Abstract
While psychosocial factors are known to affect cancer progression via biobehavioral pathways in many patient populations, these relationships remain largely unexplored in hematopoietic stem cell transplant (HCT) patients. The purpose of this paper is to critically review the literature regarding psychosocial and endocrine/immune aspects of HCT, with an emphasis on exploring pathways that may mediate the associations between psychosocial factors and disease outcomes. These include the roles of catecholamines, glucocorticoids, inflammation, vascular endothelial growth factor (VEGF), immune reconstitution and infectious susceptibility, as well as the new opportunities available in genomics research. We also discuss the implications for potential immunomodulating psychosocial interventions. Elucidating the biological pathways that account for the associations between psychosocial factors and clinical course could ultimately lead to improved outcomes for this psychologically and immunologically vulnerable population.
Keywords: hematopoietic stem cell transplantation, psychooncology, psychosocial factors, psychoneuroendocrinology, psychoneuroimmunology
1. Introduction
Multiple psychosocial factors significantly affect cancer progression via biobehavioral pathways in various populations (Antoni et al., 2006; Costanzo et al., 2011). Researchers have identified several psychological processes as likely predictors of cancer progression; these include, but are not limited to, mood (depression/anxiety), social support, stress, optimism, loneliness, and socioeconomic status. Advances in mechanistic studies continue to identify biological signaling pathways that may be responsible for such effects (Costanzo et al., 2011; Lutgendorf and Sood, 2011; McGregor and Antoni, 2009). Biobehavioral psychooncology research has focused most consistently on specific solid tumors and virally-mediated cancers, yet many other types of cancers remain largely unexplored.
One such common group of cancers, hematologic malignancies, is often treated with hematopoietic stem cell transplantation (HCT). While some studies have demonstrated an association between several psychosocial factors and HCT outcomes (Hoodin et al., 2006), the biobehavioral pathways accounting for this association remain unknown. Despite the high psychological and immunological vulnerability in HCT recipients, little psychoneuroendocrinology (PNE) or -immunology (PNI) research has been conducted in this population. This may be in part because of the inherent complexity of the endocrine and immunobiologic changes occurring in the transplant setting, requiring a depth of basic and clinical knowledge to explore PNE/PNI-mediated outcomes in this population. Costanzo et al recently discussed biobehavioral influences on recovery following HCT (Costanzo et al., 2012). In this review we consider additional perspectives such as genomics, with greater focus on the implications of neuroendocrine pathways.
It is an especially salient time to expand our understanding of the biobehavioral outcomes and mechanisms of cancer and its treatments given the Institute of Medicine’s statement that providing appropriate psychosocial services to all cancer patients and their families should become standard in quality cancer care (Adler and Page, 2008). This article surveys pertinent oncologic biobehavioral literature with the goal of proposing relevant pathways of study of HCT recipients and their specific biology. We begin with a brief overview of hematopoietic stem cell transplantation. We then discuss the current state of psychosocial research in HCT patients, reviewing key findings and current conceptual and methodological limitations of published studies to date, emphasizing the rationale and direction for further PNE/PNI research in this population. We then propose candidate physiological markers for novel research based on psychooncologic principles. Finally, we consider the role of genomics as well as possible targets for interventions research.
2. Hematopoietic Stem Cell Transplantation Overview
With 7,000 allogeneic and 12,000 autologous HCTs performed in North America in 2010, the annual number of transplants has doubled over the last two decades (Center for International Blood and Marrow Transplant Research (CIBMTR), 2012). HCT has been used to treat a variety of malignant and non-malignant conditions since the late 1960’s. Due to its increased safety and efficacy for a growing range of hematologic malignancies and disorders, autoimmune diseases, and solid tumors, its use has greatly expanded in more recent years. Some of the more common diseases treated with HCT include multiple myeloma, Hodgkin’s and non-Hodgkin’s lymphoma, acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, and chronic lymphocytic leukemia.
HCTs can be quite variable, particularly with respect to the source of the transplanted cells. Allogeneic transplants (normal marrow or blood stem cells from a compatible donor) are more problematic than autologous transplants (patient-to-self) since they require higher intensity suppression of host immune system post-transplant to prevent foreign graft rejection and graft-versus-host-disease (GVHD). GVHD is donor T cell attack of host tissues and is the major complication of allogeneic transplantation. Typically, acute GVHD develops within the first 100 days post-transplant and is characterized by alloreactive donor T cells attacking skin, liver, stomach, and intestines. Chronic GVHD usually develops after 100 days post-transplant and is characterized by T lymphocyte imbalances from overexpansion of pathological subsets and/or loss of appropriate regulation as well as alloreactive T cells (Lee et al., 2003). Both acute and chronic GVHD remain significant barriers to long-term health and quality of life following allogeneic transplant, with 20–50% of recipients experiencing some form of acute GVHD (Ball et al., 2008) and 60–70% developing chronic GVHD (Lee et al., 2003).
Patients receive their own cells in autologous transplantation, therefore GVHD and graft rejection do not occur and patients do not require long-term immune suppression. Different donor sources, conditioning regimens, and infection risk may vary within and/or between transplant types. The probability of 5-year survival is 15–55% depending on pre-transplant disease status and transplant and donor type (CIBMTR, 2009). Full immune reconstitution can take two years or longer to occur; reconstitution is generally faster in autologous stem cell transplantation. Thus, it is important to consider the heterogeneity of disease and transplant type when conducting research in the HCT population.
3. Psychoneuro-Endocrine and -Immune Implications for Hematopoietic Stem Cell Transplant Patients
Patients undergoing HCT experience both unique and severe immunological and psychological conditions, making them an especially pertinent population for exploring PNE/PNI mechanisms. While most cancer treatments have significant immunosuppressive consequences, HCTs involve complete or near-complete ablation of the native marrow with very high doses of chemotherapy, potentially leaving patients more severely immunocompromised than patients with other cancers. Furthermore, the process of HCT is often rife with radical medical interventions and complications, including invasive diagnostic and treatment procedures as well as infection and GVHD. Thus, treatment often may be more distressing than other cancer treatments, although this has not been directly investigated. Compared to other transplants, successful HCT may require prolonged periods of physical isolation depending on type of transplant, institutional practice, and potential complications. For patients that do require prolonged isolation there is a high incidence of psychological disorders, including depression and anxiety (Sasaki et al., 2000). The altered endocrine milieu associated with psychological distress may play a significant role in the robustness or speed of immune reconstitution post-transplant.
It remains unclear whether psychosocial variables in general, transplant-related stressors in particular, or transplant-related exacerbation of prior psychological dysfunction may impact clinical outcomes (Hoodin et al., 2006). Differentiation of these prognostic factors is important in designing appropriate screening and interventions. An even greater limitation is that very few studies have investigated possible biological mediators and mechanisms linking psychosocial factors to observed differences in HCT morbidity and mortality. This stands in contrast to the wealth of studies examining these mechanisms in other oncology populations (Antoni et al., 2006; McGregor and Antoni, 2009). While health behavior changes such as diet, exercise, or smoking likely account for some of the observed effects of psychosocial factors on HCT outcome and survival, there is evidence in other populations of more direct stress-induced brain-immune physiological effects on outcomes (Costanzo et al., 2011; Lutgendorf and Sood, 2011).
The most recent comprehensive review of the effect of emotions on HCT mortality (Hoodin et al., 2006) uncovered 19 published reports. Out of the 15 studies investigating negative emotion and survival, 5 studies found no statistical relationship, whereas 7 studies reported that patients with more depressive symptomatology had shorter survival. Five studies examined the role of positive emotions in HCT survival and showed that pre-transplant optimism, hopefulness, social support, and ‘fighting spirit’ were positive prognostic factors for longer survival.
There have been limited published studies examining the association between psychosocial factors and mortality since Hoodin’s 2006 review. However, a recent report by Pereira et al suggests that spiritual absence, or the degree to which patients lack religious or spiritual personal resources to cope with medical stressors, as measured by the Millon Behavioral Medicine Diagnostic, is associated with poorer survival following HCT (Pereira et al., 2010).
Only a handful of studies have examined the relationship between psychological profiles and complicating clinical events that precede HCT mortality. Higher anxiety among allogeneic transplant recipients was significantly associated with the development of acute GVHD (Gregurek et al., 1996). Similarly, in a previously mentioned study (Pereira et al., 2010), infection, sepsis, and GVHD were identified as significant events contributing to increased 1-year mortality among those HCT patients with greater spiritual absence. Another study demonstrated that a high-risk psychological profile (as defined by the ‘FIT assessment’) conferred greater risk of recurrent disease or progressive major organ dysfunction (Sullivan et al., 1999), the extreme consequence of physiological stressors inducing a systemic inflammatory response syndrome (SIRS) (Rangel-Frausto et al., 1995) and amplifying the inflammatory cytokine cascade. Most recently, investigators demonstrated that higher levels of pre-transplant anxiety and depression were associated with slower white blood cell recovery among myeloablative transplant recipients (McGregor et al., 2012). The PNI/PNE implications of these events will be discussed in greater depth in the following sections.
While promising, the results from studies investigating psychosocial factors on HCT outcomes should be interpreted with caution due to lack of methodological consistency. Some studies incorporate both prospective and retrospective data, non-psychometrically validated inventories based on non-standardized psychiatric interview, retrospective chart review, and small sample sizes. The timing of psychological assessment is also important in determining well-being, as there is generally a decline in quality of life in the immediate post-transplant period with a gradual improvement over time (Norkin et al., 2012). A retrospective study of almost 3000 patients demonstrated the lowest quality of life while subjects are inpatients, with a return to pre-transplant quality of life status by about one year post-transplant (Grulke et al., 2012). A careful longitudinal study with multiple psychological assessment times may be useful for better understanding psychosocial effects on outcome. Furthermore, the assessment of psychological functioning before, during, and after transplant varies in terms of modality, instruments, timing, and examiner (e.g. student, nurse, social worker, psychologist, or physician). Efforts should be made to include reliable, validated, and commonly employed psychometric assessment tools at standard waypoints in the transplant process. Researchers conducting interviews should have standardized training with assessments done prospectively whenever possible.
4. Proposed Physiological Factors
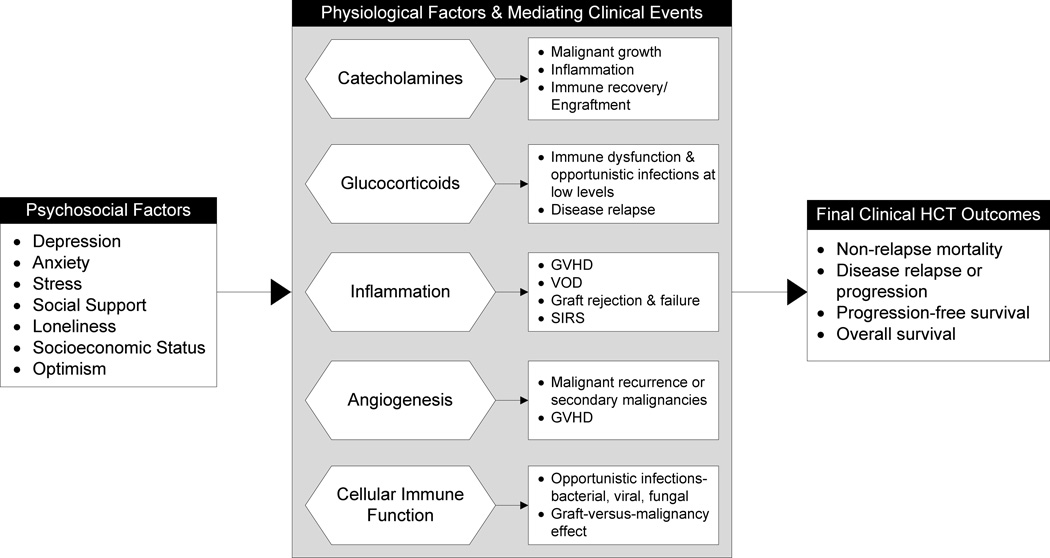
It is unknown which relevant biobehavioral pathways (Figure 1) might apply to this pathophysiologically-distinct population; therefore, further study into the unique outcomes and mechanisms involved with HCT, and not simply extrapolating from other oncology populations, is necessary. To understand the biobehavioral pathways and physiological factors involved and design appropriate interventions for these patients, it is ultimately important to determine what drives psychosocial stress effects on tumor and treatment subtype biology so we may best know when and how to intervene. We will discuss these pathways from a brain-immune system-outcomes approach, starting with more centrally derived mediators before moving to more peripherally-derived ones, concluding with discussion of immune reconstitution and infectious susceptibility. We will consider these physiological factors as they relate to mediating clinical events as well as commonly assessed final clinical outcomes in HCT recipients including non-relapse mortality, disease relapse or progression, progression-free survival, and overall survival (Figure 1).
Figure 1.
This biobehavioral model highlights physiological factors linking psychosocial factors and mediating clinical events with adverse outcomes in hematopoietic stem cell transplant patients. Psychosocial factors, both positive and negative, impact hormonal and messenger systems as well as immune function; this directly affects intermediary clinical events relevant to the overall success of HCT. Mortality, relapse or progression, and survival are commonly assessed final endpoints among the HCT population and may be influenced by these biobehavioral pathways. GVHD, graft-versus-host-disease; VOD, veno-occlusive disease; SIRS, systemic inflammatory response syndrome; VEGF, vascular endothelial growth factor
4.1. Catecholamines
The central nervous system is linked to both the bone marrow and the thymus as well as to secondary lymphoid organs; sympathetic nervous system innervation suggests one important mechanism involved in biobehavioral effects in HCT recipients. For the majority of HCT recipients, the bone marrow encompasses the tumor microenvironment. Stress increases levels of catecholamines in tumor microenvironments of other cancers, including ovarian, colon, and breast (Cole and Sood, 2012), subsequently affecting tumor biology and pathogenesis by promoting tumor growth.
Catecholamines are present in substantially higher levels in the bone marrow microenvironment than in the circulation (Maestroni et al., 2006). It is important to consider that immune cell distribution in response to stress may vary by innate vs. adaptive immune components. For example, repeated exposure to social stress in a murine model induced differential redistribution of myelocytes and lymphocytes; increased myelopoiesis occurred in the bone marrow with subsequent increased myelocytes in circulation and the spleen, while conversely, B and T cells were decreased in the bone marrow, with a selective increase in B cells in the spleen (Engler et al., 2004). More recently, an orthotopic mouse model demonstrated progression of human pre-B cell acute lymphoblastic leukemia in response to chronic stress via a beta-adrenergic signaling pathway (Lamkin et al., 2012). The effects of chronic stress have been successfully blocked in mice with use of the betaadrenergic receptor antagonist propranol (Lamkin et al., 2012; Lee et al., 2009). In the absence of chronic psychosocial stress, increased adrenergic activity alone in the bone marrow microenvironment enhanced mobilization of murine progenitor cells (Lucas et al., 2012).
Future direction in human HCT research may examine whether elevated catecholamines interfere with the engraftment process, maturation of the transplanted immune system, or disease relapse, as they may have different effects over time. Due to the complexities of the transplant process and the variable issues at different stages, evaluating these time points separately may be integral to identifying PNI/PNE mediated effects. It is also important to distinguish how the effect of adrenergic signaling on tumor growth and immune reconstitution may be confounded by additional neuroendocrine stress hormones such as glucocorticoids, given that cortisol and catecholamines may act in a synergistic manner to facilitate growth in other cancer types (Antoni et al., 2006).
4.2. Glucocorticoids
The relationship between stress, glucocorticoids, and HCT outcomes is not well understood, as there is evidence for effects leading to both better and poorer outcomes. Glucocorticoids have obvious beneficial effects for HCT recipients; high doses are a mainstay of immunosuppressive therapy following HCT (Sionov et al., 2008), treatment of GVHD (Hovi et al., 2004), and treatment of leukemias involving the lymphoid lineage (acute lymphoblastic leukemia but not acute myeloid leukemia). In mouse models, glucocorticoids are necessary for proliferation of erythroid progenitor cells under stressful conditions (Bauer et al., 1999), suggesting a possible salubrious role for glucocorticoids in the early stages of immune reconstitution following HCT.
Despite the evidence that glucocorticoid therapy may have a positive influence in the HCT setting, there is also evidence to the contrary. First, high dose steroids that many allogeneic recipients need render them susceptible to immune dysfunction and opportunistic infections. Further, glucocorticoids may have differential effects on tumor cells depending on the dose, as they have been proven to suppress tumor growth at higher levels while stimulating growth at lower levels (Kawamura et al., 1998). This could have specific relevance for disease relapse in autologous transplant recipients for whom exogenous high dose glucocorticoids are not routinely administered, yet the recipients are still vulnerable to the effects of stress-induced elevations in endogenous glucocorticoids likely inherent with this rigorous procedure. It is not known at what critical level endogenous and exogenous glucocorticoids become helpful vs. harmful. Finally, evidence from solid tumor research suggests that dysregulation of the HPA axis response is associated with poorer outcomes, earlier mortality, and suppressed immunity (Lutgendorf and Sood, 2011). Given the above conflicting findings between tumor type and glucocorticoid use, more research is needed to elucidate the specific effects of glucocorticoids in HCT recipients. In so doing, the early (immune reconstitution) vs. late (disease relapse) phases of the transplant process should be considered as separate clinical entities when examining the effects of glucocorticoids.
4.3. Inflammation
Inflammation may be a key component in the biobehavioral mechanisms involved in this population. Cytokines have been implicated in multiple adverse transplant-related complications. An imbalance in cytokines favoring the pro-inflammatory state may be related to severe GVHD (Hill et al., 1997), with serum levels of tumor necrosis factor-α (TNF-α) and soluble IL2 receptor useful markers for early detection of acute GVHD and evaluating treatment response (Holler et al., 1990; Liem et al., 1998; Lunn et al., 2005). Typically, acute GVHD is characterized by a three-step process involving interaction between the innate and adaptive immune systems: 1) tissue damage secondary to preconditioning treatment (radiation/chemotherapy), 2) donor T-cell activation and expansion, and 3) cellular and inflammatory factors including pro-inflammatory cytokines, specific anti-host cytotoxic T-lymphocytes, NK cells, and nitric oxide (Ferrara and Reddy, 2006).
Elevated pre-allogeneic transplant TNF-α levels are associated with the development of veno-occlusive disease (VOD) (Gugliotta et al., 1994), another serious complication of HCT. High levels of TNF-α and interleukin (IL)-6 are associated with graft rejection and failure as well (Nagler et al., 1995). C-reactive protein (CRP) has also been shown to be an early predictor of severe post-transplant complications and death (Artz et al., 2008), and may be another pro-inflammatory marker worthy of investigation.
The release of systemic inflammatory cytokines corresponds not only to severe morbidity and mortality in HCT patients, but also to symptom burden including fatigue, pain, and disturbed sleep (Wang et al., 2008). Proinflammatory cytokines such as IL-1, IL-6, and TNF-α are known to induce this “sickness” behavior (Dantzer et al., 2008) and likely contribute to the poorer quality of life reported by HCT survivors as compared to healthy controls (Andrykowski et al., 2005).
Inflammation may be a result of adverse psychological and physical states, including stress, depression, obesity, and atherosclerosis (Elenkov et al., 2005). Evidence that depressive disorders or symptoms may lead to inflammation (Capuron and Miller, 2011) suggests that the pro-inflammatory pathway is a biologically plausible mechanism whereby negative psychosocial states may adversely influence HCT outcome. As previously mentioned, the study by Sullivan et al (1999) provides clinical evidence for a model of psychological distress triggering cytokine-mediated processes. These processes may potentiate the systemic inflammatory response syndrome (SIRS) and ultimately lead to death from multisystem organ failure in HCT patients. The neuroendocrine relationship between depression/anxiety and inflammation is more complex, however, as the reverse is also true with inflammatory cytokines triggering psychological stress (Miller et al., 2008). It is in this context whereby inflammation may be a candidate biobehavioral pathway linking psychosocial factors with HCT outcomes.
Methodologically sound studies of these intermediary events are needed to elucidate the mechanisms involved in the relationship between psychosocial factors and clinical outcomes as there have not, however, been clinical studies investigating cytokines and inflammation as a potential mechanism or causal link. As inflammation plays a key role in varying transplant stages from early processes such as physiologic response to preconditioning regimens and engraftment, to often later complications such as infection and disease relapse, it’s appropriate to examine PNI/PNE effects at all HCT time points. Careful attention to methodology is necessary given the variability and vast array of inflammation-associated experiences or manipulations associated with the HCT recipients process, including, but not limited to, psychosocial status, demographic factors, health behaviors, chemotherapy, radiation, reason for and type of transplant, remission or relapse status, medical comorbidities, presence of GVHD, exposure to exogenous steroids, and infection. Large sample sizes may be utilized to either select or control for this wide array of inflammatory confounders.
4.4. Angiogenesis
Vascular endothelial growth factor (VEGF) is a well-studied angiogenic molecule that plays a role in tumor neovascularization and proliferation (Ferrara, 1996). Like glucocorticoids, VEGF may have differential effects in the HCT process dependent upon which outcome and point in time is considered. In solid tumors, VEGF is traditionally thought to play a negative role in outcomes, contributing to tumor invasion, growth, and metastasis, with chronic stress increasing angiogenesis and VEGF expression (Thaker et al., 2006). In terms of hematologic malignancies, this can also be true in that VEGF plays a role in the pathogenesis of acute myeloid leukemia (Abou-Shamaa and Mahmoud, 2008) and mediates increased angiogenesis induced by chronic lymphocytic leukemia B-cells (Maffei et al., 2010). This negative effect of VEGF may play a more significant role in the later stages of the transplant process when disease relapse or secondary malignancy is of greater concern. On the other hand, VEGF is a major regulator of endothelial and hematopoietic stem cell development (Ciau-Uitz et al., 2010), and therefore, like glucocorticoids, may have salubrious effects during early stages of immune reconstitution.
Though there are no studies directly evaluating the effects of psychosocial factors on VEGF in HCT recipients, chronic behavioral stress in mice results in markedly increased vascularization and VEGF expression in ovarian tumor cells (Thaker et al., 2006). In clinical models, lower VEGF levels are associated with higher levels of social well being in presurgical ovarian cancer patients (Lutgendorf et al., 2002). These effects appears to be mediated by the beta-adrenergic signaling pathway (Yang et al., 2006).
A few studies have evaluated VEGF in the HCT setting. Salivary VEGF concentrations were not different in HCT recipients as compared to controls at 100 days post-transplant, a meaningful clinical assessment point, nor were they influenced by the stage of chronic GVHD (Souza et al., 2004). Serum VEGF levels did not vary significantly over the first 6 months following autologous transplantation for multiple myeloma, although bone marrow microvascular density decreased significantly (Sucak et al., 2011); neither were significantly associated with overall survival or progression free survival. In one study, however, serum VEGF levels were significantly higher in allogeneic HCT recipients with GVHD (Lunn et al., 2005).
IL-6 and IL-8 are other proangiogenic molecules modulated by behavioral factors and the stress response pathway (Costanzo et al., 2011). Greater levels of IL-6 in the tumor microenvironment are correlated with increased social isolation among ovarian cancer patients (Costanzo et al., 2005). Among HCT recipients, increased IL-6 is associated with increased symptom burden in the first 30 days following allogeneic transplantation (Wang et al., 2008). Further, these various tumor microenvironment pathways likely work synergistically, as norepinephrine is known to upregulate VEGF and cytokines via the–adrenergic receptor pathway (Costanzo et al., 2011). Future work in understanding these pathways needs to take the synergistic activity of these angiogenic molecules and the catecholamines into account.
4.5. Cellular Immune Function and Infectious Susceptibility
Immune reconstitution is a critical goal of successful HCT, and specific immune cell populations engraft at variable rates. Clinically, the absolute neutrophil count (ANC) and platelet levels are initially most closely monitored, as they are the most sensitive indicators of susceptibility to infection and bleeding immediately post-transplant. Neutrophil recovery above neutropenic levels (defined as neutrophil count <500/mm3), when the patient is most susceptible to bacterial infections, and platelet recovery above which transfusion is indicated, typically occur an average of two weeks post-transplant (Hogge et al., 2000), though this varies depending on stem cell source and dose, age, and transplant type. Natural killer (NK) cells and dendritic subsets typically recover to normal levels within 1–2 months (Rajasekar et al., 2009). While innate immunity recovers within several weeks, B cell and CD8+ T cell numbers take several months to normalize, with CD4+ T cells taking years to recover, or, in the presence of chronic GVHD, perhaps never recover. While common for at least the first year post-HCT, a low or inverted CD4+/CD8+ ratio renders patients susceptible to infection post-transplant (Steingrimsdottir et al., 2000), with a normal CD4+/CD8+ ratio of 2:1 associated with better survival among HCT recipients 30 days post-transplant (Klyuchnikov et al., 2010). There is a paucity of published data regarding psychosocial factors and immune reconstitution in HCT recipients, although one previously mentioned study demonstrated that higher levels of pre-transplant anxiety and depression were associated with slower white blood cell recovery (McGregor et al., 2012).
The robustness of cell-mediated immune recovery has significant clinical ramifications in terms of prevention of disease recurrence. Donor NK cells can induce a graft-versusleukemia effect, with a series of studies demonstrating rapid NK cell recovery predicting less relapse and better survival (Nakamura et al., 2008; Savani et al., 2006; Savani et al., 2007). Further, day 30 NK cell counts were positively associated with rapid molecular remission in patients with myeloid malignancies. Conversely, decreased numbers of NK cells at day 28 post-transplant were associated with secondary graft rejection (Rajasekar et al., 2009) and relapse in allogeneic transplant recipients (Klyuchnikov et al., 2010). In sum, NK cells are important for transplant success and their administration has proven to be a beneficial adaptive immunotherapy (Passweg et al., 2004), preventing relapse in some HCT recipients (Miller et al., 1994).
Biobehavioral associations with infection-regulating immune processes, including NK and T cell activity, have been described in other cancer populations (Andersen et al., 1998; Sephton et al., 2009). Breast cancer patients reporting more stress and depressive symptoms have suppressed cell-mediated immunity, including impaired NK cell activity and decreased T cell responses to mitogens (Andersen et al., 1998; Sephton et al., 2009). Opportunistic infections are a major concern and source of morbidity during the transplant process, arising due to the disease itself, previous chemotherapies, preparative regimen, mucosal barrier breakdown, GVHD, and other immunosuppressive therapy. The early recovery period (first 6 weeks post-transplant) is marked by severe neutropenia and mucosal damage, rendering HCT recipients most at risk of infection with skin and gastrointestinal organisms during this time. Patients are extremely susceptible to local or disseminated bacterial fungal, and viral (reactivation or novel pathogens) infections. Nosocomial pathogens, including drug-resistant organisms, are of special consideration during this time period. From 2–3 months post-transplant, cellular and humoral immunodeficiency remains a significant clinical consideration. Finally, during the late recovery period, the patient remains at continued risk of recurrent encapsulated bacterial infection, viral reactivation, and fungal pneumonia.
Viruses – latent and novel – pose significant risk following HCT. Since biobehavioral responses affect neuroendocrine function and contribute to viral activity and regulation of the immune response (Antoni et al., 2006), the biological repercussions of psychosocial processes are likely of particular relevance to the HCT population. Stress hormones influence the activity of various human tumor viruses (Zur Hausen, 1991), modulate herpesvirus latency (Glaser et al., 1985), increase risk of illness from common respiratory viruses (Cohen et al., 1993), and cause symptomatic HSV recurrence (Chida and Mao, 2009), all significant risks for HCT recipients. Cytomegalovirus (CMV)-associated disease (a type of herpesvirus) is a major cause of morbidity and mortality following HCT (Boeckh et al., 2003). PNE/PNI effects of these psychosocial stressors are compounded by the already medication-induced immunosuppressed state of these individuals.
Immune recovery and infectious profiles vary depending on type of transplant. Autologous HCT recipients have a more rapid rate of immune recovery and thus fewer opportunistic infections than allogeneic recipients. Allogeneic recipients with less well-matched donors or GVHD also have an altered immunologic response and cell-mediated and humoral immune deficits that make them persistently vulnerable to infection. It is important to incorporate methodological considerations, such as type of transplant and GVHD, when evaluating biobehavioral effects on immune reconstitution in HCT recipients.
5. Genomics
Genetic constitution may be investigated both structurally and functionally to understand and identify genotypes at-risk for developing psychopathology and/or associated PNI/PNE mediated outcomes during the transplant process. Structural genomics approaches offer the opportunity for identification of polymorphisms that confer both physical and psychological susceptibility; genetic polymorphisms in signaling molecules and their receptors adversely impact stress adaptation responses in non-cancer populations and may have implications for HCT recipients (Agrawal et al., 2012; Zhang et al., 2012).
Certain single gene polymorphisms (SNPs) are associated with symptom burden and quality of life in other cancer populations (Rausch et al., 2010). There is one published study assessing the relationship between genetic factors and psychological stress resilience in HCT patients (Romanowicz et al., 2012); this study suggests that a polymorphism in brainderived neurotrophic growth factor (BDNF) confers risk of depressive symptomatology associated with HCT. A small sample size and p-values > .05 limit interpretation of these findings; however, this research strategy bears promise and should continue to be refined. Given the immunological and psychological vulnerability of HCT patients to any perturbation, genomics could allow us to identify individuals at risk of developing adverse outcomes in the context of coping with this stressful medical event. While the genomics analyses discussed thus far focus on structural polymorphisms conferring risk of psychopathology, the subsequent step in connecting structure with stress-mediated adverse medical outcomes is less well understood. Functional genomics research may provide further guidance in probing these questions.
In addition to assessing static properties of the genome, functional genomics research, with its dynamic assessment of transcriptional activity, may lead to a more complete understanding of PNE-mediated biobehavioral pathways. Altered genome wide expression of transcription factors consistent with stress physiology is observed in both socially isolated adults as well as adults reared in unfavorable socioeconomic conditions (Cole et al., 2007; Miller et al., 2009). Less social support and lower socioeconomic status are associated with development of psychopathology and poorer survival among HCT recipients (Baker et al., 2009; Frick et al., 2005; Rini et al., 2011; Wells et al., 2009). The biobehavioral pathways identified in these transcription factor studies involve altered glucocorticoid and proinflammatory signaling as well as altered expression of processes regulating cell growth and cell cycle inhibition, both with specific implications for HCT outcomes.
Genetic studies may aid in pre-transplant risk determination, identifying patients that might benefit from early and consistent psychosocial intervention. One benefit of genomics analysis in HCT recipients, especially those of a structural nature, is that genetic composition is less likely than other peripheral biomarkers to be altered by medical perturbations of the transplant process.
6. Possible Immunomodulating Interventions
Given that stress is a potent immunomodulator via multiple neuroendocrine pathways (Xiang et al., 2012), one effective means of altering physiology may be to use interventions to reduce the impact of psychosocial stressors. Very few studies have examined interventions in HCT recipients, and none have investigated possible immunological benefit. Music therapy was effective in decreasing pain and nausea in HCT patients (Sahler et al., 2003) and mindfulness-based interventions are effective in addressing psychological and emotional problems (Horton-Deutsch et al., 2007). It is as yet inconclusive as to what degree of psychological change is needed to translate into biological change, or to what degree differing interventions are efficacious.
A recent review emphasized the importance of cognitive, behavioral, and social factors as intervention tools to facilitate adaptation to cancer during all stages of survivorship (Antoni, 2012). This review outlined the data suggesting beneficial effects of such interventions on neuroendocrine and immune function as well as psychological systems, yet qualified such potential benefit with our as yet limited knowledge about the effects of intervention on tumor activity and growth-promoting processes. An exhaustive review of immunomodulating interventions is beyond the scope of this article, however, there are several worth mentioning due to their possible relevance to HCT patients.
Most recently, a randomized controlled trial of patients with any type of cancer demonstrated that a combined stress management and exercise intervention yielded significant improvements in anxiety and depression (Jacobsen et al., 2012). Exercise by itself is one of the only interventions known to improve cancer-related fatigue, which also occurs post-transplant. It has advantageous effects for both physical and mental health, decreasing psychological stress as well as improving the inflammatory and cytokine-associated milieu (Starkweather, 2007), of significant importance in the transplant process. Of particular relevance to HCT patients, exercise has been shown to augment the immune system with respect to infection resistance and cancer cell growth (Silveira et al., 2007). One benefit of exercise may be its applicability in the immediate post-transplant hospitalization period, for example, on a stationary bike. Without appropriate equipment this may be difficult to accomplish in the post-transplant period following discharge due to necessary isolation precautions. However, after day 100 following transplant, HCT recipients should not have additional restrictions compared to other cancer populations.
Mind-body interventions, defined as any treatment that addresses the interaction between the mind (thoughts, feelings) and body (physical processes), are increasingly used among cancer patients and survivors and often combine stress-reducing activities with physical activity. Evidence is mounting that these interventions effectively alleviate both psychological and physical suffering. There is some indication from cancer populations that yoga provides immunological benefit in addition to psychological improvement (Nunes et al., 2007). A more specific form of stress management and mind-body technique incorporating yoga, mindfulness based stress reduction (MBSR), improves quality of life as well as immune function, including decreased TNF-α as well as increased NK cell activity, in a variety of cancer patients (Carlson et al., 2007; Witek-Janusek et al., 2008). Elements of MBSR, such as yoga, could be a lost-cost, feasible, and eventually self-guided intervention for HCT recipients at multiple points in their transplant process, ranging from the preconditioning to late survivorship phases, pending any physical limitations. MBSR is a more intensive, structured intervention that might be most applicable to transplant recipients in their later stages of recovery. As a result, varying interventions may have differential impact on immune recovery and other pertinent biobehavioral processes, pending the time point of their administration.
Another promising intervention for HCT recipients might be cognitive behavioral stress management (CBSM), which resulted in a decrease in stress and neoplastic growth in HIV patients (Antoni et al., 2008). These findings suggest its usefulness as a cancer prevention or recurrence strategy among those who are immunocompromised, such as HCT recipients (Antoni et al., 2008). Due to the time intensive nature of this program, like MBSR, it may be better suited for patients following the acute recovery period.
Finally, utilization of psychopharmacologic agents not only in the treatment of active mental health issues, but perhaps as a prophylactic consideration in the HCT process, may be warranted. This could involve any of the psychotropic medications used to mitigate the neuroendocrine effects associated with stress, depression, and anxiety including, most commonly, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), benzodiazepines, and beta-adrenergic receptor antagonists (beta-blockers). Anti-psychotics or mood stabilizing medications may also be considered in a judicious manner. Beta-adrenergic signaling impacts most, if not all, of the previously discussed biobehavioral pathways (Cole and Sood, 2012); therefore, given their low cost and relatively benign side effect profile, beta-blockers may have particular relevance for administration in the HCT process. Studies have linked use of beta-blockers with decreased progression of multiple tumor types (Lamkin et al., 2012;Masur et al., 2001;Melamed et al., 2005;Palm et al., 2006;Powe et al., 2010). These effects have not yet been examined in HCT recipients.
7. Conclusions
Increased inflammation, toxic conditioning regimens, re-developing immune systems, and severe psychosocial stressors make the HCT population especially susceptible to adverse outcomes. It remains to be investigated whether and how modulation by individual psychological factors affects these interrelationships. With their integrated brain/body approaches, PNE and PNI offer invaluable tools to explore the above physiological markers in the HCT population. Numerous biobehavioral pathways whereby psychosocial states may affect HCT outcome have been proposed. The biobehavioral pathways that might mediate this relationship need to be evaluated with the same scientific rigor other cancer subtypes have received. These include, but are not limited to, the roles of catecholamines, glucocorticoids, inflammation, VEGF, and immune reconstitution and infection susceptibility as well as the new opportunities available in genomics research. Future research is needed to focus on systematically evaluating the validity and reproducibility of both positive and negative psychological prognosticators of hematopoietic stem cell transplant outcomes. This research agenda is important not only in designing interventions to aid this psychologically and immunologically vulnerable population, but for contributing to the advancement of knowledge within psychooncology.
Acknowledgements
This work was supported by NIA grant 5R24 AG031089 and NIMH grant T32 MH073452.
Abbreviations
- HCT
hematopoietic stem cell transplantation
- PNE
psychoneuroendocrinology
- PNI
psychoneuroimmunology
- GVHD
graft-versus-host-disease
- SIRS
systemic inflammatory response syndrome
- CRP
C-reactive protein
- HSV
herpes simplex virus
- NK
natural killer
- CMV
cytomegalovirus
- HPA
hypothalamicpituitary-adrenal
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest to disclose.
References
- Abou-Shamaa LA, Mahmoud GN. Leukocytic-vascular endothelial growth factor and integrin alphavbeta3 in acute myeloid leukemia: relation to clinical outcome. Egypt. J. Immunol. 2008;15:81–91. [PubMed] [Google Scholar]
- Adler NE, Page AE. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. National Academy Press; 2008. [PubMed] [Google Scholar]
- Agrawal A, Nelson EC, Littlefield AK, Bucholz KK, Degenhardt L, Henders AK, Madden PAF, Martin NG, Montgomery GW, Pergadia ML. Cannabinoid Receptor Genotype Moderation of the Effects of Childhood Physical Abuse on Anhedonia and Depression. Arch. Gen. Psychiatry. , archgenpsychiatry. 2012 doi: 10.1001/archgenpsychiatry.2011.2273. 2011.2273 v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. JNCI Journal of the National Cancer Institute. 1998;90:30. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, Horowitz MM, Sobocinski KA, Rizzo JD, Wingard JR. Long-term healthrelated quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J. Clin. Oncol. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav. Immun. 2012 doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Pereira DB, Marion I, Ennis N, Andrasik MP, Rose R, McCalla J, Simon T, Fletcher MA, Lucci J, Efantis-Potter J, O'Sullivan MJ. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J. Psychosom. Res. 2008;65:389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz AS, Wickrema A, Dinner S, Godley LA, Kocherginsky M, Odenike O, Rich ES, Stock W, Ulaszek J, Larson RA, van Besien K. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Ballen KK, Bigelow CL, Frangoul HA, Hardy CL, Bredeson C. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2009;15:1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LM, Egeler RM EBMT Paediatric. Working P. Acute GvHD: pathogenesis and classification. Bone Marrow Transplant. 2008;41:S58–S64. doi: 10.1038/bmt.2008.56. [DOI] [PubMed] [Google Scholar]
- Bauer A, Tronche F, Wessely O, Kellendonk C, Reichardt HM, Steinlein P, Schutz G, Beug H. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biology of Blood and Marrow Transplantation. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav. Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Center for International Blood and Marrow Research. Current uses and outcomes of hematopoietic stem cell transplantation. 2009 [Google Scholar]
- Chida Y, Mao X. Does psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav. Immun. 2009;23:917–925. doi: 10.1016/j.bbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Pinheiro P, Gupta R, Enver T, Patient R. Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev. Cell. 2010;18:569–578. doi: 10.1016/j.devcel.2010.02.009. CIBMTR,2012. Personal Communication. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J. Pers. Soc. Psychol. 1993;64:131–140. doi: 10.1037//0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clinical Cancer Research. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Sood AK, Lutgendorf SK. Biobehavioral influences on cancer progression. Immunology and allergy clinics of North America. 2011;31:109. doi: 10.1016/j.iac.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Juckett MB, Coe CL. Biobehavioral Influences on Recovery Following Hematopoietic Stem Cell Transplantation. Brain Behav. Immun. 2012 doi: 10.1016/j.bbi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Eur. J. Cancer. 1996;32:2413–2422. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin. Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Frick E, Motzke C, Fischer N, Busch R, Bumeder I. Is perceived social support a predictor of survival for patients undergoing autologous peripheral blood stem cell transplantation? Psycho Oncology. 2005;14:759–770. doi: 10.1002/pon.908. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J. Behav. Med. 1985;8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Gregurek R, Labar B, Mrsić M, Batinić D, Ladika I, Bogdanić V, Nemet D, Skerlev M, Jakić-Razumović J, Klain E. Anxiety as a possible predictor of acute GVHD. Bone Marrow Transplant. 1996;18:585. [PubMed] [Google Scholar]
- Grulke N, Albani C, Bailer H. Quality of life in patients before and after haematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 2012;47:473–482. doi: 10.1038/bmt.2011.107. [DOI] [PubMed] [Google Scholar]
- Gugliotta L, Catani L, Vianelli N, Gherlinzoni F, Miggiano MC, Bandini G, Tura S. High plasma levels of tumor necrosis factor-alpha may be predictive of veno-occlusive disease in bone marrow transplantation. Blood. 1994;83:2385–2386. [PubMed] [Google Scholar]
- Hill GR, Krenger W, Ferrara JL. The role of cytokines in acute graft-versus-host disease. Cytokines Cell. Mol. Ther. 1997;3:257–266. [PubMed] [Google Scholar]
- Hogge DE, Lambie K, Sutherland HJ, Benny WB, Dalal B, Currie C, Barnett MJ, Eaves AC, Eaves CJ. Quantitation of primitive and lineage-committed progenitors in mobilized peripheral blood for prediction of platelet recovery post autologous transplant. Bone Marrow Transplant. 2000;25:589–598. doi: 10.1038/sj.bmt.1702211. [DOI] [PubMed] [Google Scholar]
- Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, Lehmacher W, Ruckdeschel G, Gleixner B, Riedner C. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–1016. [PubMed] [Google Scholar]
- Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- Horton-Deutsch S, O'Haver Day P, Haight R, Babin-Nelson M. Enhancing mental health services to bone marrow transplant recipients through a mindfulness-based therapeutic intervention. Complementary Therapies in Clinical Practice. 2007;13:110–115. doi: 10.1016/j.ctcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Hovi L, Saarinen-Pihkala UM, Taskinen M, Wikstrom AM, Dunkel L. Subnormal androgen levels in young female bone marrow transplant recipients with ovarian dysfunction, chronic GVHD and receiving glucocorticoid therapy. Bone Marrow Transplant. 2004;33:503–508. doi: 10.1038/sj.bmt.1704376. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Phillips KM, Jim HSL, Small BJ, Faul LA, Meade CD, Thompson L, Williams CC, Loftus LS, Fishman M. Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psycho-Oncology. 2012 doi: 10.1002/pon.3122. [DOI] [PubMed] [Google Scholar]
- Kawamura A, Tamaki N, Kokunai T. Effect of dexamethasone on cell proliferation of neuroepithelial tumor cell lines. Neurol. Med. Chir. (Tokyo) 1998;38:633–638. doi: 10.2176/nmc.38.633. discussion 638–40. [DOI] [PubMed] [Google Scholar]
- Klyuchnikov E, Asenova S, Kern W, Kilinc G, Ayuk F, Wiedemann B, Lioznov M, Freiberger P, Zalyalov Y, Zander AR, Kroger N, Bacher U. Post- transplant immune reconstitution after unrelated allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk. Lymphoma. 2010;51:1450–1463. doi: 10.3109/10428194.2010.496015. [DOI] [PubMed] [Google Scholar]
- Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JCY, Arevalo JM, Morizono K, Cole SW. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav. Immun. 2012 doi: 10.1016/j.bbi.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK. Surgical stress promotes tumor growth in ovarian carcinoma. Clin. Cancer Res. 2009;15:2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem LM, van Houwelingen HC, Goulmy E. Serum cytokine levels after HLA-identical bone marrow transplantation. Transplantation. 1998;66:863–871. doi: 10.1097/00007890-199810150-00009. [DOI] [PubMed] [Google Scholar]
- Lucas D, Bruns I, Battista M, Mendez-Ferrer S, Magnon C, Kunisaki Y, Frenette PS. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood. 2012;119:3962–3965. doi: 10.1182/blood-2011-07-367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn RA, Sumar N, Bansal AS, Treleaven J. Cytokine profiles in stem cell transplantation: possible use as a predictor of graft-versus-host disease. Hematology. 2005;10:107–114. doi: 10.1080/10245330400001975. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom. Med. 2011;73:724–730. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Johnsen EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Maestroni GJM. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann. N. Y. Acad. Sci. 2006;917:29–37. doi: 10.1111/j.1749-6632.2000.tb05370.x. [DOI] [PubMed] [Google Scholar]
- Maffei R, Martinelli S, Castelli I, Santachiara R, Zucchini P, Fontana M, Fiorcari S, Bonacorsi G, Ilariucci F, Torelli G, Marasca R. Increased angiogenesis induced by chronic lymphocytic leukemia B cells is mediated by leukemia-derived Ang2 and VEGF. Leuk. Res. 2010;34:312–321. doi: 10.1016/j.leukres.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine- induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- McGregor BA, Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer: A review of stress pathways and biological mediators. Brain Behav. Immun. 2009;23:159–166. doi: 10.1016/j.bbi.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor BA, Syrjala KL, Dolan ED, Langer SL, Redman M. The Effect of Pre-Transplant Distress on Immune Reconstitution among Adult Autologous Hematopoietic Cell Transplantation Patients. Brain Behav. Immun. 2012 doi: 10.1016/j.bbi.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav. Immun. 2005;19:114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of Clinical Oncology. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Klingsporn S, Lund J, Perry EH, Verfaillie C, McGlave P. Large scale ex vivo expansion and activation of human natural killer cells for autologous therapy. Bone Marrow Transplant. 1994;14:555–562. [PubMed] [Google Scholar]
- Nagler A, Or R, Nisman B, Kalickman I, Slavin S, Barak V. Elevated inflammatory cytokine levels in bone marrow graft rejection. Transplantation. 1995;60:943–948. [PubMed] [Google Scholar]
- Nakamura R, Auayporn N, Smith DD, Palmer J, Sun JY, Schriber J, Pullarkat V, Parker P, Rodriguez R, Stein A, Rosenthal J, Wang S, Karanas C, Gaal K, Senitzer D, Forman SJ. Impact of graft cell dose on transplant outcomes following unrelated donor allogeneic peripheral blood stem cell transplantation: higher CD34+ cell doses are associated with decreased relapse rates. Biol. Blood Marrow Transplant. 2008;14:449–457. doi: 10.1016/j.bbmt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin M, Hsu JW, Wingard JR. Quality of life, social challenges, and psychosocial support for long-term survivors after allogeneic hematopoietic stem-cell transplantation. Semin. Hematol. 2012;49:104–109. doi: 10.1053/j.seminhematol.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Nunes DF, Rodriguez AL, da Silva Hoffmann F, Luz C, Braga Filho AP, Muller MC, Bauer ME. Relaxation and guided imagery program in patients with breast cancer undergoing radiotherapy is not associated with neuroimmunomodulatory effects. J. Psychosom. Res. 2007;63:647–655. doi: 10.1016/j.jpsychores.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Palm D, Lang K, Niggemann B, Drell TL, Masur K, Zaenker KS, Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. International journal of cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, Favre G, Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Christian LM, Patidar S, Bishop MM, Dodd SM, Athanason R, Wingard JR, Reddy VS. Spiritual Absence and 1-Year Mortality after Hematopoietic Stem Cell Transplant. Biology of Blood and Marrow Transplantation. 2010;16:1171–1179. doi: 10.1016/j.bbmt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar R, Mathews V, Lakshmi KM, George B, Viswabandya A, Chandy M, Srivastava A. Cellular immune reconstitution and its impact on clinical outcome in children with beta thalassemia major undergoing a matched related myeloablative allogeneic bone marrow transplant. Biol. Blood Marrow Transplant. 2009;15:597–609. doi: 10.1016/j.bbmt.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–123. [PubMed] [Google Scholar]
- Rausch SM, Clark MM, Patten C, Liu H, Felten S, Li Y, Sloan J, Yang P. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116:4103–4113. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini C, Redd WH, Austin J, Mosher CE, Meschian YM, Isola L, Scigliano E, Moskowitz CH, Papadopoulos E, Labay LE, Rowley S, Burkhalter JE, Schetter CD, Duhamel KN. Effectiveness of partner social support predicts enduring psychological distress after hematopoietic stem cell transplantation. J. Consult. Clin. Psychol. 2011;79:64–74. doi: 10.1037/a0022199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowicz M, Ehlers S, Walker D, Decker P, Rundell J, Shinozaki G, Litzow M, Hogan W, Mrazek D, Black JL. Testing a Diathesis-Stress Model: Potential Genetic Risk Factors for Development of Distress in Context of Acute Leukemia Diagnosis and Transplant. Psychosomatics. 2012 doi: 10.1016/j.psym.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Sahler OJ, Hunter BC, Liesveld JL. The effect of using music therapy with relaxation imagery in the management of patients undergoing bone marrow transplantation: a pilot feasibility study. Altern. Ther. Health Med. 2003;9:70–74. [PubMed] [Google Scholar]
- Sasaki T, Akaho R, Sakamaki H, Akiyama H, Yoshino M, Hagiya K, Atsumi M. Mental disturbances during isolation in bone marrow transplant patients with leukemia. Bone Marrow Transplant. 2000;25:315–318. doi: 10.1038/sj.bmt.1702117. [DOI] [PubMed] [Google Scholar]
- Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, Leitman S, Read EJ, Childs R, Barrett AJ. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani B, Mielke S, Adams S, Uribe M, Rezvani K, Yong A, Zeilah J, Kurlander R, Srinivasan R, Childs R. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behavior and Immunity. 2009 doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Silveira EM, Rodrigues MF, Krause MS, Vianna DR, Almeida BS, Rossato JS, Oliveira LP, Jr, Curi R, de Bittencourt PI., Jr Acute exercise stimulates macrophage function: possible role of NF-kappaB pathways. Cell Biochem. Funct. 2007;25:63–73. doi: 10.1002/cbf.1365. [DOI] [PubMed] [Google Scholar]
- Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E. Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv. Cancer Res. 2008;101:127–248. doi: 10.1016/S0065-230X(08)00406-5. [DOI] [PubMed] [Google Scholar]
- Souza LN, Carneiro MA, de Azevedo WM, Gomez RS. Vascular endothelial growth factor (VEGF) and chronic graft-versus-host disease (cGVHD) in salivary glands of bone marrow transplant (BMT) recipients. J. Oral Pathol. Med. 2004;33:13–16. doi: 10.1111/j.1600-0714.2004.00035.x. [DOI] [PubMed] [Google Scholar]
- Starkweather AR. The effects of exercise on perceived stress and IL-6 levels among older adults. Biol. Res. Nurs. 2007;8:186–194. doi: 10.1177/1099800406295990. [DOI] [PubMed] [Google Scholar]
- Steingrimsdottir H, Gruber A, BjOrkholm M, Svensson A, Hansson M. Immune reconstitution after autologous hematopoietic stem cell transplantation in relation to underlying disease, type of high-dose therapy and infectious complications. Haematologica. 2000;85:832. [PubMed] [Google Scholar]
- Sucak GT, Aki SZ, Yuzbasioglu B, Akyurek N, Yagci M, Bagriacik U, Haznedar R. Prognostic value of bone marrow microvessel density and angiogenic cytokines in patients with multiple myeloma undergoing autologous stem cell transplant. Leuk. Lymphoma. 2011;52:1281–1289. doi: 10.3109/10428194.2011.569695. [DOI] [PubMed] [Google Scholar]
- Sullivan AK, Szkrumelak N, Hoffman LH. Psychological risk factors and early complications after bone marrow transplantation in adults. Bone Marrow Transplant. 1999;24:1109–1120. doi: 10.1038/sj.bmt.1702028. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, Lee BN, Giralt SA. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KJ, Booth-Jones M, Jacobsen PB. Do coping and social support predict depression and anxiety in patients undergoing hematopoietic stem cell transplantation? J. Psychosoc. Oncol. 2009;27:297–315. doi: 10.1080/07347330902978947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav. Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Del Ben KS, Rehm KE, Marshall GD., Jr Effects of acute stress-induced immunomodulation on TH1/TH2 cytokine and catecholamine receptor expression in human peripheral blood cells. Neuropsychobiology. 2012;65:12–19. doi: 10.1159/000328160. [DOI] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rao F, Pablo Miramontes-Gonzalez J, Hightower CM, Vaught B, Chen Y, Greenwood TA, Schork AJ, Wang L, Mahata M, Stridsberg M, Khandrika S, Biswas N, Fung MM, Waalen J, Middelberg RP, Heath AC, Montgomery GW, Martin NG, Whitfield JB, Baker DG, Schork NJ, Nievergelt CM, O'Connor DT. Neuropeptide Y (NPY): Genetic Variation in the Human Promoter Alters Glucocorticoid Signaling, Yielding Increased NPY Secretion and Stress Responses. J. Am. Coll. Cardiol. 2012;60:1678–1689. doi: 10.1016/j.jacc.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H. Viruses in human cancers. Science. 1991;254:1167. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]