Abstract
Women are particularly more vulnerable to tobacco use than men. This review proposes a unifying hypothesis that females experience greater rewarding effects of nicotine and more intense stress produced by withdrawal than males. We also provide a neural framework whereby estrogen promotes greater rewarding effects of nicotine in females via enhanced dopamine release in the nucleus accumbens (NAcc). During withdrawal, we suggest that corticotropin-releasing factor (CRF) stress systems are sensitized and promote a greater suppression of dopamine release in the NAcc of females versus males. Taken together, females display enhanced nicotine reward via estrogen and amplified effects of withdrawal via stress systems. Although this framework focuses on sex differences in adult rats, it is also applied to adolescent females who display enhanced rewarding effects of nicotine, but reduced effects of withdrawal from this drug. Since females experience strong rewarding effects of nicotine, a clinical implication of our hypothesis is that specific strategies to prevent smoking initiation among females are critical. Also, anxiolytic medications may be more effective in females that experience intense stress during withdrawal. Furthermore, medications that target withdrawal should not be applied in a unilateral manner across age and sex, given that nicotine withdrawal is lower during adolescence. This review highlights key factors that promote nicotine use in females, and future studies on sex-dependent interactions of stress and reward systems are needed to test our mechanistic hypotheses. Future studies in this area will have important translational value toward reducing health disparities produced by nicotine use in females.
Keywords: sex, tobacco, adolescent, withdrawal, dependence, reward
1. Introduction
Tobacco use is the number one cause of preventable deaths in the United States (Center for Disease Control and Prevention, 2011). Epidemiological studies have provided important advances with regard to which populations are especially vulnerable to tobacco use. However, very little is known about the factors that lead to higher rates of tobacco use in these vulnerable populations.
The overarching goal of our laboratory is to understand the factors that contribute to tobacco use among vulnerable populations. Five years ago, we described a psychobiological hypothesis of adolescent nicotine use for the 35th anniversary of NIDA issue of this journal (O’Dell, 2009). Our hypothesis was that nicotine use in adolescence is driven by strong rewarding effects of nicotine that are inadequately balanced by the negative effects of withdrawal from this drug. This hypothesis was derived largely from rodent studies showing that the rewarding effects of nicotine are higher, whereas the aversive effects of withdrawal are lower in adolescent versus adult rats. Subsequent studies in our laboratory revealed that age differences produced by nicotine withdrawal are mediated via dopaminergic mechanisms in the mesocorticolimbic pathway (Natividad et al., 2010; 2012). This review reflects an extension of our adolescent work to a new hypothesis that describes the interaction of biological systems, including dopamine, that are believed to contribute to nicotine use in females.
Our working hypothesis is that nicotine use in females is driven by stronger positive effects of nicotine and more intense stress produced by withdrawal from this drug as compared to males. This hypothesis was derived from pre-clinical studies showing that the rewarding effects of nicotine are enhanced in female versus male rodents (see Carroll et al., 2009; Perkins et al., 1999; Pogun and Yararbas, 2009). Also, the short-term aversive effects of nicotine are lower in female versus male rats (Torres et al., 2009). The overall result is that females may seek nicotine because of strong positive effects that are unopposed by short-term aversive effects. We recently observed that nicotine withdrawal produces more intense anxiety-like behavior and stronger biological responses within stress systems in females as compared to males. Thus, females may also seek nicotine because of intense negative affective states that emerge during withdrawal that promote relapse behavior compared to males.
This review also includes a mechanistic hypothesis that describes enhanced nicotine use among females in the context of hormone and stress regulation of mesocorticolimbic dopamine systems. Our hypothesis is that physiological substrates implicated in the rewarding effects of nicotine (dopamine) as well as those that are recruited during withdrawal from this drug (stress systems) promote nicotine use to a greater extent in females than males. Specifically, the enhanced drive to use nicotine in females is mediated, at least in part, by estrogen that facilitates dopamine release in terminal regions of the mesocorticolimbic pathway. During withdrawal, the drive to use nicotine is intensified by the emergence of stress that is stronger in females than males.
Lastly, this review considers the clinical implications of our hypothesis describing nicotine use in females. First, the finding that females experience strong rewarding effects of nicotine suggests that strategies to prevent smoking initiation in females are critical. Second, following chronic nicotine use, females display intense stress during nicotine withdrawal that likely promotes relapse behavior during abstinence. Thus, cessation medications that alleviate stress produced by nicotine withdrawal may be more effective in females versus males. Third, recent studies in our laboratory have shown that nicotine withdrawal is lower in adolescent versus adult females. Thus, medications that target withdrawal should not be applied in a unilateral manner across age and sex.
2. Clinical research
It is widely accepted that the motivational properties of tobacco are due, in large part, to the presence of nicotine (see Benowitz, 2010; Picciotto and Kenny, 2013). Nicotine use is motivated by the positive effects of this drug and avoiding negative affective states induced by withdrawal from long-term exposure to nicotine. We hypothesize that in females, strong positive effects of nicotine fuel this balance to a greater extent than males. In addition, we postulate that females experience more intense negative affective states during nicotine withdrawal than males. Thus, nicotine use in females is motivated by the avoidance of intense withdrawal states that promote relapse behavior during abstinence. Below clinical evidence is provided that supports our hypothesis regarding enhanced vulnerability to nicotine use in females.
2.1. Enhanced rewarding effects of nicotine in females
Clinical studies suggest that women are more sensitive to the rewarding effects of nicotine than men (see Greenfield et al., 2010; Mendelsohn, 2011; Perkins et al., 2009; Pogun and Yararbas, 2009; Van Voorhees et al., 2012). Women consume more tobacco products and have a harder time quitting smoking than males (Perkins et al., 1999; Zilberman et al., 2003). Moreover, self-reports of positive mood effects following cigarette use are higher in women relative to men smokers (Perkins et al., 2006). In addition, women that use tobacco regularly display higher rates of responding for smoking-related cues than men (Perkins et al., 1999; 2001).
Epidemiological studies have shown that young females are especially vulnerable to nicotine use. Adolescent females are more likely to initiate smoking and are less likely to quit than adolescent males (Anderson and Burns, 2000; James-Walke et al., 2007; Perkins, 2001). External factors also appear to contribute to nicotine use in adolescent females, including appetite suppression and a desire to project a more appealing self-image (Seguire and Chalmers, 2000). The recent rise in smoking initiation among female teenagers is believed to explain the slower decline in smoking prevalence rates in women relative to men, a phenomenon that has been referred to as the telescoping effect (Zilberman et al., 2003). In summary, these studies suggest that nicotine is more reinforcing in females relative to males regardless of age.
2.2. Enhanced aversive effects of nicotine withdrawal in females
Clinical studies have shown that women are also more sensitive to the aversive effects of nicotine withdrawal. For example, women exhibit lower rates of quitting and are less likely to benefit from tobacco cessation therapies than men (Cepeda-Benito et al., 2004; Cropsey et al., 2008; Hammond, 2009; Perkins et al., 2001; Perkins and Scott, 2008; Piper et al., 2010; Schnoll et al., 2007). During smoking abstinence, women report greater levels of anxiety, depression, and stress than men (Perkins and Scott, 2008; Schnoll et al., 2007; Xu et al., 2008). Women also report less relief from negative affective states with nicotine replacement therapy than men (Perkins et al., 2001). During abstinence from smoking, women also display higher levels of cortisol (a biological marker of stress in humans) as compared to men (Hogle and Curtin, 2006). Lastly, women report more often than men that the anxiety-reducing effects of cigarettes are the main reason for continued smoking and relapse (Perkins and Scott, 2008; Perkins et al., 2012; 2013; Piper et al., 2010). Adolescent females also report higher levels of stress and depression during nicotine abstinence as compared adolescent males (Colby et al., 2000; Nichter et al., 1997). Taken together, these studies suggest that intense stress experienced during nicotine withdrawal may be a significant factor contributing to relapse behavior in females.
On a related note, much work has illustrated that women use nicotine to cope with anxiety to a larger extent than men (Perkins, 2009; Perkins et al., 2012; Piper et al., 2010). In fact, nicotine has been shown to decrease anxiety elicited by a moderate stressor in women (File et al., 2001). Interestingly, the latter report demonstrated that nicotine increased anxiety following presentation of the moderate stressor in men. There is also evidence that pre-existing stress disorders lead to nicotine use in females. For example, there is a stronger co-morbid association between anxiety disorders and smoking rates in women than men (Mykletun et al., 2008). In addition, women with a prior history of an anxiety disorder are more likely to develop nicotine dependence later in life than men (Brook et al., 2012). These studies suggest that stress may be a more critical factor contributing to nicotine use and relapse in females as compared to males.
2.3. Other factors that contribute to nicotine use in females
There are a variety of external factors that may also contribute to nicotine use in females. For example, social factors such as having a friend who smokes contributes to smoking initiation in women (Holahan et al., 2012). Hunger suppression and weight control have also been shown to play an important role in smoking initiation among women. In fact, women consider nicotine use as a tool to control appetite and weight gain more than men (French et al., 1995; Meyers et al., 1997; Mendelson et al., 2011; Torchalla et al., 2012). This is consistent with the finding that adolescent females with a stronger desire to be thin are more likely to use nicotine later in life (Austin and Gortmaker, 2001). Lastly, one might argue that nicotine use is higher in females than males given that the marketing of tobacco products targets women. This is based on findings showing that cigarette packages with bright colors are rated as more attractive and associated with positive attributes such as glamour and sophistication among women compared to men (Doxey and Hammond, 2011; White et al., 2012). An online survey also showed that women rate tobacco products with female-oriented packaging as having more appeal, style, and glamour (Hammond et al., 2011). These findings highlight the importance of regulating marketing strategies that target young women, especially given that females initiate smoking to increase sociability, attractiveness and sophistication (Kaufman and Augustson, 2008; Oh et al., 2011).
2.4. Implications for extrapolating from clinical findings
A fundamental assumption in the review is that females are more susceptible to tobacco addiction than males. Our assessment of the literature is based on data collected in the US and other developed nations. It is recognized; however, that epidemiological evidence from around the world suggests that the prevalence of tobacco use is higher in men as compared to women (Ernster et al., 2000; Mackay, 1996; Slama, 2008). This discrepancy is likely related to where the data are collected, as smoking prevalence rates in women are higher in developed versus underdeveloped countries (Mackay, 1994). Nonetheless, regardless of nationality, women find it harder to quit smoking than men (Mackay and Amos, 2003). The smoking prevalence rates in developed versus under-developed countries is likely influenced by cultural norms, socio-economic status, access to tobacco products, and marketing strategies that promote tobacco use in women from developed countries. These factors that influence tobacco use prevalence rates should be carefully considered when extrapolating the patterns of sex differences found in clinical findings to the interpretation of pre-clinical studies using animal models.
3. Pre-clinical rodent models of nicotine reward and aversive effects
Rodent models have been widely used to assess the motivational factors that contribute to nicotine use (Le Foll and Goldberg, 2009; O’Dell and Khroyan, 2009). Intravenous self-administration (IVSA) procedures have been widely used to study the reinforcing effects of nicotine (see Caille et al., 2012; Le Foll and Goldberg, 2009; Levin et al., 2010). The IVSA model is based on reinforcement principles in which the behavioral response is strengthened by presentation of nicotine after the operant response is performed. Place-conditioning procedures have also been used to study the rewarding effects of nicotine (Brielmaier et al., 2008; Calcagnetti and Schechter, 1994; LeFoll and Goldberg, 2005). These procedures involve passive administration of drug on one side of a conditioning apparatus and saline on an adjacent side. Following repeated drug-environment pairings, animals are allowed free access to both compartments simultaneously in the absence of drug. The nature of the affective properties of the drug is evident on the test day when the environmental cues elicit either conditioned place preference (CPP) or aversion (CPA) to the drug-paired side relative to the neutral side. This is a major advantage of place-conditioning procedures, as the short-term rewarding and aversive effects of nicotine can be assessed in the same study.
Nicotine withdrawal has been widely studied in rats using chronic nicotine administration via subcutaneous osmotic mini-pumps for 5–7 days (Kenny and Markou, 2001; Malin, 2001). Withdrawal from nicotine is produced via removal of the nicotine pump (spontaneous withdrawal) or administration of a nicotinic receptor antagonist (precipitated withdrawal). Using either spontaneous or precipitated methods, nicotine withdrawal produces a behavioral profile comprised of both physical and affective components. The physical signs of nicotine withdrawal in male adult rats include abdominal constrictions, facial fasciculation, writhes, gasps, eye blinks, and ptosis (O’Dell et al., 2004; Shram et al., 2008; Watkins et al., 2000).
The affective properties of nicotine withdrawal have been assessed in procedures that measure anxiety-like behavior (for a review see Bruijnzeel et al., 2012). When rodents experience stress, they spend more time in the enclosed arms of an elevated plus maze or the peripheral areas of an open field as compared to control rats. Previous studies have demonstrated that male adult rats display an increase in time spent in the enclosed arms of the elevated plus maze following precipitated (Tejeda et al., 2012; Wilmouth and Spear, 2006) and spontaneous (Chae et al., 2007; Irvine et al., 2001; Jonkman et al., 2008) withdrawal from nicotine. Male adult rats experiencing nicotine withdrawal also display an increase in the amount of time spent in the peripheral areas of an open field compared to controls (Tzavara et al., 2002). The effects of nicotine withdrawal on anxiety-like behavior in male rodents have also been assessed in the defensive burying paradigm (George et al., 2007), the light/dark exploration test (Jonkman et al., 2005; Stoker et al., 2008) and the startle-response test (Helton et al., 1993).
The negative affective properties of nicotine withdrawal have also been assessed using intracranial self-stimulation procedures (see Malin and Goyarzu, 2009; Paterson and Markou, 2007). Withdrawal from chronic nicotine produces an increase in the brain reward threshold that is thought to reflect a decrease in brain reward function in male adult rats (Bauzo and Bruijnzeel, 2012; Epping-Jordan et al., 1998; Panagis et al., 2000). The need for higher current levels is believed to reflect a decrease in brain reward function during withdrawal. The affective properties of nicotine withdrawal have been assessed using CPA procedures. During conditioning, nicotine-treated animals receive a nicotinic receptor antagonist to precipitate withdrawal in one side of the apparatus. On alternating days they receive saline in the other compartment. Following conditioning, nicotine-dependent male adult rats display a CPA for the compartment where they experienced withdrawal (Ise et al., 2000; O’Dell et al., 2007; Suzuki et al., 1996; Watkins et al., 2000). Collectively, these studies demonstrate that nicotine withdrawal induces negative affective states in rodents.
There are several issues to consider when examining the effects of nicotine in female rodents in the models described above. First, ovarian hormones such as estrogen and progesterone fluctuate across the 4-day estrous cycle in adult female rodents (see McCarthy et al., 2012). The fluctuation of hormones levels may influence the behavioral outcome assessed by these rodent models. To address this issue, some studies test female adult rats during each phase of the estrous cycle. However, this approach quadruples the size of the study. Another approach involves ovariectomy (OVX) procedures and testing the female rats following replacement of key ovarian hormones, such as estrogen and/or progesterone. Special consideration must be taken when employing OVX procedures in rodent models of motivational behavior, given that OVX procedures alter anxiety-like behavior (Pandaranandaka et al., 2006) and suppress dopamine systems (Bosse and Di Paolo, 1996; Garcia-Munoz et al., 1989; Morissette and Di Paolo, 1993; Russo et al., 2003). Nicotine has also been shown to facilitate learning tasks to a greater extent in females as compared to males (Taylor and Maloney, 2010). A final issue to consider is that female rats have a higher fat/muscle ratio than males, which may influence the absorption, elimination and metabolism of nicotine. These issues should be considered when comparing sex differences in rodent models of nicotine use.
4. Pre-clinical research on the short-term effects of nicotine in females
When examining the abuse liability of nicotine, one must consider that this drug produces initial short-term effects that likely influence whether or not the drug is used in the future. This is particularly important with regards to nicotine, as nicotine produces both positive and negative short-term effects.
4.1. Enhanced rewarding effects of nicotine in females
Similar to the clinical research reviewed above, several lines of pre-clinical evidence suggest that the rewarding effects of nicotine are enhanced in female versus male rodents (see Carroll et al., 2009; Perkins et al., 1999; Pogun and Yararbas, 2009). One of the first major studies in this area demonstrated that female adult rats display faster acquisition rates of nicotine IVSA at lower doses than males (Donny et al., 2000). This study also showed that adult females reach a higher break point for nicotine infusions on a progressive ratio (PR) schedule of reinforcement than males. Subsequent work in the same laboratory demonstrated that female rats display twice as much nicotine intake in the presence of a visual stimulus as compared to males (Chaudhri et al., 2005). In contrast to these reports, another recent study demonstrated that female and male rats display similar levels of nicotine intake, extinction, and stress-induced reinstatement of drug seeking-behavior (Feltenstein et al., 2011). The authors suggest that the lack of sex differences in their study may be related to lower reinforcement requirements as compared to previous reports. Another report showed that nicotine intake is more robust in female rats, as they shown higher and more stable levels of oral nicotine intake than males (Nesil et al., 2011). The latter study also showed that female rats display greater weight suppressant effects of nicotine than males.
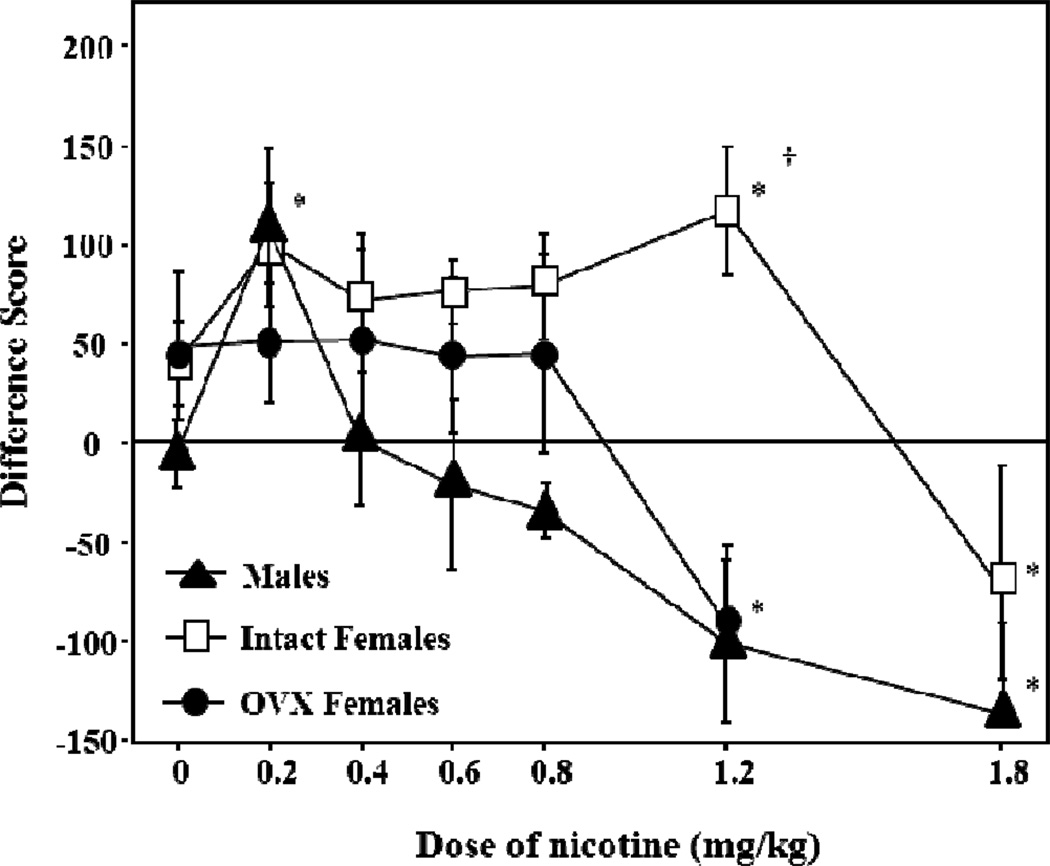
Female rodents also display greater rewarding effects of nicotine than males in studies employing place-conditioning procedures. Work in our laboratory demonstrated that female rats display CPP that is larger in magnitude and observed across a wider range of nicotine doses as compared to males (see Figure 1; Torres et al., 2009). The latter study also revealed that OVX females did not display CPP produced by nicotine. These results suggest that ovarian hormones facilitate the rewarding effects of nicotine. Our results are consistent with the finding that nicotine produces CPP in female mice that is larger in magnitude as compared to males (Kota et al., 2008). However, we also acknowledge a report showing that nicotine-induced CPP is larger in male versus female rats (Yararbas et al., 2010). The discrepancy in these reports may be related to differences in the rat strain and/or conditioning parameters. In general, the IVSA and CPP studies provide support for the hypothesis that the rewarding effects of nicotine are enhanced in female versus male rodents.
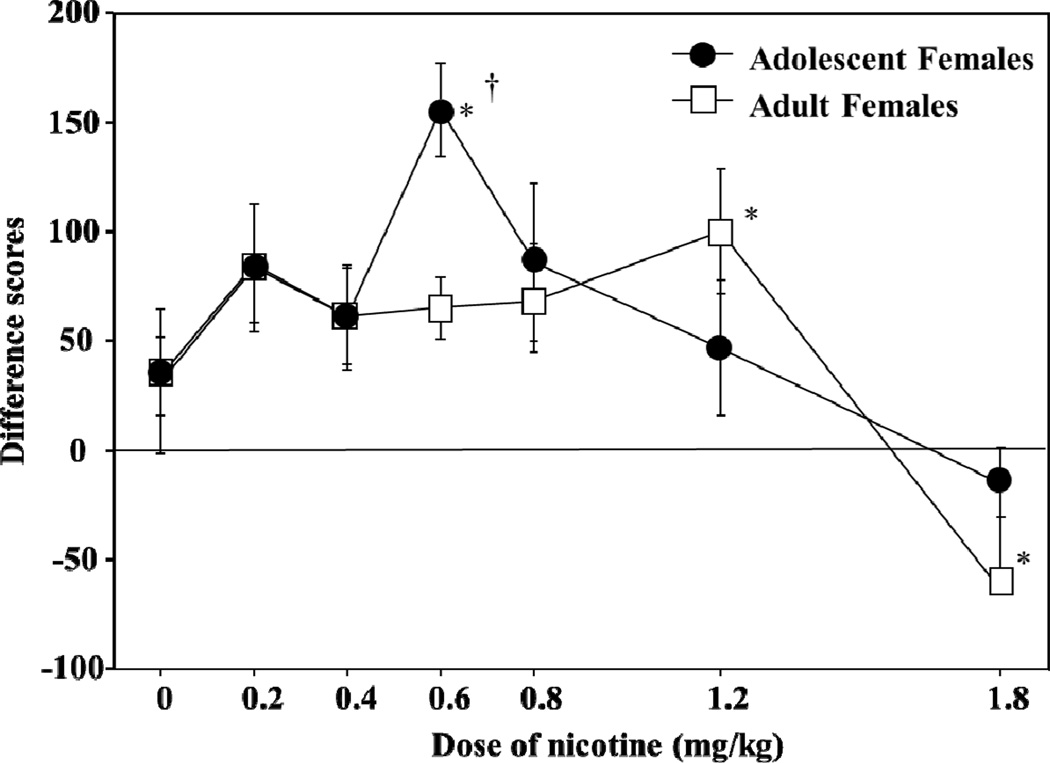
Fig. 1. Enhanced rewarding and reduced aversive effects of nicotine in female versus male rats.
This graph reflects place conditioning produced by various doses of nicotine (0, 0.2, 0.4, 0.6, 0.8, 1.2, or 1.8 mg/kg, base, sc) in adult male, intact female and OVX female rats. These data are presented as difference scores (±SEM), which reflect time spent in the initially non-preferred side after conditioning minus before conditioning such that values above “0” reflect a positive shift in preference (i.e., CPP); whereas, values below “0” represent a negative shift in preference (i.e., CPA). The asterisks denote a significant difference from their respective saline controls (p≤0.05) and daggers denote a significant difference between treatment groups (p≤0.05). These data were previously published in Torres et al. (2008).
4.2. Reduced aversive effects of nicotine in females
Emerging lines of evidence suggest that the aversive effects of nicotine are lower in female rodents. Work in our laboratory has shown that high doses of nicotine produce CPA in male rats; however, the magnitude of this effect is lower in females (Figure 1; Torres et al., 2009). Interestingly, the aversive effects of nicotine were similar in male and OVX female rats. This suggests that ovarian hormones protect against the aversive effects of nicotine. Sex differences in the aversive effects nicotine have also been studied using conditioned taste aversion (CTA) procedures. In contrast to the sex differences observed in CPA studies, a study using CTA procedures demonstrated that female and male rats display a similar aversion to a flavor that was previously paired with a high dose of nicotine (Rinker et al., 2008). More work may be needed to resolve the discrepancy between studies comparing the aversive effects of nicotine with different behavioral procedures in female and male rats.
5. Pre-clinical research on the long-term effects of nicotine in females
Two relevant issues arise when considering the effects of long-term nicotine exposure. First, chronic nicotine exposure produces neuroendocrine effects that alter stress systems. Second, following the removal of chronic nicotine exposure, a withdrawal syndrome emerges that shows clear negative effects, both physical and affective. Both of these long-term effects of nicotine are relevant to our hypothesis regarding greater nicotine use in females.
5.1. Enhanced stress produced by repeated nicotine administration in females
Behavioral studies have demonstrated that females are more sensitive to the effects of nicotine exposure on anxiety-like behavior. For example, female mice display more anxiety-like behavior compared to their male counterparts following chronic oral nicotine intake (Caldarone et al., 2008). Female rats also display a greater increase in nicotine IVSA following a pharmacological stressor as compared to males (Li et al., 2012). The authors of the latter report suggest that females are more sensitive to the effects of stress on nicotine intake as compared to males. Consistent with this, a recent report showed that chronic administration of nicotine in combination with immobilization stress produced a larger decrease in feeding and body weight in females as compared to males (Faraday et al., 2005).
The main neuroendocrine substrate of the stress response is the hypothalamic-pituitary-adrenal (HPA) axis (for a review see Vale et al., 1981). When a stressor is experienced, corticotropin-releasing factor (CRF) is secreted from the hypothalamus and stimulates adrenocorticotropic hormone (ACTH) release from the pituitary gland. ACTH then simulates the release of corticosterone and other glucocorticoids from the adrenal cortex. Corticosterone serves as a major negative feedback signal that terminates HPA axis activity. Within the hypothalamus, corticosterone binds to nuclear glucocorticoid receptor II subunits causing an inhibition of CRF mRNA synthesis.
Studies comparing biological indices of stress are consistent with behavioral reports. For example, plasma corticosterone levels are increased to a greater extent in female versus male rats following repeated injections (Gentile et al., 2011; Moidel et al., 2006; Rhodes et al., 2001; 2004; Skwara et al., 2012) and continuous delivery (Faraday et al., 2005) of nicotine. In an in vitro perfusion system, the presence of nicotine increased CRF and ACTH levels to a greater extent in hypothalamic tissue that was collected from female versus male rats (Mcklveen et al., 2010; Moidel et al., 2006). Overall, these studies suggest that the behavioral and biological effects of nicotine on stress systems are greater in females as compared to males.
5.2. Enhanced effects of nicotine withdrawal in females
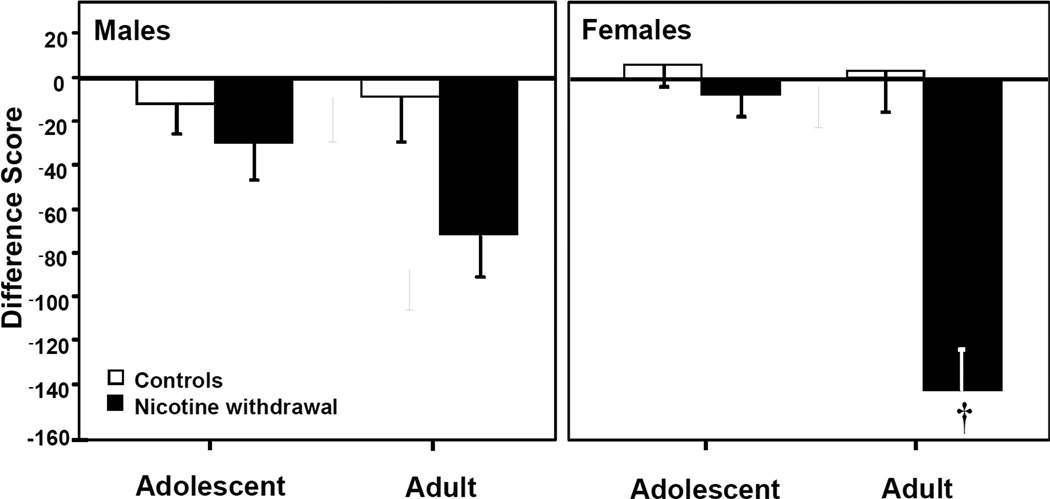
Pre-clinical studies have shown that the behavioral effects of nicotine withdrawal are larger in female versus male rats. Although few studies have compared sex differences in the physical signs of withdrawal, one report showed that female adult rats display more physical signs of nicotine withdrawal relative to males (Hamilton et al., 2009). The affective properties of withdrawal also appear to be higher in females. Recent unpublished observations in our laboratory revealed that the ability of nicotine withdrawal to produce CPA is greater in female versus male rats (Figure 2). These studies suggest that the physical and negative affective properties of nicotine withdrawal are greater in female versus male rats.
Fig. 2. Enhanced negative affective states produced by nicotine withdrawal in female versus male rats.
This graph reflects place aversion produced by nicotine withdrawal in adolescent and adult female and male rats. Rats (n=7–14) were tested for initial preference and were then prepared with saline or nicotine pumps. A dose of nicotine was used that produced equivalent plasma cotinine levels (a metabolite of nicotine) across age and sex treatment groups. After 7 days of nicotine exposure, the rats were conditioned with repeated injections of mecamylamine to precipitate withdrawal in their initially preferred side. On alternate days, they received saline in their initially non-preferred side. Following 8 days of conditioning, the rats were re-tested for any shifts in preference behavior. The data reflect difference scores (±SEM) that were calculated as time spent in the initially preferred side after minus before conditioning, such that negative values reflect the aversive effects of nicotine withdrawal (i.e., CPA). Female rats displayed a significant decrease in time spent in their withdrawal-paired side that was larger than all other treatment groups († p≤0.05).
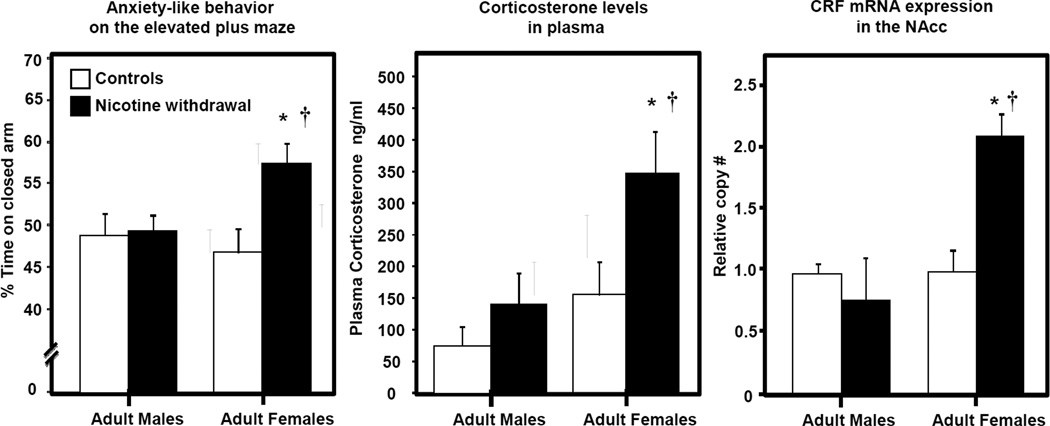
Recent work in our laboratory has compared sex differences in the behavioral and biological markers of stress during nicotine withdrawal (Figure 3). This work has revealed that females display both an increase in anxiety-like behavior and corticosterone levels that are higher as compared to males. Female rats also displayed an increase in CRF gene expression in the nucleus accumbens (NAcc) as compared to male rats experiencing withdrawal from nicotine. This sex difference in CRF gene expression was not observed in the amygdala or hypothalamus of these rats, suggesting that the NAcc plays a central role in modulating sex differences produced by withdrawal. Our behavioral results are consistent with the finding that female adult mice display more anxiety-like behavior on the elevated plus maze during nicotine withdrawal as compared to males (Kota et al., 2007; 2008). Similarly, Caldarone et al. (2008) showed that female mice display a higher level of anxiety-like behavior following chronic oral nicotine intake as compared to males. Two recent reports also showed that adult female rats display higher plasma corticosterone and ACTH levels during nicotine withdrawal as compared to males (Gentile et al., 2011; Skwara et al., 2012). Taken together, there is strong pre-clinical evidence to suggest that females experience greater stress during nicotine withdrawal as compared to males.
Fig. 3. Enhanced behavioral and biological indices of stress produced by nicotine withdrawal in adult female versus male rats.
This graph reflects anxiety-like behavior, plasma corticosterone levels and changes in CRF gene expression in the NAcc during spontaneous withdrawal from nicotine in adult female and male rats (n=6–8). Control rats were not exposed to nicotine and received a sham surgery. All nicotine-treated rats displayed similar plasma cotinine levels (a metabolite of nicotine) prior to the removal of nicotine, suggesting that our effects are not confounded by sex differences in nicotine metabolism. The asterisks (*) denote a significant difference from their respective sham controls and the dagger (†) denotes a significant difference between males and females (p≤0.05). These data are under review for publication in another journal.
The pre-clinical evidence described above may elucidate some of the factors that contribute to nicotine use in humans. Namely, we hypothesize that the strong rewarding effects of nicotine and robust negative affective states that emerge during nicotine withdrawal both contribute to greater nicotine use in females as compared to males. Our hypothesis relies on strong positive effects of nicotine that promote initial use and activate stress systems during withdrawal to a larger extent in females versus males. Over time, intense stress produced by withdrawal may contribute to a higher incidence of relapse behavior in women.
6. Opponent-process theory as a framework for nicotine use in females
Organisms are equipped with homeostatic mechanisms that regulate internal parameters within upper and lower limits. Opponent processes correct for deviations outside of normal parameters to maintain stability. These adaptive responses oppose the disruption in homeostasis, and are therefore referred to as opponent processes. Organisms are also equipped with opponent processes that limit the excessive activation of brain reward systems. This is adaptive to limit an organism from being continuously immersed in activities that activate reward systems, such as eating or sexual behavior.
The opponent-process theory of motivation was originally applied to tobacco use by Solomon and Corbit in (1973). These researchers suggested that tobacco usurps opponent processes that modulate motivated behavior. More recent extensions of this theory postulate that nicotine produces strong motivational effects via activation of brain reward systems that are opposed by stress systems. Following chronic nicotine use, Koob and LeMoal, (2001) suggest that the opponent processes that limit reward function fail to return to a normal homeostatic range. These changes are counteradaptive because they reflect a chronic deviation from homeostasis in which reward is regulated around a drug-modified set point outside of the normal limits. This alteration is referred to as allostasis, which represents a chronic deviation of the reward set point that causes an enhanced state of vulnerability and a loss of control over drug use. The term allostatic load refers to the excessive demand on the organism caused by defending a set point that is outside of a normal range.
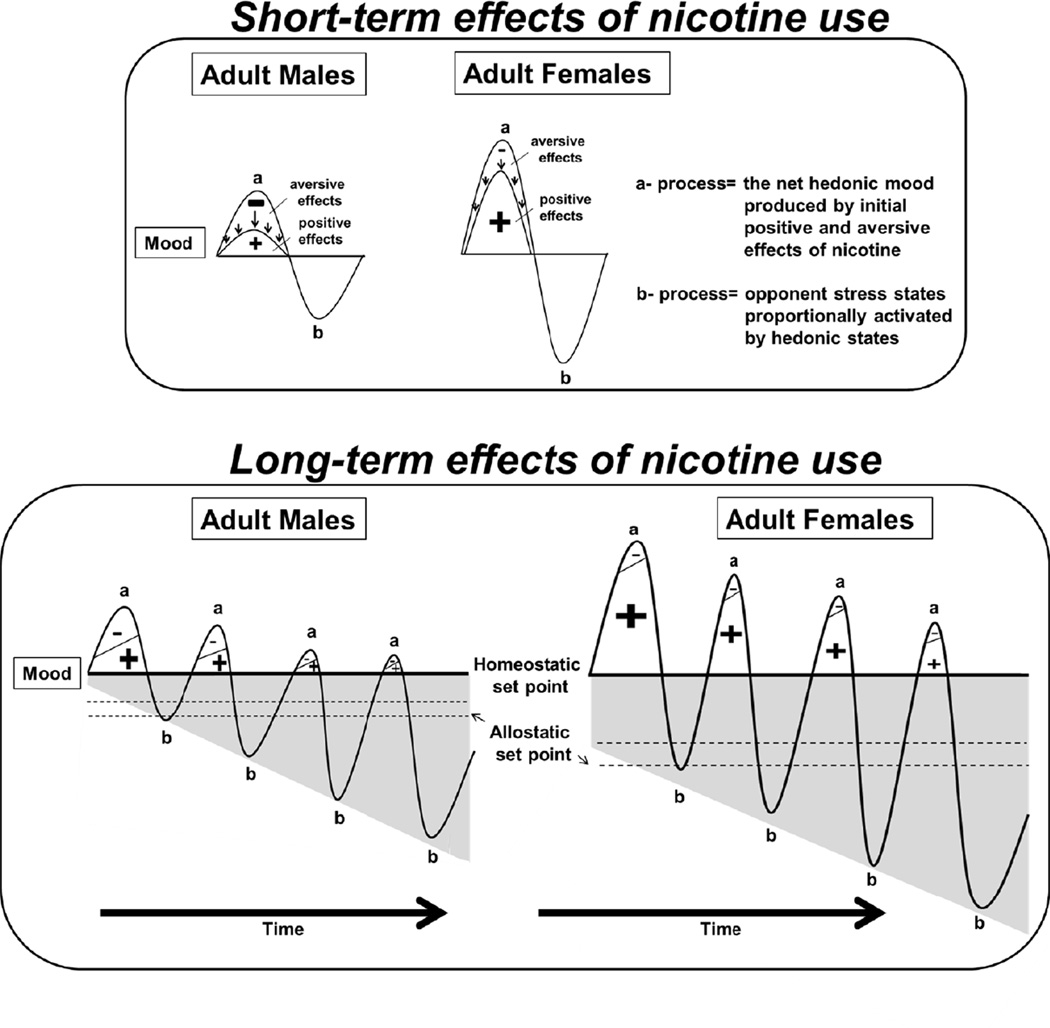
The opponent-process theory of addiction postulates that there are two opposing motivational processes (George et al., 2012). The short- and long-term effects of nicotine use are illustrated in Figure 4. The a-process has a quick onset but quick offset, and it decreases with repeated drug exposure. Nicotine produces short-term rewarding effects as well as initial aversive effects. Thus, we propose that the a-process reflects the cumulative mood state that is the net result of the initial positive effects of nicotine minus any aversive effects. Given that nicotine has short-term aversive effects, the intake of this drug is carefully titrated in order to achieve positive mood states. Our interpretation of the a-process may be unique to nicotine, given that other drugs of abuse produce less short-term aversive effects and typically used in a binge-like pattern. Thus, a female that experiences primarily strong rewarding effects of nicotine would display a larger positive hedonic state (larger a-process) as compared to males that experience more initial aversive effects of this drug.
Fig. 4. Opponent-process theory of nicotine use in females.
The diagrams denote changes in mood states induced by short-term (top) and long-term (bottom) nicotine use. Our hypothesis regarding enhanced vulnerability to nicotine use in females is derived from the opponent-process theory of addiction that was developed by Koob and Le Moal (2001). These researchers hypothesize that there are two processes that influence the development of drug dependence. These a- and b-processes oppose each other and change over the course of long-term drug use. Following initial nicotine use, the net hedonic state (a-process) induced by nicotine reflects the initial positive effects of nicotine minus any aversive effects. We hypothesize that females experience greater positive mood states (denoted with a larger plus sign) due to stronger rewarding effects of nicotine and minimal initial aversive effects of this drug as compared to males. The b-process reflects the activation of stress systems that oppose the initial hedonic state elicited by nicotine. Furthermore, the magnitude of the a-process determines the extent to which the opponent b-process is recruited. Thus, we hypothesize that because females experience greater initial hedonic responses to nicotine, they will also experience greater recruitment of opponent stress systems than males. Koob and Le Moal also postulate that following initial drug use, with no prior drug history; ample time is provided for the a- and b-processes to return to a normal homeostatic set point. However, repeated drug use produces a dysregulation in the opponent processes that maintain homeostasis, as reflected by the downward shift in the homeostatic set point to a new allostatic set point. We suggest that chronic nicotine exposure produces a greater sensitization of stress systems in females than males. Thus, during nicotine withdrawal, the cumulative stress effects lower the allostatic set point to a greater extent in females than males. As a result, long-term nicotine use in females is driven to a larger extent by avoidance of intense stress states produced by nicotine withdrawal than males.
On the other hand, it is postulated that the b-process opposes the a-process, and appears after the a-process has terminated (Koob and Le Moal, 2001). The b-process is sluggish in onset and becomes sensitized with long-term drug exposure. The b-process involves the recruitment of stress systems, which are activated by positive hedonic states produced by nicotine. The recruitment of stress systems is hypothesized to modulate the behavioral and biochemical consequences of withdrawal from chronic nicotine use, and have been referred to as the dark side of addiction (Koob, 2010). The magnitude of the positive hedonic state is hypothesized to determine the extent to which the ensuing opponent process is recruited to counter the hedonic state. We propose that females display stronger positive effects of nicotine (a-process) that will recruit stress systems (b-process) to a larger extent than males. Thus, long-term nicotine use produces a larger recruitment of opponent systems in females that results in a greater stress response during nicotine withdrawal as compared to males.
The opponent-process theory of addiction has provided a useful framework for studying the underlying factors that contribute to drug dependence. We postulate that there are important differences in the processes that mediate nicotine use in females as compared to what has been described for nicotine dependence in males. A comprehensive hypothesis describing the rapid downward spiral into drug abuse has been described for females (Becker et al., 2012). Here we extend the opponent-process theory of addiction to include a hypothesis of chronic nicotine use in females of different ages.
6.1. Opponent processes mediating the short-term effects of nicotine in females
It is hypothesized that stronger positive mood states (a-process) contribute to higher nicotine use in females than males (Figure 4). This is based on a large body of evidence showing that the rewarding effects of nicotine are enhanced in females as compared to males, as described previously. The stronger rewarding effects of nicotine may promote nicotine use in females during the initiation, maintenance and relapse phases of nicotine use. It is presently unclear whether the positive rewarding effects of nicotine change with repeated exposure in females. Given that the rewarding effects of drugs of abuse generally decrease over time in adult drug users, we denote the change in the a-process as a gradual decrease over time. However, the possibility exists that the rewarding effects of nicotine increase over time in females, but not males. This is based on the finding that adolescent female rats display an increase in nicotine intake as they enter adulthood, an effect that was not observed in males (Levin et al., 2011).
During short-term use, nicotine can also induce aversive effects. Work in our laboratory demonstrated that adult female rats display less aversive effects of nicotine as compared to males (Figure 1). This finding suggests that females display strong rewarding effects of nicotine that are only slightly offset by aversive effects. Thus, the positive mood state produced by nicotine is larger in magnitude in females as compared to males.
6.2. Opponent processes mediating nicotine withdrawal in females
We suggest that females who experience stronger hedonic mood states following nicotine administration also exhibit a larger recruitment of opponent processes than males. During nicotine withdrawal, we postulate that chronic activation of stress systems produces a greater downward shift in the allostatic set point of females versus males (Figure 4). Thus, the chronic activation of stress systems results in a sensitized endocrine response during nicotine withdrawal, that is larger in females relative to males. This hypothesis is based largely on pre-clinical studies showing that the behavioral and neuroendocrine effects of nicotine withdrawal are greater in females versus males, as described previously. In clinical studies, females also report more than males that the anxiety-reducing effects of nicotine are the main reason for relapse behavior (Perkins and Scott, 2008; Perkins et al., 2009; 2012; Piper et al., 2010). Taken together, these studies support our hypothesis that females are more likely to use nicotine because of the strong rewarding effects of nicotine in combination with an intense desire to avoid stress produced withdrawal from this drug.
Below we provide a biochemical hypothesis regarding the neural substrates that mediate nicotine use in females versus males. Although this framework is derived from rodent models, it is intended to provide a possible explanation for why females are different from males with regard to the role of estrogen and stress systems on dopamine transmission in the mesocorticolimbic system.
7. Hypothesized substrates mediating nicotine use in females
It has been suggested that the allostatic state is fueled by a dysregulation in the brain circuits within the extended amygdala, which includes the NAcc (Koob and Volkow, 2010). Changes within these brain structures are believed to modulate the net hedonic effects of nicotine (a-process), as well as changes in counter-adaptive processes that counteract this response (b-process). Recently, Koob and colleagues postulated that within-system changes in a-processes are modulated by the dopamine system, whereas between-system alterations in b-processes are modulated via CRF stress systems (George et al., 2012). Drugs of abuse are postulated to elicit an opposing neutralizing reaction within the same system in which the drug elicits its primary reinforcing actions. Accordingly, nicotine produces an increase in dopamine in the NAcc that is reversed during withdrawal from this drug. Thus, the pattern of changes in dopamine reflects within-system changes. The hedonic states induced by nicotine activate between system changes in CRF systems that sensitize with repeated nicotine exposure. Our hypothesis regarding nicotine use in females is focused on the circuitry within the NAcc that regulates dopamine and CRF release in this region.
7.1. Within-system changes in dopamine
The behavioral effects of nicotine are modulated, in large part, by dopamine transmission in the mesocorticolimbic pathway (Corrigall et al., 1992; Mansvelder and McGehee, 2002). This pathway originates in the ventral tegmental area (VTA) and terminates in the frontal cortex as well as several forebrain structures including the amygdala and NAcc. Behavioral work has also shown that nicotine reward is mediated, in large part, by increasing dopamine levels in the NAcc (Balfour, 2002; Mansvelder et al., 2003). The neurochemical effects of nicotine are reversed during withdrawal from this drug, such that NAcc dopamine levels are decreased (Carboni et al., 2000; Di Chiara et al., 2000; Hildebrand et al., 1998; Rada et al., 2001). Thus, dopamine in the NAcc is critically important to both nicotine reward and withdrawal from this drug.
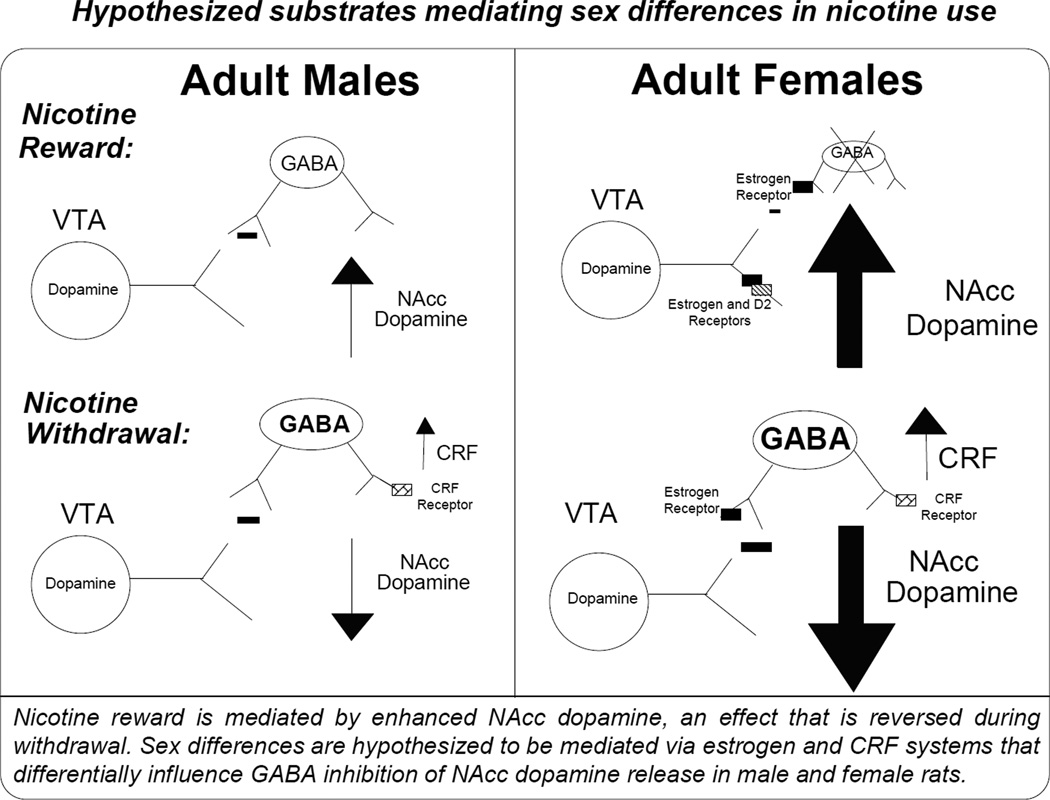
With regard to the rewarding effects of nicotine, an estrogen-based mechanism is offered to explain sex differences in the ability of nicotine to increase dopamine levels in the NAcc based on the work described in (Becker, 1999 and 2012). Estrogen receptors are located on the local terminals of medium spiny striatal neurons that have recurrent collaterals on dopamine terminals (Mermelstein et al., 1996). We suggest that estrogen inhibits the activity of the intrinsic medium spiny striatal neurons that modulate dopamine. Medium spiny striatal neurons are primarily of gamma-aminobutyric acid origin (GABA). Thus, it is speculated that the inhibitory effects of estrogen decrease GABA levels via a reduction of calcium currents. This results in a decreased response to GABA at the dopamine terminals that results in increased stimulated dopamine release in the NAcc.
Another mechanism by which estrogen may facilitate dopamine release in the NAcc is that estrogen may act directly on dopamine terminals to down-regulate D2 auto-receptors (Bazzett et al., 1994). In support of our hypothesis, estrogen has been shown to increase nicotine-induced striatal dopamine levels in female, but not male rats (Dluzen and Anderson, 1997). Consistent with this and most relevant to our hypothesis, Pogun (2001) describes data from his laboratory showing that adult female rats display a greater increase in NAcc dopamine following nicotine administration as compared to males. Taken together, these data suggest that nicotine may produce greater increases in NAcc dopamine in the presence of estrogen. Future studies are needed to fully assess the proposed mechanisms.
With regard to within-system changes during withdrawal, dopamine is decreased in the NAcc of adult male rats experiencing nicotine withdrawal (Carboni et al., 2000; Hildebrand et al., 1998; Rada et al., 2001). Recent work in our laboratory has shown that dopamine levels in the NAcc are reduced to a greater extent in adult versus adolescent rats (Natividad et al., 2010). These data suggest that NAcc dopamine modulates age-dependent differences produced by nicotine withdrawal. Here, we extend our hypothesis regarding the difference between adolescents and adults to sex differences, by suggesting that changes in dopamine systems also modulate enhanced nicotine withdrawal in females. Specifically, we suggest that the changes in dopamine transmission produced by withdrawal in females are potentiated by stress systems within the NAcc, as described below.
7.2. Between-system changes in CRF
Pre-clinical studies have shown that CRF systems modulate the motivational effects of stress produced by nicotine withdrawal (for a review see Bruijnzeel, 2012). Work in this area has focused on the effects of nicotine withdrawal in adult male rats. For example, blockade of CRF-1 receptors reduces the escalation of nicotine IVSA in rodents given extended access to this drug (George et al., 2007). Also, nicotine-withdrawal produces a deficit in brain reward function that was prevented by activation of CRF receptors (Bruijnzeel et al., 2007). Activation of CRF receptors also reverses hyperphagia and weight gain produced by withdrawal from chronic nicotine (Kamdi et al., 2009). Blockade of CRF-1 receptors in NAcc reverses the deficits in brain reward function produced by nicotine withdrawal (Marcinkiewcz et al., 2009). This suggests that CRF systems play a role in modulating stress produced by nicotine withdrawal, and that the NAcc is an important neuronal structure implemented in these effects.
The interaction between CRF and dopamine in the NAcc appears to change over time following as a function of chronic stress. For example, initial activation of CRF systems increases dopamine transmission in this region (Chen et al., 2012; Lemos et al., 2012). This suggests that the relationship between CRF and dopamine in the NAcc is facilitative. However, another report suggests that effects of CRF in the NAcc undergo a dynamic shift following chronic stress. Specifically, intra-NAcc infusions of CRF produce a place preference in naïve rats (Lemos et al., 2012). However, following chronic stress intra-NAcc infusions of CRF produce a place aversion. The later report also showed that the ability of CRF to increase NAcc dopamine is reversed in rats that received chronic stress, an effect that persisted for 90 days after stress exposure. These data suggest that the modulatory role of CRF on dopamine systems within the NAcc is switched such that following chronic stress, CRF undergoes a dynamic change from facilitating hedonic states to inducing negative aversive states.
It is our hypothesis that the shift in the relationship between CRF and dopamine is modulated via GABA systems. Within the NAcc, GABAergic interneurons inhibit dopamine release. We suggest that activation of the CRF −1 receptors results in the increase of GABA levels that inhibit dopamine release in this region. This is based on the finding that activation of CRF −1 receptors increases GABAergic inhibitory post-synaptic potentials in dopamine neurons of the mesocorticolimbic pathway (Beckstead et al., 2009). Thus, chronic exposure to nicotine is expected to enhance the release of CRF. Therefore, we suggest that a greater activation of CRF-1 receptors produces an increase in local GABA levels, which in turn leads to a decrease in dopamine during withdrawal. This hypothesis relies heavily on enhanced GABA levels in the NAcc during withdrawal from nicotine, and future studies are needed to examine the influence of CRF systems on GABA levels in the NAcc during withdrawal. However, there is evidence supporting our hypothesis in cortical terminals of the mesocorticolimbic pathway where GABA levels are increased following chronic CRF administration (Kirby et al., 2008; Sirinathsinghji and Heavens, 1989).
With regard to sex differences during nicotine withdrawal, we suggest the ability of CRF to modulate dopamine systems is disproportionally greater in females. We furthermore propose that sex-dependent differences in CRF modulation of dopamine occur within the local circuits of the NAcc. This is based on recent work in our laboratory showing that changes in CRF gene expression were increased in the NAcc of female versus male rats (Figure 3). There are several ways in which females may be more susceptible to stress during withdrawal. Given that females display less negative feedback inhibition of the CRF systems, nicotine withdrawal may produce a stronger and more sustained activation of stress systems as compared to males. This may involve higher numbers of CRF receptors or a higher ratio of CRF-1 receptor to coupling of G-proteins in female versus male rats (Bangasser et al., 2010). Females also display lower levels of beta-arrestin2, an intracellular protein that internalizes CRF-1 receptors (Bangasser et al., 2010). Thus, females may be more responsive to CRF stimulation due to reduced internalization of CRF-1 receptors. By extension, the female CRF system may be more activated by CRF release during nicotine withdrawal as compared to males.
Another possibility for explaining greater female susceptibility to nicotine use is that estrogen may potentiate the effects of stress produced by nicotine withdrawal in females versus males. Viau et al. (2005) showed that CRF mRNA levels are higher in the hypothalamus of female versus male rats. Also, the highest levels of CRF have been observed during the proestrus phase of the estrous cycle, where estrogen levels are highest (Bohler et al., 1990; Nappi et al., 1997). Direct activation of estrogen-beta receptors (ERβ) also increases CRF mRNA expression in vitro (Chen et al., 2008; Lalmansingh and Uht, 2008; Zhu and Zhou, 2008). The estrogen gene sequence has also been shown to promote CRF gene transcription (Vamvakopoulos and Chrousos, 1993). These studies suggest that estrogen may also potentiate the female response to stress.
Taken together, we suggest that repeated withdrawal from nicotine produces hyper-activation of CRF stress systems in females. This may occur via a sensitization mechanism or an inability to return to normative stress levels. Given that females display a stronger recruitment of CRF systems during withdrawal, these changes represent an even greater allostatic load for females that display heightened stress during withdrawal as compared to males.
To summarize, we suggest that the acute rewarding effects of nicotine are greater in females via estrogen receptors that reduce GABAergic inhibition of dopamine as well as a down-regulation of D2 auto receptors in the NAcc. However, following chronic activation of stress systems by repeated nicotine withdrawal episodes, CRF systems in the NAcc switch from facilitating dopamine to inhibiting dopamine release in the NAcc. We suggest that this change in CRF modulation on dopamine transmission is the result of increased GABA levels within the NAcc. Furthermore, we hypothesize that this effect is larger in females due to a greater activation of GABA via CRF-1 receptors as compared to males. During withdrawal, one might expect that in females estrogen may serve to produce a larger inhibition of GABA. However, we suggest that the larger effect of CRF in females overrides the inhibitory effects of estrogen on GABA within the NAcc. Our hypothesis with regard to GABA is supported by a recent report suggesting that drug reward and aversive effects are modulated via GABAergic medium spiny neurons that tonically inhibit dopamine release in the NAcc (Carlezon and Thomas, 2009).
8. Hypothesis for nicotine use in adolescent females
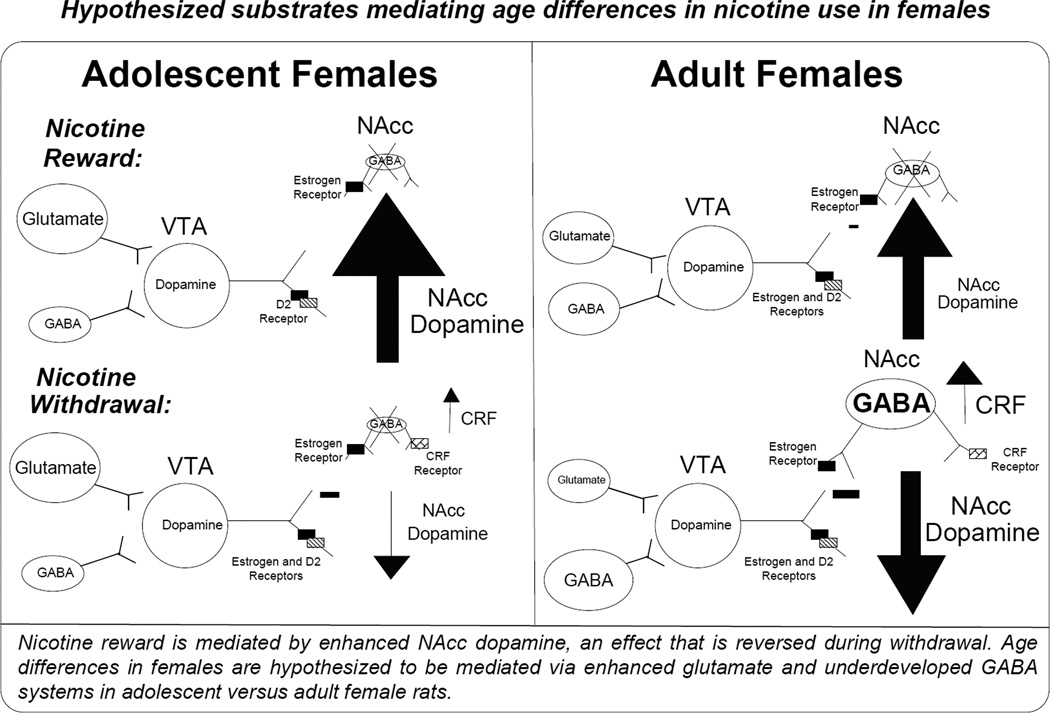
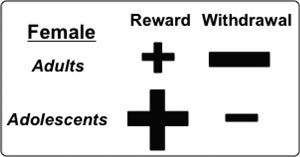
An important question for our research program has been whether adolescent females are particularly more vulnerable to nicotine use because they are both adolescent and female. In general, our assessment has been that adolescent females follow an addiction profile that is more closely akin to what has been described for adolescents as opposed to adult females. With regard to the rewarding effects of nicotine, adolescent females display greater rewarding effects of nicotine than adult females (see Figure 7), consistent with other reports in mice (Kota et al., 2008). With regard to nicotine withdrawal, female adults display aversive effects produced by nicotine withdrawal that are absent in adolescent female rats (Figure 2). In addition, female adults also display behavioral and biological indices of stress produced by nicotine withdrawal that are also lower in female adolescents. These data suggest that adolescent females experience stronger rewarding effects of nicotine (denoted as a larger plus sign), but fewer negative effects of withdrawal (denoted with a smaller minus sign) relative to adult females.
Fig. 7. Hypothesized mechanisms mediating nicotine use in adolescent females.
The VTA is under inhibitory control via GABA and excitatory regulation via glutamate release that regulates dopamine transmission in the NAcc. During nicotine withdrawal, dopamine levels in the NAcc are decreased, an effect that is hypothesized to be related to enhanced GABA and reduced glutamate in the VTA. We hypothesize that adolescent rats will display higher increases in NAcc dopamine following nicotine and fewer changes in this measure during withdrawal. We also hypothesize that these age differences are related to overdeveloped glutamate and underdeveloped GABA systems that modulate dopamine cell bodies in the VTA that project to the NAcc.
Here, we propose a mechanistic hypothesis describing why adolescent females may experience stronger rewarding effects of nicotine and are impervious to the negative effects of withdrawal as compared to adult females. Our hypothesis is that GABA and glutamate systems in the VTA are organized in a manner that protects adolescents from the decreases in dopamine in NAcc produced by nicotine withdrawal in adult rats (Figure 7). Our work with adult male rats demonstrated that nicotine withdrawal produced a decrease in NAcc dopamine that was the result of an increase in GABA levels and a decrease in glutamate levels in the VTA (Natividad et al., 2010; 2012). However, adolescent rats experienced lower withdrawal-related deficits in NAcc dopamine than adults. The lack of changes in dopamine levels appears to be related to an absence of changes in GABA and glutamate in the VTA of adolescent rats. To explain the differences between adolescents and adults, we postulated that excitatory regulation of dopamine is enhanced whereas inhibitory regulation of this pathway is reduced. This is based on previous literature showing that adolescence is characterized by heightened excitatory glutamatergic influence in the cortex (de Graaf-Peters and Hadders-Algra, 2006; McDonald and Johnston, 1990; Dunah et al., 1996; Herlenius and Lagercrantz, 2004) and diminished inhibitory GABAergic influences (Cherubini et al., 1991; Paysan et al., 1994; Yu et al., 2006). Thus, it is possible that this age-related imbalance toward excitation serves to buffer against withdrawal-associated decreases in excitatory drive and enhancements in inhibitory signaling.
Our neurochemical hypothesis regarding the special vulnerability of adolescent females to nicotine use also focuses on the contribution of GABA and glutamate systems in the VTA that regulate dopamine release in the NAcc. With regard to nicotine reward, it is suggested that adolescent females display a greater increase in NAcc dopamine as compared to adults following nicotine administration. We hypothesize that this effect is modulated via enhanced excitatory glutamate and reduced inhibitory GABA release in the VTA following nicotine administration. Our hypothesis regarding the role of glutamate systems in age-dependent differences in nicotine use has been described (Placzek et al., 2009). Also, the ability of estrogen to decrease GABA is greater in adolescents because the GABA system at this stage in development is still underdeveloped. Thus, the net result is that nicotine produces a greater increase in NAcc dopamine as compared to adult females.
With regard to nicotine withdrawal, we postulate that adolescent females will display a smaller decrease in NAcc dopamine than adults. We hypothesize that this effect may be modulated via higher glutamate and reduced GABA release in the VTA of adolescent females, as was shown in adolescent males. Moreover, we suggest that in adult females GABA systems play an intermediary role between CRF and decreases in NAcc dopamine produced by withdrawal. However, adolescent female GABA systems are underdeveloped, which means that the ability of CRF to decrease GABA in the NAcc is reduced. In fact, we recently observed that nicotine withdrawal produced an up-regulation of CRF gene expression in the NAcc of female adult, but not female adolescent rats. Given that CRF mRNA in the NAcc is synthesized in GABA interneurons, we suggest that adolescents displayed fewer changes in CRF gene expression due to underdeveloped GABA systems. The GABA interneurons that control CRF production are absent and/or underdeveloped in adolescent female rats. Thus, adolescents may be less sensitive to the decreases in NAcc dopamine due to enhanced excitation of glutamate in the VTA and reduced GABAergic inhibition of dopamine transmission in the VTA and NAcc. This neurochemical profile is consistent with our behavioral finding that adolescent females are less sensitive to stress produced by nicotine withdrawal than adult females.
9. Do the factors that contribute to nicotine use overlap in females of different ages?
A common finding among adolescent rats of both sexes and female adult rats is that they both display enhanced rewarding effects of nicotine compared to their respective controls. Adolescent and adult female rats also display reduced short-term aversive effects of nicotine as compared to age-matched control males (Torres et al., 2008; 2009). These findings suggest that populations that are susceptible to nicotine use experience strong rewarding effects of nicotine that promote continued nicotine use. Also, a lack of initial aversive effects of nicotine may facilitate intake of higher amounts of nicotine that would also promote dependence on this drug.
However, a major point of divergence in our work with female adolescent and adult rats has been with regard to nicotine withdrawal. Adolescent females are less sensitive to nicotine withdrawal, whereas adult females display intense stress during withdrawal from this drug. In our neurochemical analyses, different biological substrates have emerged as important for modulating age and sex differences produced by nicotine withdrawal. Our work with adolescent rats has demonstrated that GABA and glutamate systems in the VTA play an important role in modulating age differences to nicotine. In contrast, our work with female adults suggests that stress is a critical factor that modulates sex differences to nicotine. These findings imply that different mechanisms modulate nicotine use in females of different ages. Namely, during adolescence GABA and glutamate systems promote nicotine reward while at the same time they protect from the consequences of withdrawal from this drug. When a female reaches adulthood, the systems that promote nicotine reward are fully developed, such as estrogen facilitation of NAcc dopamine release. Also, adult females outgrow the mechanisms that protected them from withdrawal during adolescence. Importantly, adult females also have developed stress systems that promote nicotine withdrawal, such as CRF systems. Future studies are needed to examine sex differences in the VTA and age differences in the NAcc the may also promote nicotine use in females.
It is difficult to find a single explanation for enhanced vulnerability for nicotine use across age and sex. This is due, in large part, to the paucity of studies that assess the effects of nicotine in female and male rats at different stages of development. However, a study assessing this question revealed that adolescent female rats increase nicotine IVSA as they enter adulthood, whereas males do not display this effect (Levin et al., 2011). Consistent with this, adolescent female rats display a greater increase in nicotine/acetaldehyde IVSA as they enter adulthood as compared to males (Park et al., 2007). These studies suggest that adolescence is a unique period of development characterized by enhanced rewarding effects of nicotine. Adolescent females may also display an increase in nicotine use as they age with fully developed estrogen systems that facilitate dopamine and promote stress responses elicited by nicotine withdrawal.
10. Are the hypothesized mechanisms sufficient to understand sex differences to nicotine?
The proposed framework is intended to provide a testable hypothesis regarding sex differences to the behavioral effects of nicotine. This mechanism involves dopamine transmission in the mesocorticolimbic pathway and its regulation via stress systems in the NAc. With regard to sex differences in adolescent rats, our proposed mechanism involves excitatory and inhibitory mechanism in the cell body region of this pathway. Although this framework focuses on one particular neural system, it is recognized that the proposed mechanisms are not likely sufficient to explain the complex sex and age differences to the behavioral effects of nicotine.
One important factor to consider with regard to the addictive properties of tobacco is the presence of monoamine oxidase (MAO) inhibitors that suppress the breakdown of monoamines, such as dopamine (Fowler et al., 2003). Indeed, MAO inhibitors have been shown to enhance the rewarding effects of nicotine in rodents (Guillem et a., 2006; Kapelewski et al., 2011). With regard to age differences, MAO inhibitors have been demonstrated to promote the anti-depressant effects of nicotine in adolescent rats (Villegier et al., 2010). With regard to sex differences, female rats display greater sensitivity to MAO-B inhibition following L-deprenyl administration (Murphy et al., 1993). Thus, the possibility exists that MAO inhibition may contribute to the anti-depressant effects of tobacco that promote smoking behavior in women.
Another factor to consider with regard to age and sex differences to the behavioral effects of nicotine is the density of nicotine binding sites in the brain. Nicotine binds to a family of cholinergic receptors consisting of pentameric membrane proteins of homomeric or heteromeric complexes of α or β subunits. To date, 12 subunits have been identified (α2–10 and β2–4), and the various combinations of these subunits leads to differences in channel activation and desensitization in the presence of nicotine (Leslie et al., 2013). With regard to age differences, it is well established that the emergence of the various nicotine acetylcholine receptors (nAChRs) is developmentally different, and the expression and/or functional properties of these subunits are likely to contribute to behavioral differences to nicotine in adolescent and adults (see Slotkin, 2002). Furthermore, there are several reported long-term effects of adolescent nicotine exposure on cholinergic regulation of transmitter release, neurite outgrowth, cell survival and synaptogenesis (see Dwyer et al., 2008). With regard to sex differences, females display higher nAChR densities in various brain regions; however, chronic exposure to nicotine produces a larger up-regulation of nAChRs in males as compared to female rats (Koylu et al., 1997) and mice (Mochizuki et al, 1998). Accordingly, male smokers display an up-regulation of b2 receptors in the striatum during abstinence from chronic tobacco use, an effect that was not observed in females (Cosgrove et al., 2012). However, it is acknowledged that when rats self-administer nicotine, no sex differences in nAChRs were observed (Donny et al., 2000). Future work is needed to systematically examine the contribution of different nAChRs in modulating sex differences produced by nicotine.
11. Concluding statements regarding clinical implications
The hypothesis that vulnerable populations display strong rewarding effects of nicotine implies that prevention strategies are important for discouraging experimentation with tobacco products in young persons. Legislative approaches are also important to limit marketing strategies that promote nicotine use in vulnerable populations, such as tobacco packaging designed to entice women. In fact, a recent report showed that cigarettes in branded packaging were rated as more appealing, better tasting, and more closely associated with style and sophistication in female smokers (White et al., 2012). There have been some limitations on the tobacco industry with regard to marketing to adolescents; however, more legislation is needed to limit marketing strategies that target women.
The hypothesis that nicotine withdrawal is qualitatively different between adolescents and adults implies that medications targeting withdrawal should not be prescribed in a unilateral manner across smokers of different ages. One might also predict that treatments that target withdrawal may be less effective in adolescent versus adult smokers, regardless of sex. Moreover, nicotine replacement therapies in adolescent smokers may increase the probability of nicotine use later in life. Our observation that adult female rats experience intense stress during nicotine withdrawal implies that cessation treatments that are given in combination with anxiolytics medications may be more effective in women versus men smokers. In fact, a recent report showed that sazetidine-A, a selective nicotinic receptor desensitizing agent and partial agonist is less effective at reducing nicotine IVSA in female versus male rats (Johnson et al., 2012). This drug may be less effective in women smokers because it does not target stress systems that are critical in modulating nicotine use in females.
In conclusion, serious consideration should be given to specialized medicines for tobacco cessation in different vulnerable populations. It is important to recognize and respond to differences in the factors that motivate nicotine use in distinct populations. This review posited several empirical questions for future studies. This basic research has translational value toward developing more effective cessation medications in populations that are especially vulnerable to nicotine use.
Fig. 5. Hypothesized mechanisms mediating nicotine use in females.
The rewarding effects of nicotine are mediated via enhanced dopamine transmission in the NAcc. The dopamine terminals in this region are under inhibitory control via GABA interneurons that have estrogen receptors on them. Estrogen receptor activation is postulated to reduce GABA inhibition of dopamine, producing a greater increase in NAcc dopamine following nicotine administration. During nicotine withdrawal, dopamine levels in the NAcc are decreased, an effect that is hypothesized to be related to enhanced GABA release in this region. We hypothesize that female rats will display a greater suppression of dopamine that is modulated via CRF systems in this region. Thus, sex differences are related to overactive CRF systems that modulate dopamine release in the NAcc.
Fig. 6. Enhanced rewarding effects of nicotine in adolescent versus adult female rats.
This graph reflects place conditioning produced by various doses of nicotine (0, 0.2, 0.4, 0.6, 0.8, 1.2, or 1.8 mg/kg, base, sc) in adolescent and adult female rats. These data are presented as difference scores (±SEM), which reflect time spent in the initially non-preferred side after conditioning minus before conditioning such that values above “0” reflect a positive shift in preference (i.e., conditioned place preference); whereas, values below “0” represent a negative shift in preference (i.e., conditioned place aversion). The asterisks denote a significant difference from their respective saline controls (p≤0.05) and the dagger denotes a significant difference between treatment groups (p≤0.05). These data were previously published in Torres et al. (2008).
Highlights.
Females display enhanced nicotine reward and stress during withdrawal than males.
Females display enhanced nicotine reward via estrogen.
Adolescent females display enhanced nicotine reward and reduced withdrawal.
Medications targeting withdrawal should not be applied in a unilateral manner.
Pre-clinical work has translational value for reducing health disparities in females.
Acknowledgements
The author acknowledges financial support from the National Institute of Drug Abuse (R01-DA021274; R25-DA033613; R24-DA029989), the American Diabetes Association (7-12-BS-135), and the National Institute on Minority Health and Health Disparities (G12MD007592). The authors also appreciate the insightful suggestions provided by Drs. Donald Moss, Kristin Gosselink and Luis Carcoba. The valuable contribution of the members of our laboratory is also recognized, Luis Natividad, Joseph Pipkin, Ivan Torres and Jesus Jurado.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson C, Burns DM. Patterns of adolescent smoking initiation rates by ethnicity and sex. Tobacco Control. 2000;9:4–8. doi: 10.1136/tc.9.suppl_2.ii4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SB, Gortmaker SL. Dieting and smoking initiation in early adolescent girls and boys: a prospective study. Am J Public Health. 2001;91:446–450. doi: 10.2105/ajph.91.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour DJ. Neuroplasticity within the mesoaccumbens dopamine system and its role in tobacco dependence. Curr Drug Targets CNS Neurol Disord. 2002;1:413–421. doi: 10.2174/1568007023339076. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Molecular Psychiatry. 2010;15:896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Bruijnzeel AW. Animal models of nicotine withdrawal: intracranial self-stimulation and somatic signs of withdrawal. Methods in Molecular Biology. 2012;829:257–268. doi: 10.1007/978-1-61779-458-2_16. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;8:259–262. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Bosse R, DiPaolo T. The modulation of brain dopamine and GABAA receptors by estradiol: a clue for CNS changes occurring at menopause. Cell Mol Neurobiol. 1996;16:199–212. doi: 10.1007/BF02088176. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav. 2008;89:94–100. doi: 10.1016/j.pbb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Brook JS, Zhang C, Brook DW, Koppel J, Whiteman M. Psychosocial predictors of nicotine dependence among women during their mid-sixties. The American Journal on Addiction. 2012;21:302–312. doi: 10.1111/j.1521-0391.2012.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, Alexander JC. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacology Biochemistry and Behavior. 2012;101:62–68. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF central nervous system: an autoradiographic study. Journal of Neuroscience. 2007;5:3189–3103. [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods in Molecular Biology. 2012;829:243–256. doi: 10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:925–933. doi: 10.1016/0278-5846(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neuroscience Letters. 2008;439:187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug and Alcohol Dependence. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104:S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥ 18 Years—United States. [[accessed 2012 Nov 7]];Morbidity and Mortality Weekly Report. 2011 :2005–2010. [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. Journal of Consulting and Clinical Psychology. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Chae Y, Yeom M, Han JH, Park HJ, Hahm DH, Shim I, Lee HS, Lee H. Effect of acupuncture on anxiety-like behavior during nicotine withdrawal and relevant mechanisms. Neuroscience Letters. 2007;430:98–102. doi: 10.1016/j.neulet.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Chen XN, Zhu H, Meng QY, Zhou JN. Estrogen receptor-alpha and -beta regulate the human corticotropin-releasing hormone gene through similar pathways. Brain Research. 2008;1223:1–10. doi: 10.1016/j.brainres.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends in Neuroscience. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Dependence. 2000;59:83–95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, Krishnan-Sarin S, Staley JK, Picciotto MR, O’Malley SS. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69:418–427. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, Best A. Smoking cessation intervention for female prisoners: addressing an urgent public health need. American Journal of Public Health. 2008;98:1894–1901. doi: 10.2105/AJPH.2007.128207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: What is happening when? Early Human Development. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Behavioral pharmacology and neurobiology of nicotine reward and dependence. Handbook of experimental pharmacology. 2000;144:603–750. [Google Scholar]
- Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230:140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell P, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Doxey J, Hammond D. Deadly in pink: the impact of cigarette packaging among young women. Tob Control. 2011;20:353–360. doi: 10.1136/tc.2010.038315. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang YH, Luo J, Davila-Garcia M, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. Journal of Neurochemistry. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ernster V, Kaufman N, Nichter M, Samet J, Yoon SY. Women and tobacco: moving from policy to action. Bull World Health Organ. 2000;78:891–901. [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacology Biochemistry and Behavior. 2005;80:577–589. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2011;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. Int J Neuropsychopharmacol. 2001;4:371–376. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- French SA, Jeffery RW, Klesges LM, Forster JL. Weight concerns and change in smoking behavior over two years in a working population. Am J Public Health. 1995;85:720–722. doi: 10.2105/ajph.85.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Munoz M, Johnson M, MacLeod N, Arbuthnott G. The influence of the estrous cycle on the activity of striatal neurons recorded from freely moving rats. Neurosci Lett. 1989;107:233–238. doi: 10.1016/0304-3940(89)90823-9. [DOI] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Research Bulletin. 2011;85:145–152. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF–CRF 1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiology and Behavior. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in men. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, Grunberg NE. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacology Biochemistry and Behavior. 2009;92:51–59. doi: 10.1016/j.pbb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Hammond SK. Global patterns of nicotine and tobacco consumption. Handbook of Experimental Pharmacology. 2009;192:3–28. doi: 10.1007/978-3-540-69248-5_1. [DOI] [PubMed] [Google Scholar]
- Hammond D, Doxey J, Daniel S, Bansal-Travers M. Impact of female-oriented cigarette packaging in the United States. Nicotine Tob Res. 2011;13:579–588. doi: 10.1093/ntr/ntr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton DR, Modlin DL, Tizzano JP, Rasmussen K. Nicotine withdrawal: a behavioral assessment using schedule controlled responding, locomotor activity, and sensorimotor reactivity. Psychopharmacology. 1993;113:205–210. doi: 10.1007/BF02245698. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Experimental Neurology. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Research. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Holahan CK, Holahan CJ, North RJ, Hayes RB, Powers DA, Ockene JK. Smoking Status, Physical Health-Related Quality of Life, and Mortality in Middle-Aged and Older Women. Nicotine Tob Res Sep. 2012;10 doi: 10.1093/ntr/nts182. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacology Biochemistry and Behavior. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Ise Y, Narita M, Nagase H, Suzuki T. Modulation of opioidergic system on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Psychopharmacology. 2000;151:49–54. doi: 10.1007/s002130000482. [DOI] [PubMed] [Google Scholar]
- James-Walke NL, Williams HL, Taylor DA, McMillen BA. Periadolescent nicotine exposure produces sensitization to reinforcement by diazepam in the rat. Neurotoxicology and Teratology. 2007;29:31–36. doi: 10.1016/j.ntt.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology. 2012;222:269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. European Journal of Pharmacology. 2005;516:40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33:2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdi SP, Nakhate KT, Dandekar MP, Kokare DM, Subhedar NK. Participation of corticotropin-releasing factor type 2 receptors in the acute, chronic and withdrawal actions of nicotine associated with feeding behavior in rats. Appetite. 2009;53:354–362. doi: 10.1016/j.appet.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Kapelewski CH, Vandenbergh DJ, Klein LC. Effect of the monoamine oxidase inhibition on rewarding effects of nicotine in rodents. Curr Drug Abuse Rev. 2001;4:110–121. doi: 10.2174/1874473711104020110. [DOI] [PubMed] [Google Scholar]
- Kaufman AR, Augustson EM. Predictors of regular cigarette smoking among adolescent females: does body image matter? Nicotine Tob Res. 2008;10:1301–1309. doi: 10.1080/14622200802238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacology Biochemistry Behavior. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. Journal of Neuroscience. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkowm ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj M. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 2008;198:201–120. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. The Journal of Pharmacology and Experimental Therapeutics. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Koylu E, Demirgören S, London ED, Pöğün S. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci. 1997;61:185–190. doi: 10.1016/s0024-3205(97)00665-6. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and beta recruitment to a 3',5'-cyclic adenosine 5'-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149:346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handbook of Experimental Pharmacology. 2009;192:335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2013;83:753–758. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Levin ED, Hampton D, Rose JE. IV nicotine self-administration in rats using the consummatory operant licking response. Physiol Behav. 2010;101:755–758. doi: 10.1016/j.physbeh.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Johnson M, Williams P, Horton K, Rezvani AH. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res. 2011;225:473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li Z, Pei L, Le AD, Liu F. The α7nACh-NMDA receptor complex is involved in cue-induced reinstatement of nicotine seeking. J Exp Med. 2012;209:2141–2147. doi: 10.1084/jem.20121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay JL. The fight against tobacco in developing countries. Tuber Lung Dis. 1994;75:8–24. doi: 10.1016/0962-8479(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Mackay J. Women and tobacco: international issues. J Am Med Womens Assoc. 1996;51:48–51. [PubMed] [Google Scholar]
- Mackay JL, Amos A. Women and tobacco. Respirology. 2003;8:123–130. doi: 10.1046/j.1440-1843.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- Malin DH. Nicotine dependence: studies with a laboratory model. Pharmacology Biochemistry Behavior. 2001;70:551–559. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Malin DH, Goyarzu P. Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol. 2009;192:401–434. doi: 10.1007/978-3-540-69248-5_14. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. European Journal of Pharmacology. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex Differences in the Brain: The Not So Inconvenient Truth. The Journal of Neuroscience 15. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Research Reviews. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Wilson JM, Rubin RT, Rhodes ME. Sexually diergic, dose-dependent hypothalamic-pituitary-adrenal axis responses to nicotine in a dynamic in vitro perfusion system. Journal of Pharmacology and Toxicology Methods. 2010;61:311–318. doi: 10.1016/j.vascn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. Women who smoke - A review of the evidence. Aust Fam Physician. 2011;40:403–407. [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons through a membrane receptor. J. Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Villemagne VL, Scheffel U, Dannals RF, Finley P, Zhan Y, Wagner HN, JR, Musachio JL. Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse. 1998;30:116–118. doi: 10.1002/(SICI)1098-2396(199809)30:1<116::AID-SYN15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Moidel MA, Belz EE, Czambel RK, Rubin RT, Rhodes ME. Novel in vitro perfusion system for the determination of hypothalamic-pituitary-adrenal axis responses. J Pharmacol Toxicol Methods. 2006;53:2642–2671. doi: 10.1016/j.vascn.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Wu PH, Milgram NW, Ivy GO. Monoamine oxidase inhibition by L-deprenyl depends on both sex and route of administration in the rat. Neurochem Res. 1993;18:1299–1304. doi: 10.1007/BF00975051. [DOI] [PubMed] [Google Scholar]
- Mykletun A, Overland S, Aarø LE, Liabø HM, Stewart R. Smoking in relation to anxiety and depression: evidence from a large population survey: the HUNT study. European Psychiatry. 2008;23:77–84. doi: 10.1016/j.eurpsy.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Bonneau MJ, Rivest S. Influence of the estrous cycle on c-fos and CRH gene transcription in the brain of endotoxin-challenged female rats. Neuroendocrinology. 1997;65:29–46. doi: 10.1159/000127162. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Parsons LH, Torres OV, O'Dell LE. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. Journal of Neurochemistry. 2012;123:578–588. doi: 10.1111/j.1471-4159.2012.07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Tejeda HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichter M, Nichter M, Vuckovic N, Quintero G, Ritenbaugh C. Smoking experimentation and initiation among adolescent girls. Tobacco Control. 1997;6:185–195. doi: 10.1136/tc.6.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56:263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacology Biochemistry and Behavior. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicology Teratology. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DL, Heck JE, Dresler C, Allwright S, Haglund M, Del Mazo SS, Kralikova E, Stucker I, Tamang E, Gritz ER, Hashibe M. Determinants of smoking initiation among women in five European countries: a cross-sectional survey. BMC Public Health. 2010;10:74. doi: 10.1186/1471-2458-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G, Hildebrand BE, Svensson TH, Nomikos GG. Selective c-fos induction and decreased dopamine release in the central nucleus of amygdala in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Synapse. 2000;35:15–25. doi: 10.1002/(SICI)1098-2396(200001)35:1<15::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav. 2006;87:828–835. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Park MJ, Breland D. Alcohol and cigarette use among adolescent and young adult males. American Journal of Men’s Health. 2007;1:339–346. doi: 10.1177/1557988307306753. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Paysan J, Bolz J, Mohler H, Fritschy JM. GABA(A) receptor alpha 1 subunit, an early marker for area specification in developing rat cerebral cortex. J Comp Neurol. 1994;350:133–149. doi: 10.1002/cne.903500110. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr Symp Motiv. 2009;55:143–169. doi: 10.1007/978-0-387-78748-0_9. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. Review. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Giedgowd GE, Karelitz JL, Conklin CA, Lerman C. Smoking in response to negative mood in men versus women as a function of distress tolerance. Nicotine Tob Res. 2012;14:1418–1425. doi: 10.1093/ntr/nts075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addictive Behavior. 2013;38:1527–1531. doi: 10.1016/j.addbeh.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine and Tobacco Research. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3:a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine and Tobacco Research. 2010;12:647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–692. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogun S. Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. Int J Psychophysiol. 2001;42:195–208. doi: 10.1016/s0167-8760(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Pogun S, Yararbas G. Sex differences in nicotine action. Handb Exp Pharmacol. 2009;192:261–291. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Kennell JS, Belz EE, Czambel RK, Rubin RT. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine. Brain Research Bulletin. 2004;64:205–213. doi: 10.1016/j.brainresbull.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Research Bulletin. 2001;54:681–688. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Busse GD, Riley AL. An assessment of sex differences in nicotine-induced conditioned taste aversions. Pharmacol Biochem Behav. 2008;88:427–431. doi: 10.1016/j.pbb.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. Womens Health. 2007;16:1211–1218. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- Seguire M, Chalmers KI. Late adolescent female smoking. J Adv Nurs. 2000;31:1422–1429. doi: 10.1046/j.1365-2648.2000.01450.x. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF, Lê AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology. 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Sirinathsinghji DJ, Heavens RP. Stimulation of GABA release from the rat neostriatum and globus pallidus in vivo by corticotropin-releasing factor. Neuroscience Letters. 1989;100:203–209. doi: 10.1016/0304-3940(89)90685-x. [DOI] [PubMed] [Google Scholar]
- Skwara AJ, Karwosk TE, Czambel RK, Rubin RT, Rhodes ME. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behavioral Brain Research. 2012;234:1–10. doi: 10.1016/j.bbr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama K. Global perspective on tobacco control. Part I. The global state of the tobacco epidemic. Int J Tuberc Lung Dis. 2008;12:3–7. [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. European Journal of Pharmacology. 2010;314:281–284. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Maloney S. Gender differences and the role of estrogen in cognitive enhancements with nicotine in rats. Pharmacol Biochem Behav. 2010;95:139–145. doi: 10.1016/j.pbb.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Natividad LA, Orfila JE, Torres OV, O’Dell LE. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology. 2012;224:289–301. doi: 10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchalla I, Okoli CT, Bottorff JL, Qu A, Poole N, Greaves L. Somking cessation programs targeted to women: a systematic review. Women Helath. 2012;52:32–54. doi: 10.1080/03630242.2011.637611. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology Biochemistry and Behavior. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Monory K, Hanoune J, Nomikos GG. Nicotine withdrawal syndrome: behavioural distress and selective up-regulation of the cyclic AMP pathway in the amygdala. European Journal of Neuroscience. 2002;16:149–153. doi: 10.1046/j.1460-9568.2002.02061.x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. Journal of Clinical Investigations. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhees EE, Mitchell JT, McClernon FJ, Beckham JC, Kollins SH. Sex, ADHD symptoms, and smoking outcomes: an integrative model. Med Hypotheses. 2012;78:585–593. doi: 10.1016/j.mehy.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Gallager B, Heston J, Belluzzi JD. Leslie FM. Age influences the effects of nicotine and monoamine oxidase inhibition on mood-related behaviors in rats. Psychopharmacology (Berl) 2010;208:593–601. doi: 10.1007/s00213-009-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tobacco Research. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- White CM, Hammond D, Thrasher JF, Fong GT. The potential impact of plain packaging of cigarette products among Brazilian young women: an experimental study. BMC Public Health. 2012;12:737. doi: 10.1186/1471-2458-12-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2006;85:648–657. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, London ED. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine and Tobacco Research. 2008;10:1653–1661. doi: 10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58:374–382. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABA(A) receptor subunit distribution during brain maturation and aging Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhou JN. SUMO1 enhances 17-beta estradiol's effect on CRH promoter activation through estrogen receptors. Neuroendocrinology Letters. 2008;29:230–234. [PubMed] [Google Scholar]
- Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol-and other substance-related disorders. J Addict Dis. 2003;22:61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]