Abstract
Pre-exposure prophylaxis (PrEP) is a novel HIV prevention strategy which requires high adherence. We tested the use of daily short message service (i.e., SMS/text message) surveys to measure sexual behavior and PrEP adherence in Kenya. Ninety-six HIV-uninfected adult individuals, taking daily oral PrEP in a clinical trial, received daily SMS surveys for 60 days. Most participants (96.9 %) reported taking PrEP on ≥80 % days, but 69.8 % missed at least one dose. Unprotected sex was reported on 4.9 % of days; however, 47.9 % of participants reported unprotected sex at least once. Unprotected sex was not correlated with PrEP use (OR = 0.95). Participants reporting more sex were less likely to report PrEP non-adherence and those reporting no sex were most likely to report missing a PrEP dose (adjusted OR = 1.87). PrEP adherence was high, missed doses were correlated with sexual abstinence, and unprotected sex was not associated with decreased PrEP adherence.
Keywords: HIV prevention, Pre-exposure prophylaxis, Sexual behavior, Adherence, HIV serodiscordant couples
Background
Antiretroviral pre-exposure prophylaxis (PrEP) is a novel HIV prevention strategy that is being investigated for optimal use. Recent clinical trials have demonstrated the efficacy of PrEP for HIV prevention, and implementation projects are underway to determine uptake of and adherence to PrEP outside of clinical trial settings [1-3]. Adherence to PrEP is key for effective HIV prevention, and understanding patterns of adherence related to sexual behavior may be especially important [4-6]. In addition, different options for effective PrEP delivery, including intermittent or coital-dependent dosing, are being investigated, but little is known about the potential for PrEP users to anticipate sexual activity, which would be necessary for non-daily PrEP use.
Mobile technologies offer promising possibilities in Africa and other low-income country settings for promoting and measuring health behaviors, with mobile phone text messaging, otherwise known as short message service (SMS), representing an emerging low-technology and low-cost option. Mobile phone ownership is common and rapidly growing in Africa—for example, of 43 million persons in Kenya, an estimated 29.2 million are mobile phone subscribers and 89 % of the population has access to mobile phones [7]. Two randomized controlled trials conducted in Kenya have demonstrated increased antiretroviral therapy (ART) adherence through SMS interventions [8, 9]. SMS has also been used to conduct data collection, including medication adherence data [10-12] and for clinic visit reminders [13]. Health data collected through mobile devices can be received immediately and more frequently, allowing for medical providers to monitor behavior and outcomes remotely in “real time” [14].
We assessed the use of daily SMS surveys to measure sexual behavior and PrEP use in real time among HIV-uninfected members of HIV serodiscordant couples.
Methods
Participants
HIV-uninfected participants were recruited from the Thika, Kenya site of the Partners PrEP Study. Thika is a peri-urban and farming community 45 km north of Nairobi. The Partners PrEP Study is a randomized controlled trial of daily oral tenofovir and emtricitabine–tenofovir PrEP among 4,758 HIV serodiscordant couples in 9 sites in Kenya and Uganda; study procedures and eligibility criteria have been previously described [15]. In July 2011, the study demonstrated daily oral PrEP to be highly efficacious in preventing HIV [2]; participants were informed of the study results and the placebo arm was offered active PrEP. Data for this pilot evaluation study were collected between December 2011 and April 2012, when all HIV-uninfected participants were taking active PrEP (either tenofovir or emtricitabine–tenofovir).
Eligible participants were HIV-uninfected, taking PrEP, literate, owned a mobile phone that they did not share, knew how to send and receive SMS, had regular access to an electrical outlet to charge their phone, and had to respond to ≥5 of 7 daily surveys during the 1st week run-in of the SMS study.
Study Design and Procedures
The target sample size for this pilot evaluation was 100 participants. Study staff briefly described this study and offered enrollment to participants during clinical trial visits. At enrollment, after eligibility was confirmed and participants were trained on SMS study questions and procedures, participants selected a time for survey delivery (8:00, 12:00, or 20:00), language (English, Kiswahili, or Kikuyu), and an alpha or numeric password <8 digits. Participants then completed a practice survey on their phone. Surveys were automated to send daily for 60 days. The survey began with a password question (“what is your secret password?”) to ensure the intended user was completing the survey. An SMS encouraging participants to retry entering their password was sent up to two times upon receipt of incorrect passwords or non-response (after a 15 min delay). After successful password completion, participants received sequential questions on sexual activity (question 1: “did you have sex yesterday?”), condom use (if “yes” to 1, question 2: “did you or your partner use a condom when you had sex?”), expectation of sex (question 3: “do you think you will have sex tomorrow?”), and PrEP use (question 4: “did you remember to take your study pill yesterday?”). Accepted response options included 1 for “yes,” 2 for “no,” and 3 for “I choose not to respond.” The survey question was resent if the participant responded with anything other than “1”, “2”, or “3.” Participants received an SMS thanking them for their participation after successfully completing each survey. SMS responses received after survey completion prompted an SMS with a brief message and the number to a study mobile phone managed by staff. Participants were compensated with delivery of phone credit to their mobile phone twice a week: 5 Kenyan Shillings (KSH) (~0.05 USD) for each SMS response and 50 KSH (~0.50 USD) for each completed survey. On days selected by convenience, study staff conducted quality assurance interviews by phone after participant completion of the SMS survey to ask the exact same questions to later evaluate concordance; participants were randomly chosen to complete the interview and were contacted after the next SMS survey completed if they were unable to complete the interview on the first attempt.
SMS Survey System
An SMS system for automated delivery and recording of SMS surveys and responses was developed (Dimagi, Inc., Cambridge, USA). The software was written using the RapidSMS open source framework. The SMS web database was hosted in an Amazon Elastic Compute Cloud (EC2) and messages were sent through the MACH SMS gateway. Study investigators could access the SMS web database to view survey responses, track survey completion by participant, day, and time, as well as send messages to participants and edit participant information (i.e., phone numbers, passwords) as needed.
Statistical Analysis
Participant characteristics and survey responses were analyzed using descriptive statistics. Chi square tests of homogeneity (frequency) and Wilcoxon–Mann–Whitney test (medians) were used to compare characteristics of enrolled participants to those from the Thika site who did not participate in the study.
To assess correlates of unprotected sex and missed PrEP doses, univariate and multivariate generalized estimating equation (GEE) logistic models with robust standard errors were used to calculate odds ratios and 95 % confidence intervals, taking into account within-individual correlation over time in the longitudinal dataset. Logistic regression analyses were restricted to surveys with “yes” or “no” responses; responses of “choose not to respond” were treated as missing data and no attempt was made to impute missing responses. An independent correlation structure was used for greatest flexibility, although GEE is not sensitive to the specification of correlation structure [16]. Covariates that were significant at P < 0.10 in univariate analyses were included in the respective multivariate models.
To compare reports of anticipated sex and actual sex, percent agreement and Kappa correlation coefficients, which correct for the proportion of agreement due to chance, were calculated. Lag variables were used to link reports of anticipated sex with reports of actual sex when participants had data for three consecutive days. Accurate sexual prediction was defined as agreement (“yes” or “no”) between expectation of sex (“tomorrow”) and sexual activity (“yesterday”) provided on SMS surveys 2 days apart.
Data were analyzed using STATA version 11.0 (College Station, TX, USA).
Ethics
The University of Washington Human Subjects Review Committee and the Kenyatta National Hospital Ethics Review Committee approved the study protocol. The Partners PrEP Study is registered with ClinicalTrials.gov (NCT00557245). All participants provided written informed consent.
Results
Participant Enrollment and Characteristics
A total of 206 HIV-uninfected participants were approached for the study: 79 were ineligible, 17 declined, and 110 eligible participants were enrolled, of whom fourteen failed to meet the criterion of high response (≥5 of 7 daily surveys completed) during the 1st week run-in, resulting in 96 participants followed with daily SMS surveys for 60 days (Fig. 1). The most common reasons for ineligibility were not knowing how to send SMS (42/79, 53.2 %) and not owning a phone (28/79, 35.4 %). One participant became pregnant and discontinued PrEP (a requirement of the parent clinical trial protocol), and thus stopped participation after 28 days in this SMS study.
Fig. 1.
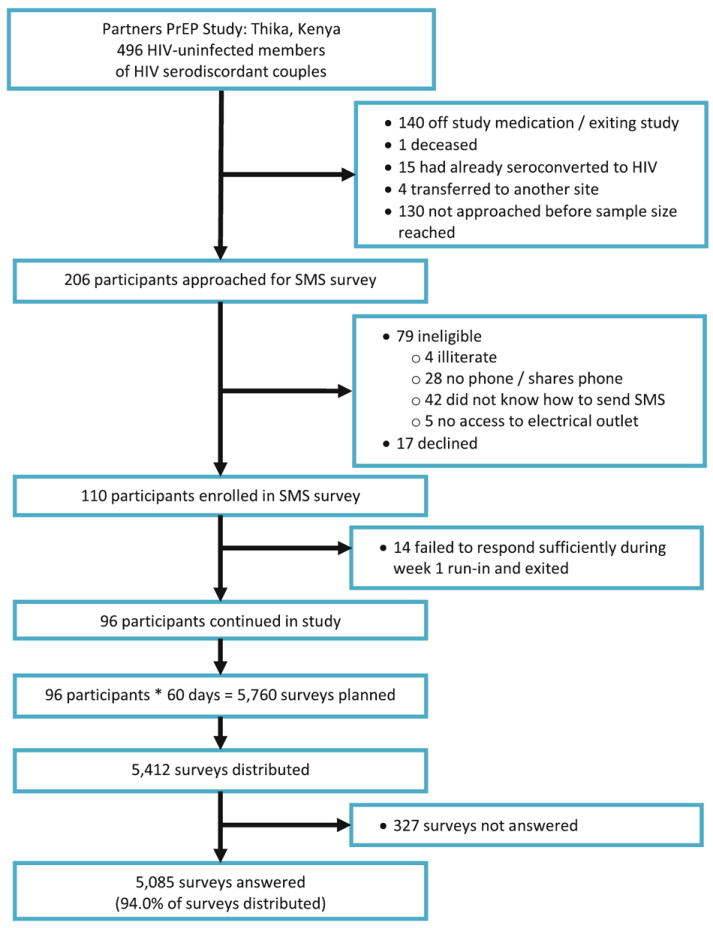
Flow chart of participant enrollment, survey distribution, and response rates. The target sample size for this study was 100. Study staff approached Partners PrEP clinical trial participants for enrollment during their monthly clinic visits. Some participants had already completed maximum follow-up for the Partners PrEP Study clinical trial at the time this pilot evaluation was conducted. A total of 110 participants were enrolled in the study, of which 96 participants responded to ≥5 of 7 daily surveys during the 1st week run-in. Of 5,760 surveys planned for 96 participants over 60 days, 5,412 surveys were distributed, 32 surveys were cancelled after one participant exited due to pregnancy, and 316 surveys were not delivered due to a mid-study block by a local mobile phone provider
The majority of the 96 participants were male (75.0 %), married (96.9 %), and earning some type of income (93.8 %) (Table 1). The median age of participants was 33.3 (interquartile range [IQR]: 30.9–37.3) years and the median years in school was 11.0 (IQR: 8.0–12.0). When participants enrolled in the SMS study, they had been in the Partners PrEP Study clinical trial for a median of 2.1 years (IQR: 1.7–2.4). Based on monthly interviewer-administered questionnaires, only 8 (8.3 %) reported unprotected sex with their HIV-infected study partner in the month prior to starting the SMS surveys and 7 (7.3 %) reported sex with someone other than their study partner. The median number of sex acts in the prior month was 4.0 (IQR: 2.0–8.0).
Table 1.
Socio-demographic and behavioral characteristics of participants (N = 96)
| N (%) or Median (IQR) | |
|---|---|
| Male | 72 (75.0 %) |
| Age, years | 33.3 (30.9–37.3) |
| Married | 93 (96.9 %) |
| Living with study partner | 91 (94.8 %) |
| Number of children with study partner | 1 (0–2) |
| Number of years in school | 11 (8–12) |
| Earning any income | 90 (93.8 %) |
| Electricity available in the home | 46 (48.9 %) |
| Time spent in Partners PrEP Study, years | 2.1 (1.7–2.4) |
| HIV-infected study partner on antiretroviral therapya | 29 (30.2 %) |
| Number of sex acts, prior montha | 4 (2–8) |
| Any unprotected sex with study partner, prior montha | 8 (8.3 %) |
| Any sex with other partners, prior montha | 7 (7.3 %) |
| Any unprotected sex with any sexual partner, prior montha | 13 (13.5 %) |
Reported through interviewer-administered questionnaire at clinic visit prior to enrollment in text message survey
SMS survey participants (n = 96) tended to be younger (33.3 vs. 37.4 years, P = 0.003), had significantly more education (11 vs. 7 years, P < 0.001), and were more likely to have electricity (47.9 vs. 11.4 %, P < 0.001) compared to other HIV-uninfected participants who were in the Partners PrEP Study clinical trial but were not eligible for the SMS study (n = 79). Eligible participants who declined to participate or had poor response the 1st week (n = 31) did not significantly differ from SMS survey participants (n = 96) for any factors in Table 1.
Survey Response Rates
There were 5,760 daily surveys planned for delivery to the 96 participants over 60 days (Fig. 1). Of these planned surveys, 5,412 were distributed, with the remainder failing to distribute due to technical errors (and, in the case of the participant who became pregnant, study exit). A total of 5,085/5,412 (94.0 %) distributed surveys were answered. The median number of unanswered surveys during the 60-day study period was 2 (IQR: 1–4); 20.8 % (20/96) of participants completed every survey, 7.3 % (7/96) had >10 unanswered surveys, and one participant had >20 unanswered surveys (1/96, 1.0 %). Unanswered surveys were more likely to occur during later weeks of the study (test for trend, P = 0.02).
Sexual Behavior
Among 5,085 answered surveys, sex was reported on 1,686 days (33.2 %) and unprotected sex was reported on 251 days (4.9 %) (Table 2). Nearly half of participants (46/96, 47.9 %) reported sex unprotected by a condom at least once during the 60-day study period. The proportion of participants reporting sex without a condom in the first 30 days of the SMS survey was significantly greater than the proportion who reported unprotected sex in the previous month (with any partner) at the monthly clinic visit prior to enrollment in the SMS study (36.5 vs. 13.5 %, P = 0.004).
Table 2.
Text message survey questions, responses, and response rates among answered surveys (N = 5,085)
| Survey questions & responses | N (%) |
|---|---|
| Did you have sex yesterday? | |
| Yes | 1,686/5,085 (33.2 %) |
| No | 3,300/5,085 (64.9 %) |
| Choose not to respond | 92/5,085 (1.8 %) |
| Missing (question not answered) | 7/5,085 (0.1 %) |
| Did you or your partner use a condom when you had sex?a | |
| Yes | 1,399/1,686 (83.0 %) |
| No | 251/1,686 (14.9 %) |
| Choose not to respond | 8/1,686 (0.5 %) |
| Missing (question not answered) | 28/1,686 (1.7 %) |
| Do you think you will have sex tomorrow? | |
| Yes | 1,463/5,085 (28.8 %) |
| No | 2,678/5,085 (52.7 %) |
| Choose not to respond | 860/5,085 (16.9 %) |
| Missing (question not answered) | 84/5,085 (1.7 %) |
| Did you remember to take your study pill yesterday? | |
| Yes | 4,651/5,085 (91.5 %) |
| No | 215/5,085 (4.2 %) |
| Choose not to respond | 71/5,085 (1.4 %) |
| Missing (question not answered) | 148/5,085 (2.9 %) |
Survey question dependent on “yes” response to previous question
In multivariate analyses, unprotected sex was significantly correlated with not living with study partner, having 8 or more years of education, and reporting any weekly alcohol consumption (Table 3). Unprotected sex was not significantly associated with whether or not participants reported taking their PrEP medication that same day.
Table 3.
Crude and adjusted odds ratios for reports of unprotected sex, for survey days when sex was reported
| Correlates | Survey days N (%)a | Unprotected sex N (%)a | OR (95 % CI) | P | AOR (95 % CI)b | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 1,254 (76.0) | 205 (16.4) | 1.49 (0.55, 4.01) | 0.4 | ||
| Female | 396 (24.0) | 46 (11.6) | Ref | |||
| Age (years) | ||||||
| <30 | 402 (24.4) | 47 (11.7) | Ref | |||
| 30–35 | 591 (35.8) | 56 (9.5) | 0.79 (0.23, 2.69) | 0.7 | ||
| 35+ | 657 (39.8) | 148 (22.5) | 2.20 (0.73, 6.61) | 0.2 | ||
| Lives with partner | ||||||
| Yes | 1,580 (95.8) | 216 (13.7) | Ref | Ref | ||
| No | 70 (4.2) | 35 (50.0) | 6.31 (1.24, 32.23) | 0.03 | 3.88 (1.10, 13.67) | 0.04 |
| Children together | ||||||
| Yes | 966 (58.6) | 167 (17.3) | Ref | |||
| No | 684 (41.5) | 84 (12.3) | 0.67 (0.26, 1.75) | 0.4 | ||
| Education (years) | ||||||
| 5–8 | 725 (43.9) | 53 (7.3) | Ref | Ref | ||
| >8 | 925 (56.1) | 198 (21.4) | 3.45 (1.26, 9.46) | 0.02 | 3.05 (1.16, 8.00) | 0.02 |
| Any monthly income | ||||||
| Yes | 1,540 (93.3) | 232 (15.1) | 0.85 (0.16, 4.60) | 0.9 | ||
| No | 110 (6.7) | 19 (17.3) | Ref | |||
| Electricity at home | ||||||
| Yes | 803 (48.7) | 129 (16.1) | Ref | |||
| No | 847 (51.3) | 122 (14.4) | 0.88 (0.35, 2.19) | 0.8 | ||
| Alcohol use (weekly) | ||||||
| None | 1,234 (74.8) | 143 (11.6) | Ref | Ref | ||
| Any | 416 (25.2) | 108 (26.0) | 2.68 (1.01, 7.10) | 0.05 | 2.63 (1.00, 6.90) | 0.05 |
| HIV-infected study partner on ART | ||||||
| Yes | 496 (30.1) | 70 (14.1) | 0.88 (0.30, 2.64) | 0.8 | ||
| No | 1,154 (69.9) | 181 (15.7) | Ref | |||
| PrEP use (same day) | ||||||
| Yes | 1,540 (97.2) | 227 (14.7) | Ref | |||
| No | 45 (2.8) | 10 (22.2) | 1.65 (0.83, 3.28) | 0.2 | ||
OR odds ratio, CI confidence interval, AOR adjusted odds ratio, P p value, ART antiretroviral therapy, PrEP pre-exposure prophylaxis
There were 1,650 surveys with “yes” or “no” responses for condom use; 8 “choose not to respond” responses and 28 unanswered questions were treated as missing data
Multivariate model includes lives with study partner, education, and alcohol use
Prediction of Sex
The question about the expectation of sex was answered “choose not to respond” for 16.9 % of days—a substantially higher proportion than any other survey question (Table 2). Among 3,716 surveys with a “yes” or “no” response to anticipated sex and subsequent report of sexual activity, there was 71.7 % agreement (2,666/3,716 response matches) and a Kappa indicating fair agreement (Kappa = 0.38, Z = 22.93, P < 0.001) between expected sexual activity and subsequently-reported sexual activity. Participants’ reported sexual expectations had a positive predictive value of 57.4 % (95 % confidence interval [CI] 54.7–60.1 %; 760/1,325) and a negative predictive value of 79.7 % (95 % CI 78.1–81.3 %; 1,906/2,391).
PrEP Adherence
Among 5,085 answered surveys, participants reported missing a daily PrEP dose on 215 surveys (4.2 %) (Table 2). However, of the 96 participants, 67 (69.8 %) reported missing a PrEP dose at least once during the survey period. The median number of days that participants reported not taking PrEP during the 60-day study period was 2 (IQR: 0–4). Most participants reported taking PrEP on at least 80 % (93/96, 96.9 %) or 90 % (80/96, 83.3 %) of days. Most reports of non-adherence to PrEP (178/215, 82.8 %) were isolated, with only 17.2 % (37/215) of missed PrEP doses occurring on ≥2 consecutive days. Participants reporting no sex (odds ratio [OR] = 3.29, 95 % CI 1.42–7.60, P = 0.005), 1–10 sex acts (OR = 2.93, 95 % CI 1.76–4.87, P < 0.001), or 11–20 sex acts (OR = 1.96, 95 % CI 1.09–3.56, P = 0.03) were significantly more likely to miss a PrEP dose than participants reporting 20 or more sex acts during the study period (test for trend, P < 0.001).
In multivariate analysis, sexual activity and living with study partner were predictors of PrEP adherence. Specifically, participants who did not report sex that day were significantly more likely to have missed that day’s PrEP dose than those who had reported sex (adjusted odds ratio [AOR] = 1.87, 95 % CI 1.35–2.60, P < 0.001) (Table 4). Participants who were not living with their study partner were also significantly more likely to report non-adherence (AOR = 2.09, 95 % CI 1.15–3.78, P = 0.02). Having a study partner on ART and having unprotected sex were not correlated with missing a PrEP dose.
Table 4.
Crude and adjusted odds ratios for reports of missing doses of PrEP
| Correlates | Survey days N (%)a | Missed PrEP N (%)a | OR (95 % CI) | P | AOR (95 % CI)b | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 3,685 (75.7) | 145 (3.9) | Ref | Ref | ||
| Female | 1,181 (24.3) | 70 (5.9) | 1.54 (0.99, 2.40) | 0.06 | 1.39 (0.90, 2.14) | 0.1 |
| Age (years) | ||||||
| <30 | 1,025 (21.1) | 51 (5.0) | Ref | |||
| 30–35 | 1,842 (37.9) | 79 (4.3) | 0.86 (0.46, 1.60) | 0.6 | ||
| 35+ | 1,999 (41.1) | 85 (4.3) | 0.85 (0.49, 1.45) | 0.5 | ||
| Lives with partner | ||||||
| Yes | 4,635 (95.3) | 196 (4.2) | Ref | Ref | ||
| No | 231 (4.8) | 19 (8.2) | 2.03 (1.03, 4.02) | 0.04 | 2.09 (1.15, 3.78) | 0.02 |
| Children together | ||||||
| Yes | 3,154 (64.8) | 153 (4.9) | Ref | |||
| No | 1,712 (35.2) | 62 (3.6) | 0.74 (0.40, 1.35) | 0.3 | ||
| Education (years) | ||||||
| 5–8 | 1,969 (40.5) | 82 (4.2) | Ref | |||
| >8 | 2,897 (59.5) | 133 (4.6) | 1.11 (0.71, 1.73) | 0.7 | ||
| Any monthly income | ||||||
| Yes | 4,572 (94.0) | 194 (4.2) | Ref | Ref | ||
| No | 294 (6.0) | 21 (7.1) | 1.74 (0.93, 3.25) | 0.09 | 1.52 (0.78, 2.95) | 0.2 |
| Electricity at home | ||||||
| Yes | 2,362 (48.5) | 98 (4.2) | Ref | |||
| No | 2,504 (51.5) | 117 (4.7) | 1.13 (0.71, 1.80) | 0.6 | ||
| Alcohol use (weekly) | ||||||
| None | 3,711 (76.3) | 170 (4.6) | Ref | |||
| Any | 1,155 (23.7) | 45 (3.9) | 0.84 (0.50, 1.43) | 0.5 | ||
| HIV-infected study partner on ART | ||||||
| Yes | 1,543 (31.7) | 80 (5.2) | 1.29 (0.82, 2.03) | 0.3 | ||
| No | 3,323 (68.3) | 135 (4.1) | Ref | |||
| Sex (same day) | ||||||
| Yes | 1,598 (32.8) | 46 (2.9) | Ref | Ref | ||
| No | 3,182 (65.4) | 168 (5.3) | 1.88 (1.34, 2.64) | <0.001 | 1.87 (1.35, 2.60) | <0.001 |
| Unprotected sex | ||||||
| Yes | 237 (4.9) | 10 (4.2) | 0.95 (0.53, 1.72) | 0.9 | ||
| No | 4,629 (95.1) | 205 (4.4) | Ref | |||
OR odds ratio, CI confidence interval, AOR adjusted odds ratio, P p value, ART antiretroviral therapy
There were 4,866 survey responses with “yes” or “no” responses for PrEP use; 71 “choose not to respond” responses and 148 unanswered questions were treated as missing data
Multivariate model includes gender, lives with study partner, income, and sex
Quality Assurance Interviews
Study staff completed 101 quality assurance interviews by phone among 68 participants. The interviewer-administered question “did you have sex yesterday?” had 90.0 % agreement (90/101 response matches) with the SMS response and a Kappa indicating substantial agreement (Kappa = 0.78, Z = 7.97, P < 0.001). Responses to the condom use question had 100 % agreement (29/29) and excellent agreement according to Kappa (Kappa = 1.00, Z = 5.39, P < 0.001). The PrEP adherence question had 97.0 % agreement (96/101) and an uninterpretable Kappa (Kappa = −0.01, Z = −0.14, P = 0.6) due to the highly skewed distribution of responses (e.g., 96 “yes” responses and 2 “no” responses).
Discussion
In this study, we demonstrated that SMS surveys elicited high response rates (>90 %) and captured daily sexual behavior and PrEP adherence in an African heterosexual population. Our results suggest that SMS data collection of sensitive health information, such as sexual behavior and pill taking, is feasible and acceptable. Participants reported high adherence to daily oral PrEP—with higher sexual activity a strong predictor of greater daily PrEP adherence. Over a third of participants reported unprotected sex during the first 30 days of the study (and nearly half over the 60-day study period), which was over twice as many than at the monthly clinic visit prior to the study start. Notably, sex without a condom was not associated with reduced PrEP use. Finally, participants had difficulty predicting when they would have sex. Thus, SMS data collection provided frequent and timely accounts of health behaviors and appeared to provide high-quality self-reported data.
Previous studies employing two-way SMS communication in Africa have had varying response levels [8, 10, 12]. In a randomized controlled trial of weekly SMS questions to Kenyan ART patients over 1 year, approximately 76 % of questions were answered in time [8]. That trial also reported high acceptance of SMS communication [8]. However, a study using daily SMS to collect sexual behavior data among HIV-uninfected men who have sex with men and female sex workers in Kenya had a median daily response rate of 33 % [12]. In that study, participants were given mobile phones, SIM cards, and airtime, though many of them already owned phones and were familiar with SMS [12]. The high response rate found in the present study could reflect a selection only of adherent responders, a shorter survey period, use of own phone rather than study phone, a more stable telecom network, and/or a more technologically-savvy or motivated population.
Notably, participants more frequently reported unprotected sex in daily SMS surveys compared to monthly clinic reports. The sense of privacy and anonymity provided by mobile technology may have minimized social desirability bias, the tendency of respondents to report more favorable behaviors [17]. This has been noted for other technology-based sexual self-reported data collection, though not always consistently [18, 19]. Also, the reliability of data on sex frequency diminishes with longer periods of recall or more frequent sexual behaviors [17]. Daily SMS data collection thus may be both acceptable and collect more accurate data regarding sexual behavior in this population than periodic and face-to-face surveys.
Sex was strongly correlated with adherence to PrEP: there was higher PrEP use with daily sexual activity. In addition, participants who were not sexually active or having sex less frequently in the study were significantly more likely to report missing doses of PrEP than those who reported more frequent sex. Thus, PrEP adherence appeared to be greatest among participants most at risk of HIV infection. Importantly, there was high agreement between SMS responses to sexual behavior and PrEP adherence questions in the quality assurance interviews.
Results from this SMS survey and quality assurance interviews indicated that it was difficult for participants to accurately predict sexual activity, even within the context of daily behavioral surveys. The sexual expectations question resulted in the highest proportion of “choose not to respond” answers (16.9 %). In addition, for those who answered the question in the affirmative, there was relatively low agreement between participants’ expectation of sex and reports of sexual activity and a positive predictive value below 60 %. This population of HIV serodiscordant couples, with moderate frequency of sex, were better at accurately predicting when they would not have sex (negative predictive value = 79.7 %) than when they would have sex (positive predictive value = 57.4 %). These data suggest that daily PrEP dosing may be better at achieving optimal adherence than intermittent or peri-coital PrEP dosing regimens that depend on the ability to anticipate sex.
There were limitations and implementation challenges to this pilot study. The greatest limitation is the small, selected population—participants were enrolled in a clinical trial, literate, owned a mobile phone, and had a high response rate during the 1st week. Daily surveys may have served as reminders for condom use and adherence. Conduct of the study among clinical trial participants, who received monthly counseling on HIV risk reduction and PrEP adherence and who were highly motivated, may limit the generalizability of study findings to a broader research-naïve population. However, it should be noted that despite frequent counseling and high awareness of HIV prevention methods, nearly half of participants reported unprotected sex during the 2 months of the study. Resources did not allow for a comparison of self-reported PrEP adherence to biological measures of adherence and thus the accuracy of self-reported behaviors is unknown and a possible limitation. The frequent reporting of less socially desirable behaviors, such as missed PrEP doses (69.8 % ever during the study period) and unprotected sex (47.9 % ever during the study period), is notable. Also, the use of an existing cohort of clinical trial provided additional insight into PrEP use and sexual risk behavior among participants taking a known active drug. Missing data in bivariate analysis may have not been missing at random and may have introduced bias. However, the total proportion of missing and “choose not respond” replies was low (2.1 % for condom use, 4.3 % for PrEP use) and should not substantially affect the results. Finally, in future studies, systematic approaches to conducting quality assurance interviews may be valuable.
The study also faced a few implementation challenges: 1–2 h twice a week of staff time for manual reimbursement for SMS responses, a few mobile phones reported lost by participants, and a temporary, mid-study block on survey delivery by one of the major mobile phone providers in Kenya. To our knowledge, the lost phones did not lead to any breach of sensitive information or other harmful consequences. Automated reimbursement for SMS responses and a locally hosted messaging system (versus a Kenyan phone card hosted in Europe, as was used in this study) could eliminate these challenges and increase response rates. Inclusion of individuals who are illiterate or do not own mobile phones should be considered when planning mobile health interventions.
Conclusion
This study of PrEP use and sexual behavior among HIV-uninfected Kenyan men and women highlights the strengths and growing potential of daily SMS data collection. Reported PrEP adherence rates were high and sexual risk behavior was greater than anticipated from monthly interviewer-administered questionnaires. High response rates and participant retention provides evidence that SMS can be used to collect health data in this setting. Two-way SMS communication between research clinics or health facilities and patients may eliminate the need for certain types of visits, promptly identify potential health problems, and ultimately save time and money.
Acknowledgments
We thank the study participants, Partners PrEP Study staff in Thika, Kenya, and our technical partner Dimagi for all their time and efforts. The National Institutes of Health (R21 NR012663) and the Bill and Melinda Gates Foundation (Grant OPP47674) provided financial support for this study. KC was a scholar in the International AIDS Research and Training Program, supported by the Fogarty International Center (D43 TW000007), and in the University of Washington STD/AIDS Research Training Program (T32 AI007140).
Contributor Information
Kathryn Curran, Email: kgcurran@uw.edu, Department of Epidemiology, University of Washington, Seattle, WA, USA; Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA.
Nelly R. Mugo, Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA Department of Obstetrics and Gynaecology, Kenyatta National Hospital, Nairobi, Kenya.
Ann Kurth, Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA; School of Nursing, University of Washington, Seattle, WA, USA; College of Nursing, New York University, New York, NY, USA.
Kenneth Ngure, Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA; Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya.
Renee Heffron, Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA.
Deborah Donnell, Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Connie Celum, Department of Epidemiology, University of Washington, Seattle, WA, USA; Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
Jared M. Baeten, Department of Epidemiology, University of Washington, Seattle, WA, USA Department of Global Health, University of Washington, 325 Ninth Avenue, Box 359927, Seattle, WA 98104, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
References
- 1.Karim AQ, Karim ASS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26(7):F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 5.Donnell D, Baeten J, Hendrix C, Bumpus N, Bangsberg D, Haberer J, et al. Tenofovir Disoproxil Fumarate drugs levels indicate PrEP use is strongly correlated with HIV-1 protective effects: Kenya and Uganda. 19th conference on retroviruses and opportunistic infections; Seattle. 2012. paper 30. [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Communications Commission of Kenya. Mobile subscribers in the country inch towards 30 million. [17 Nov 2012];2012 http://www.cck.go.ke/news/2012/Mobile_Subscribers.html.
- 8.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on anti-retroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 9.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25(6):825–34. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberer JE, Kiwanuka J, Nansera D, Wilson IB, Bangsberg DR. Challenges in using mobile phones for collection of antiretroviral therapy adherence data in a resource-limited setting. AIDS Behav. 2010;14(6):1294–301. doi: 10.1007/s10461-010-9720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark D, Kibengo F, Mutuo G, Sanders E, Priddy F, Haberer J, et al. Mobile phones for self-report: lessons learned from an intermittent PrEP trial. AIDS Vaccine Conference; Atlanta. 2010. poster 1505. [Google Scholar]
- 12.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odeny TA, Bailey RC, Bukusi EA, Simoni JM, Tapia KA, Yuhas K, et al. Text messaging to improve attendance at post-operative clinic visits after adult male circumcision for HIV prevention: a randomized controlled trial. PLoS One. 2012;7(9):e43832. doi: 10.1371/journal.pone.0043832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker I, Sigouin C, Sek J, Almonte T, Carruthers J, Chan A, et al. Comparing hand-held computers and paper diaries for haemophilia home therapy: a randomized trial. Haemophilia. 2004;10(6):698–704. doi: 10.1111/j.1365-2516.2004.01046.x. [DOI] [PubMed] [Google Scholar]
- 15.Mujugira A, Baeten JM, Donnell D, Ndase P, Mugo NR, Barnes L, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 17.Fenton KA, Johnson AM, McManus S, Erens B. Measuring sexual behaviour: methodological challenges in survey research. Sex Trans Infect. 2001;77(2):84–92. doi: 10.1136/sti.77.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips AE, Gomez GB, Boily MC, Garnett GP. A systematic review and meta-analysis of quantitative interviewing tools to investigate self-reported HIV and STI associated behaviours in low- and middle-income countries. Int J Epidemiol. 2010;39(6):1541–55. doi: 10.1093/ije/dyq114. [DOI] [PubMed] [Google Scholar]
- 19.Hensel DJ, Fortenberry JD, Harezlak J, Craig D. The feasibility of cell phone based electronic diaries for STI/HIV research. BMC Med Res Methodol. 2012;12:75. doi: 10.1186/1471-2288-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]


