix derived from fibroblasts or ESCs induced to express DE markers and grown in either NP or DE inductive medium. Lineage specific transcription factor expression was then measured to assess the population's fate. In all conditions and for both CCE and R1 cells, Oct4 expression decreased (Figure 5). NP medium was sufficient to induce up to a 2-fold increase in Pax6 mRNA expression and inhibit expression of mesoderm or endoderm lineage markers after 6 days of culture on ESC-derived matrix (black data, Figure 5). On the other hand, DE medium inhibited Pax6 and induced mesoderm and endoderm marker expression; the mesoderm marker Brachyury peaked at day 6 coincident with or followed by endoderm markers Foxa2 and GATA4 (green data, Figure 5). At least during a portion of the time course, Foxa2 and GATA4 were differentially expressed on ESC-derived matrix compared to on fibroblast-derived matrix, suggesting that ECM differences may enhance DE differentiation. No detectable levels of mRNA were measured from decellularized ECMs prior to reseeding (data not shown), indicating that these results were not affected by residual mRNA transcript.
To compare matrices that differ only in the presence or absence of a single ECM component, fibroblasts were grown in the presence of exogenous laminin-111, which was incorporated into the DOC-insoluble matrix in a dose-dependent manner (Figure 6A). Naïve CCE and R1 ESCs cultured on decellularized, fibroblast-derived ECM with varying amounts of laminin again showed induction media-dependent expression of Pax6 or Brachyury, Foxa2, and GATA4 in NP or DE induction medium, respectively. More specifically, a laminin dose-dependent increase in Foxa2 and GATA4 was observed after 6 and 10 days for the R1 and CCE cell lines, respectively (Figure 6B). R1 and CCE cells also showed a 2-fold laminin-dependent increase in the percentage of cell nuclei expressing the endoderm marker SOX17 after 6 or 10 days, respectively (Figure 6C & Supplemental Figure 2). The number of population doublings was similar for all conditions (Supplemental Figure 3), indicating that proliferative differences could not account for laminin-111-dependent increase in DE marker expression.
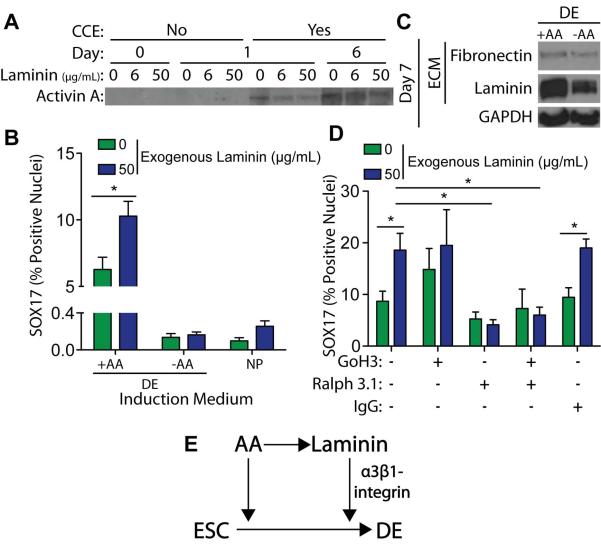
Laminin-111 Enhances Endoderm Differentiation through α3-integrin mediated Signaling
To understand how ECM-incorporated laminin might enhance DE differentiation, we considered three mechanisms: laminin-mediated Activin A sequestration, Activin A-independent integrin signaling, and an Activin A-laminin feed-forward loop. First, decellularized fibroblast-derived ECM, grown with 0 to 50 μg/mL laminin-111, was exposed to Activin A-containing induction medium, either in presence or absence of CCE cells, to determine if the laminin-containing matrix was regulating the presentation of soluble cues as has been shown with FN [7]. The DOC-insoluble matrix fractions contained Activin A after 1 day only when CCE cells were reseeded onto the decellularized matrices; however, Activin A sequestration in the matrix was laminin-independent (Figure 7A), which suggests that laminin is not enhancing DE differentiation through ECM-sequestration of Activin A. To assess whether laminin-mediated integrin signaling induced DE differentiation independent of Activin A, R1 cells were cultured on fibroblast-derived ECM with 50 μg/mL laminin-111 but in DE medium that did or did not contain Activin A. ESCs in DE medium on laminin-containing matrix had an almost 2 orders of magnitude increase in Sox17 positive nuclei versus cells grown on the same substrate but in medium lacking Activin A (Figure 7B). Cells exposed to Activin A but lacking laminin within their matrix showed ~2-fold less Sox 17 compared with cells plus Activin A and laminin, consistent with previous data (Figure 6C) and indicating that laminin does not act independently of but rather in concert with Activin A. Since Activin A is a TGF-β family protein and stimulates FN production [6, 38], it is possible that Activin A signaling could be sufficient to increase endogenous laminin expression and drive DE marker expression. The matrix of R1 cells grown in the presence of exogenous Activin A contained more laminin-111 and similar amounts of fibronectin as matrix from cells grown without Activin A (Figure 7C).
Figure 7. Laminin Enhances DE Induction through α3-integrin mediated signaling.
(A) Decellularized 3T3-derived ECM, grown with the indicated concentration of exogenous mouse laminin-111, was incubated in DE induction medium, containing 100 ng/mL Activin A, either in the presence (Yes) or absence (No) of mouse ESCs (CCE cell line) for the indicated number of days. The DOC-insoluble matrix was separated by SDS-PAGE and blotted for bound Activin A. (B) 3T3-derived ECMs, grown with the indicated concentration of exogenous laminin-111, were decellularized and reseeded for 6 days with naïve mouse ESCs (R1 cell line) in either neural progenitor (NP) or definitive endoderm (DE) induction, with or without 100 ng/mL exogenous Activin A. Immunofluorescent images of SOX17 and DAPI stain nuclei were taken and the percentage of SOX17 positive nuclei was quantified (>103 nuclei/group) (mean ± standard error). (C) Mouse ESCs (R1 cell line) were grown for 7 days on a gelatin-coated substrate in DE induction medium, with or without 100 ng/mL Activin A. A DOC-solubility assay was used to isolate the DOC-soluble cell lysis fraction from the DOC-insoluble extracellular matrix. Both fractions were separated by SDS-PAGE and blotted for GAPDH and extracellular matrix proteins, respectively. (D) 3T3-derived ECMs, grown with the indicated concentration of exogenous laminin, were decellularized and reseeded for 6 days with naïve mouse ESCs (R1 cell line) in DE medium containing Activin A and the indicated integrin function-blocking antibodies. Immunofluorescent images of SOX17 and DAPI stained nuclei were taken and the percentage of SOX17 positive nuclei was quantified (mean ± standard error). (E) Schematic of proposed feed-forward mechanism in which Activin A enhances endogenous laminin expression and laminin-mediated alpha-3 integrin signaling enhances the DE induction efficiency of Activin A. * p < 0.05 as determined by ANOVA.
While Activin A enhanced matrix incorporation of endogenous laminin-111, its role in inducing DE marker expression could act via non-matrix mediated pathways. To examine whether the effects of Activin A on laminin-111 matrix induce DE marker expression as a result of integrin signaling, R1 cells were cultured in DE induction medium on fibroblast-derived ECM, with or without 50 μg/mL laminin-111, and also with function-blocking antibodies to disrupt common laminin receptors α6-integrin (GoH3 antibody [39]) or α3-integrin (Ralph 3.1 antibody [40, 41]) [42]. The laminin-111 associated increase in Sox17 positive nuclei was not observed when the α3-integrin blocking antibody was included, either alone or in conjunction with an α6-integrin blocking antibody, despite the presence of Activin A (Figure 7D). Together these data indicate that laminin-111 enhances DE differentiation through a feed-forward mechanism in which Activin A enhances endogenous laminin matrix production and laminin-mediated α3-integrin signaling enhances the DE induction efficiency of Activin A (Figure 7E).
Discussion
This study sought to determine the effects of stem cell derived matrix on mouse ESC differentiation. Fibronectin was found to inhibit pluripotency and correlate with definitive endoderm (DE) differentiation in embryoid bodies. In monolayer culture, mouse ESCs exposed to DE-inducing growth factors, i.e. Activin A and Wnt3a, assembled a DOC-insoluble matrix that contained more laminin-111 than the matrix derived from other ESC lineages. Laminin-111 was found to enhance Activin A-mediated DE differentiation and appeared to depend on α3β1-integrin signaling.
Our results suggest that conventional growth factor cocktails used to induce neural progenitors (NP) [26], mesoderm (MD) [27], and definitive endoderm lineages [3] each may be effective in part due to production of and signaling from the ECM; indeed we observed that each cocktail produced a distinct matrix. We also found that specific components, e.g. FN, are required for embryoid bodies to lose pluripotency marker expression and acquire lineage specific differentiation markers; in fact, there is a direct temporal correlation between the onset of FN production and the loss of Nanog as well as a spatial correlation between FN and the endoderm marker GATA4. Human ESCs differentiated on a FN-coated substrate have shown improved DE differentiation in comparison to collagen and laminin substrates [21], which together suggests that the ECM may be an early regulator of cell fate by causing ESCs to exit pluripotency.
Beyond pluripotency, ECM composition has also been suggested as a lineage-specific regulator of differentiation by high throughput screening and tissue-specific matrix peptide coatings on planar substrates [21, 43, 44]. However using these defined ECM compositions is complicated by the matrix produced by ESCs themselves, which depends on the exogenous cues presented, i.e. serum proteins and growth factors. We found that maintaining pluripotency inhibited ECM assembly, but as most stem cell methods focus on differentiation, ESC-produced matrix is likely to complicate which differentiation pathways protocols employ, e.g. matrix- vs. growth factor-mediated. That said, ESC-produced matrix could be advantageous as it defines specific components likely to drive lineage-specific differentiation; indeed, we observed that ESCs exposed to Activin A and Wnt3a [3] produced an ECM that was distinct from other ESC-derived or fibroblast-derived ECM, which contained substantially less or completely lacked laminin, respectively. Moreover in matrix that originally lacked laminin, e.g. fibroblast-derived ECM, ESCs still expressed Foxa2 and GATA4, likely because they produced the laminin that was not already present. On the other hand, substrates where laminin-111 was titrated into the matrix exhibited a dose-dependent expression of these markers. These data suggest that laminin is not only supportive of DE expression in the presence of Activin A and Wnt3a, but it can also augment growth factor signaling. Composition specific matrix has been implicated not just in differentiation but also in patterning; laminin-111 expression within embryoid bodies establishes a polarized basement membrane [45]. Progenitor cells also have been found to increase ECM production in response to TGF-β family proteins, including Activin A [6, 38, 46, 47], indicating that growth factor-induced matrix expression is likely conserved, though the cell response is context specific.
Matrix production caused by induction medium can initiate integrin-mediated signaling, which may enhance cell fate changes brought on by growth factors. For example, we found that laminin incorporated into a fibroblast-derived ECM enhanced DE differentiation in the presence of DE induction medium, but that chronic treatment with an α3 integrin function blocking antibody prevented this response. Other laminin receptors, e.g. α6 integrin, did not exhibit the same blocking capabilities, though in addition to laminin-111 binding, both are receptors for other ECM proteins including fibronectin, collagen I, and thrombospondin [40, 42]. Integrin specific signaling also appears to extend to fate choices between visceral and definitive endoderm [14], suggesting that adhesion complex composition may play a larger role in matrix-mediated signaling than previously thought. We also noted that differentiation was not caused by matrix sequestration of growth factors, and laminin-containing FN matrix in the absence of exogenous Activin A was insufficient to induce DE marker expression, and thus neither Activin A binding nor integrin-signaling alone were responsible for the improved differentiation observed on laminin-containing ECM. These results suggest that Activin A directs DE differentiation and laminin production and that laminin-mediated α3β1 integrin signaling improves DE differentiation efficiency.
These findings that ECM composition augments growth factor signaling fit into a larger body of work showing that stem cell differentiation is affected by ECM properties, including stiffness [48, 49], topography [50, 51] and ligand composition [21, 44]. These findings also demonstrate that defined ECM composition, regardless of whether it was initially presented to ESCs or is the result of subsequent matrix production, should be considered when evaluating differentiation protocols.
Supplementary Material
Supplemental Figure 1: Actin localization in embryoid bodies. (A) Immunofluorescent image of antibody-stained actin (green) and phalloidin (red) made at the widest confocal cross-section of an EB (CCE cell line) cultured in complete media. (B) Protein distribution of actin, as measured by both labeling techniques, was similar as shown by correlation analysis. (C–E) Immunofluorescent image of heparin-treated EB stained for GATA4 (green) and FN (red) were made at the widest confocal cross-section of an EB. Note that the FN stained in the image is not assembled into a fibrillar structure as in Figure 2. Protein distribution of the two proteins was uncorrelated similar as shown by correlation analysis. (F–H) Immunofluorescent image of the integrin alpha-5 blocking antibody, BIIG2, treated EB stained for GATA4 (green) and FN (red) were made at the widest confocal cross-section of an EBs. Protein distribution of the two proteins was uncorrelated similar as shown by the correlation assay. Scale bars are 100 μm.
Supplemental Figure 2: Laminin-containing ECM promotes DE Induction after 10 days of culture. Immunofluorescent images of SOX17 and DAPI stained nuclei were taken after CCEs and R1s had been differentiated on decellularized fibroblast-derived ECM for 10 days. The percentage of SOX17 positive nuclei was quantified (>103 nuclei/group) (mean ± standard error). * p < 0.05 as determined by an unpaired t-test and is a comparison of the percentage of SOX17 positive nuclei in the indicated substrate and culture medium, within the same mouse ESC cellline.
Supplemental Figure 3: ESC Proliferation under defined matrix conditions. The number of population doublings was assessed for R1 and CCE cells when cultured on the indicated matrices.
Acknowledgments
The authors thank Dr. Ihor Lemischka for kindly providing the Nanog-GFP ESCs, Dr. Melissa Micou for assistance with statistical analyses, and Dr. Maike Sanders for fruitful discussions. This work was supported by grants from the National Institutes of Health (NIH R01CA044627 to J.E.S. and NIH R21EB011727 to A.J.E.). Pre-doctoral fellowship support was also provided by a National Science Foundation Graduate Research Fellowship (to H.T.-W.).
Footnotes
Author Contributions Hermes Taylor-Weiner: Conception and design, collection/assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
Jean E. Schwarzbauer: Conception and design, financial support, data analysis and interpretation, final approval of manuscript
Adam J. Engler: Conception and design, financial support, collection/assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. CELL. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Stainier DYR. A glimpse into the molecular entrails of endoderm formation. GENES & DEVELOPMENT. 2002;16(8):893–907. doi: 10.1101/gad.974902. [DOI] [PubMed] [Google Scholar]
- 3.D'Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. NATURE BIOTECHNOLOGY. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 4.Roszell B, Mondrinos MJ, Seaton A, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. TISSUE ENGINEERING. 2009;15(11):3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and isceral endoderm differentiation of mouse ES cells. NATURE BIOTECHNOLOGY. 2005;23(12):1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 6.Sales VL, Engelmayr GC, Mettler B a, et al. Transforming growth factor-beta1 modulates extracellular matrix production, proliferation, and apoptosis of endothelial progenitor cells in tissue-engineering scaffolds. CIRCULATION. 2006;114:I193–I199. doi: 10.1161/CIRCULATIONAHA.105.001628. [DOI] [PubMed] [Google Scholar]
- 7.Vlodavsky I, Folkman J, Sullivan R, et al. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 1987;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt FM. Out of Eden: Stem Cells and Their Niches. SCIENCE. 2000;287(5457):1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 9.Wartiovaara J, Leivo I, Virtanen I, et al. Appearance of fibronectin during differentiation of mouse teratocarcinoma in vitro. NATURE. 1978;272:355–356. doi: 10.1038/272355a0. [DOI] [PubMed] [Google Scholar]
- 10.Hay E. Cell Biology of Extracellular Matrix. Plenum Press; New York: 1991. [Google Scholar]
- 11.Snyder JM, O'Brien JA, Rodgers HF. Localization and accumulation of fibronectin in rabbit fetal lung tissue. DIFFERENTIATION. 1987;34(1):32–39. doi: 10.1111/j.1432-0436.1987.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 12.Uscanga L, Kennedy R, Stocker S. Immunolocalization of Collagen Types, Laminin and Fibronectin in the Normal Human Pancreas. DIGESTION. 1984;30(3):158–164. doi: 10.1159/000199100. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Edgar D, Fässler R, et al. The role of laminin in embryonic cell polarization and tissue organization. DEVELOPMENTAL CELL. 2003;4(5):613–24. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, He X, Corbett SA, et al. Integrins are required for the differentiation of visceral endoderm. JOURNAL OF CELL SCIENCE. 2009;122(Pt 2):233–242. doi: 10.1242/jcs.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Y, Schwarzbauer JE. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. JOURNAL OF CELL SCIENCE. 2005;118(Pt 19):4427–4436. doi: 10.1242/jcs.02566. [DOI] [PubMed] [Google Scholar]
- 16.Prowse ABJ, Chong F, Gray PP, et al. Stem cell integrins: Implications for ex-vivo culture nd cellular therapies. STEM CELL RESEARCH. 2011;6(1):1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Hunt GC, Singh P, Schwarzbauer JE. Endogenous production of fibronectin is required for self-renewal of cultured mouse embryonic stem cells. EXPERIMENTAL CELL RESEARCH. 2012;318(15):1820–1831. doi: 10.1016/j.yexcr.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domogatskaya A, Rodin S, Boutaud A, et al. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. STEM CELLS (DAYTON, OHIO) 2008;26(11):2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Furue MK, Okamoto T, et al. Integrins regulate mouse embryonic stem cell self-enewal. STEM CELLS (DAYTON, OHIO) 2007;25(12):3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 20.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. NATURE METHODS. 2005;2(2):119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 21.Brafman DA, Phung C, Kumar N, et al. Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions. CELL DEATH AND DIFFERENTIATION. 2012:1–13. doi: 10.1038/cdd.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. SCIENCE (NEW YORK, N.Y.) 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 23.Robertson E, Bradley A, Kuehn M, et al. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. NATURE. 1986;323(2):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- 24.Nagy A, Rossant J, Nagy R, et al. Derivation of completely cell culture-derived mice from arly-passage embryonic stem cells. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 1993;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaniel C, Li F, Schafer XL, et al. Delivery of short hairpin RNAs--triggers of gene silencing--into mouse embryonic stem cells. NATURE METHODS. 2006;3(5):397–400. doi: 10.1038/nmeth0506-397. [DOI] [PubMed] [Google Scholar]
- 26.Bibel M, Richter J, Lacroix E, et al. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. NATURE PROTOCOLS. 2007;2(5):1034–1043. doi: 10.1038/nprot.2007.147. [DOI] [PubMed] [Google Scholar]
- 27.Taha MF, Valojerdi MR. Effect of bone morphogenetic protein-4 on cardiac differentiation from mouse embryonic stem cells in serum-free and low-serum media. INTERNATIONAL JOURNAL OF CARDIOLOGY. 2008;127(1):78–87. doi: 10.1016/j.ijcard.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 28.Galante LL, Schwarzbauer JE. Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. THE JOURNAL OF CELL BIOLOGY. 2007;179(5):999–1009. doi: 10.1083/jcb.200707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huhtala P, Humphries M. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. THE JOURNAL OF CELL BIOLOGY. 1995;129(3):867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wierzbicka-Patynowski I, Mao Y, Schwarzbauer JE. Analysis of fibronectin matrix assembly. CURRENT PROTOCOLS IN CELL BIOLOGY. 2004;Chapter 10(Unit 10.12) doi: 10.1002/0471143030.cb1012s25. [DOI] [PubMed] [Google Scholar]
- 31.Wierzbicka-patynowski I, Mao Y, Schwarzbauer JE. Continuous Requirement for pp60-Src and Phospho-Paxillin During Fibronectin Matrix Assembly by Transformed Cells. JOURNAL OF CELLULAR PHYSIOLOGY. 2007;756(April 2006):750–756. doi: 10.1002/jcp.20886. [DOI] [PubMed] [Google Scholar]
- 32.Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. THE JOURNAL OF BIOLOGICAL CHEMISTRY. 1994;269(45):27863–27868. [PubMed] [Google Scholar]
- 33.Williams CM, Engler AJ, Slone RD, et al. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. CANCER RESEARCH. 2008;68(9):3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George EL, Georges-Labouesse EN, Patel-King RS, et al. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. DEVELOPMENT. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 35.Darribère T, Schwarzbauer JE. Fibronectin matrix composition and organization can regulate cell migration during amphibian development. MECHANISMS OF DEVELOPMENT. 2000;92(2):239–50. doi: 10.1016/s0925-4773(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 36.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. NATURE. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 37.Chen LB, Murray A, Segal R, et al. Studies on Intercellular LETS Glycoprotein Matrices. CELL. 1978;14(June):377–391. doi: 10.1016/0092-8674(78)90123-x. [DOI] [PubMed] [Google Scholar]
- 38.Caniggia I, Lye SJ, Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. ENDOCRINOLOGY. 1997;138(9):3976–3986. doi: 10.1210/endo.138.9.5403. [DOI] [PubMed] [Google Scholar]
- 39.Aumailley M, Timpl R, Sonnenberg A. Antibody to integrin alpha 6 subunit specifically inhibits cell-binding to laminin fragment 8. EXPERIMENTAL CELL RESEARCH. 1990;188(1):55–60. doi: 10.1016/0014-4827(90)90277-h. [DOI] [PubMed] [Google Scholar]
- 40.DeFreitas MF, Yoshida CK, Frazier WA, et al. Identification of integrin alpha 3 beta 1 as a neuronal thrombospondin receptor mediating neurite outgrowth. NEURON. 1995;15(2):333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 41.Demyanenko GP, Schachner M, Anton E, et al. Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. NEURON. 2004;44(3):423–437. doi: 10.1016/j.neuron.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Nishiuchi R, Ohoshi M, Fujiwara H, et al. Characterization of the Ligand-Binding Specificities of Integrin α3β1 and α6β1 Using a Panel of Purified Laminin Isoforms Containing Distinct Chains. JOURNAL OF BIOCHEMISTRY. 2003;134(4):497–504. doi: 10.1093/jb/mvg185. [DOI] [PubMed] [Google Scholar]
- 43.Baharvand H, Azarnia M, Parivar K, et al. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. JOURNAL OF MOLECULAR AND CELLULAR CARDIOLOGY. 2005;38(3):495–503. doi: 10.1016/j.yjmcc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 44.DeQuach J, Yuan S, Lawrence S, et al. Decellularized Porcine Brain Matrix for Cell Culture and Tissue Engineering Scaffolds. TISSUE ENGINEERING. 2011;17(21):2583–2592. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Harrison D, Carbonetto S, et al. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. THE JOURNAL OF CELL BIOLOGY. 2002;157(7):1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. BIOMATERIALS. 2001;22(5):439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 47.Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. EXPERIMENTAL CELL RESEARCH. 2001;268(2):189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 48.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. BIOMATERIALS. 2011;32(4):1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. CELL. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 50.Oh S, Brammer KS, Li YSJ, et al. Stem cell fate dictated solely by altered nanotube dimension. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 2009;106(7):2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee MR, Kwon KW, Jung H, et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. BIOMATERIALS. 2010;31(15):4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Actin localization in embryoid bodies. (A) Immunofluorescent image of antibody-stained actin (green) and phalloidin (red) made at the widest confocal cross-section of an EB (CCE cell line) cultured in complete media. (B) Protein distribution of actin, as measured by both labeling techniques, was similar as shown by correlation analysis. (C–E) Immunofluorescent image of heparin-treated EB stained for GATA4 (green) and FN (red) were made at the widest confocal cross-section of an EB. Note that the FN stained in the image is not assembled into a fibrillar structure as in Figure 2. Protein distribution of the two proteins was uncorrelated similar as shown by correlation analysis. (F–H) Immunofluorescent image of the integrin alpha-5 blocking antibody, BIIG2, treated EB stained for GATA4 (green) and FN (red) were made at the widest confocal cross-section of an EBs. Protein distribution of the two proteins was uncorrelated similar as shown by the correlation assay. Scale bars are 100 μm.
Supplemental Figure 2: Laminin-containing ECM promotes DE Induction after 10 days of culture. Immunofluorescent images of SOX17 and DAPI stained nuclei were taken after CCEs and R1s had been differentiated on decellularized fibroblast-derived ECM for 10 days. The percentage of SOX17 positive nuclei was quantified (>103 nuclei/group) (mean ± standard error). * p < 0.05 as determined by an unpaired t-test and is a comparison of the percentage of SOX17 positive nuclei in the indicated substrate and culture medium, within the same mouse ESC cellline.
Supplemental Figure 3: ESC Proliferation under defined matrix conditions. The number of population doublings was assessed for R1 and CCE cells when cultured on the indicated matrices.