Summary
To investigate the influence of menstrual cycle phase and the presence of severe premenstrual symptoms on cardiac autonomic control during sleep, we performed heart rate variability (HRV) analysis during stable non-rapid eye movement (NREM) and REM sleep in 12 women with severe premenstrual syndrome and 14 controls in the mid-follicular, mid-luteal, and late-luteal phases of the menstrual cycle. Heart rate was higher, along with lower high frequency (HF) power, reflecting reduced vagal activity, and a higher ratio of low frequency (LF) to high frequency power, reflecting a shift to sympathetic dominance, in REM sleep compared with NREM sleep in both groups of women. Both groups of women had higher heart rate during NREM and REM sleep in the luteal phase recordings compared with the mid-follicular phase. HF power in REM sleep was lowest in the mid-luteal phase, when progesterone was highest, in both groups of women. The mid-luteal phase reduction in HF power was also evident in NREM sleep in control women but not in women with PMS, suggesting some impact of premenstrual syndrome on autonomic responses to the hormone environment of the mid-luteal phase. In addition, mid-luteal phase progesterone levels correlated with HF power and LF/HF ratio in control women in NREM sleep and with the LF/HF ratio during REM sleep in both groups of women. Our findings suggest the involvement of female reproductive steroids in cardiac autonomic control during sleep in women with and without premenstrual syndrome.
Keywords: heart rate variability, menstrual cycle, premenstrual syndrome, REM sleep
1. Introduction
The menstrual cycle is characterized by fluctuations in several hormones, most notably the gonadal steroids, estrogen and progesterone. When menses occurs, the levels of progesterone and estradiol are low. Thereafter, through coordination by gonadotropin-releasing hormone and the gonadotropins released from the anterior pituitary, estradiol levels gradually rise during the follicular phase, peaking before ovulation. After ovulation, in the luteal phase, progesterone and estradiol are secreted by the corpus luteum, reaching peak levels about seven days after ovulation, after which levels start to decline if fertilization and implantation do not occur (Guyton and Hall, 2006). In addition to their critical roles in reproductive function, reproductive hormones also influence other systems including the autonomic nervous system (Gibson, 1981; Charkoudian, 2001). Experimental studies in animals have shown that estrogens act centrally to modulate the autonomic nervous system, increasing vagal and decreasing sympathetic activity (Saleh and Connell, 2007), thus providing a cardiovascular protective function. Progesterone, on the other hand, appears to have an opposing effect, elevating central noradrenaline release (Genazzani et al., 2000). Given these effects, changes in progesterone and estradiol across the menstrual cycle may be associated with changes in autonomic nervous system function.
Spectral analysis of heart rate variability (HRV) provides a sensitive non-invasive measure of cardiac autonomic regulation via the parasympathetic (vagal) and sympathetic nervous systems. Beat-to-beat variability in the heart’s rhythm is predominantly caused by modulation of the intrinsic cardiac pacemakers by the autonomic nervous system (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). High frequency (HF) power reflects vagal activity and low frequency (LF) power is thought to measure a combination of vagal and sympathetic nervous system activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). As such, the LF component is less easily interpretable, although the LF/HF ratio is accepted as an indicator of sympathovagal balance (Trinder, 2007).
Several studies have investigated HRV in the frequency domain during short periods of supine rest in awake women at different phases of the normal menstrual cycle. Most studies have found either decreased HF power, reflecting decreased vagal activity, and/or an increased LF/HF ratio in the mid-luteal compared with the mid-follicular phase (Sato et al., 1995; Guasti et al., 1999; Hirshoren et al., 2002; Yildirir et al., 2002; Princi et al., 2005). One study found no menstrual phase related change in HRV components although estrogen levels were positively correlated with HF and LF power during ovulation (Leicht et al., 2003).
Recent studies have shown that the presence of severe premenstrual symptoms may impact autonomic nervous system function and, in particular, measures of HRV. Premenstrual syndrome (PMS) describes a range of emotional, behavioral, and physical symptoms that occur during the luteal phase, particularly the premenstrual week, of the ovulatory menstrual cycle and abate following menstruation (Freeman, 2003). Up to 18 % of women have severe PMS that causes significant impairment in term of family/social relationships and quality of life (Wittchen et al., 2002). Between 3-8 % of women qualify for a diagnosis of premenstrual dysphoric disorder (PMDD), a severe form of PMS defined in the DSM-IV (Halbreich, 2003). For a diagnosis of PMDD, at least five of 11 symptoms must be endorsed, of which one must be dysphoric-related. Symptoms need to be rated daily over at least two months to confirm the diagnosis (American Psychiatric Association, 1994). Some studies show higher heart rate, greater skin conductance, or greater norepinephrine levels throughout the menstrual cycle (Palmero and Choliz, 1991; Asso and Magos, 1992; Girdler et al., 1998) or just in the late-luteal phase in women with severe PMS (Woods et al., 1994) and others show no differences in these measures compared to controls (Van den Akker and Steptoe, 1987, 1989; Girdler et al., 1993). Studies of HRV in PMS have also produced inconsistent results. One study of HRV during supine rest found that HF power was lower in women with PMDD compared to controls in the asymptomatic follicular phase but not in the symptomatic late-luteal phase, with no menstrual phase variation in HF power reported for either group (Landen et al., 2004). In contrast, another study found that HF power during supine rest was lower in the late-luteal phase than in the early follicular phase in women with severe PMS (the severity was determined by the self-reported rate of increase in scores on the Menstrual Distress Questionnaire (Moos, 1968) from the follicular to the late luteal phase) but not in controls or in women with moderate symptoms of PMS (Matsumoto et al., 2006). In a second study by that group (Matsumoto et al., 2007), women with PMDD had reduced HF power in both the follicular and late-luteal phases of the menstrual cycle compared to controls and women with PMS.
Sleep represents a condition relatively free of external disruptive events that can affect measurement of HRV while awake (Brandenberger et al., 2005), allowing a more accurate measure of basal autonomic activity (Orr et al., 2000). In support of sleep being a favorable condition in which to measure HRV, a recent study in rats found that estrous-cycle variation in HF power was best demonstrated during quiet non-rapid eye movement (NREM) sleep (Kuo et al., 2010). However, cardiovascular activity is not uniform across all sleep stages. NREM sleep is characterized by a lower heart rate and higher HF power compared to presleep-wakefulness (Trinder et al., 2001). REM sleep is characterized by a higher heart rate and lower HF power compared to NREM sleep, with values tending to be closer to those recorded during presleep-wakefulness (Trinder et al., 2001). To our knowledge only one study has investigated HRV specifically during sleep in women at two phases of the menstrual cycle (mid-follicular and late-luteal) and in relation to the presence of premenstrual symptoms. The study was conducted by our group and we found no significant variation in any time or frequency domain variables recorded during sleep between the mid-follicular and late-luteal phases of the menstrual cycle in controls (Baker et al., 2008). However, women with severe PMS had lower HF power during sleep in the late-luteal phase compared with their own follicular phase, suggesting an influence of PMS symptoms on autonomic cardiac activity during sleep. This study did not include a mid-luteal phase recording, when progesterone levels are highest and most likely to have an impact on autonomic cardiac activity.
The aim of the current study was to investigate further the influence of menstrual cycle phase, reproductive hormone levels, and the presence of premenstrual symptoms on HRV assessed during sleep. We measured HRV during the mid-follicular, mid-luteal, and late-luteal phases in groups of women with and without severe PMS. We hypothesized that both groups of women would show reduced HF power in both NREM and REM sleep in the mid-luteal phase compared with the follicular phase. The reduction in HF power is hypothesized to persist from the mid-luteal to late-luteal phase in women with PMS, but not in controls.
2. Methods
2.1. Subjects
Participants gave written informed consent for participation, and the study protocol was conducted according to the Helsinki declarations and was approved by the Institutional Review Boards of SRI International and Palo Alto Medical Foundation Research Institute. Potential participants, aged 18 to 44 years, were recruited from the community. About 20% of potential PMS participants were recruited from community outpatient clinics after referral from their physician. Screening and assessment procedures are fully described in (Sassoon et al., 2011). Sleep and hormone data collected in the follicular and late-luteal phases of the menstrual cycle from a larger sample of women including the women presented here have been published elsewhere (Baker et al., 2012). Data from a subset of women from this sample who had follicular, mid-luteal, and late-luteal phase recordings are presented here. Inclusion criteria for both groups were regular menstrual cycles lasting between 24 – 35 days with no more than 4-days reported variability between cycles, regular sleep-wake schedules, no chronic illnesses, no regular medication over the previous three months (including hormonal contraceptives). With the exception of the PMS group, no participants met for a current Axis I psychopathology. All women in the PMS group met criteria for a diagnosis of current “depressive disorder, not otherwise specified (PMDD, provisional)”. Women suffering from sleep disorders (e.g. sleep disorder breathing, restless leg syndrome) were excluded from the study.
2.2. Symptoms
Women completed the Penn Daily Symptom Rating Form (DSR), a validated diagnostic tool for PMS (Freeman et al., 1996) over a 2-month period to track the severity of PMS symptoms and to confirm or deny the presence of PMDD. The DSR lists 17 common PMS symptoms including the 11 symptoms listed in the DSM-IV as criteria for a PMDD diagnosis: depression; anxiety; mood swings; irritability; decreased interest; difficulty concentrating; fatigue; food cravings; hypersomnia or insomnia; feeling overwhelmed; and physical symptoms (American Psychiatric Association, 1994). Subjects rate each item on a five-point scale (0 = none to 4 = extreme). To qualify for severe PMS, women need to score 80 or greater on the DSR in their late-luteal phase (total score for the 6 premenstrual days) and show an increase of at least 50% from their follicular phase score (total score for cycle days 5-10, where Day 1 is the first day of bleeding) for both screening months. To meet DSM-IV criteria for PMDD, women have to rate at least five of the 11 PMDD symptoms as severe (3 or 4) on at least two premenstrual days, with the same symptoms being rated as absent or minimal (0 or 1) postmenstrually (Freeman et al., 2004). During both the screening and recording phases, women used a commercially available self-testing kit that detects the presence of luteinizing hormone (LH) in urine (One Step Ovulation Test, IND Diagnostic, Inc, CA) to confirm ovulation and to allow accurate scheduling of luteal phase recordings. Twelve women with severe PMS, confirmed with 2 months of prospective ratings on the DSR (Age: 32.7 ± 5.8 y, Body mass index: 23.1 ± 4.4 Kg.m-2), seven of whom met criteria for PMDD, were included in the final sample. 14 women with minimal symptoms (controls Age: 31.3 ± 6.0 y, Body mass index: 22.6 ± 2.0 Kg.m-2) were included in the control group. The two groups did not differ according to age (p = .463) or BMI (p = .662).
2.3. Study procedures
Following the screening period, participants had an adaptation/screening night with clinical polysomnography (PSG) to confirm the absence of a clinically-significant sleep disorder. They returned to the laboratory for recordings at three phases of the menstrual cycle: mid-follicular phase (6 – 11 days after the onset of menses), mid-luteal phase (5 – 9 days after the LH surge, on average 8 ± 2 days before menses onset), and late-luteal phase (10 – 14 days after the LH surge, on average 3 ± 2 days before menses onset). Women entered the study at different phases of the menstrual cycle and recordings were not necessarily completed across one menstrual cycle (eight controls and five women with PMS had recordings in the same cycle). Six women with PMS and four controls had their first recording in the follicular phase. Four women with PMS and five controls had their first recording in the mid-luteal phase. The remaining women had their first recording in the late-luteal phase. On study days, the women were requested to refrain from drinking caffeinated beverages after 1500h, not to drink any alcoholic beverages, and not to take naps. All participants registered 0 on the breathalyzer and tested negative on a urine drug test at each recording. On each recording night before bedtime, women rated their daily mood on a 100 mm visual analogue scale ranging from ‘worst mood’ to ‘best mood’. Participants slept in sound-attenuated bedrooms, where ambient temperature was maintained between 20 – 22 °C. Lights-out and lights-on times were self-selected by participants.
2.4. Data acquisition and analysis
Electrocardiographic, electroencephalographic, electrooculographic, and electromyographic recordings were made using E-series amplifiers and Profusion software (Compumedics, Abbotsford, Victoria, Australia) linked to appropriate transducers. The ECG was recorded through Meditrace Ag/AgCl spot electrodes placed on the subject’s lower left rib cage and on the right clavicula notch. The ECG signal was digitized at a sampling rate of 512 Hz. Electrodes for EEG recordings were placed at F3, F4, C3, C4, O1, and O2 according to the international 10-20 system and cross-referenced to A1 or A2. EEG signals were digitized at a sampling rate of 256 Hz, high-pass filtered at 0.3 Hz, and low-pass filtered at 30 Hz. Thirty-second epochs were scored from C3-A2 or C4-A1 according to standard criteria (Rechtschaffen and Kales, 1968) by two scorers. Interrater reliability of sleep scoring was set at 0.90, and discrepancies were resolved by a third scorer. Total sleep time is the time spent asleep minus in-bed wakefulness during the time in bed. Sleep efficiency was calculated as a percentage of total sleep time during time in bed. Sleep-onset latency was taken as the time from lights-out to the first epoch of any stage of sleep. The time between sleep onset and the first epoch of rapid eye movement (REM) sleep was defined as REM sleep-onset latency.
2.5. Hormone analysis
Blood samples collected from subjects on each visit were analyzed for progesterone and estradiol using standard radioimmunoassay kits. Hormone levels were used to verify menstrual cycle phase. The intraassay and interassay coefficients of variations were 4.0% and 5.7%, respectively, for the progesterone assay, and 4.3% and 6.8%, respectively, for the estradiol assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Blood samples were not obtained from one woman with PMS in her follicular and late-luteal phase recordings, due to her reluctance to have a blood draw. A blood sample was not obtained from another woman with PMS in the late-luteal phase and from a control in the follicular phase due to unavailability of a phlebotomist.
2.6. Heart rate variability analysis
The ECG across the first seven hours after lights-out was subjected to spectral analysis of HRV according to the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). The analyses were conducted as previously described in detail (Baker et al., 2008) using software developed at the University of Melbourne (Trinder et al., 2001). During initial analyses of the ECG, R waves were detected using an automated algorithm, allowing inter-beat interval to be calculated by the program. The detection of R waves was visually checked and edited where the automatic detection was incorrect.
As HRV resulting from sleep disturbance masks autonomic control effects, two-min periods of stable artifact free sleep were selected for these analyses. Results are reported as the averages for all 2-min bins selected in NREM sleep (Stage 2, 3 and 4) and in REM sleep, separately. The inter-beat interval time series for each 2-minute data epoch selected for analysis was first re-sampled at a frequency of 4 Hz. The time series was then de-trended using a third order polynomial with a 20 second (81 point) window. The effect of this size window was to filter out any DC component, but leave intact low frequency (LF) activity. Power spectrum analysis was then applied to the time series. The program calculated the power spectrum density estimate for frequency bins that were then combined to form frequency bands 0.02 Hz wide. Thus, the total power spectrum ranged from 0 to 0.5 Hz in 0.02 Hz bands. The LF component was identified using the peak value in the frequency bands from 0.03 to 0.15 Hz. The width of the LF component was defined by the first frequency bands either side of the peak to fall to 50% of the peak value. The area between and including these frequency bands was then integrated. The HF component was defined as the peak value between 0.15 and 0.40 Hz. Power within the LF and HF bands was quantified by the absolute integrated power in arbitrary units. The LF/HF ratio was then calculated. Peak frequency of the high frequency band (HFpf), which is a measure of respiratory rate (Trinder et al., 2001), was also calculated. Total and HF power values were transformed with a natural log transform before statistical analysis.
2.7. Statistical Analysis
All results are reported as means (SD). Hormone levels, mood and polysomnographic variables were analyzed with repeated-measures two-way ANOVAs at a 95% confidence interval, with “menstrual phase (follicular, mid-luteal and late-luteal)” as the within factor and “group” as the between factor. A two-way repeated-measures ANOVA was used to analyze daily symptom ratings according to phase (follicular and late-luteal) and group. HRV variables in the frequency domain were analyzed with repeated-measures three-way ANOVAs with “menstrual phase (follicular, mid-luteal and late luteal)” and “sleep stage (NREM or REM sleep)” as within factors and “group” as the between factor. Newman-Keuls tests were conducted on significant interactions and where more than two levels were involved to find the origin of significance. In addition, we examined correlations between pre-sleep mood, plasma progesterone and estradiol concentrations and HRV variables in the mid-luteal phase separately for PMS and control groups of women using Spearman correlations.
3. Results
3.1. Premenstrual symptoms and mood
As expected, women with severe PMS had higher symptom scores on the DSR compared with controls in the late-luteal phase (p < .001) and compared to their own follicular phase (p < .001) (Table 1). Women with PMS also had higher DSR scores than controls in the follicular phase (p < .001). Women with PMS had, on average, 4 out of 6 postmenstrual days with absent or minimal symptoms as rated on the DSR. Women with PMS reported worse mood ratings on visual analogue scales completed on the evenings of their mid- (p < .05) and late-luteal (p < .001) phase recordings compared to controls and compared to their own follicular phase (p < .05). Pre-sleep mood did not vary significantly over the menstrual cycle in controls (Table 1).
Table 1.
Plasma hormone levels, mood and polysomnographic variables together with significant ANOVA results for 12 women with severe PMS and 14 controls in three phases of the menstrual cycle.
| Variable | Group | Follicular | Mid-luteal | Late-luteal | ANOVA |
|---|---|---|---|---|---|
| Daily symptom rating§ | PMS | 50.2 (20.8) | / | 141.0 (39.1) | Group: F(1, 21) = 123.32, p < 0.001 |
| Controls | 8.5 (9.4) | / | 12.7 (11.4) | Phase: F(1, 21) = 61.16, p < 0.001 | |
| Phase × Group: F(1, 21) = 47.60, p < 0.001 | |||||
| Daily mood (mm on a 100 mm visual analogue scale, where 0 = worst mood) | PMS | 75.8 (15.7) | 56.5 (24.5) | 47.1 (20.7) | Group: F (1, 24) = 13.11, p = 0.004 |
| Controls | 72.6 (17.8) | 75.7 (13.3) | 77.9 (12.3) | Phase: F (2, 48) = 3.97, p = 0.025 | |
| Phase × Group: F(2, 48) = 8.25, p < 0.001 | |||||
| Progesterone (ng.ml-1)# | PMS | 0.78 (0.51) | 10.48 (4.84) | 6.88 (4.80) | Phase: F (2, 40) = 42.19, p < 0.011 |
| Controls | 0.53 (0.28) | 11.04 (4.18) | 5.17 (5.00) | ||
| Estradiol (pg.ml-1)# | PMS | 76.54 (53.85) | 101.42 (41.56) | 86.38 (50.38) | |
| Controls | 80.03 (34.60) | 124.92 (60.59) | 72.00 (62.71) | ||
| Total recording time (min) | PMS | 453.3 (58.2) | 448.3 (48.9) | 478.9 (42.1) | |
| Controls | 450.8 (53.0) | 447.1 (65.0) | 458.6 (50.6) | ||
| Total sleep time (min) | PMS | 407.7 (68.0) | 397.7 (65.2) | 433.0 (47.5) | Phase: F (2, 48) = 4.94, p = 0.011 |
| Controls | 405.2 (63.9) | 382.7 (71.3) | 413.9 (53.4) | ||
| Sleep efficiency (%) | PMS | 89.6 (6.7) | 88.7 (10.7) | 90.5 (6.3) | |
| Controls | 89.7 (6.8) | 86.1 (13.2) | 90.3 (6.5) | ||
| Wake after sleep onset (min) | PMS | 31.1 (20.0) | 31.9 (31.1) | 30.2 (17.9) | |
| Controls | 32.2 (19.2) | 57.7 (63.2) | 31.9 (22.8) | ||
| Number of awakenings | PMS | 14.4 (5.1) | 11.8 (7.3) | 14.2 (4.9) | Group: F (1, 24) = 7.25, p = 0.013 |
| Controls | 20.1 (8.4) | 21.3 (9.4) | 22.4 (12.5) | ||
| Arousal index (No./h) | PMS | 6.7 (3.1) | 6.8 (2.9) | 7.4 (2.2) | |
| Controls | 6.0 (3.0) | 8.3 (4.8) | 7.7 (3.4) | ||
| Sleep onset latency (min) | PMS | 14.8 (13.4) | 18.4 (21.5) | 15.6 (16.4) | |
| Controls | 14.3 (14.7) | 7.8 (6.7) | 13.4 (12.1) | ||
| REM latency (min) | PMS | 89.4 (33.5) | 80.9 (32.0) | 76.0 (23.2) | |
| Controls | 83.1 (24.2) | 101.2 (64.1) | 75.5 (12.6) | ||
| REM sleep (%) | PMS | 22.5 (5.9) | 20.9 (5.0) | 21.9 (3.0) | Phase: F (2, 48) = 3.47, p = 0.039 |
| Controls | 22.4 (3.2) | 20.1 (4.0) | 24.0 (3.9) | ||
| Stage 1 sleep (%) | PMS | 3.7 (1.4) | 3.2 (1.4) | 4.2 (1.9) | Group: F (1, 24) = 9.63, p = 0.005 |
| Controls | 6.4 (2.9) | 6.1 (2.4) | 4.4 (2.0) | Phase × Group: F (2, 48) = 5.18, p = 0.009 | |
| Stage 2 sleep (%) | PMS | 57.4 (7.5) | 58.5 (8.5) | 57.6 (5.2) | |
| Controls | 55.4 (6.0) | 59.1 (4.8) | 60.3 (4.5) | ||
| Slow wave sleep (%) | PMS | 16.3 (6.6) | 17.4 (5.2) | 16.2 (5.0) | Phase: F (2, 48) = 3.26, p = 0.047 |
| Controls | 16.1 (5.8) | 14.3 (5.4) | 11.3 (2.9) |
Sleep stages are shown as a % of total sleep time;
n = 12 controls and 10 PMS; PMS = premenstrual syndrome; NREM = non-rapid eye movement; REM = rapid eye movement;
scores are averaged for 6 postmenstrual follicular phase days and 6 premenstrual late-luteal phase days over two menstrual cycles.
3.2. Reproductive steroid hormones
There were no group effects for progesterone or estradiol. As expected, progesterone levels varied according to menstrual phase, being highest in the mid-luteal phase and lowest in the follicular phase (phase main effect: p <.001). Estradiol levels did not significantly differ between menstrual phases. There was no group by menstrual phase interaction for either hormone.
3.3. Polysomnographic sleep variables
As shown in Table 1, there were few differences in sleep composition between groups or between the three menstrual phases. Women with PMS had fewer awakenings than controls regardless of menstrual phase (group main effect: p < .05). There were significant group (p < .05), and group-phase (p < .01) interaction effects for %Stage 1 sleep. Post-hoc tests revealed that controls had more %Stage 1 sleep in the follicular (p < .05) and mid-luteal phases (p < .01) than women with PMS.
Significant menstrual phase main effects were seen for total sleep time, % REM sleep and % slow wave sleep (SWS; Stages 3 and 4) (p < .05). Post hoc tests indicated less total sleep time in the mid-luteal compared with the late-luteal phase (p < .01), less %REM sleep in the mid-luteal phase compared to the late-luteal and follicular phases (p < .05), and reduced %SWS in late luteal compared to the follicular and mid luteal phases (p < .05). There was no significant group by menstrual phase interaction for any of these variables.
3.4. Heart rate and heart rate variability
Variables derived from spectral analysis of HRV are shown in Figure 1.
Figure 1.
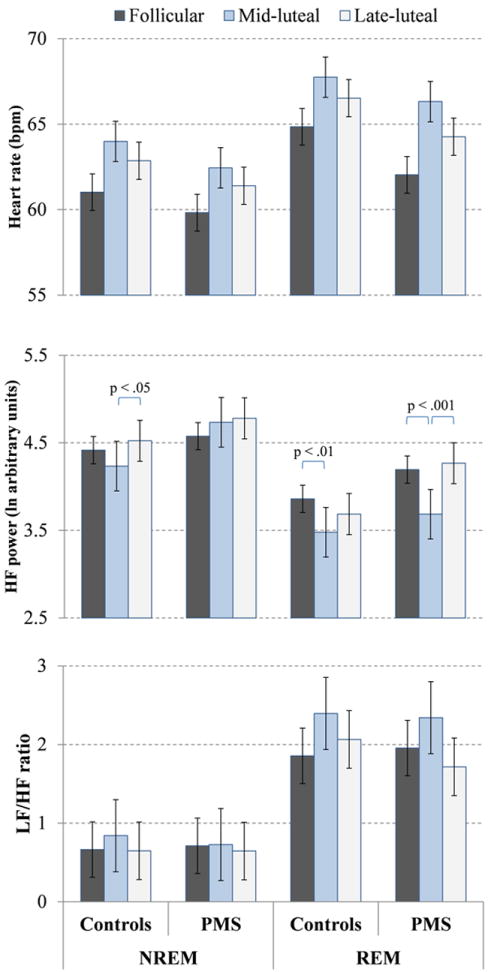
Means and standard errors for heart rate, high frequency (HF) power, and low-to-high frequency ratio (LF/HF) across phases of the menstrual cycle in REM and NREM sleep in 12 women with PMS and 14 controls. HR was higher, HF power was lower, and LF/HF ratio was higher in REM sleep than NREM sleep (stage effects, p < .001). HR was highest in the mid-luteal phase (phase effect p < .001). Significant stage-phase-group interaction effects for HF power are shown on the Figure.
3.4.1. Heart Rate
There was a significant effect of sleep stage (F (1, 24) = 46.36, p < .001) with a higher heart rate in REM sleep compared with NREM sleep. There was also a significant effect of menstrual phase (F (2, 48) = 7.83, p < .01). Post hoc tests indicated that heart rate was higher in the mid- (p < .001) and late-luteal (p < .05) phases than the follicular phase. There was no overall effect of group and no significant interactions between factors in the ANOVA model.
3.4.2. High Frequency Power (vagal tone)
There was no significant effect of group for HF power. There was a significant effect of sleep stage (F (1, 24) = 41.12, p < .001) with lower HF power in REM sleep than NREM sleep. There was also a significant effect of menstrual phase (F (1, 24) = 4.26, p < .05) and significant sleep stage by menstrual phase (F (2, 48) = 8.02, p < .001) and sleep stage by menstrual phase by group (F (2, 48) = 4.45, p < .05) interaction effects. As shown in Figure 1, women with PMS had lower HF power during REM sleep in the mid-luteal phase compared with the mid-follicular and late-luteal phases (post-hoc tests, p < .001). There were no significant phase effects in the PMS group for NREM sleep. Control women had reduced HF power in the mid-luteal phase compared with the late-luteal phase in NREM sleep (post-hoc test, p < .05) and compared with the follicular phase in REM sleep (post-hoc test, p < .01).
3.4.3. Low frequency power to high frequency power ratio (LF/HF, sympathovagal balance)
There were no significant group or menstrual phase effects for the ratio of LF/HF. However, the LF/HF ratio was higher during REM sleep than NREM sleep (stage main effect: F (1, 24) = 44.95, p < .001). There were no interactions between factors in the model.
3.4.4. Total power
The total power did not display significant main effects of group, sleep stage or menstrual phase. There was however a significant sleep stage by menstrual phase (F (2, 48) = 4.76, p < .05) interaction. Post hoc tests indicated that total power was lower during REM sleep in the mid-luteal phase compared with late-luteal phases (p < .05).
3.4.5. Peak frequency of the HF power (respiratory rate)
There were no effects of group or sleep stage on HF peak frequency, but there was a significant menstrual phase effect (F (2, 48) = 7.74, p < .01). Post hoc tests indicated higher values in the mid- and late-luteal (p < .01) phases than in the follicular phase.
3.5. Relationship between mid-luteal phase steroid hormones and autonomic heart rate variability measures
Spearman’s rank correlation coefficients are provided in Table 2. In the PMS group, plasma progesterone levels correlated with the LF/HF ratio in REM sleep (r = .59, p < .05). In the control group, progesterone correlated with the LF/HF ratio in NREM sleep (r = .93, p < .001) and in REM sleep (r = .71, p < .01) and with HF power in NREM sleep (r = -.58, p < .05). Estradiol was not significantly correlated with any variables apart from HF peak frequency during REM sleep (r = -.70, p < .01) and NREM sleep (r = -.54, p < .05) in control women. Presleep mood correlated with LF/HF ratio in REM sleep in PMS women (r = -.62, p < .05) and with total power in NREM sleep in controls (r = .54, p < .05).
Table 2.
Correlations (Spearman’s rank coefficients) for the relations between hormones, pre-sleep mood and heart rate variability spectral indices during the mid-luteal phase in PMS and control groups of women. Significant correlations (p < .05) in bold.
| NREM | REM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Spearman Rank | Heart rate | HF peak frequency | HF power | LF/HF ratio | Total Power | Heart rate | HF peak frequency | HF power | LF/HF ratio | Total Power | |
| PMS | Progesterone | -0.44 | -0.46 | 0.15 | 0.40 | 0.28 | -0.48 | -0.36 | 0.36 | 0.59 | 0.47 |
| Estradiol | -0.16 | -0.41 | -0.03 | -0.01 | -0.01 | -0.21 | -0.26 | -0.03 | 0.54 | 0.10 | |
| Pre-sleep Mood | 0.50 | 0.18 | -0.11 | -0.35 | -0.27 | 0.45 | -0.04 | -0.06 | -0.62 | -0.17 | |
|
| |||||||||||
| Control | Progesterone | 0.32 | -0.13 | -0.58 | 0.93 | -0.24 | 0.31 | -0.07 | -0.49 | 0.71 | -0.15 |
| Estradiol | 0.45 | -0.54 | -0.39 | 0.21 | -0.33 | 0.32 | -0.70 | -0.37 | 0.30 | -0.22 | |
| Pre-sleep Mood | -0.25 | 0.25 | 0.50 | -0.06 | 0.54 | -0.15 | 0.42 | 0.37 | -0.22 | 0.33 | |
HF = high frequency; LF = low frequency; NREM = non-rapid eye movement sleep; REM = rapid eye movement sleep
4. Discussion
In this study we have shown that measures derived from HRV analysis, analyzed during stable arousal-free NREM and REM sleep, vary with menstrual cycle phase. Heart rate and breathing frequency (assessed by the high frequency peak frequency) were higher in the mid- and late-luteal phase compared with the follicular phase in groups of women with and without PMS. High frequency power, a marker of vagal activity, was lowest in the mid-luteal phase during REM sleep, in both groups of women. While a mid-luteal phase reduction in HF power compared to the late-luteal phase was also evident in NREM sleep in controls, it was absent in women with PMS, suggesting reduced impact of the mid-luteal phase hormone environment on the parasympathetic nervous system in NREM sleep in women with PMS. Overall, the presence of severe premenstrual symptoms had no significant impact on HRV measures; there were no group differences in HRV indices in the symptomatic late-luteal phase.
Our finding of reduced HF power in the mid-luteal phase in the controls is consistent with previous studies that have used frequency domain analysis of HRV over 24 hours or short periods of wakefulness in healthy women (Sato et al., 1995; Guasti et al., 1999; Yildirir et al., 2002; Princi et al., 2005). It is unlikely that the decrease in HF power in the mid-luteal phase is due to the small increase in breathing frequency that we found (as indicated by the peak frequency of the HF band), which would have little effect on HF power (Brown et al., 1993). In addition, breathing frequency did not differ between the mid- and late-luteal phases whereas HF power was significantly reduced in the mid-luteal compared to late-luteal phase. It is also unlikely that the decrease in HF power is related to changes in sleep across the menstrual cycle. We found no change in wakefulness after sleep onset or in the arousal index and only a small decrease in REM sleep in the mid-luteal phase, confirming previous results that REM sleep is sensitive to the hormonal changes of the menstrual cycle (reviewed in Baker and Driver, 2007; Shechter and Boivin, 2010). In addition, analysis of heart rate variability was performed on two-minute epochs of undisturbed sleep, reducing the impact of nocturnal arousals on HRV indices.
Both groups showed the expected NREM-REM sleep difference in HRV indices, regardless of menstrual cycle phase, with a higher HR, lower HF power, and higher LF/HF ratio in REM sleep, as shown previously (Trinder et al., 2001). Despite REM sleep already being a state of relatively low vagal activity, vagal activity was even further reduced in the mid-luteal phase relative to other phases in both groups of women.
The changes in HF power associated with the menstrual cycle may be mediated directly by the changes in the gonadal hormone environment. There are several lines of evidence implicating estrogen as a modulator of vagal and sympathetic regulation of heart rate (Saleh and Connell, 2007). Postmenopausal women taking estrogen replacement therapy have higher HF power than postmenopausal women not taking estrogen (Liu et al., 2003). Also, women have reduced HF power following oophorectomy that is restored to pre-surgical values with estrogen therapy (Mercuro et al., 2000) and women undergoing acute estrogen treatment for ovulation induction have increased HF power compared to before treatment (Weissman et al., 2009). There is less evidence for the influence of progesterone on the autonomic nervous system. Postmenopausal women on combined progestogen-estrogen replacement therapy show reduced HF power, along with increased heart rate, reduced total power, and increased urinary excretion of catecholamines compared to women not taking hormone replacement, leading the authors to conclude that progesterone may increase sympathetic activity (Christ et al., 2002). High levels of progesterone in the mid-luteal phase, therefore, may oppose the positive effects of estradiol on the autonomic nervous system.
Our finding of reduced HF power specifically in the mid-luteal phase, when progesterone levels are highest and estradiol levels are not significantly different from the other phases, supports the involvement of progesterone in modulating HF power. However, progesterone was significantly correlated with HF power in the mid-luteal phase only in control women during NREM sleep. Possibly, the decrease in HF power could be driven by changes in other physiological variables such as body temperature, which is increased during sleep in the mid-luteal phase compared to the follicular phase (Driver et al., 1996; Baker et al., 2002). Interestingly, although we found no significant change in the LF/HF ratio across phases, progesterone concentrations in the mid-luteal phase were positively correlated with the LF/HF ratio in REM sleep in both groups of women and also in NREM sleep in controls: women with higher levels of progesterone were likely to have higher sympathovagal balance, i.e. relative sympathetic dominance. Thus, progesterone may not only influence vagal activity but also cardiac sympathetic activity. Indeed, others have shown that muscle sympathetic nerve activity, a direct measurement of sympathetic output, is increased in the mid-luteal phase compared to the follicular phase at rest (Minson et al., 2000; Middlekauff et al., 2012) as well as in response to orthostatic stress (Carter et al., 2009), in women without PMS, although the involvement of particular reproductive hormones in mediating this effect is currently unclear.
We found no group differences in HRV measures when women with PMS were most symptomatic in the late-luteal phase, which contrasts with our previous study, in which we found that HF power was decreased in the late-luteal phase compared with the mid-follicular phase in women with severe PMS but not in controls (Baker et al., 2008). Methodology and sample groups were similar between studies except that the first study did not include a mid-luteal phase recording. Possibly, some women with severe PMS show a prolonged decrease in HF power throughout the luteal phase including the late-luteal phase (Baker et al., 2008). If this is the case, it would point towards there being subgroups of women with PMS who have abnormalities in autonomic regulation in association with their symptoms. In support of this possibility, three other studies that measured HRV during 24-hours (time-domain) or short periods of supine rest (frequency-domain) have found effects of PMS or PMDD on HRV, although findings varied between studies and were not necessarily related to the presence of symptoms. One study found that supine HF power was significantly lower in women with PMDD compared with controls in the follicular phase but no group difference in the symptomatic late-luteal phase and no differences in HRV between menstrual phases in either group (Landen et al., 2004). Matsumoto and colleagues (2006) found that women with more severe premenstrual distress had lower HF power in the late-luteal phase compared to their follicular phase and controls. In a subsequent study, Matsumoto and colleagues (2007) found that women with PMDD had lower HF power and total power compared to controls in the follicular and late-luteal phases, whereas women with moderate PMS had lower total power and HF power in the late-luteal phase compared with their follicular phase but no difference compared to controls. No menstrual-phase difference was found in controls.
In the current study, the only group difference we found was specific to NREM sleep in the mid-luteal phase before premenstrual symptoms reach their maximum severity. It is unclear why HF power was not reduced during NREM sleep as it was during REM sleep in women with PMS in the mid-luteal phase. There is evidence of disturbed circadian rhythms in women with PMS or PMDD relative to controls, with decreased melatonin secretion and possibly also phase-advanced rhythms (Shechter and Boivin, 2010). Given that vagal activity is also under circadian regulation (Burgess et al., 1997), regulation of vagal activity might differ in women with PMS such that it is only sensitive to the impact of reproductive hormones in the early morning, when REM sleep predominates. Interestingly, presleep-mood was negatively correlated with the LF/HF ratio, again only in REM sleep, in women with PMS in the mid-luteal phase, indicating a relative sympathetic dominance in the women with a poorer mood. Studies of larger groups of women with a range of severity of premenstrual symptoms are needed to determine whether specific symptoms and/or severity of symptoms are associated with altered autonomic function and whether the impact of PMS is modulated by sleep-wake or circadian state. Also, to fully understand the contributions of normal menstrual cycle variability as well as premenstrual syndrome-related effects on HRV in women, future studies are needed to monitor changes in HRV at more frequent time points across the menstrual cycle.
Our findings need to be considered within the context of the limitations of the study. Recordings were made at only three discrete time points in the menstrual cycle and were not necessarily made within the same menstrual cycle for each woman. A strength of our study is that we measured frequency domain variables of HRV during undisturbed episodes of NREM and REM sleep, during which time HRV measures are less likely to be influenced by arousals or external events.
In conclusion, we have shown that the menstrual cycle impacts autonomic regulation during sleep, causing a shift in heart rate and vagal activity in the mid-luteal phase when progesterone levels are highest in women with and without severe premenstrual syndrome.
Acknowledgments
We thank Sharon Turlington, Lindsay Hoffman, Amanda Wagstaff, Benjamin Mayer, and Rebecca Carr for ensuring smooth coordination and execution of study procedures.
Footnotes
Contributors
Fiona Baker, Ian Colrain and John Trinder designed the study. Fiona Baker wrote the protocol and wrote the first draft of the manuscript. Massimiliano de Zambotti performed the data reduction and analysis of the data and wrote the manuscript. Christian Nicholas and John Trinder performed part of the heart rate variability analysis and they were involved in helping understanding the dynamic of the autonomic variables adopted. All authors contributed in writing the paper and in understanding the results. All authors have approved the final manuscript.
Conflict of Interest
None of the authors has any conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Asso D, Magos A. Psychological and physiological changes in severe premenstrual syndrome. Biol Psychol. 1992;33:115–132. doi: 10.1016/0301-0511(92)90027-r. [DOI] [PubMed] [Google Scholar]
- Baker F, Driver H. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Baker F, Driver H, Paiker J, Rogers G, Mitchell D. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J Appl Physiol. 2002;92:1684–1691. doi: 10.1152/japplphysiol.00919.2001. [DOI] [PubMed] [Google Scholar]
- Baker F, Sassoon S, Kahan T, Palaniappan L, Nicholas C, Trinder J, Colrain I. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21:535–545. doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Colrain IM, Trinder J. Reduced parasympathetic activity during sleep in the symptomatic phase of severe premenstrual syndrome. J Psychosom Res. 2008;65:13–22. doi: 10.1016/j.jpsychores.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger G, Buchheit M, Ehrhart J, Simon C, Piquard F. Is slow wave sleep an appropriate recording condition for heart rate variability analysis? Auton Neurosci. 2005;121:81–86. doi: 10.1016/j.autneu.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- Burgess H, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009;297:E85–91. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N. Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin Auton Res. 2001;11:295–301. doi: 10.1007/BF02332974. [DOI] [PubMed] [Google Scholar]
- Christ M, Seyffart K, Tillmann HC, Wehling M. Hormone replacement in postmenopausal women: impact of progestogens on autonomic tone and blood pressure regulation. Menopause. 2002;9:127–136. doi: 10.1097/00042192-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology. 2003;28(Suppl 3):25–37. doi: 10.1016/s0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Res. 1996;65:97–106. doi: 10.1016/s0165-1781(96)02929-0. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M, Xiao S. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. Am J Psychiatry. 2004;161:343–351. doi: 10.1176/appi.ajp.161.2.343. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Stomati M, Morittu A, Bernardi F, Monteleone P, Casarosa E, Gallo R, Salvestroni C, Luisi M. Progesterone, progestagens and the central nervous system. Hum Reprod. 2000;15(Suppl 1):14–27. doi: 10.1093/humrep/15.suppl_1.14. [DOI] [PubMed] [Google Scholar]
- Gibson A. The influence of endocrine hormones on the autonomic nervous system. J Auton Pharmacol. 1981;1:331–358. doi: 10.1111/j.1474-8673.1981.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Stern RA, Light KC. Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychol. 1993;12:180–192. doi: 10.1037//0278-6133.12.3.180. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81:163–178. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- Guasti L, Grimoldi P, Mainardi LT, Petrozzino MR, Piantanida E, Garganico D, Diolisi A, Zanotta D, Bertolini A, Ageno W, Grandi AM, Cerutti S, Venco A. Autonomic function and baroreflex sensitivity during a normal ovulatory cycle in humans. Acta Cardiol. 1999;54:209–213. [PubMed] [Google Scholar]
- Guyton A, Hall J. Textbook of medical physiology. 11. Elsevier Inc; Philadelphia, PA: 2006. [Google Scholar]
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology. 2003;28(Suppl 3):55–99. doi: 10.1016/s0306-4530(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab. 2002;87:1569–1575. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Lai CT, Hsu FC, Tseng YJ, Li JY, Shieh KR, Tsai SC, Yang CC. Cardiac neural regulation oscillates with the estrous cycle in freely moving female rats: the role of endogenous estrogens. Endocrinology. 2010;151:2613–2621. doi: 10.1210/en.2009-1410. [DOI] [PubMed] [Google Scholar]
- Landen M, Wennerblom B, Tygesen H, Modigh K, Sorvik K, Ysander C, Ekman A, Nissbrandt H, Olsson M, Eriksson E. Heart rate variability in premenstrual dysphoric disorder. Psychoneuroendocrinology. 2004;29:733–740. doi: 10.1016/S0306-4530(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Leicht AS, Hirning DA, Allen GD. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp Physiol. 2003;88:441–446. doi: 10.1113/eph8802535. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol. 2003;285:H2188–2193. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ushiroyama T, Kimura T, Hayashi T, Moritani T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. Biopsychosoc Med. 2007;1:24. doi: 10.1186/1751-0759-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Ushiroyama T, Morimura M, Moritani T, Hayashi T, Suzuki T, Tatsumi N. Autonomic nervous system activity in the late luteal phase of eumenorrheic women with premenstrual symptomatology. J Psychosom Obstet Gynaecol. 2006;27:131–139. doi: 10.1080/01674820500490218. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Rosano GM. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol. 2000;85:787–789. A789. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- Middlekauff H, Park J, Gornbein J. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol. 2012;302:H2560–H2566. doi: 10.1152/ajpheart.00579.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson C, Halliwill J, Young T, Joyner M. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- Moos R. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30:853–867. doi: 10.1097/00006842-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Orr WC, Elsenbruch S, Harnish MJ. Autonomic regulation of cardiac function during sleep in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:2865–2871. doi: 10.1111/j.1572-0241.2000.02318.x. [DOI] [PubMed] [Google Scholar]
- Palmero F, Choliz M. Resting heart rate (HR) in women with and without premenstrual symptoms (PMS) J Behav Med. 1991;14:125–139. doi: 10.1007/BF00846175. [DOI] [PubMed] [Google Scholar]
- Princi T, Parco S, Accardo A, Radillo O, De Seta F, Guaschino S. Parametric evaluation of heart rate variability during the menstrual cycle in young women. Biomed Sci Instrum. 2005;41:340–345. [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, Md: U. S. National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. NIH publication. [Google Scholar]
- Saleh TM, Connell BJ. Role of oestrogen in the central regulation of autonomic function. Clin Exp Pharmacol Physiol. 2007;34:827–832. doi: 10.1111/j.1440-1681.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- Sassoon SA, Colrain IM, Baker FC. Personality disorders in women with severe premenstrual syndrome. Arch Womens Ment Health. 2011;14:257–264. doi: 10.1007/s00737-011-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Miyake S, Akatsu J, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosom Med. 1995;57:331–335. doi: 10.1097/00006842-199507000-00004. [DOI] [PubMed] [Google Scholar]
- Shechter A, Boivin DB. Sleep, Hormones, and Circadian Rhythms throughout the Menstrual Cycle in Healthy Women and Women with Premenstrual Dysphoric Disorder. Int J Endocrinol. 2010 doi: 10.1155/2010/259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Trinder J. Cardiac activity and sympathovagal balance during sleep. Sleep Med Clin. 2007;2:199–208. [Google Scholar]
- Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, Kim Y. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- Van den Akker O, Steptoe A. Psychophysiological responses in women with premenstrual and menstrual symptoms. J Psychophysiol. 1987;1:149–158. [Google Scholar]
- van den Akker O, Steptoe A. Psychophysiological responses in women reporting severe premenstrual symptoms. Psychosom Med. 1989;51:319–328. doi: 10.1097/00006842-198905000-00006. [DOI] [PubMed] [Google Scholar]
- Weissman A, Lowenstein L, Tal J, Ohel G, Calderon I, Lightman A. Modulation of heart rate variability by estrogen in young women undergoing induction of ovulation. Eur J Appl Physiol. 2009;105:381–386. doi: 10.1007/s00421-008-0914-4. [DOI] [PubMed] [Google Scholar]
- Wittchen H, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32:119–132. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- Woods NF, Lentz MJ, Mitchell ES, Kogan H. Arousal and stress response across the menstrual cycle in women with three perimenstrual symptom patterns. Res Nurs Health. 1994;17:99–110. doi: 10.1002/nur.4770170205. [DOI] [PubMed] [Google Scholar]
- Yildirir A, Kabakci G, Akgul E, Tokgozoglu L, Oto A. Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Ann Noninvasive Electrocardiol. 2002;7:60–63. doi: 10.1111/j.1542-474X.2001.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


