Abstract
ENMD-2076 is an aurora kinase inhibitor that also has multi-target tyrosine kinase inhibitor properties. In this study, the mRNA and the protein expression of aurora-A and aurora-B were evaluated in three canine mast cell tumor cell lines. Dose dependent cytotoxicity was seen in the cells treated, and it affected the cell cycle with cells in the G2/M phase being selectively killed. The cells were also evaluated for radiosensitivity with/without ENMD-2076, and radiosensitization was seen after 3 Gy and 6 Gy exposures with ENMD-2076 for 48 hours. Protein expression of caspase-3 was gradually increased, and the expression intensity was highest at 24 hours post irradiation in cells without ENMD-2076 treatment, which indicates that radiation exposure with ENMD-2076 induced cell death faster than radiation treatment alone. Our study results suggest the potential usefulness of treating canine mast cell tumors with aurora kinase inhibitors alone or in conjunction with radiation therapy.
Keywords: Aurora kinase inhibitors, ENMD-2076, canine mast cell tumors, radiosensitivity
Introduction
Canine mast cell tumors are the most common cutaneous malignant tumors in dogs.1 While histologic grades, I, II, and III provide useful information regarding a patient’s prognosis,2,3 inter-observer variations have been reported 4, as well as the observation that grade II tumors, which represent the majority of canine mast cell tumors, have varied outcomes. Other factors such as mitotic index, Ki67, nucleolar organizing regions (AgNOR), and/or c-KIT expression also have been reported to correlate with prognosis.1,5-7 Treatment options depend on staging, but in general surgery and radiation therapy are very effective.1,8-10 It has been reported that for even one loco-regional lymph node metastasis with an incompletely excised mast cell tumor in dogs, definitive radiation therapy for both primary surgically excised and metastatic lymph node sites still can provide long term disease control and a disease-free survival time of 1,240 days.10 Chemotherapy is recommended if the patient has either systemic disease, a grade II mast cell tumor with negative histologic prognostic factors, or a grade III mast cell tumor. 11,12,13 However, no clear evidence of prolonging survival time has been reported yet. The most commonly used chemotherapy drugs for mast cell tumors are CCNU and vinblastine.1,13,14 Targeted therapy also has been attempted for canine mast cell tumors because this tumor expresses c-kit and contains a mutated constitutively activated form.15,16 The c-kit receptor is a one of a family of tyrosine kinase receptors that regulate cell signal transduction, cell survival, cell proliferation, and differentiation.17 Tyrosine kinase inhibitors have been reported to be radiosensitizers in humans. Some of the most well-known radiosensitizing agents are epidermal growth factor receptor (EGFR) inhibitors.18 It has been reported that the presence of EGFR inhibitors enhances DNA damage, which could lead to lethal damages.19 Since tyrosine kinase inhibitors are starting to be used intensively in veterinary medicine,16,20, it is very important to investigate their potential use as radiosensitizing agents. Another justification of investigating radiosensitizng agents would be that, although radiation therapy alone for gross mast cell tumors in dogs has a favorable response duration (reported as 12 months)21, using a radiosensitizing agent for non-surgical canine mast cell tumors might result in a longer tumor control and survival time over radiation therapy alone.
Aurora kinases are serine/threonine kinases that regulate the cell cycle and mitotic spindle assembly by centrosome duplication and separation.22 There are three types of aurora kinases: aurora-A, aurora-B and aurora-C. Aurora-A and aurora-B have been investigated in human medicine. Aurora-A and aurora-B are expressed in most normal cells, but the localization and the timing of the activation of aurora-A and aurora B are different during the cell cycle within normal cells. Aurora-A is ubiquitously expressed and regulates cell cycle events occurring from late S-phase through the M phase. 23 Aurora-B localizes to the centromere during the early stages of mitosis, and then after anaphase begins, Aurora-B relocates gradually to the midzone and persists throughout cytokinesis.22 Overexpression of the protein and mRNA of both aurora-A and aurora-B has been documented in multiple human tumors.22 We recently confirmed both the protein and mRNA expression of aurora-A and aurora-B in normal canine endothelial cells and canine malignant lymphoid cell lines in our laboratory.24 We have reported that canine aurora-A has a 90% homology to human aurora-A, and canine aurora-B has a 92% homology to human aurora-B by performing sequence alignment.24 This result indicates that these genes are highly conserved between humans and canine species. ENMD-2076 is a novel, orally bioavailable, aurora kinase inhibitor that also targets various tyrosine kinases such as Flt-3 (FMS-like tyrosine kinase-3), Src (Sarcoma), VEGFR-2 (Vascular endothelial growth factor receptor-2) and FGR1 (Fibroblast growth factor receptor-1).25
In this study, we evaluate the protein and mRNA expression of aurora-A and aurora-B in the canine mast cell tumors cell lines (CoMS, CM-MC1, and VI-MC1). We also investigate the cell survival, the drug’s effect on the cell cycle, and the enhancement of radiosensitivity with aurora kinase inhibitors. Our hypothesis is that ENMD-2076 enhances radiosensitivity and leads to cell death through apoptosis for canine mast cell tumor cells in vitro.
Materials and Methods
Cell culture
Three malignant canine mast cell tumor cell lines, CoMS, CM-MC1, VI-MC1, were used in this study, and all three cell lines have been previously characterized.26-28 CoMS originated from a mucosal mast cell tumor in a dog.26 CM-MC1 and VI-MC1 originated from visceral mast cell tumors in dogs.27,28 All three canine mast cell tumors were kindly donated by Dr. Nakagawa from Tokyo University, Tokyo (Japan) and Dr. Kadosawa from Rakuno Gakuen University, Hokkaido (Japan). All three cell lines were maintained in RPMI-1640 (American Type Cell Culture Collection, ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS, ATCC, Manassas, VA) and 2mM L-glutamine (ATCC, Manassas, VA). All cells were maintained in 5% CO2 at 37°C in a humidified incubator. The same incubation conditions were used for the MTT and cell cycle assays.
Drug
ENMD-2076 (aurora kinase and multi-targeted tyrosine kinase inhibitor, Selleck chemicals, Houston, TX) was purchased as a powder. ENMD-2076 was dissolved in dimethyl sulfoxide (DMSO) as a 10 mM stock solution and stored at −20°C.
RNA preparation
RNA was extracted from malignant canine mast cell tumor cells (CoMS, CM-MC1, VI-MC1) using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA) following the manufacturer’s protocol. The cells were counted manually with a hemocytometer, and 5 × 106 cells were used. Approximately 500 ng/μl of RNA was collected and the concentration of the RNA was measured by NanoDrop® ND-1000 (Wilmington, DE). The total RNA was treated with TURBO DNA-free™ to remove contaminating residual genomic DNA. For cDNA synthesis, the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Carlsbad, CA) was used following the manufacturer’s protocol. Briefly, 10X reverse transcription (RT) buffer, 25X dNTP mix, 10X RT random primers, MultiScribe reverse transcriptase, Nuclease-free H2O, and 20μl of total RNA were mixed gently in a total volume of 40 μl in a PCR tube, and then incubated in a GeneAmp® PCR System 9700 thermocycler (Applied Biosystems, Carlsbad, CA) under the following conditions: 25°C for 10 minutes, 37°C for 2 hours followed by enzyme inactivation at 85°C for 5 minutes.
RT-PCR
Canine specific aurora-A and aurora-B cDNA sequences were determined by our group previously.24 The cDNA gene sequence of canine specific aurora-A (GeneBank Accession: KC127668), canine specific aurora-B (GeneBank Accession: KC137547), and canine specific GAPDH (GeneBank Accession: NM_001003142.1) were used to generate primers using the Primer 3 program (http://frodo.wi.mit.edu/primer3/). The primers of aurora-A, aurora-B and GAPDH are as follows; aurora-A (forward/reverse sequences/amplicon size; CAGCCATAAACCGGCTCAGA / TCTCCTGGAGGATGGAGCAT/661 bp), aurora-B (CCTCATGGAGCCGCTCCAAT / CCTCCATGATCGTGGCTGTT/ 428 bp), and GAPDH (TCCATCTTCCAGGAGCGAGA / ATACATTGGGGGTGGGGACA/499 bp). The PCR conditions were 95°C for the initial denaturation, followed by 30 cycles of 45 seconds at 95°C (denaturing), 45 seconds at 57°C (annealing), and 60 seconds at 72°C (extension), then maintained at 4°C. The PCR was performed with 0.5 μl of cDNA, 2 μl each of forward and reverse primers, 20.5 μl of nuclease-free H2O, and 25 μl of PCR master mix (Qiagen Inc., Calencia, CA) in a total volume of 50 μl. The PCR was performed with the same thermocycler that was used for the cDNA synthesis. The amplified PCR products were separated by electrophoresis on 2% agarose gels with 0.01% ethidium bromide, and then analyzed by FluorChem® Q (Alpha Innotech, Santa Clara, CA) under UV light.
Irradiation
The irradiation source in these experiments was a 6 MV x-ray beam produced by a linear accelerator (Clinac 600C, Varian Medical Systems, Palo Alto, CA) at a dose rate of 2.5Gy/minute at the Cancer Treatment Unit of the Department of Veterinary Clinical Sciences, Louisiana State University. Beam dosimetry is part of routine maintenance. Cell culture flasks were irradiated at a 100 cm source-to-surface distance with a 1.5cm of tissue equivalent bolus material to obtain maximum dose at the level of bottom of the flasks. The flasks were irradiated with a single dose of either 3Gy or 6Gy with a 6MV x-ray beam at 180 degree of gantry of the linac. Doses at the bottom of flasks were determined using standard formulas from dose output calibrated at a 100 cm source-to-surface protocol following the American Association of Physicists in Medicine (AAPM) TG-51 protocol.
Western blot
Protein expression of aurora-A and aurora-B in canine mast cell tumors cell lines
To assess the protein expression of aurora-A and aurora-B in canine mast cell tumors, 2 × 107 CoMS, CM-MC1, and VI-MC1 cells were harvested from T-75 flasks, washed twice with phosphate-buffered saline (PBS, ATCC, Manassas, VA), and lysed with RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer’s protocol. Briefly, the harvested cells were lysed with the RIPA buffer containing 10 μl of PMSF (phenylmethylsulfonyl fluoride), 10 μl of sodium orthovandate, and 10 μl of protease inhibitor cocktail per ml of buffer for 30 minutes on ice. The lysed cells were then disrupted with a 21-gauge needle, incubated another 30 minutes, and then centrifuged at 10,000 X g for 10 minutes at 4°C. Protein concentrations were determined by the BCA protein assay according to the manufacturer’s protocol (Pierce Biotechnology, Rockford, IL). Western blot analysis was performed with 16% SDS-PAGE. The samples were mixed with a gel loading buffer, heated at 95°C for 5 minutes, and then 20 μg of protein was loaded into each lane of the SDS-PAGE gel. The SDS-PAGE gel was transferred to nitrocellulose membranes by electroblotting. Five percent non-fat dry milk in PBS with 0.2% TWEEN-20 was used to block the blots. The blots were probed with anti-rabbit Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2000 dilution; monoclonal mouse antibody, Abcam, Cambridge, MA), anti-human aurora-A (1:1000 dilution; polyclonal goat antibody , Tocris Bioscience, Ellisville, MO), and anti-human aurora-B (1:1000 dilution; polyclonal rabbit antibody, Abcam, Cambridge, MA) antibodies at room temperature for 1 hour. The membranes were then incubated with the appropriate horseradish peroxidase conjugated secondary antibody (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour. The proteins were visualized using Amersham ECL plus substrate (GE Healthcare, Buckinghamshire).
To detect caspase-3 expression for evaluation of apoptosis, canine mast cell tumor cell lines were irradiated with/without ENMD-2076, harvested and lysed as described above, but harvested at different time points (control, 0.5 hour, 6 hour, and 24 hour). Briefly, CoMS and VI-MC1 cells were seeded into T-75 flasks, and then 5ml of this culture, containing 1 ×107 cells, was allocated to 10 T-25 flasks. One flask served as a control (no radiation or drug), and was treated with 0.01% DMSO. The remaining flasks were exposed to 3 Gy of radiation using the 6MV linac. Three had no drug, three were treated with the IC10 of ENMD-2076, and three were treated with the IC50 of ENMD-2076. The cells were harvested after 0.5, 6, and 24 hours of incubation. The protein concentrations were determined as before, blots were made, and the blots were probed with anti-human caspase-3 (1:1000 dilution; polyclonal rabbit antibody, Cell Signaling Technology, Danvers, MA), incubated at 4°C overnight with shaking. The blots were visualized in the same manner as described above.
MTT assay
The cytotoxicity of ENMD-2076 was evaluated by MTT assay (Roche Applied Science, Indianapolis, IN) following the manufacturer’s protocol. Briefly, 5 × 104 cells of CoMS, CM-MC1, and VI-MC1 were counted manually with a hemocytometer, and seeded in 96 well plates. The plated cells were incubated with various concentrations of ENMD-2076 for 48 hours. The ENMD-2076 concentrations ranged from 0 to up to 5 μM (0, 25 nM, 50nM, 100 nM, 250 nM, 500 nM, 1 μM, 2.5 μM, and 5 μM). Ten microliters of MTT labeling reagent was applied to each well, incubated for 4 hours, and then one hundred microliters of solubilization solution were added. The plates were incubated at least 18 hours or until complete solubilization of purple formazan crystal, and then the absorbance at 570 nm was measured on a microplate reader. Cell viability was calculated as a percentage of the absorbance of the treated cells relative to the absorbance of the control cells (cell viability % = absorbance of treated cells/absorbance of control). Non-ENMD-2076 treated cells were incubated only with vehicle (0.01% DMSO), and served as controls. Cell survival fraction curves were generated, and the IC10 and IC50 were calculated for CoMS, CM-MC1, and VI-MC1 using Graph Pad Prism version 5 (Graph Pad Prism Software Inc., La Jolla, CA). The samples were run in duplicate and the experiments were repeated at least three times.
Additionally, to evaluate the concurrent effect of ENMD-2076 and radiation exposure, MTT assays were performed using the same protocol described above. Only CoMS and VI-MC1 cell lines were used for this experiment because the effect of ENMD-2076 on the cell cycle was not obvious in CM-MC1 cells, so this cell line was not used. CoMS and VI-MC1 were grown in T-75 flasks, and then treated with no-drug (treated only with 0.01% DMSO), IC10, or IC50 of ENMD-2076 with 0 Gy, 3 Gy, or 6 Gy of radiation using 6MV x-rays. After treatment, 5 × 104 cells were plated in 96 well plates, and were incubated for 48 hours. Again, the survival fraction was calculated. The samples were run in duplicate and the experiments were repeated at least three times.
Cell cycle analysis
To evaluate the effect of ENMD-2076 on the cell cycle phases in canine mast cell tumors, cell cycle analysis was performed. Briefly, 2 × 105 CoMS, CM-MC1, and VI-MC1 cells were cultured in 48-well plates. The IC10 and IC50 of ENMD-2076 was added to each well and incubated for 48 hours. The treated cells were washed twice with cold-PBS and then resuspended in 100 μl of cold-PBS. Ice-cold methanol was added slowly and mixed well, then incubated at 4°C for 30 minutes. The cells were then washed with cold-PBS and resuspended in 450 μl of PBS containing 25 μl of RNAse A (Sigma-Aldrich, St. Louis, MO) and 25 μl of propidium iodide and incubated for 30 minutes at room temperature in the dark. The stained cells were gated and counted by flow cytometry (BD Biosciences, San Jose, CA). The data was analyzed using Verity Modfit LT software (Verity Software House, Topsham, ME) and reported as a percentage of each cell-cycle phase, which are G1, S and G2/M. Untreated cells served as controls, and were treated with 0.01% DMSO. The samples were kept in the dark, and analyzed within 3 hours. The samples were run in duplicate, and the experiments were repeated at least three times.
Statistical and Data analysis
For the MTT and cell cycle assay, the data were reported as the mean and standard deviation (SD). The Shapiro-Wilk test was used to analyze the normal distribution of theMTT assay, cell cycle assay, and the MTT assay survival fraction after radiation and ENMD-2076 treatments. To compare outcome in group, analysis of variance (ANOVA) was used, and then post hoc comparisons were conducted with Tukey’s test for pairwise comparisons of significant overall effects. A p value < 0.05 was considered significant. The collected cell survival fractions (SF) and their propagated standard errors (1σ) were plotted versus dose (D) and fit to the Linear Quadratic (LQ) hypothesis:
| (1) |
where α and β are fitting parameters related to the dual radiation action theory. For cross-comparison purpose, the sensitization enhancement ratio was utilized at the 50% cell survival level (SER50). The SER50 is defined as the ratio of doses required to reach 50% cell inactivation in each of the mast cell tumor cell lines without ENMD-2076 treatment to those treated with the IC10 and IC50 of ENMD-2076. The SER50 values were determined for CoMS and VI-MC1 cells. All data was analyzed using either Graph Pad Prism version 5 (Graph Pad Prism Software Inc., La Jolla, CA) or SPSS version 18 (IBM, Armonk, NY). Graph Pad Prism was used to calculate the IC10 and IC50 for each cell line.
Results
Expression of aurora kinase in malignant canine mast cells
RT-PCR
RT-PCR was performed to detect the expression of aurora-A and aurora-B in canine mast cell tumors. The expression of the mRNA of aurora-A and aurora-B was detected in all canine mast cell tumor cell lines (CoMS, CM-MC1, and VI-MC1; Fig. 1). The intensity of the GAPDH bands was the same throughout all three cell lines and served as a positive control. Both aurora-A and aurora-B were identified and sequenced from normal canine aortic endothelial cells (CnAOECs) in a previous study24 and they also served as a positive control (Data not shown here). RNA extracted from each canine mast cell tumor cell line was used as a template in negative control reactions. No bands were detected from the negative control (Data not shown here).
Figure 1.
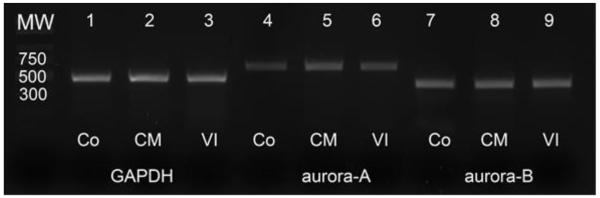
Results of RT-PCR of GAPDH, aurora-A and aurora-B from canine malignant mast cell tumor cell lines (CoMS, CM-MC1 and VI-MC1). GAPDH served as the positive control. Amplicons in lanes 1, 2, 3 are GAPDH, amplicons in lanes 4, 5, 6 are aurora-A, and amplicons in lanes 7, 8, 9 are aurora-B. Bands of the size of aurora-A and aurora-B were clearly detected from all canine mast cell tumor cell lines (CoMS, CM-MC1, and VI-MC1). MW, Co, CM, and VI represent molecular weight, CoMS, CM-MC1, and VI-MC1, respectively. The size of amplicons for each target gene is identical to the size predicted from the primers.
Protein expression of aurora kinases
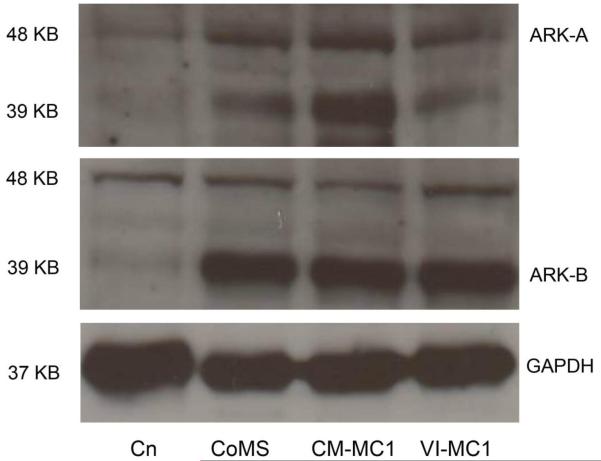
To evaluate the protein expression of aurora-A and aurora-B kinase in canine malignant mast cell tumors, western blot analysis was performed on protein extracts from CoMS, CM-MC1, and VI-MC1 cells. The protein expression of aurora-A and aurora-B kinase was detected in all canine mast cell tumor cell lines (CoMS, CM-MC1 and VI-MC1; Fig. 2). Cross reactivity between aurora-A and aurora-B antibodies was seen in all three cell lines. The molecular weights of aurora-A and aurora-B are 48 and 39KD, respectively. GAPDH served as a loading control, and the intensity of the bands of GAPDH in all three mast cell tumor cell lines are identical. The protein expression of aurora-A and aurora-B kinase from normal canine aortic endothelial cells (CnAOECs) was identified in a previous study,24 and it also served as a positive control in the same manner as the RT-PCR.
Figure 2.
The protein expression of aurora-A and aurora-B kinase in canine mast cell tumor cell lines (CoMS, CM-MC1 and VI-MC1). Canine aortic endothelial cells served as a positive control. The aurora-A and aurora-B kinases were detected in all three canine malignant mast cell tumor cell lines. There is cross reactivity between the aurora-A and aurora-B antibodies. The protein size of aurora-A, aurora-B, and GAPDH are approximately 48, 39, and 37 KD, respectively. GAPDH was used as a loading control, and the intensity of bands of GAPDH in all three mast cell tumor cell lines are identical. Cn, ARK-A, and ARK-B represent canine aortic endothelial cells, aurora-A kinase and aurora-B kinase, respectively.
Cell viability with ENMD-2076 in canine malignant mast cell tumor cells (CoMS, CM-MC1, and VI-MC1)
The cytotoxic effect of ENMD-2076 was evaluated by MTT assay after 48 hours of incubation with a variety of concentrations of ENMD-2076 in the canine malignant mast cell tumor cell lines (CoMS, CM-MC1, and VI-MC1; Fig. 3). The IC10 (10% decrease of cell viability) and IC50 (50% decrease of cell viability) of ENMD-2076 were calculated. The IC10 and IC50 for CoMS are 117 nM and 360 nM, respectively. The IC10 and IC50 for CM-MC1 are 17.5 nM and 404 nM, respectively. The IC10 and IC50 for VI-MC1 are 291 nM and 539 nM, respectively. The cytotoxic effect of ENMD-2076 was observed as dose dependent. These three canine mast cell tumors cell lines are susceptible to ENMD-2076 even though some variation of response was seen among those cell lines.
Figure 3.

Cytotoxic effect and cell viability of the canine malignant mast cell tumor cell lines (CoMS, CM-MC1, and VI-MC1). CoMS, CM-MC1, VI-MC1 were cultured with various concentrations of ENMD-2076 for 48 hours. The IC10 and IC50 for CoMS are 117 nM and 360 nM, respectively. The IC10 and IC50 for CM-MC1 are 17.5 nM and 404 nM, respectively. The IC10 and IC50 for VI-MC1 are 291 nM and 539 nM, respectively. The cytotoxic effect of ENMD-2076 was observed to be dose dependent. The three canine mast cell tumor cell lines are susceptible to ENMD-2076 even though some variation of the responses was seen among the cell lines. The mean ± SD cell viability was measured by MTT assay. The absorbance was measured at 570 nm and the cell viability was calculated as the mean absorbance of the treated cells divided by the mean absorbance of the control. The samples were run in duplicate and the experiments were repeated three times. *Values are significantly different (p<0.05) for each control.
Cell cycle analysis and DNA content change with ENMD-2076 in canine malignant mast cell tumor cells (CoMS, CM-MC1, and VI-MC1)
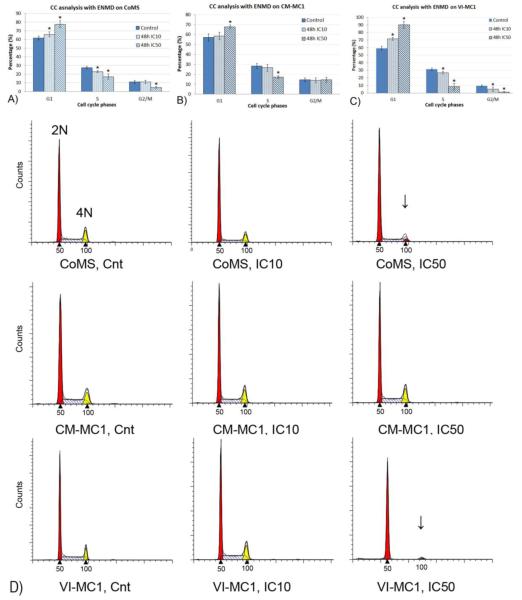
We evaluated the effect of ENMD-2076 on the cell cycle phases of mast cell tumors cells because aurora kinases are involved with the regulation of the cell cycle. The cell cycle analysis was evaluated by flow cytometry after 48 hours of incubation with the IC10 and IC50 of ENMD-2076 in canine malignant mast cell tumor cell lines (CoMS, CM-MC1, and VI-MC1). The percentage of G1, S, and G2/M phases was represented as the mean ± standard deviation (SD), then the bar graphs were generated (Figs. 4. A, B, and C). The most affected cell cycle phases were the S and G2/M phases in CoMS, and VI-MC1 cells, and a significant effect was seen after 48 hours of incubation with the IC50 (p<0.05). The CoMS and VI-MC1 cells in the G2/M phase incubated with the IC50 for 48 hours were selectively killed. The DNA content of the CoMS, CM-MC1, and VI-MC1 cells were present in the diploid (2N), and tetraploid (4N) state, and a histogram of cell cycle phases was generated (Fig. 4. D). A dramatic decrease of the DNA content of the 4N cells is obvious in the G2/M phase when incubated with the IC50 for 48 hours in CoMS and VI-MC1 cells. The comparison was performed between treated and non-treated cells within each phase in each cell line.
Figure 4.
The cell cycle effect of ENMD-2076 in CoMS, CM-MC1, and VI-MC1 cells. The cell cycle analysis was evaluated by flow cytometry after 48 hours of incubation with the IC10 and IC50 of ENMD-2076. The percentage of G1, S, and G2/M phases were calculated as the mean ± standard deviation (SD), and then bar graphs were generated. The bar graphs show the percentage of cells in each cell cycle phase within each cell line (Figs. 4A, 4B, and 4C). An obvious decrease was seen in the S and G2/M phases in CoMS and VI-MC1 cells incubated with the IC50 of ENMD-2076. The histogram of the cell cycle shows the change of DNA content in CoMS, CM-MC1, and VI-MC1 cells. The arrows point out the dramatic distribution change of the diploid (2N), and tetraploid (4N) cells (Fig. 4D). The comparison was done between the control and either the IC10 or IC50 of each group. The samples were run in duplicate and the experiments were repeated at least three times. *Values are significantly different (p<0.05) for each control. CC and Cnt are abbreviations for the cell cycle and control.
Enhancement of radiosensitivity with ENMD-2076 in CoMS and VI-MC1
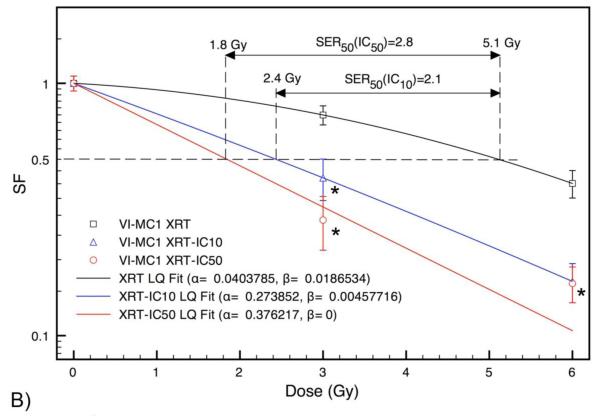
We evaluated the potential radiosensitizing effect of ENMD-2076 on canine mast cell tumors. CoMS and VI-MC1 were selected because the effect on the cell cycle distribution in CM-MC1 cells is less than with the CoMS and VI-MC1 cells. The cells were irradiated using a 6 MV linac at the dose rate of 2.5Gy/minute with 3 Gy and 6 Gy with/without the IC10 or the IC50 of ENMD-2076, and then incubated for 48 hours in 96-well plates. Figures 5A and 5B show the radiation response of CoMS and VI-MC1 cells with and without treatment using ENMD-2076 at levels IC10 and IC50. The LQ curve fits have root mean square (RMS) and chi-squared values of zero in all cases, except for IC50. For CoMS with IC50 the RMS and chi-squared values are 1.7E-8 and 5.6E-10, respectively, which indicate a very good fit. For VI-MC1 with IC50 RMS=3.9E-2 and χ2=1.52. Sensitization enhancement values from these fits at 50% survival for CoMS cells are 1.4 and 2.3 with IC10 and IC50, respectively. In the case of VI-MC1 cells, SER50 (IC10)=2.1 and SER50 (IC50)=2.8. These results indicate significant radiosensitizing effects.
Figure 5.
Cell survival curves for CoMS and VI-MC1 cells after irradiation by 6 MV x-rays. Error bars are ±1σ uncertainty. Treatment with ENMD-2076 enhances the radiation sensitivity of the canine malignant mast cell tumor cell lines CoMS (Fig. 5A) and VI-MC1 (Fig. 5B). CoMS and VI-MC1 were irradiated with 3 Gy and 6 Gy in the presence of either the IC10 or IC50 of ENMD-2076, and then incubated for 48 hours. The survival fraction (SF) was fitted with linear-quadratic curves on the survival data for CoMS and VI-MC1. The samples were run in duplicate and the experiments were repeated at least three times. *Values are significantly different (p<0.05) for each control. Goodness of fit: CoMS: root mean square (RMS) and χ2 values are zero except for IC50 where RMS=1.7E-8 and χ2=5.6E-10. VI-MC1: RMS and χ2 values are zero except for IC50 where RMS=3.9E-2 and χ2=1.52.
Induced apoptosis with ENMD-2076 in CoMS and VI-MC1
Caspase-3 expression
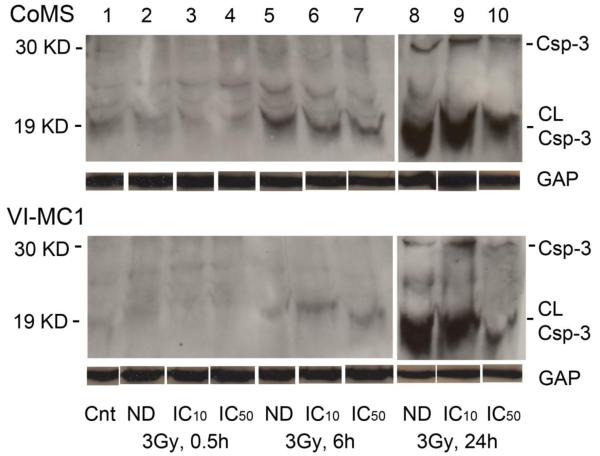
To evaluate caspase-3 expression and the mechanism of cell death induced by ENMD-2076, western blot analysis was performed in the same manner described above, but harvested at different time points (control, 0.5 hour, 6 hour, and 24 hour; Fig. 6). Minimum detection of caspase-3 was seen at 0.5 hour. However, the intensity of the caspase-3 bands gradually increased, and maximum intensity of expression was observed at the final 24 hour time point following the 3 Gy exposure in the presence of ENMD-2076. The maximum expression of caspase-3 in cells that were not treated with the drug was also observed at the 24 hour time point. Moreover, in the cells that received the maximum dose of ENMD-2076 (IC50), there was lower expression of caspase-3 after 24 hrs. of incubation than with the IC10 of ENMD-2076. In other words, the expression of caspase-3 in the cells treated with the IC50 of ENMD-2076 peaked before 24 hrs. All taken together, the results indicate that apoptosis was induced earlier than 24 hours with 3 Gy radiation exposure in the presence of the IC50 of ENMD-2076.
Figure 6.
The caspase-3 expression at 24 hours after 3 Gy exposure with concurrent treatment either with/without the IC10 or IC50 of ENMD-2076. The caspase-3 activity was evaluated at different time points (control, 0.5 hour, 6 hour, and 24 hour). GAPDH was used as a loading control, and the intensity of bands of GAPDH in the CoMS and VI-MC1 cells are identical. The protein size of cleaved caspase-3, caspase-3, and GAPDH are approximately 19, 30, and 37 KD, respectively. GAP, Csp-3, CL-Csp-3, Cnt, and ND indicate GAPDH, Caspase-3, cleaved caspase-3, Control, and Non-drug, respectively.
Discussion
One of the main functions of aurora kinases is mitotic spindle formation and cell separation.22 Due to the fact that aurora kinases are involved with cell cycle regulation, targeting the aurora kinases in cancer therapy would be a promising approach. Scant information exists regarding aurora kinases in veterinary medicine,24,29 but our group has shown the existence of mRNA and protein expression of aurora kinases in both normal canine endothelial cells and malignant canine lymphoid cells.24 Our group has also shown that the aurora-A and aurora-B genes are highly conserved between humans and dogs. We have started to evaluate the mRNA and protein expression of aurora-A and aurora-B in canine malignant mast cell tumors. The expression of mRNA (Fig. 1) and protein expression (Fig. 2) of aurora-A and aurora-B were clearly detected by RT-PCR and western blots. In humans, over-expression of aurora-A and/or aurora-B has been suggested as prognostic markers in some types of malignant tumors.30-33 Quantification of the mRNA between malignant tumors, and investigating its relationship to the treatment response and/or prognosis would be a useful next step.
We also evaluated the cytotoxic effect of ENMD-2076 in canine mast cell tumors. All three cell lines used in this study are susceptible to ENMD-2076 (Fig. 3). More importantly, the IC50 for CoMS, CM-MC1, and VI-MC1 ranges from 360 nM to 539 nM; which is much lower than in a previous study using malignant canine lymphoid cells.24 This indicates that ENMD-2076 is effective on canine mast cell tumors. The common side effects of ENMD-2076 in humans have been reported in phase I clinical trials as hypertension, nausea, vomiting, fatigue and/or diarrhea. The severity of these side effects appears to be dose dependent,34 therefore, using less of the drug may avoid these unfavorable side effects. The response curves with ENMD-2076 in canine malignant mast cell tumors are very similar to those reported in human studies using small molecules, which have very narrow therapeutic windows and steep dose-response curves.35,36 Clinically, it might be a challenge to find the optimum dose of small molecule drugs because patients exhibit a variation of tumor sensitivity to the drug relative to normal tissues. Toceranib phosphate (Palladia, SU11654) has been approved for the treatment of canine mast cell tumors, and it is beginning to be widely used to treat a variety of tumors in dogs.16,20,21 The current recommended dose of toceranib phosphate is 3.25mg/kg, every other day (3 times/week), and with this dosage, optimum plasma concentrations of 40 ng/ml were attained.37 However, in actual clinical situations, we still see some unacceptable side effects even with the recommended dosage, and it is not rare to be forced use a lower dose of toceranib phosphate in veterinary medicine, which may also be a less effective dose.
The histograms of cell cycle analysis allow us to evaluate any change of the distribution of cell cycle phases by ENMD-2076 (Fig. 4D). The majority of aurora kinases induce polyploidy (increase to 4N and/or 8N) and apoptosis.38,39 Wang, et al showed the change of cell cycle phase distribution and increased polyploidy with ENMD-2076 using human multiple myeloma cells.40 However, in this study, we did not observe the same response in two canine mast cell tumor cell lines (CoMS and VI-MC1). In the cell cycle analysis, the G2/M phases were selectively depleted with ENMD-2076 after a 48 hour incubation, which indicates that the cells in the G2/M phase of two canine malignant mast cell tumor cell lines were selectively killed by ENMD-2076, and/or the cells did not move into G2/M. It is unknown why no accumulations of 4N or 8N cells were seen with the canine mast cell tumor cells as is seen with other aurora kinases. Most of the Wang study was conducted using human cell lines, and therefore different results may be observed in the canine cells and with a different tumor type. ENMD-2076 is a multi-target aurora kinase inhibitor, which also can inhibit the activity of tyrosine kinases and/or their receptors. The multiple target mechanisms might explain why the ENMD-2076 did not cause polyploidy. It also did not appear that there was a sub G1 accumulation of cells even with evidence of undergoing apoptosis. It is known that sub G1 accumulation would be seen if apoptosis is present due to condensation of chromatin.41 However, G1 accumulation could be obscured or invisible due to various reasons, such as DNA loss due to the washing process, the generation of bleb, debris, and necrotic cells.41,42 Because there is the potential failure to detect a discreet peak of chromatin condensation, assessing apoptosis using G1 sub accumulation by flow cytometry analysis would not always be ideal. Because apoptosis pathways are extremely complicated processes, it has been suggested that multiple assays would best assess apoptosis pathways.
Using small molecules as radiation-sensitizers is currently getting a lot of attention in veterinary medicine. Epidermal growth factor receptor (EGFR) inhibitors are some of the best known of the small molecules, and have been investigated as potential radiosensitizers for head and neck tumors in human medicine. Many phase II clinical trials of chemoradiation treatment in the presence of cetuximab have been conducted, and most of the results are encouraging moving to a higher stage of clinical trial due to the improvement of clinical responses and tumor control duration even with an increased incidence of acceptable acute toxicity.43-45 Multi-institutional clinical trials of canine nasal adenocarcinoma using radiation therapy and Palladia (tyrosine kinase inhibitors) are being conducted by the VRTOG (Veterinary Radiation Therapy Oncology Group). We evaluated the effect of ENMD-2076 with concurrent radiation exposure to 6 MV therapy x-rays and observed a radiosensitizing effect in CoMS, and VI-MC1 cells. The LQ fits of the IC50 data in both CoMS and VI-MC1 cells show the lack of a shoulder region in the survival curves (Figs. 5A and 5B), indicating that at this level the effect of ENMD-2076 aurora kinase inhibitor is significant. In the case of VI-MC1, the LQ model shows a nearly pure exponential decrease even at the IC10 level, which suggests that this cell line is more sensitive to radiation at this level of ENMD-2076 compared to CoMS. In this study, the evaluation of the shoulder region was hindered by the low number of dose points. In the case of VI-MC1, the survival fraction of IC50 at 6 Gy exhibits a pseudo-radiation hardening, which is due to overkill, and it is not well reproduced by the LQ curve. However, this is not the case with the CoMS line, which also shows a pure exponential decrease with the IC50 without a shoulder. Because of the general similarities between the two sets of survival curves and because of the evidently higher sensitization in VI-MC1, it is likely that the current LQ fit correctly predicts the lack of a shoulder in the VI-MC1 IC50. To further evaluate the degree of sensitization, the sensitivity enhancement ratio was evaluated at the 50% survival fraction (SER50) using the fitted LQ models. The 50% endpoint was selected because the assessment at this degree of cell inactivation did not require extrapolation. Significant radiosensitization was seen with the IC10 and the IC50 in both CoMS and VI-MC1 cells (Figs. 5A and 5B). In CoMS cells treated with the IC10, the SER50=1.4 whereas when treated with the IC50, the SER50=2.3. A much greater radiosensitizing effect was seen in the VI-MC1 cells, where the SER50 (IC10)=2.1 while the SER50 (IC50)=2.8. Our results are consistent with various sensitization enhancement experiments conducted with mammalian cells. For example, using the chemical agent iododeoxyuridine (IUdR) in conjunction with 4 MV clinical beams has been shown to yield SER10 values ranging from 1.5 to 2.6 depending on the tissue IUdR concentration and on the uptake by the DNA.46 Our data show a comparatively higher sensitization because in our case it is the SER50 that has similar values. Because of the divergent nature of the survival curves, the SER is higher at lower survival fractions. The IC10 of ENMD-2076 can decrease cell survival 30% without a concurrent 3 Gy radiation treatment in VI-MC1 cells, which suggests that an even lower dose of small molecules might be able to cause a similar response in a clinical setting. When using toceranib phosphate as a radiation sensitizer in veterinary medicine, there exists the possibility of life threatening side effects (such as gastrointestinal bleeding and/or perforation). The ability to lower the dose to acquire a raidosensitizing effect allows the clinician to more easily use the drug without worrying about causing life-threatening side effects. Using a lower dose of toceranib phosphate may result in T-regulatory cell suppression, immunomodulatory effects, and anigiogenesis inhibition, which could lead to a superior tumor control rate and/or longer survival time.47-49
Caspase-3 expression was also evaluated to investigate the mechanism and timing of cell death with ENMD-2076 treatment concurrent with radiation exposure. Caspase-3 expression was highest after 24 hours of incubation without ENMD-2076 after a 3 Gy exposure (Fig. 6). Due to the radiosensitizing effects of ENMD-2076 treatment with radiation exposure seen in CoMS and VI-MC1 cells, the results indicate that the cell death was induced before 24 hours. The expression of caspase-3 in both CoMS and VI-MC1 cells at control and after 0.5 hours is low. The intensity of expression gets stronger at 6 hours and reaches a maximum at 24 hours. The stronger expression of caspase-3 at 6 hours in VI-MC1 cells in the presence of ENMD-2076 rather than at 6 hours with radiation treatment alone indicates that ENMD-2076 accelerates cell death through apoptosis earlier than with radiation alone.
There are some limitations to this study. We have not investigated the actual mechanism of radiosensitivity with ENMD-2076. It would be useful to know how the ENMD-2076 enhances the radiosensitivity of canine mast cell tumors. Because we do not know the importance of the expression of aurora-A and aurora-B regarding the tumor activity, treatment response, and prognosis, it would be ideal to quantitate the mRNA expression level with quantitative RT-PCR. This is an in vitro study; the results of this study might not be seen in an in vivo study. In order to better assess the shoulder region of the survival curve, multiple radiation exposures at low doses will be required. However, when comparing the two cell lines, we feel that our general conclusion with respect to the lack of pronounced shoulder at the IC50 is likely accurate. No other definitive conclusions pertaining to the shoulder of the survival curve can be drawn. In this current experiment, the more sensitive cell line was a visceral mast cell tumor, which is likely a more aggressive variant and generally not treatable with radiation therapy given the location. Also 2 of the 3 cell lines were of visceral origin and the third was of mucosal origin. These may behave differently than cutaneous mast cell tumors.
In conclusion, we have detected mRNA and protein expression of aurora-A and aurora-B in canine mast cell tumor cells lines. We also observed a dose-dependent cytotoxic effect by ENMD-2076. The cell cycle distribution and DNA content changes were seen particularly in CoMS and VI-MC1 cells. The ENMD-2076 caused a radiosensitizing effect in the tested cells yielding sensitization enhancement ratios at 50% survival ranging from 1.4 to 2.8, with VI-MC1 cells being much more sensitive than CoMS. These results suggest the general potential for treating canine mast cell tumors with aurora kinase inhibitors, and specifically warrant further in vitro and in vivo studies using ENMD-2076 treatment in conjunction with radiotherapy.
Acknowledgments
This study was supported by a NIH T35 grant (NIH student training grant) and the Louisiana State University CORP foundation. The authors would like to thank Marilyn Dietrich, Roxanne Coffey, and Kyle Waite for technical help. Finally, the authors would like to thank Dr. Takayuki Nakagawa (Tokyo University, Tokyo, Japan) and Dr. Tsuyoshi Kadosawa (Rakuno Gakuen University, Hokkaido, Japan) for kindly donating the canine mast cell tumor cell lines.
References
- 1.Thamm DH, Vail DM. Mast cell tumors. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 4th edn Elsevier Saunders; Edinburgh: 2007. pp. 402–424. [Google Scholar]
- 2.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Veterinary Pathology. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 3.Preziosi R, Sarli G, Paltrinieri M. Multivariate survival analysis of histological parameters and clinical presentation in canine cutaneous mast cell tumours. Veterinary Research Communications. 2007;31:287–296. doi: 10.1007/s11259-006-3427-9. [DOI] [PubMed] [Google Scholar]
- 4.Northrup NC, Harmon BG, Gieger TL, Brown CA, Carmichael KP, Garcia A, Latimer KS, Munday JS, Rakich PM, Richey LJ, Stedman NL, Cheng AL, Howerth EW. Variation among pathologists in histologic grading of canine cutaneous mast cell tumors. Journal of Veterinary Diagnostic Investigation. 2005;17:245–248. doi: 10.1177/104063870501700305. [DOI] [PubMed] [Google Scholar]
- 5.Maglennon GA, Murphy S, Adams V, Miller J, Smith K, Blunden A, Scase TJ. Association of Ki67 index with prognosis for intermediate-grade canine cutaneous mast cell tumours. Veterinary Comparative Oncology. 2008;6:268–274. doi: 10.1111/j.1476-5829.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JJ, Yager JA, Best SJ, Pearl DL, Coomber BL, Torres RN, Kiupel M, Foster RA. Canine subcutaneous mast cell tumors: cellular proliferation and KIT expression as prognostic indices. Veterinary Pathology. 2011;48:169–181. doi: 10.1177/0300985810390716. [DOI] [PubMed] [Google Scholar]
- 7.Webster JD, Yuzbasiyan-Gurkan V, Miller RA, Kaneene JB, Kiupel M. Cellular proliferation in canine cutaneous mast cell tumors: association with c-KIT and its role in prognostication. Veterinary Pathology. 2007;44:298–308. doi: 10.1354/vp.44-3-298. [DOI] [PubMed] [Google Scholar]
- 8.Fulcher RP, Ludwig LL, Bergman PJ, Newman SJ, Simpson AM, Patnaik AK. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. Journal of the American Veterinary Medical Association. 2006;228:210–215. doi: 10.2460/javma.228.2.210. [DOI] [PubMed] [Google Scholar]
- 9.Schultheiss PC, Gardiner DW, Rao S, Olea-Popelka F, Tuohy JL. Association of histologic tumor characteristics and size of surgical margins with clinical outcome after surgical removal of cutaneous mast cell tumors in dogs. Journal of the American Veterinary Medical Association. 2011;238:1464–1469. doi: 10.2460/javma.238.11.1464. [DOI] [PubMed] [Google Scholar]
- 10.Chaffin K, Thrall DE. Results of radiation therapy in 19 dogs with cutaneous mast cell tumor and regional lymph node metastasis. Veterinary Radiology & Ultrasound. 2002;43:392–395. doi: 10.1111/j.1740-8261.2002.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 11.Thamm DH, Turek MM, Vail DM. Outcome and prognostic factors following adjuvant prednisone/vinblastine chemotherapy for high-risk canine mast cell tumour: 61 cases. The Journal of Veterinary Medical Sciences. 2006;68:581–587. doi: 10.1292/jvms.68.581. [DOI] [PubMed] [Google Scholar]
- 12.Hume CT, Kiupel M, Rigatti L, Shofer FS, Skorupski KA, Sorenmo KU. Outcomes of dogs with grade 3 mast cell tumors: 43 cases (1997-2007) Journal of American Animal Hospital Association. 2011;47:37–44. doi: 10.5326/JAAHA-MS-5557. [DOI] [PubMed] [Google Scholar]
- 13.Rassnick KM, Bailey DB, Russell DS, Flory AB, Kiselow MA, Intile JL, Malone EK, Balkman CE, Barnard SM. A phase II study to evaluate the toxicity and efficacy of alternating CCNU and high-dose vinblastine and prednisone (CVP) for treatment of dogs with high-grade, metastatic or nonresectable mast cell tumours. Veterinary Comparative Oncology. 2010;8:138–152. doi: 10.1111/j.1476-5829.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper M, Tsai X, Bennett P. Combination CCNU and vinblastine chemotherapy for canine mast cell tumors: 57 cases. Veterinary Comparative Oncology. 2009;7:196–206. doi: 10.1111/j.1476-5829.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- 15.London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, Henry CJ, Mitchener KL, Klein MK, Hintermeister JG, Bergman PJ, Couto GC, Mauldin GN, Michels GM. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clinical Cancer Research. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 16.Robat C, London C, Bunting L, McCartan L, Stingle N, Selting K, Kurzman I, Vail DM. Safety evaluation of combination vinblastine and toceranib phosphate (Palladia®) in dogs: a phase I dose-finding study. Veterinary Comparative Oncology. 2012;10:174–183. doi: 10.1111/j.1476-5829.2011.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.London C. Kinase inhibitors in cancer therapy. Veterinary Comparative Oncology. 2004;2:177–193. doi: 10.1111/j.1476-5810.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukutome M, Maebayashi K, Nasu S, Seki K, Mitsuhashi N. Enhancement of radiosensitivity by dual inhibition of the HER family with ZD1839 (“Iressa”) and trastuzumab (“Harceptin”) International Journal of Radiation Oncology, Biology, Physics. 2006;66:528–536. doi: 10.1016/j.ijrobp.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Morsbach F, Sander D, Gheorghiu L, Nanda A, Benes C, Kriegs M, Krause M, Dikomey E, Baumann M, Dahm-Daphi J, Settleman J, Willers H. EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Research. 2011;71:6261–6269. doi: 10.1158/0008-5472.CAN-11-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.London C, Mathie T, Stingle N, Clifford C, Haney S, Klein MK, Beaver L, Vickery K, Vail DM, Hershey B, Ettinger S, Vaughan A, Alvarez F, Hillman L, Kiselow M, Thamm D, Higginbotham ML, Gauthier M, Krick E, Phillips B, Ladue T, Jones P, Bryan J, Gill V, Novasad A, Fulton L, Carreras J, McNeill C, Henry C, Gillings S. Preliminary evidence for biologic activity of toceranib phosphate (Palladia(®)) in solid tumours. Veterinary Comparative Oncology. 2012;10:194–205. doi: 10.1111/j.1476-5829.2011.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaDue T, Price GS, Dodge R, Page RL, Thrall DE. Radiation therapy for incompletely resected canine mast cell tumors. Veterinary Radiology and Ultrasound. 1998;39:57–62. doi: 10.1111/j.1740-8261.1998.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 22.Mountzios G, Terpos E, Dimopoulos MA. Aurora kinases as targets for cancer therapy. Cancer Treatment Reviews. 2008;34:175–182. doi: 10.1016/j.ctrv.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W. Aurora kinase inhibitors--rising stars in cancer therapeutics? Molecular Cancer Therapeutics. 2010;9:268–278. doi: 10.1158/1535-7163.MCT-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiomitsu K, Xia X, Sehgal I, Li S. Evaluation of the aurora kinase inhibitor, ZM447439, in canine malignant lymphoid cells in vitro. Open Journal of Veterinary Medicine. 2013;3:29–38. [Google Scholar]
- 25.Tentler JJ, Bradshaw-Pierce EL, Serkova NJ, Hasebroock KM, Pitts TM, Diamond JR, Fletcher GC, Bray MR, Eckhardt SG. Assessment of the in vivo antitumor effects of ENMD-2076, a novel multitargeted kinase inhibitor, against primary and cell line-derived human colorectal cancer xenograft models. Clinical Cancer Research. 2010;16:2989–2998. doi: 10.1158/1078-0432.CCR-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishiguro T, Kadosawa T, Mori K, Takagi S, Okumura M, Fujinaga T. Establishment and characterization of a new canine mast cell tumor cell line. The journal of veterinary medical science. 2001;63:1031–1034. doi: 10.1292/jvms.63.1031. 2001. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Kitani S, Nagase M, Mochizuki M, Nishimura R, Morita Y, Sasaki N. IgG-mediated histamine release from canine mastocytoma-derived cells. International archives of allergy and immunology. 2001;125:228–235. doi: 10.1159/000053820. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi Y, Fujino Y, Watanabe M, Nakagawa T, Ohno K, Sasaki N, Sugano S, Tsujimoto H. Aberrant autophosphorylation of c-kit receptor in canine mast cell tumor cell lines. Veterinary immunology and immunopathology. 2010;137:208–216. doi: 10.1016/j.vetimm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CM, Pozniak J, Scott MC, Ito D, Gorden BH, Graef AJ, Modiano JF. Canine osteosarcoma cells exhibit resistance to aurora kinase inhibitors. Veterinary Comparative Oncology. 2013 doi: 10.1111/vco.12018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendiola M, Barriuso J, Mariño-Enríquez A, Redondo A, Domínguez-Cáceres A, Hernández-Cortés G, Pérez-Fernández E, Sánchez-Navarro I, Vara JA, Suárez A, Espinosa E, González-Barón M, Palacios J, Hardisson D. Aurora kinases as prognostic biomarkers in ovarian carcinoma. Human Pathology. 2009;40:631–638. doi: 10.1016/j.humpath.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Lassusn H, Staff S, Leminen A, Isola J, Butzow R. Aurora-A overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecologic oncology. 2011;120:11–17. doi: 10.1016/j.ygyno.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 32.García-Fernández E, De Diego JI, Collantes-Bellido E, Mendiola M, Prim MP, Pérez-Fernández E, Miguel-Martín M, Nistal M, Hardisson D. Aurora B kinase expression in laryngeal squamous cell carcinoma and its prognostic implications. Histopathology. 2011;58:368–376. doi: 10.1111/j.1365-2559.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 33.Nadler Y, Camp RL, Schwartz C, Rimm DL, Kluger HM, Kluger Y. Expression of Aurora A (but not Aurora B) is predictive of survival in breast cancer. Clinical Cancer Research. 2008;14:4455–4462. doi: 10.1158/1078-0432.CCR-07-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamond JR, Bastos BR, Hansen RJ, Gustafson DL, Eckhardt SG, Kwak EL, Pandya SS, Fletcher GC, Pitts TM, Kulikowski GN, Morrow M, Arnott J, Bray MR, Sidor C, Messersmith W, Shapiro GI. Phase I safety, pharmacokinetic, and pharmacodynamic study of ENMD-2076, a novel angiogenic and Aurora kinase inhibitor, in patients with advanced solid tumors. Clinical Cancer Research. 2011;17:849–860. doi: 10.1158/1078-0432.CCR-10-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, Borge L, Hajem B, Lermet A, Sippl W, Voisset E, Arock M, Auclair C, Leventhal PS, Mansfield CD, Moussy A, Hermine O. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikezoe T, Yang Y, Nishioka C, Bandobashi K, Nakatani H, Taguchi T, Koeffler HP, Taguchi H. Effect of SU11248 on gastrointestinal stromal tumor-T1 cells: enhancement of growth inhibition via inhibition of 3-kinase/Akt/mammalian target of rapamycin signaling. Cancer Science. 2006;97:945–951. doi: 10.1111/j.1349-7006.2006.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yancey MF, Merritt DA, Lesman SP, Boucher JF, Michels GM. Pharmacokinetic properties of toceranib phosphate (Palladia, SU11654), a novel tyrosine kinase inhibitor, in laboratory dogs and dogs with mast cell tumors. Journal of Veterinary Pharmacology and therapeutics. 2010;33:162–171. doi: 10.1111/j.1365-2885.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K, Togitani K, Koeffler HP, Taguchi H, Yokoyama A. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–2040. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 39.Ikezoe T, Takeuchi T, Yang J, Adachi Y, Nishioka C, Furihata M, Koeffler HP, Yokoyama A. Analysis of Aurora B kinase in non-Hodgkin lymphoma. Laboratory investigation. 2009;89:1364–1373. doi: 10.1038/labinvest.2009.106. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Sinn AL, Pollok K, Sandusky G, Zhang S, Chen L, Liang J, Crean CD, Suvannasankha A, Abonour R, Sidor C, Bray MR, Farag SS. Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. British Journal of Hematology. 2010;150:313–325. doi: 10.1111/j.1365-2141.2010.08248.x. [DOI] [PubMed] [Google Scholar]
- 41.Tounekti O, Belehradek J, Jr, Mir LM. Relationships between DNA fragmentation, chromatin condensation, and changes in flow cytometry profiles detected during apoptosis. Experimental Cell Research. 1995;217:506–516. doi: 10.1006/excr.1995.1116. [DOI] [PubMed] [Google Scholar]
- 42.Darzynkiewicz Z, Bedner E. Analysis of apoptotic cells by flow and laser scanning cytometry. Methods in Enzymology. 2000;322:18–39. doi: 10.1016/s0076-6879(00)22005-3. [DOI] [PubMed] [Google Scholar]
- 43.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, Chua YJ, Wong R, Barbachano Y, Oates J, Chau I. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) Journal of Clinical Oncology. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Wu X, Bu S, He C, Wang W, Liu J, Guo W, Tan B, Wang Y, Wang J. Promising outcomes of definitive chemoradiation and cetuximab for patients with esophageal squamous cell carcinoma. Cancer Science. 2012;103:1979–1984. doi: 10.1111/j.1349-7006.2012.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keil F, Selzer E, Berghold A, Reinisch S, Kapp KS, De Vries A, Greil R, Bachtiary B, Tinchon C, Anderhuber W, Burian M, Kasparek AK, Elsäßer W, Kainz H, Riedl R, Kopp M, Kornek G. Induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil followed by radiotherapy with cetuximab for locally advanced squamous cell carcinoma of the head and neck. European Journal of Cancer. doi: 10.1016/j.ejca.2012.08.004. in press. [DOI] [PubMed] [Google Scholar]
- 46.Dugas JP, Varnes ME, Sajo E, Welch CE, Ham K, Hogstrom KR. Dependence of cell survival on iododeoxyuridine concentration in 35-keV photon-activated Auger electron radiotherapy. International Journal of Radiation Oncology Biology, Physics. 2011;79:255–261. doi: 10.1016/j.ijrobp.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell L, Thamm DH, Biller BJ. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. Journal of Veterinary Internal Medicine. 2012;26:355–362. doi: 10.1111/j.1939-1676.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 48.Desar IM, Jacobs JFM, Hulsbergen-vandeKaa CA, Oyen WJ, Mulders PF, van der Graaf WT, Adema GJ, van Herpen CM, de Vries IJ. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. International Journal of Cancer. 2011;129:507–512. doi: 10.1002/ijc.25674. [DOI] [PubMed] [Google Scholar]
- 49.Murray A, Little SJ, Stanley P, Maraveyas A, Cawkwell L. Sorafenib enhances the in vitro anti-endothelial effects of low dose (metronomic) chemotherapy. Oncology Report. 2010;24:1049–1058. doi: 10.3892/or.2010.1049. [DOI] [PubMed] [Google Scholar]