Abstract
Background
Diagnosis of Parkinson’s disease (PD) currently relies on assessment of motor symptoms. Recently, sensitive, specific and readily available splice variant-specific biomarkers were identified in peripheral blood from participants in the Diagnostic and Prognostic Biomarkers in Parkinson Disease study.
Methods
Here we test for an association between candidate splice variant biomarkers and PD in blood of an independent population of cases and controls nested in the Harvard NeuroDiscovery Center Biomarker Study.
Results
Expression of seven out of thirteen candidate biomarkers was dysregulated in whole cellular blood of patients with PD.
Conclusions
These results support the view that differential expression of a subset of splice variant markers in blood is associated with PD. Further evaluation in untreated, de novo patients and at-risk subjects is warranted.
Keywords: Parkinson’s disease, biomarker, neurodegeneration, splicing, gene expression
INTRODUCTION
Diagnosis of PD has classically relied on motor symptoms including resting tremor, rigidity, bradykinesia and postural instability. The availability of accessible diagnostic biomarkers would be beneficial for identifying pre-symptomatic patients and for following the progression of the disease. In this regard, several studies have examined gene expression profiling in blood to identify molecular signatures associated with PD {Scherzer, 2007 #4;Molochnikov, 2012 #5;Soreq, 2012 #12}. Likewise, we previously identified 13 splice variant biomarkers in whole blood that could be used to distinguish PD patients from healthy and neurological controls {Potashkin, 2012 #1}. In order to confirm the association between biomarkers levels in blood and PD we tested them in an independent cross-sectional cases-control study nested in the Harvard NeuroDiscovery Center Biomarker Study (HBS). We confirm associations between seven of the candidate biomarkers and PD in the HBS population.
METHODS
Study populations
The Institutional Review Boards of Rosalind Franklin University of Medicine and Science, and Brigham and Women’s Hospital approved the study protocol. Written informed consent was received from all participants. 96 individuals including 50 PD patients (mean Hoehn and Yahr scale 2, Table 1) and 46 healthy HC age-matched controls were enrolled in the HBS. Patient and control recruitment, clinical assessments, and biobanking in the Harvard Biomarker Study population have been reported in part elsewhere {Ding, 2011 #2} and at http://www.neurodiscovery.harvard.edu/research/biomarkers.html.
Table 1. Clinical characteristics of study participants.
BMI is body mass index, N is normal, OW is overweight and OB is obese. Body mass index (BMI) was defined by standard measures as normal (N)=18.5–24.9, overweight (OW)=25–29.9 and obese (OB)= 30 or greater.
| Disease status | PD | HC | p-value |
|---|---|---|---|
|
| |||
| Number | 50 | 46 | >0.5 |
|
| |||
| Age at enrollment (Mean±SD) | 63.12±8.96 | 64.28±10.42 | >0.5 |
|
| |||
| Age of onset (Mean±SD) | 58.75±10.17 | N/A | |
|
| |||
| Male | 31 | 26 | >0.5 |
|
| |||
| Female | 19 | 20 | >0.5 |
|
| |||
| BMI (Mean±SD) | N (16); 22.81±1.54 | N (19); 22.26±2.09 | >0.5 |
| OW (22); 27.08±1.35 | OW (12); 26.92±1.42 | 0.0001 | |
| OB (12); 35.65±3.43 | OB (15); 33.14±2.98 | >0.5 | |
| OW+OB (34); 30.36±4.82 | OW+OB (27); 29.77±3.86 | 0.01 | |
|
| |||
| Hypertension | 18 | 15 | >0.5 |
|
| |||
| Diabetes | 5 | 5 | >0.5 |
|
| |||
| Hoehn & Yahr (Mean±SD) | 1.97±0.62 | N/A | |
RNA preparation and gene expression analysis
Blood was collected and prepared as described using the PAXgene Blood RNA system (Qiagen, Valencia, CA){Scherzer, 2007 #4}. Samples with RNA integrity values > 7.0 (indicating excellent RNA integrity) and ratio of absorbances at 260/280 nm between 1.7 and 2.4 were used in the current study. The High Capacity RNA transcription kit (Applied Biosystems, Foster City, CA) was used to reverse transcribe 1μg of total RNA according to the manufacturer’s protocol. The primers and amplification conditions have been previously published {Potashkin, 2012 #1}.
A stepwise multivariate discriminant linear regression was performed on the expression data adjusting for covariates including BMI, sex and age, and a correlation analysis was used to determine if individual variables correlate with each other using Statistica 8.0. Network analysis was done using the GeneMania prediction server {Warde-Farley, 2010 #3}. A two-tailed Student t test was used to estimate the significance between PD and controls (GraphPad Software, La Jolla, CA).
RESULTS
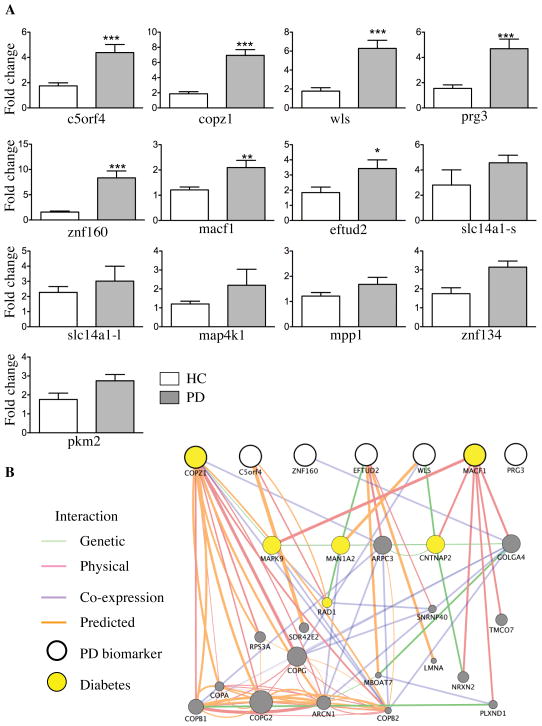
The mean age of onset and Hoehn and Yahr rating of the PD patients in this study was 58.75±10.17 and 1.97±0.62, respectively (Table 1). Relative mRNA expression levels revealed a significant up-regulation of expression of splice variants of c5orf4 (p=0.006), copz1 (p=0.00003), eftud2 (p=0.0001), macf1(p=0.03), prg3 (p=0.002), wls (p=0.006) and znf160 (p=0.00008) in whole blood of PD patients compared to HC in the univariate analysis (Figure 1A). The direction of the gene expression change of each confirmed splice variant is consistent with that previous reported{Potashkin, 2012 #1}. Six splice variants including slc14a1-s, slc14a1-l, map4k1, mpp1, znf134 and pkm2 did not show significant association in this study population (p>0.05). A priori power analysis was carried out using the results from the previous study {Potashkin, 2012 #1} to demonstrate that a fold change of 1.5 or higher could be determined with a 90% power using 40 samples per group with a significance level of 0.05. Correlation analysis revealed that none of the variables correlate with each other, with correlation values ranging from R=0.27–0.52. Regression analysis revealed that expression of each biomarker was independent from BMI (p=0.55), age at enrollment (p=0.50), age of onset (p=0.30) and sex (p=0.48). Correlation of biomarker expression with drug dose was not evaluated since most of the patients with PD were medicated with several drugs and the number of untreated patients was to small to reliably detect a significant change.
Figure 1.
A. Relative mRNA expression levels of biomarkers in samples obtained from participants of the HBS study.
Expression levels at the enrollment visit are shown. Fold change of each splice variant was calculated using gapdh as a reference gene and expression levels in the HC as a calibrator. P-values (* p=0.01 ** p<0.001, *** p< 0.0001) HC indicates healthy controls and PD indicates patients with Parkinson’s disease. N= 50 PD patients and 46 healthy controls. Error bars represents standard error. B. Network analysis of the biomarkers. Candidate PD biomarkers are shown in bold-faced white circles. Genes associated with diabetes are displayed in yellow. Interaction is shown by color-coded lines (green=genetic, pink=physical, purple=co-expression and orange=predicted)
In order to build a model with the highest predictive accuracy, a stepwise multivariate linear discriminant regression (LDA) was performed on the gene expression data adjusting for covariates. This type of analysis evaluates the discriminant power of each interrogated variable in each step, thus building a prediction model by progressively adding the variables with the most significant, individual p-value (p≤0.05) at each step. Based on this analysis, a seven-gene panel was found to discriminate PD patients from controls. The resulting canonical discriminant equation is DPD = 0.170 *Xcopz1 + 0.130* Xc5orf4 + 0.106*Xznf160 − 0.288* Xeftud2 + 0.081*Xwls +0.070* Xprg3 − 0.133*Xmacf1 −1.28, where DPD is the discriminant score value (raw canonical coefficients) and Xi is the mRNA expression level of each biomarker. The four most significant predictors were znf160 (0.75), copz1 (0.70), c5orf4 (0.45), and wls (0.38) (standardized coefficients). Although the number of overweight (BMI >25) and obese (BMI>30) participants was significantly higher in PD than HC (p<0.01), it had no impact on the prediction model (p=0.90). Other covariates including sex and age were also removed from the prediction model by stepwise analysis (sex p=0.88; age p=0.65).
LDA was also used to determine the predictive accuracy of the biosignature to discriminate between PD patients and HC. The average discriminant score for PD patients and HC controls was −0.92 ±0.17 and 1.0±0.09 respectively. Patients with discriminant scores below D≤0.20 were classified as PD and patients above the cut-off value were classified as HC. Based on this analysis, PD patients were identified with 78% sensitivity and 90% specificity and HC controls with 94% sensitivity and 93% specificity.
Network analysis indicated that six of the biomarkers were connected and the most significant canonical pathways dysregulated in PD were the golgi vesicle transport and RNA processing (Figure 1B).
DISCUSSION
Environmental factors play a key role in regulating many steps of gene expression including alternative splicing. The consequence of regulated splicing is the production of several splice variants from a single pre-messenger ribonucleic acid (pre-mRNA). Because of the rapid response of the splicing machinery to environmental factors, which play a key role in the development of PD, we tested the hypothesis that PD patients may be identified using splice variant-specific biomarkers. Here we replicated an association between expression levels of seven splice variants previously identified{Potashkin, 2012 #1}and PD in a new case-control study. Six of the splice variants showed no statistically significant association with PD in this cohort. However, these markers may be useful for distinguishing PD patients from other atypical parkinsonian disorders {Potashkin, 2012 #1}. Network analysis revealed that macf1 and copz1, both genes associated with diabetes {Santiago, 2013 #11}, interact with the mitogen activated protein kinase 9 (mapk9) (Figure 1B). Interestingly, disruption of the mapk9 gene, which encodes the cJun N-terminal kinase 2 (JNK2), reduced insulitis, hyperglycemia and disease progression in diabetic mice {Jaeschke, 2005 #8}. In addition, JNK2 expression is associated with insulin resistance and inflammation and plays a key role in obesity {Han, 2013 #7}. Another central node within the network was mannosidase, alpha, class 1A, member 2 (man1a2). Recent evidence suggests that man1a2 is targeted by the peroxisome proliferator-activated receptor (PPAR-γ) in a novel anti-inflammatory mechanism in vascular endothelial cells {Chacko, 2011 #9} (Figure 1B). This finding is interesting in light of the fact that PPAR-γ co-activators and anti-diabetic drugs targeting PPARs are neuroprotective in models of PD {Zheng, 2010 #13;Schintu, 2009 #14}.
Given the links between PD and diabetes discussed above, future research directed at understanding common dysregulated pathways may enable novel therapeutic strategies for PD {Santiago, 2013 #11}. Although these biomarkers have been replicated in an independent cohort of patients, the results from this cross-sectional study may be vulnerable to bias from unanticipated confounds. For example, differences in blood counts and Parkinson’s medications may bias gene expression results. Thus, evaluation of these biomarkers in patients not treated with PD medications and in a large well-characterized prospective study will be important to determine the clinical utility of these findings. Determining whether these markers are usefully for distinguishing individuals at risk for PD, for progression of PD and/or for distinguishing sub-categories of PD patients will be important for future research.
Acknowledgments
Funding sources: This study was funded by the US Army Medical Research and Materiel Command under awards number W81XWH-09-0708 and W81XWH13-1-0025 to J.A.P. The Harvard Biomarker Study is supported by the Harvard NeuroDiscovery Center. C.R.S. is funded by NIH grants U01 NS082157 and R01 NS064155. Opinions, conclusions, interpretations and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Harvard NeuroDiscovery Center Biomarker Study: We thank the HBS investigators: Co-Directors: Harvard NeuroDiscovery Center: Clemens R. Scherzer, Bradley T. Hyman, Adrian J. Ivinson; Investigators and Study Coordinators: Harvard NeuroDiscovery Center: Ana Trisini-Lipsanopoulos, Kaltra Dhima, Stephen Bayer, Kaitlin C. Lockhart; Brigham and Women’s Hospital: Lewis R. Sudarsky, Michael T. Hayes, Reisa Sperling; Massachusetts General Hospital: John H. Growdon, Michael A. Schwarzschild, Albert Y. Hung, Alice W. Flaherty, Deborah Blacker, Anne-Marie Wills, U. Shivraj Sohur, Vivek K. Unni, Nicte I. Mejia, Anand Viswanathan, Stephen N. Gomperts, Vikram Khurana, Mark W. Albers, Kyleen E. Swords, Rebecca K. Rudel; University of Ottawa: Michael G. Schlossmacher; Scientific Advisory Board: Massachusetts General Hospital: John H. Growdon, Brigham and Women’s Hospital: Lewis R. Sudarsky, Dennis J. Selkoe, Reisa Sperling; Harvard School of Public Health: Alberto Ascherio; Data Coordination: Harvard NeuroDiscovery Center: Thomas Yi, Massachusetts General Hospital: Joseph J. Locascio; Biobank Management Staff: Harvard NeuroDiscovery Center: Zhixiang Liao, Ashley N. Hoesing, Karen Duong, Sarah Roderick.
Footnotes
JAS: Research project (organization and execution), statistical analysis (design and execution), manuscript (writing of the first draft, review and critique); CRS: Research project (organization of patient recruitment, assessment, and biobanking), manuscript (review and critique); JAP: Research project (conception and organization), statistical analysis (design and review), manuscript (writing of the first draft, review and critique)
Financial Disclosure
This study was funded by the US Army Medical Research and Materiel Command under awards number W81XWH-09-0708 and W81XWH13-1-0025 to J.A.P. The Harvard Biomarker Study is supported by the Harvard NeuroDiscovery Center. C.R.S. is funded by NIH grants U01 NS082157 and R01 NS064155.
Financial Disclosure
Jose A. Santiago- Employment: Rosalind Franklin University of Medicine and Science
Judith A. Potashkin- Employment: Rosalind Franklin University of Medicine and Science, Grants: US Army Medical Research and Materiel Command
Clemens R. Scherzer- Employment: Brigham and Women’s Hospital, has participated in collaborations with DiaGenic, Pfizer, Opko, and Proteome Sciences, and is funded by NIH grants R01 NS064155, R01 AG044113, U01 NS082157, U01 AT000613, P01 NS058793, the Harvard NeuroDiscovery Center, the Michael J. Fox Foundation, and the M.E.M.O. Hoffman Foundation.
References
- 1.Scherzer CR, Eklund AC, Morse LJ, Liao Z, Locascio JJ, Fefer D, et al. Molecular markers of early Parkinson’s disease based on gene expression in blood. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):955–60. doi: 10.1073/pnas.0610204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molochnikov L, Rabey JM, Dobronevsky E, Bonucelli U, Ceravolo R, Frosini D, et al. A molecular signature in blood identifies early Parkinson’s disease. Molecular neurodegeneration. 2012;7:26. doi: 10.1186/1750-1326-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soreq L, Bergman H, Israel Z, Soreq H. Exon arrays reveal alternative splicing aberrations in Parkinson’s disease leukocytes. Neuro-degenerative diseases. 2012;10(1–4):203–6. doi: 10.1159/000332598. [DOI] [PubMed] [Google Scholar]
- 4.Potashkin JA, Santiago JA, Ravina BM, Watts A, Leontovich AA. Biosignatures for Parkinson’s disease and atypical parkinsonian disorders patients. PloS one. 2012;7(8):e43595. doi: 10.1371/journal.pone.0043595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding H, Sarokhan AK, Roderick SS, Bakshi R, Maher NE, Ashourian P, et al. Association of SNCA with Parkinson: replication in the Harvard NeuroDiscovery Center Biomarker Study. Movement disorders : official journal of the Movement Disorder Society. 2011;26(12):2283–6. doi: 10.1002/mds.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic acids research. 2010;38:W214–20. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends in molecular medicine. 2013 doi: 10.1016/j.molmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke A, Rincon M, Doran B, Reilly J, Neuberg D, Greiner DL, et al. Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(19):6931–5. doi: 10.1073/pnas.0502143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–22. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchner DA, Geisinger JM, Glazebrook PA, Morgan MG, Spiezio SH, Kaiyala KJ, et al. The juxtaparanodal proteins CNTNAP2 and TAG1 regulate diet-induced obesity. Mammalian genome : official journal of the International Mammalian Genome Society. 2012;23(7–8):431–42. doi: 10.1007/s00335-012-9400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chacko BK, Scott DW, Chandler RT, Patel RP. Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor gamma ligands. The Journal of biological chemistry. 2011;286(44):38738–47. doi: 10.1074/jbc.M111.247981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Science translational medicine. 2010;2(52):52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. The European journal of neuroscience. 2009;29(5):954–63. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]