Abstract
Traditional Chinese herbal medications (TCHM) are frequently used in conjunction with western pharmacotherapy for treatment of chronic kidney diseases (CKD) in China and many other Asian countries. The practice of traditional Chinese medicine is guided by cumulative empiric experience. Recent in vitro and animal studies have confirmed the biological activity and therapeutic effects of several TCHM in CKD. However, the level of evidence supporting TCHM is limited to small, non-randomized trials. Due to variations in the prescription pattern of TCHM and the need for frequent dosage adjustment, which are inherent to the practice of traditional Chinese medicine, it has been challenging to design and implement large randomized clinical trials of TCHM. Several TCHM are associated with significant adverse effects, including nephrotoxicity. However, reporting of adverse effects associated with TCHM has been inadequate. To fully realize the therapeutic use of TCHM in CKD we need molecular studies to identify active ingredients of TCHM and their mechanism of action, rigorous pharmacologic studies to determine the safety and meet regulatory standards required for clinical therapeutic agents, and well-designed clinical trials to provide evidence-based support of their safety and efficacy.
Keywords: Kidney disease, Chinese herb medications, toxicity, alternative medicine, drug discovery
Introduction
Corticosteroid and immunosuppressive medications are cornerstone therapies for glomerular diseases. However, these medications are associated with serious side effects. Furthermore, resistance to therapy and relapse of disease after medication discontinuation are common. For patients with chronic kidney disease (CKD), modulation of the renin-angiotensin axis provides only partial salutary effects and does not necessarily prevent the progression to end stage renal disease and the need for renal replacement therapy1. The lack of therapeutic options for CKD has prompted patients in China and other Asian countries to seek out alternative treatment such as traditional Chinese herbal medications (TCHM).
The practice of traditional Chinese medicine (TCM) is largely guided by the cumulative empirical experience of its practitioners. Although many small clinical studies suggest a therapeutic potential for TCHM in CKD, large randomized trials are lacking. Some TCHM are known to cause nephrotoxicity, which are often overlooked by some physicians and patients because of the incorrectly held belief that herbal medications are innocuous. Another major concern is that the active component(s) in most TCHM formulae and their underling mechanism of action remain unidentified. Despite these concerns, significant progress has been made in the past decade. However, recent reviews of this topic in the English language are limited2-4.
In the current review, we reviewed the current knowledge of TCHM for treatment of CKD based on publications in peer-reviewed journals of the English language. We also render a prospective on the direction of future investigation. Our target audience includes clinical nephrologists who care for patients being treated with TCHM and basic scientists who are interested in the drug discovery pertaining to TCHM.
General therapeutic principles of traditional Chinese medicine
The overarching principle in the practice of TCM is the focus on individual assessment and treatment to coordinate the natural balance of the Ying and Yang, which are two major opposing forces of the body represented in the ancient Chinese Taoism philosophy. TCM posits that disease of the body arises from an imbalance within the body and between the body and the nature leading to an alteration in the entire body system.
The syndrome differentiation and treatment (SDAT) approach is a principle that has been used to understand, diagnose, and treat diseases based on the theories of TCM. The diagnostic procedure involves an analysis of the clinical data regarding symptoms, physical signs, and disease history, together with information obtained from application of the four diagnostic methods, which consist of inspection, auscultation and smelling, inquiry, and pulse-taking and palpation (see Table 1). These four diagnostic methods allow the differentiation of syndromes, which in term dictates the approach to clinical treatment. The patient’s response to a specific clinical treatment plan then informs the correctness of the deduced differentiation. These three key aspects of the SDAT approach form the basis of diagnosis and treatment in TCM.
Table 1.
Comparison between Western Medicine and Traditional Chinese Medicine
| Traditional Chinese Medicine | Western Medicine | |
|---|---|---|
| Development | Based on empirical experience of practicing TCM |
Based on the scientific knowledge and practice experience. |
| Principles of practice |
Balancing Yin-Yang and the five major elements |
Understanding the molecular and cellular pathophysiology of disease and targeting therapy to normalize the underlying disease process |
| Disease perception |
Disease is the result of interactions among different parts of the body and the environment |
Disease is engendered by alterations in the cellular and molecular processes |
| Nomenclature of the organ |
Organ system is named by the organ and its related tissues. e.g., kidney usually means the kidney and related organs such as bone, ear, etc. |
Each organ has a unique name. |
| Diagnostic approach |
Diagnosis involves inspection, auscultation and smelling, inquiry, pulse-taking and palpation*. |
Diagnosis relies on history, physical examination and diagnostic testing of biological samples (pathology, blood tests, and radiographic imaging) |
| Principle of therapy |
Apart from treatment of disease, TCM also focuses on the reaction of body to herbs. Prescription is adjusted frequently based on signs and symptoms during follow-up visits. |
Emphasis is on targeted therapy that normalizes the underlying disease pathogenesis. Efficacy of treatment is evidence based. The dose of medications is adjusted or medications are changed if the effect is not achieved. |
| Therapy with medications |
Prescription is based on the combination of multiple herbs and their interaction in order to regulate whole body system and minimize toxic effects of herbs. |
Drugs are designed based on the individual target with defined molecular identify. Most patients are first treated with one drug and a second drug is added if the first medication is not efficacious. |
| Physician training |
Based on individual experiences which are accumulated over years’ practice. |
Understanding of basic medical knowledge and familiarity with clinical trials and guidelines. |
inspection means observation of patient’s general condition including physical appearance and activity, body movement, skin color and condition, color and appearance of the tongue, and body secretions (urine and feces). Auscultation means listening the voice, respiration, coughing, et al. Smelling means to smell the odors from patients’ body, urine and feces. Inquiry is to get patients’ current and past medical history. Pulse-taking is a diagnostic method performed by pressing patients’ radial artery to examine the strength and variability of the pulse. Palpitation is to touch, feel, and press the skin and muscle, hand and foot, and chest and abdomen in order to detect pathological changes.
In the practice of TCM, it is generally considered that multiple herbal medications are more effective than a single herbal agent. Therefore, prescriptions of TCHM usually combine several herbs in which a few components contribute to the main effect of the prescription; and these herbs are referred to as “ruler drugs”. In addition, another group of herbs known as “minister drugs” provide an additive effect to the “ruler drugs”, and the remaining constituents of the herbal prescription are called “assistant drugs and messenger drugs”. Physicians practicing TCM usually prescribe a formulas that combines several types of herbs or minerals where one herb represents the principal component and others serve as adjunctive agents assisting the effects or facilitating the delivery of the principal component. In China, more than 3,200 herbs and 300 mineral and animal extracts are used in more than 400 different formulae 5. Adjustment of individual components of the herbal prescription by the practitioner is based on patients’ signs and symptoms with an overall goal of restoring the balance of Ying and Yang. At each follow-up visit, physicians usually change either the herbal components or their relative percentage in the prescription.
Differences in the practice and principles of TCM and western medicine are summarized in the Table 1. These differences largely stemmed from their different historical background. Concepts of TCM related to organ function, disease pathogenesis, and treatment approach were formulated in the absence of our current molecular understanding of disease and deeply influenced by social, religious and cultural factors in these historical periods. One can debate the validity and scientific merits of the TCM approach. However, one should not immediately discount the empiric knowledge accumulated over centuries of treating patients with different decoctions of herbal mixtures. With recent integration of TCM and western medicine, diagnosis of CKD by practitioner of TCM has supplemented traditional diagnostic approach with western molecular and imaging diagnostic tools. The current treatment of CKD in TCM is often achieved by combining TCHM and western pharmacologic agents. Due to the rapid economic growth and scientific development over last decade, Chinese government has supported studies to examine the scientific basis of TCM using advanced cell and molecular biology approaches. A rapid development of TCM is expected over the next decade.
Treatment of kidney disease with traditional Chinese herbal medications
The therapeutic principles of TCM for CKD include “replenishing vital energy and nourishing blood”, “clearing heat and eliminating dampness”, and “coordinating Yin and Yang in the body” 6. Hundreds of herbs used in prescriptions of a single herb, decoctions of multiple herbs, or patent medicines have been used to treat patients with CKD. These prescriptions have effects including promotion of diuresis, reduction of proteinuria, and improvement of renal function 2,7. Mechanisms of action have been studied for some herbs. Their effects are mainly related to anti-inflammation, anti-oxidative, anti-fibrosis, regulation of immune system, anticoagulation, and improvement of metabolic disturbance 2,3. Active ingredient purified from herbs that been studied in CKD include saikosaponin a/d and triptolide. However, the active compounds in many decoctions or patent medications are still unknown and clinical trials demonstrating their efficacy for treatment of CKD are limited. We have summarized current evidence supporting TCHM in treatment of CKD. Key findings from in vitro studies, animal models, and human trials are included in Table 2.
Table 2.
Summary of the mechanisms, animal studies, and human trials of the most commonly used TCHM for kidney disease (DOX-N: Doxorubicin-induced nephropathy; DN: diabetic nephropathy; UUO: unilateral ureteral obstruction; PAN: puromycin-induced nephrosis)
| Cellular mechanisms |
Animal studies | Human studies | |
|---|---|---|---|
| Astragalus and A&A |
Regulation of immune system, diuresis, antioxidation, and anti- inflammation |
Reduction of proteinuria and kidney injury in 5/6 nephrectomy, DOX-N and DN animal models. A&A exhibited an anti-fibrosis effect in PAN and UUO models |
Low-to-moderate level of evidence in DN based on meta-analysis and systematic review of multiple small clinical studies |
| Rheum and it’s components( e modin, rhein) |
Promotes waste product excretion through intestines and regulation of inflammation and immune response |
Reduction of proteinuria and improvement in renal function and histology in 5/6 nephrectomized rats and db/db diabetic mice. Rheum and emodin have antioxidant effects in rat AKI models. |
Low level of evidence in CKD based on meta- analysis and systematic review of multiple small and low quality clinical studies |
| The decoction (ST) contain Radix bupleuri and Radix bupleuri’s SSa and SSd |
Anti-inflammation, Immune- modulation, Anti- mesangial cell proliferative effects |
ST inhibits mesangial cell proliferation in IgA and mesangio-proliferation GN models. It also decreases urinary protein excretion and kidney injury in subtotal nephrectomy model, rat model of gentamicin nephrotoxicity and mouse model of MRL/lpr. SSd reduces proteinuria and extracellular matrix deposition in mesangio-proliferative GN and DOX-N models. |
Moderate level of evidence for ST in patients with IgA nephropathy, likely by inhibiting mesangial cell proliferation |
| Cs and it’s component H1-A |
Antioxidant and anti-mesangial cell proliferative effects |
Cs improves renal function in animal models of ischemia/reperfusion, immunocomplex GN, MRL lpr/lpr, and 5/6 nephrectomy. Reduction of mesangial expansion in diabetic kidneys. Cs and it’s component H1-A reduce hematuria and proteinuria in a murine model of IgA nephropathy. |
Moderate level of evidence for Cs on cyclosporine- induced nephrotoxicity and CAN |
| Triptolide | Immune suppression and modulation, anti- inflammation, and anti-oxidant stress |
Reduction of proteinuria and improve renal function by protecting podocyte from injury in PAN rats. Inhibition of cyst growth in PKD mice. Prevention of renal injury in murine models of DN and lupus nephritis. |
Published clinical studies are only in Chinese medical journals A large clinical trial is ongoing in China |
1. Astragalus and decoction of Astragalus with Angelica sinensis
The medicinal herb Astragalus is derived from the root of Leguminosae plant Astragalus membranaceus or Astragalus mongholicus. It contains more than 60 components including polysaccharides, saponins (astragalosides I~VII), flavonoids, amino acids, and trace elements 8. Astragalus is traditionally used either alone or in conjunction with another Chinese herb, Angelica sinensis to treat patients with CKD 2.
Mechanisms of action
Pharmacological studies have shown that several compounds from Astragalus exhibit multiple effects including stimulation of the immune system, diuresis, antioxidation, and anti-inflammation 9,10,11. In addition, Astragalus membranaceus has been shown to attenuate podocyte injury induced by complement membranous attack complex 12. In a recent study, effects of Astragaloside IV were analyzed systemically using a computer-assisted target identification program, which identified 39 putative targets including calcium influx inhibition, vasodilatation, anti-thrombosis, anti-oxidation, anti-inflammation, and immune regulation 13.
Animal studies
The biological effects of Astragalus has been investigated in several animal models of kidney disease including 5/6 nephrectomy 14, doxorubicin-induced nephropathy 15, unilateral ureteral obstruction 16, glomerulonephritis 17, and streptozotocin-induced diabetic nephropathy (DN) 18. The results from these studies suggest that Astragalus treatment reduces proteinuria and attenuates kidney injury. These effects are associated with inactivation of free radicals, inhibition of nitric oxide synthesis, and reduction of tumor necrosis factor α (TNF-α) production 15,17,18. A decoction of Astragalus with Angelica sinensis has also been shown to attenuate renal interstitial fibrosis in rats with chronic puromycin aminonucleoside nephrosis and obstructive uropathy by suppressing TGF-β1 expression, macrophages infiltration, and ROS production, and promoting ECM degradation 14,19-21.
Clinical studies
Astragalus has been used by itself or as one of the “ruler drugs” in decoctions to treat CKD. Several small clinical studies published in Chinese language journals suggest that Astragalus decreases proteinuria and improves the plasma levels of total cholesterol and albumin in patients with nephrotic syndrome. In a systematical review of randomized and semi-randomized trials using Astragalus for the treatment of DN, 21 randomized-controlled and 4 case-controlled studies with a total of 1804 patients (945 in the treatment group and 859 in the control group) were included. The authors of the systematic review concluded that Astragalus is able to improve renal function and reduce proteinuria in patients with DN 22. In another meta-analysis of patients with DN, significant beneficial effects on glomerular filtration rate, urinary albumin excretion rate, and thickness of the glomerular basement membrane were observed in the Astragalus-treated group compared to the control group 23. Unfortunately, in these two studies the authors could not carry out a systematic review on adverse effects due to the lack of report on severe side effects in all the clinical trials involved. Success treatment of patients with nephrotic syndrome using Astragalus has also been reported. For example, a 77-year-old woman with idiopathic membranous nephropathy and nephrotic syndrome who had developed treatment failure with angiotensin converting enzyme inhibitors, angiotensin receptor blockers, cyclosporine A, and mycophenolate mofetil, responded to Astragalus membranaceus at a dose of 15 g/d with remission of proteinuria 24. No side effects were reported in this patient.
Side effects
Astragalus is generally considered safe for most adults. Side effects that can be exclusively ascribed to astragalus, however, are not well characterized because it is generally used in combination with other herbs. Astragalus is known to inhibit CYP3A4 and can affect the metabolism of certain drugs metabolized by this enzyme 25. For example, astragalus was reported to reduce cyclophosphamide-induced immunosuppression 26. From the above meta-analysis, it is important to note that adverse effects of TCHM have been underreported in clinical trials and this should be corrected in the future studies.
2. Rheum palmatum L and its components: emodin and rhein
The medicinal herb rhubarb is derived from the root of the Rheum palmatum L plant. Studies of inorganic elements in rootstocks of Rheum australe by atomic absorption spectrophotometry identified 19 elements including more than 20 types of anthraquinones, in which emodin (3-methyl-1,6,8-carboxyl-anthraquinone), rhein, and aloe-emodin have been studied extensively 27,28.
Mechanisms of action
The strong cathartic actions of rhubarb are thought to increase the excretion of waste products, including nitrogenous waste accumulated in patients with renal failure, through the intestines 29. Hence, rhubarb has been used to treat patients with renal failure. Recently, emodin, an active compound of rhubarb, has been shown to inhibit the lipopolysaccharide (LPS)-induced expression of TLR4 and down-regulate TNFα and IL-6 synthesis in renal tubular epithelial cells 30. Emodin has also been shown to inhibit the differentiation and maturation of dendritic cells and increase the number of regulatory T cells 31. These studies suggest that emodin has a major role in the regulation of inflammation and immune response. Rhein, another active compound of rhubarb, has been shown to improve cell metabolism through glucose transporter 1 and decreases mesangial cell hypertrophy and extracellular matrix synthesis 32. These results indicate that active ingredients in rhubarb have multiple mechanisms of action that could improve CKD.
Animal studies
Zhang and El Nahas examined the effect of a rhubarb extract on the development of renal failure in Wistar rats with 5/6 nephrectomy 33. Rhubarb extract treatment decreased proteinuria and glomerulosclerosis compared with no treatment. Treatment of db/db diabetic mice using rhein, an active compound of rhubarb, has been shown to decrease the levels of extracellular matrix (ECM) and expression of transforming growth factor (TGF) β-1 and fibronectin in the kidney 34. In addition, combination therapy with rhein and an angiotensin converting enzyme inhibitor (ACEI) in db/db mice provided additional renal protection that was more than either therapy by itself, as reflected in the reduction of urinary albumin excretion and improvement of renal function and histology 35.
Clinical studies
Clinical studies on the effects of rhubarb in patients with CKD have been reported in Chinese medical journals and summarized in an English review article 27. These studies suggest that rhubarb is able to reduce proteinuria and improve renal function by itself and may also cause further reduction of proteinuria and improvement of renal function when used together with ACEIs. A recent meta-analysis of clinical studies of rhubarb, which included seven studies that compared Rheum officinale (rhubarb root) against no treatment and two studies that compared rhubarb against captopril, found that Rheum officinale had a beneficial effect on renal function compared with no treatment, but was not superior to captopril treatment 36. The effect of treatment on all-cause mortality was not available from those studies. Only minor adverse events were reported in association with Rheum officinale. Diarrhea was reported in two-thirds of patients when the initial dose of Rheum officinale was greater than 3 g/day but resolved when the dosage was reduced. Standardized monitoring or voluntary self-reporting was not undertaken. Authors of the meta-analysis concluded that all nine studies are low quality and available evidence supporting the use of Rheum officinale in patients with CKD is scant.
Side effects
The major side effects of Rhem include nausea, vomiting, diarrhea, and abdominal pain. Long-term use of Rheum could cause electrolyte disorders and liver toxicity 37.
3. Decoctions of Radix bupleuri and components of Radix bupleuri (Saikosaponin a and d)
Saireito is a combination of twelve herbs of which Radix bupleuri is a major component. Saireito has been used in China and Japan for treatment of kidney diseases. Saikosaponin a (SSa) and its epimer saikosaponin d (SSd) are major triterpenoid saponin derivatives found in Radix bupleuri, which has been used for treatment of various inflammation-related diseases 38. The phytochemistry and pharmacology of Radix bupleuri has been reviewed 39.
Mechanism of action
Saireito suppresses inflammatory 40 and proliferation of mesangial cells 41. Both SSa and SSd have been shown to inhibit the expression of inducible nitric-oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 in lipopolysaccharide (LPS)-stimulated RAW264.7 cells 42. SSd inhibited mesangial cell proliferation and matrix synthesis 43. Saikosaponins also suppressed the proliferation of human T cells by inhibiting nuclear factor (NF)-κB, NFAT, and AP-1 (c-Fos) signaling pathways 44. In addition, both in vivo and in vitro studies confirmed that saikosaponins exhibit potent anti-inflammatory and immune-modulatory activities 39.
Animal studies
Saireito prevented mesangial cell proliferation in a rat model of mesangio-proliferation glomerulonephritis 45. Saireito also decreased urinary protein excretion in a rat model of subtotal nephrectomy 46,47. However, Saireito did not provide any additional benefit when added to enalapril for renal protection in rats with subtotal nephrectomy 48. Saireito has also been shown to reduce gentamicin-induced nephrotoxicity in rats 49 and in MRL/lpr mice 50. In a rat model of mesangioproliferative glomerulonephritis induced by anti-Thy1 monoclonal antibody, SSd attenuated the amount of proteinuria, increase in systolic blood pressure, accumulation of extracellular matrix, formation of glomerular crescents and infiltration of macrophages and CD8+ T lymphocytes 51. SSd was also able to lessen proteinuria in a rat model of aminonucleoside-induced nephropathy 52.
Clinical studies
In a randomized controlled study investigating the efficacy of saireito in a pediatric population with IgA nephropathy, 101 patients received either saireito or no treatment for 2 years 53. Urinary protein excretion and hematuria were significantly reduced in the saireito group, but remained unchanged in those who did not receive saireito. Proteinuria normalized in 46% of the patients in the saireito group compared with 10% in those in the untreated group. So far no clinical study of SSa or SSd for treatment of CKD has been published.
Side effects
Radix bupleuri use is known to cause interstitial pneumonia, elevated LFTs and jaundice, edema and hypertension, cystitis, and gastrointestinal symptoms such as nausea, vomiting and diarrhea. However, these symptoms are often associated with overdose and prolonged use of Radix bupleuri 54. It has also been reported that Saireito occasionally causes side effects such as immunodeficiency, gastro-duodenal ulcer and osteoporosis that are often associated with long-term administration of corticosteroids 55. Drug-induced pneumonitis and drug-induced acute hepatitis have been also reported with Saireito treatment 56,57. However, the side effects of these TCHM are usually underreported in clinical trials. Saireito is a combination of twelve herbs, which immediately raises the question of pharmacological interactions among them and toxicity induced by each of them. Therefore, this drug will be difficult to be approved by the current regulation of drug administration.
4. Cordyceps sinensis and its component H1-A
Cordyceps sinensis (Cs) is a blade-shaped fungus that derives its nutrients from larvae of Lepidoptera 58. Its secondary metabolites are cyclic peptides and H1-A 59. Cs was described in the old Chinese TCM textbook to treat patients suffering from urinary disturbance and edema 2000 years ago. Although kidney disease was not described at that time, these symptoms are considered to be kidney specific based on the TCM theory.
Mechanism of action
In vitro studies showed that both aqueous extracts of the whole fruiting body of Cs and purified polysaccharides from Cs have potent antioxidant activity 60. Cs extracts also inhibited proliferation of cultured mesangial cell 61-63.
Animal studies
Shahed et al examined the renal protective effects of a hot water extract of the powdered Cs in a rat model of kidney ischemia/reperfusion injury 64. Intraperitoneal injection of the Cs extract before surgery significantly mitigated the rise in serum creatinine and the increase in mRNA transcripts of inflammatory cytokines, monocyte chemoattractant protein-1, and TNF-α associated with ischemia/reperfusion kidney injury. In a separate study, Cs treatment improved the renal function of a murine model for immunocomplex glomerulonephritis (MRL lpr/lpr) 65. In an IgA nephropathy model, a fractionated crude methanolic extract of the fruiting bodies of Cs significantly lessened hematuria and proteinuria and improved kidney histology. Compound H1-A of Cs, purified by silica gel column chromatography and high-performance liquid chromatography, also demonstrated renal protection against IgA nephropathy 61. The mechanism of renal protection of Cs in the rat 5/6 nephrectomy model of renal injury was examined using nuclear magnetic resonance spectral analysis of the kidneys. Cs treatment attenuated glomerulosclerosis and urinary albumin/Cr ratio in 5/6 nephrectomized rats. Metabolic analysis of kidney tissues from 5/6 nephrectomized rats showed changes in the levels of TCA cycle intermediates (fumarate, succinate and malate) and suppression of branched-chain amino acids (valine, leucine and isoleucine) metabolism, which were reversed by Cs treatment 66.
Clinical studies
In a study of 69 renal allograft recipients, Cs reversed cyclosporine nephrotoxicity 67. In another study of 202 patients receiving standard immunosuppressive medications that were treated with or without Cs, the incidences of chronic allograph nephropathy (CAN) and total urinary protein excretion were significantly lower in the Cs group as compared to the group without Cs treatment 68. Others have also reported the beneficial effects of Cs in CAN 69. Several studies in the Chinese medical literature have reported that Cs treatment of patients with AKI or CKD improved kidney function. However, these studies have major methodological flaws including inadequate information on patient follow up, lack of a control group with standardized care, and small number of patients.
Side effects
There are very few reports of adverse reactions in human to Cs, which is available as a dietary supplement. Some patients have reported nausea, dry mouth and stomach discomfort with Cs 70,71. However, these adverse reactions are extremely rare.
5. Triptolide (PG-90)
Extracts of Tripterygium wilfordii Hook F have been used to treat glomerulonephritis for more than 30 years in China with remarkable antiproteinuric effects. Triptolide, a diterpene triepoxide, is one of the major active components of these extracts. Structural modifications, structure-activity relationships, pharmacology, and clinical development of triptolide have been recently reviewed 72.
Mechanism of action
Triptolide has potent immunosuppressive, immunomodulatory, and anti-inflammatory effects. Molecular and cellular effects of triptolide have been reviewed 73-75.
Animal studies
Triptolide effectively reduced proteinuria in rats with puromycin-induced nephropathy without affecting the glomerular filtration rate 76. The antiproteinuric effect was associated with improvement in foot process effacement, a decrease in the podocyte injury marker desmin as well as the restoration of nephrin and podocin expression and distribution. In addition, generation of reactive oxygen species and activation of p38 mitogen-activated protein kinase were suppressed by tripolide in podocytes 76. Triptolide has also been shown to attenuate kidney injury in an experimental rat model of passive Heymann nephritis 77. Triptolide also improved renal function by inhibition of cyst growth in kidney-specific Pkd1(flox−/−);Ksp-Cre mouse model of autosomal dominant polycystic kidney disease 78. It is hypothesized that triptolide arrests cellular proliferation and attenuates overall cyst formation by restoring Ca2+ signaling in these cells 79. In db/db diabetic mice, albuminuria was markedly attenuated by triptolide treatment, accompanied by alleviation of glomerular hypertrophy and podocyte injury 80. In addition, inflammation and oxidative stress in the kidneys were also attenuated. Effect of triptolide on glomerular hypertrophy was similar to valsartan, but the effects of triptolide on renal inflammation and oxidative stress were more profound than those of valsartan 80. In addition, triptolide significantly ameliorated lupus nephritis in (NZB × NZW) F1 mice through suppression of cytokine and chemokine production 81.
Clinical studies
Although extracts of Tripterygium wilfordii Hook F and triptolide have been used to treat patients with CKD for many years and multiple successful cases reports and small clinical studies have been published in Chinese medical journals, no randomized clinical trials have been ever published in English peer-reviewed journals.
Side effects
The major side effects of Tripterygium wilfordii Hook F include gastrointestinal disorders, liver toxicity, infertility, and hematopoietic disorder 82,83. Animal studies suggest that triptolide treatment is associated with nephrotoxicity 84. However, in two clinical studies to examine the effects of Tripterygium wilfordii Hook F in kidney transplant patients, no significant side effects were observed including nephrotoxicity 85,57. These data suggest that Tripterygium wilfordii Hook F, when administered at therapeutic dose, is relatively safe. There is currently no data on the adverse events in patients treated with triptolide.
The toxicity of Traditional Chinese herbal Medications
Although the TCHM described above are relatively safe and usually do not cause major toxicity at the therapeutic doses, it is critical for us to recognize that some herbal medications can cause significant toxicity including kidney toxicity 86-88. The toxicity of these herbal medications could be caused by inherent herb-induced toxicity or contamination of the herb or extract, or both. It was reported that about 10% of the incident end stage renal disease population in Taiwan is due to Chinese herb nephropathy 89. The most well described renal toxicity associated with traditional Chinese herbal medication is aristolochic acid induced nephropathy (AAN) 90,91. Despite awareness of the toxicity of aristolochic acid, some Chinese herbal preparations may still contain traces of this compound. The pathology of AAN is characterized by extensive renal interstitial fibrosis and tubular atrophy without obvious glomerular injury. Uroepithelial malignancies are commonly observed as a long-term sequelae associated with AAN. A retrospective study of 86 patients with AAN found that 19 patients (22.0%) presented with acute kidney injury, while 67 patients (78%) presented as CKD 92. Eleven (57.9%) patients with acute kidney injury regained renal function and 27 patients (40.2%) with CKD progressed to end-stage renal disease.
Certain TCHM, including Dioscorea bulbifera, Trichosanthes kirilowii, Melia toosendan, Cassia angustifolia, and Polygonum multiflorum, are known to cause liver toxicity 93. TCHM-induced liver toxicity usually occurs after one to four weeks of therapy and is manifested clinically by fatigue, jaundice, and poor appetite. Significant hematopoietic toxicity has been associated with Sinomenium acutum, mercury sulfide, and Psychotria rubra, which are known to cause thrombocytopenia and hemolytic anemia 94. However, these side effects are uncommon and mostly occur in susceptible patients with overdose and prolonged use of these herbs.
In addition, concomitant use of herbal prescriptions with western medications could engender serious herb-drug interactions leading to complications by increasing or decreasing the pharmacologic or toxicologic effects of either component. For instance, herbs traditionally used to lower glucose concentrations in diabetes could precipitate hypoglycemia if taken in combination with conventional oral hypoglycemic medications 95. It is known that reported bleeding complications could occur when warfarin is combined with either Angelica sinensis 96 or Salvia miltiorrhiza 97. Healthcare providers should also caution patients against mixing herbs and pharmaceutical drugs 98,99.
Perspective
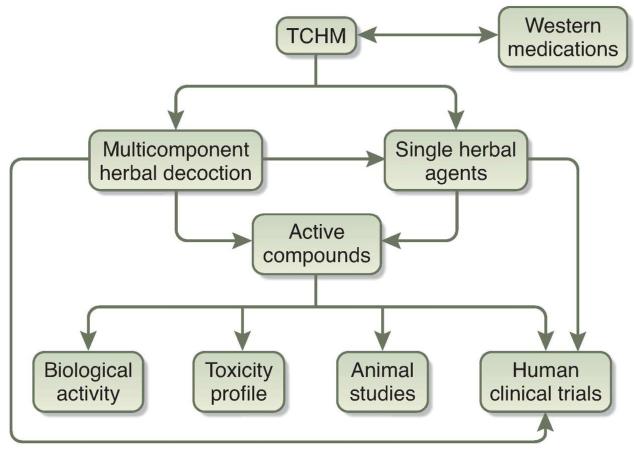
TCM is a valuable heritage of human experience. Therapeutic potential of these agents is supported by numerous animal studies. However, available clinical studies are small and well-designed randomized clinical trials are lacking to validate these therapies in patients with CKD. Although the therapeutic efficacy of herbs and herb-derived components has not been rigorously tested in large randomized controlled trials, the cumulative experience of treatment success supports more detail examination of the therapeutic potential of herbal medications in complementing or expanding existing therapies for CKD. Currently, there is inadequate evidence to support the use of traditional Chinese herbal medications in patients with CKD. Major challenges and the direction of future studies in this field are described below and summarized in Figure 1.
Figure 1. Schematic outlining evidence-based investigation of traditional Chinese herbal medications (TCHM).
Future studies are required to identify active compounds from TCHM, and determine their molecular mechanism of action. Well-designed animal studies and randomized clinical trials are needed to validate the physiological and pathological roles of these agents for treatment of patients with kidney disease. Complementary use of western and eastern medicines for treatment of kidney disease will also need to be further defined and proven.
1. Isolation of active components of herbs
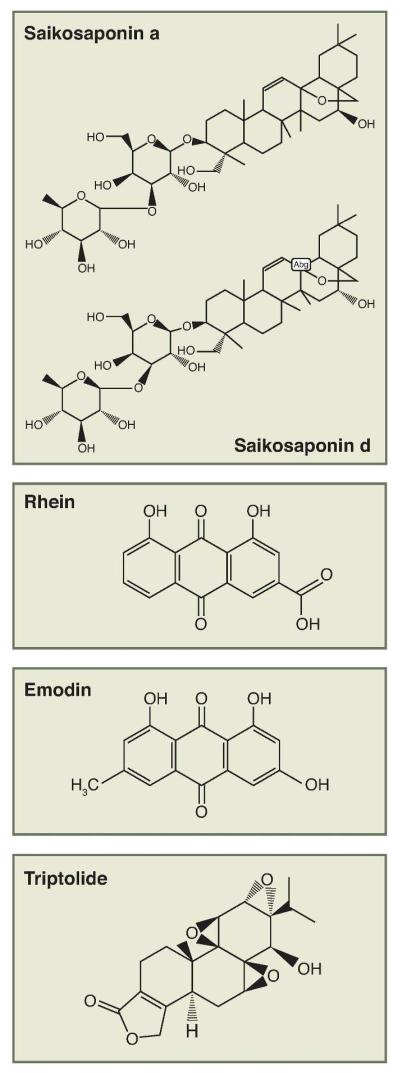
Natural occurring compounds from plants/herbs are of interest to researchers both in their natural forms and as parent compounds for additional synthetic modification. More than a hundred prescribed drugs in the United States are obtained from natural sources and represent one fourth of the total drugs used 100. Natural compounds currently used in medicine exhibit a wide chemical diversity. These compounds, together with their analogues, demonstrate the importance of compounds from natural sources in modern drug discovery efforts. Isolation of active compounds from herbs used in TCM and study of their molecular mechanisms are highly important in the development of novel, clinically useful drugs for medical therapy including CKD. Many laboratories in China and other countries are actively involved in drug discovery efforts based on herbal medication used in TCM. For example, recent studies using capillary gas chromatography and gas chromatography-mass spectrometry have identified several active compounds with strong anti-oxidant and anti-inflammation activities from Bupleurum marginatum101. Another example is the identification of a water-soluble analog of triptolide, named Minnelide, which was recently shown to effectively reduce pancreatic tumor growth and spread, and improving survival in patients 102. The therapeutic effect of Minnelide has not been determined in patients with kidney disease. The structures of some commonly used compounds for CKD are shown in Figure 2.
Figure 2. Chemical structures of active compounds in herbal medications used for treatment of kidney diseases in TCM.
Rhein and emodin are isolated from rhubarb. Saikosaponin a (SSa) and its epimer saikosaponin d (SSd) are major triterpenoid saponin derivatives from Radix bupleuri. Triptolide, a diterpene triepoxide, is one of the major active components isolated from the extracts of Tripterygium wilfordii Hook F.
2. Lack of high quality clinical trials:
Controlled randomized clinical studies of traditional Chinese herbal medications are difficult to design and execute for at least two reasons. One, there is a wide variation in the prescription of herbal medicine between physicians for the treatment of the same disease condition. This lack of standardization in prescription precludes direct comparison of treatment efficacy. Two, frequent adjustments of the herbal prescription are made based on patients’ symptoms and signs; and these adjustments in prescription are not standardized practice. Although this practice reflects more personalized care, it also renders the application of the gold standard in assessment of therapeutic efficacy, randomized controlled trials, nearly impossible. The only solution is to standardized prescription pattern and dosage adjustment among physicians. At least in the context of a clinical trial, these standardized approaches should be taken during the study. Furthermore, a recommendation has been developed to improve the reporting of clinical trials using herbal medicine interventions 103. This should help to consolidate efforts and track available clinic studies. A well-designed prospective randomized clinical trial for TCM was published recently to compare the efficacy and safety of oseltamivir and maxingshigan-yinqiaosan in treating uncomplicated H1N1 influenza 104. Recently, a multicenter double-blinded, randomized controlled clinical trial was published to compare the effects of TCHM (a mixed herbal decoction) with benazepril in 578 Chinese patients with CKD stage 3 caused by primary glomerulonephritis 105. Patients were randomly assigned to three groups: patients received TCHM, benazepril, or TCHM combined with benazepril. After 24 week follow up, the authors reported that TCHM combined with benazepril can ameliorate renal function and decrease proteinuria synergistically in these patients. Several well-designed prospective randomized clinical trials are currently ongoing in China to study TCHM in patients with CKD.
3. Mechanistic analysis of active compounds using modern approaches
Experiences from TCM practices strongly suggest that single agents are less effective than multiple herbal formulae. The basic concept of TCM consists of multiple drug therapy including rule drug, minister drug, assistant drug, and messenger drug. It is believed that herbal medications target different pathways to restore the balance of the body between Yin and Yang. This concept is quite similar to the current concept of systems pharmacology, which views therapeutic targets of drugs as parts of cellular networks that control physiological responses. Systems pharmacology aims to link genome-wide measurements and biological networks with the effects of drugs on cells, tissues, and organisms 106. In addition, the SDAT in TCM is an initial form of individualized treatment in modern medicine. Therefore, we believe that the modern systems biology concept might be inspired from TCM theory. However, the modern systems biology approach could be used to study the interactions among active compounds from herbal extracts in cells or tissues along with newly developed molecular biology technologies. A pioneer study in this field was published by Wang et al 107, in which they described the basic mechanism of Realgar-Indigo naturalis formula in treating human acute promyelocytic leukemia (APL) using systems biology and molecular biology approaches. They found that the main components of Realgar-Indigo naturalis formula are realgar, Indigo naturalis, and Salvia miltiorrhiza and their major active ingredients are tetraarsenic tetrasulfide, indirubin, and tanshinone IIA. They reported that the combination of all three ingredients yields synergy in the treatment of a murine APL model in vivo and in the induction of APL cell differentiation in vitro. This combination causes increased ubiquitination/degradation of promyelocytic leukemia-retinoic acid receptor alpha oncoprotein and enhanced G(1)/G(0) arrest in APL cells through hitting multiple targets compared with the effects of mono- or bi-agents. These data support the concept that tetraarsenic tetrasufide serve as the principal component of the formulas, whereas indirubin and tanshinone IIA serve as adjuvant ingredients. In addition, mechanistic studies of active compounds from herbal extracts should be also performed to identify the direct drug targets and downstream signaling pathways. The role of these compounds in renal physiology and pathology should also be examined using animal models. Together, these studies could improve our understanding of the mechanism of action for individual compounds and identify combinations of compounds that target different but complementary processes in hoped of developing a more effective therapy.
Conclusion
Therapeutic efficacy of TCHM is mainly supported by physicians’ clinical experiences and small clinical studies. However, large randomized clinical trials are urgently needed to validate these therapies. Many promising therapeutic compounds could be identified from herbal decoctions and developed as anti-inflammation, anti-oxidative, or immunomodulatory pharmaceutical agents. More detailed mechanistic studies using modern scientific methodology and approaches are needed to elucidate the therapeutic potential of TCHM for CKD. Clinicians who practice TCM should be aware of the limitations of TCHM as well as their toxicity profile. The reporting system for TCHM toxicity need to be improved and pharmacologic studies are required to assess the safety profile of TCHM. The combined wisdom of modern and traditional medical physicians will be needed to develop a new strategy to assess the efficacy and the safety of traditional Chinese herbal medications in patients with CKD.
Acknowledgement
YF Zhong is supported by National Natural Science Foundation of China for Young Investigators (1999-30901944) and Shanghai Bureau of Health for Young Investigators (2011-XYQ2011059); JCH is supported by NIH 1R01DK088541, Chinese 973 fund 2012CB517601, and VA Merit Award; PYC is supported by NIH 5K08DK082760.
Footnotes
Competing interest statement:
The authors declare that they have no competing financial interests.
References
- 1.de Zeeuw D. Unmet need in renal protection--do we need a more comprehensive approach? Contrib Nephrol. 2011;171:157–160. doi: 10.1159/000327337. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Wang H. Chinese herbal medicine in the treatment of chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:276–281. doi: 10.1016/j.ackd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Wojcikowski K, Johnson DW, Gobe G. Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. J Lab Clin Med. 2006;147:160–166. doi: 10.1016/j.lab.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Peng A, Gu Y, Lin SY. Herbal treatment for renal diseases. Ann Acad Med Singapore. 2005;34:44–51. [PubMed] [Google Scholar]
- 5.Liu CC, C X, Fang XW. Essentials of Traditional Chinese Medicine. In: XC X, editor. The English-Chinese Encyclopedia of Practical Traditional Chinese Medicine. Vol. 1. Higher Education Press of China; Beijing: 1991. pp. 1–4. [Google Scholar]
- 6.Xie Z, Liang A. New theoretical study on the biological origins of Chinese medicinal herbs. Zhongguo Zhong Yao Za Zhi. 1995;20:259–261. 318. [PubMed] [Google Scholar]
- 7.Li LD, Jing YZ, Li YO. Integrating traditional and Western medicine to research and develop Chinese new drugs. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16:301–304. [PubMed] [Google Scholar]
- 8.Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861–4866. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 9.Huang LF, et al. The effect of Astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia. 2012;83:1514–1522. doi: 10.1016/j.fitote.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Qin Q, et al. Astragalus membranaceus Inhibits Inflammation via Phospho-P38 Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor (NF)-kappaB Pathways in Advanced Glycation End Product-Stimulated Macrophages. Int J Mol Sci. 2012;13:8379–8387. doi: 10.3390/ijms13078379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbantsoy A, et al. Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J Ethnopharmacol. 2012;139:574–581. doi: 10.1016/j.jep.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 12.Zheng R, et al. Astragaloside IV attenuates complement membranous attack complex induced podocyte injury through the MAPK pathway. Phytother Res. 2012;26:892–898. doi: 10.1002/ptr.3656. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, et al. Therapeutic effects of astragaloside IV on myocardial injuries: multi-target identification and network analysis. PLoS One. 2012;7:e44938. doi: 10.1371/journal.pone.0044938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J, Meng L, Li S, Qu L, Li X. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, improved renal microvascular insufficiency in 5/6 nephrectomized rats. Vascul Pharmacol. 2009;50:185–193. doi: 10.1016/j.vph.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 15.You H, et al. Aqueous extract of Astragali Radix ameliorates proteinuria in adriamycin nephropathy rats through inhibition of oxidative stress and endothelial nitric oxide synthase. J Ethnopharmacol. 2011;134:176–182. doi: 10.1016/j.jep.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 16.Zuo C, et al. Astragalus mongholicus ameliorates renal fibrosis by modulating HGF and TGF-beta in rats with unilateral ureteral obstruction. J Zhejiang Univ Sci B. 2009;10:380–390. doi: 10.1631/jzus.B0820230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Zhang Y, Zhao J. Preparation and suppressive effect of astragalus polysaccharide in glomerulonephritis rats. Int Immunopharmacol. 2007;7:23–28. doi: 10.1016/j.intimp.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YW, Wu CY, Cheng JT. Merit of Astragalus polysaccharide in the improvement of early diabetic nephropathy with an effect on mRNA expressions of NF-kappaB and IkappaB in renal cortex of streptozotoxin-induced diabetic rats. J Ethnopharmacol. 2007;114:387–392. doi: 10.1016/j.jep.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Li J, Yu L, Zhao Y, Ding W. Antifibrotic effect of the Chinese herbs, Astragalus mongholicus and Angelica sinensis, in a rat model of chronic puromycin aminonucleoside nephrosis. Life Sci. 2004;74:1645–1658. doi: 10.1016/j.lfs.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Meng L, et al. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vascul Pharmacol. 2007;47:174–183. doi: 10.1016/j.vph.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Meng L, et al. Altered expression of genes profiles modulated by a combination of Astragali Radix and Angelicae Sinensis Radix in obstructed rat kidney. Planta Med. 76:1431–1438. doi: 10.1055/s-0029-1240943. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Wang W, Xue J, Gu Y, Lin S. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J Ethnopharmacol. 2011;133:412–419. doi: 10.1016/j.jep.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Xie X, Li C, Fu P. Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. J Ethnopharmacol. 2009;126:189–196. doi: 10.1016/j.jep.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed MS, Hou SH, Battaglia MC, Picken MM, Leehey DJ. Treatment of idiopathic membranous nephropathy with the herb Astragalus membranaceus. Am J Kidney Dis. 2007;50:1028–1032. doi: 10.1053/j.ajkd.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Pao LH, et al. Herb-drug interaction of 50 Chinese herbal medicines on CYP3A4activity in vitro and in vivo. Am J Chin Med. 2012;40:57–73. doi: 10.1142/S0192415X1250005X. [DOI] [PubMed] [Google Scholar]
- 26.Guo L, Bai SP, Zhao L, Wang XH. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: effects on quality of life and survival. Med Oncol. 2012;29:1656–1662. doi: 10.1007/s12032-011-0068-9. [DOI] [PubMed] [Google Scholar]
- 27.Li L. Rheum officinale: a new lead in preventing progression of chronic renal failure. Chin Med J (Engl) 1996;109:35–37. [PubMed] [Google Scholar]
- 28.Singh P, Negi JS, Rawat MS, Nee Pant GJ. Quantification of mineral elements of Rheum emodi Wallr. (Polygonaceae) Biol Trace Elem Res. 2010;138:293–299. doi: 10.1007/s12011-009-8603-7. [DOI] [PubMed] [Google Scholar]
- 29.Yokozawa T, Suzuki N, Zheng PD, Oura H, Nishioka I. Effect of orally administered rhubarb extract in rats with chronic renal failure. Chem Pharm Bull (Tokyo) 1984;32:4506–4513. doi: 10.1248/cpb.32.4506. [DOI] [PubMed] [Google Scholar]
- 30.Zhu XL, et al. Suppression of lipopolysaccharide-induced upregulation of toll-like receptor 4 by emodin in mouse proximal tubular epithelial cells. Mol Med Report. 2012;6:493–500. doi: 10.3892/mmr.2012.960. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, et al. Emodin inhibits the differentiation and maturation of dendritic cells and increases the production of regulatory T cells. Int J Mol Med. 2012;29:159–164. doi: 10.3892/ijmm.2011.820. [DOI] [PubMed] [Google Scholar]
- 32.Zheng JM, Zhu JM, Li LS, Liu ZH. Rhein reverses the diabetic phenotype of mesangial cells over-expressing the glucose transporter (GLUT1) by inhibiting the hexosamine pathway. Br J Pharmacol. 2008;153:1456–1464. doi: 10.1038/bjp.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, el Nahas AM. The effect of rhubarb extract on experimental renal fibrosis. Nephrol Dial Transplant. 1996;11:186–190. [PubMed] [Google Scholar]
- 34.Gao Q, et al. Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med. 2010;76:27–33. doi: 10.1055/s-0029-1185948. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZC, et al. Effect of astragaloside on cardiomyocyte apoptosis in murine coxsackievirus B3 myocarditis. J Asian Nat Prod Res. 2007;9:145–151. doi: 10.1080/10286020412331286506. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, et al. Rheum officinale (a traditional Chinese medicine) for chronic kidney disease. Cochrane Database Syst Rev. 2012;7:CD008000. doi: 10.1002/14651858.CD008000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J. Clinical use and side effects of Rheum palmatum L. China Practical medicine. 2012;7:230–131. [Google Scholar]
- 38.Wu MF, et al. Cordyceps sobolifera extract ameliorates lipopolysaccharide-induced renal dysfunction in the rat. Am J Chin Med. 2011;39:523–535. doi: 10.1142/S0192415X11009007. [DOI] [PubMed] [Google Scholar]
- 39.Ashour ML, Wink M. Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. 2011;63:305–321. doi: 10.1111/j.2042-7158.2010.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishida Y, et al. Therapeutic effects of Saireito (TJ-114), a traditional Japanese herbal medicine, on postoperative edema and inflammation after total hip arthroplasty. Phytomedicine. 2007;14:581–586. doi: 10.1016/j.phymed.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Awazu M, Fujita H, Omori S, Hida M. The herbal medicine Sairei-to inhibits proliferation of rat mesangial cells. Nephron. 2002;92:652–659. doi: 10.1159/000064112. [DOI] [PubMed] [Google Scholar]
- 42.Lu CN, et al. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-kappaB signaling pathway. Int Immunopharmacol. 2012;14:121–126. doi: 10.1016/j.intimp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Zu N, Li P, Li N, Choy P, Gong Y. Mechanism of saikosaponin-d in the regulation of rat mesangial cell proliferation and synthesis of extracellular matrix proteins. Biochem Cell Biol. 2007;85:169–174. doi: 10.1139/O07-002. [DOI] [PubMed] [Google Scholar]
- 44.Wong VK, Zhou H, Cheung SS, Li T, Liu L. Mechanistic study of saikosaponin-d (Ssd) on suppression of murine T lymphocyte activation. J Cell Biochem. 2009;107:303–315. doi: 10.1002/jcb.22126. [DOI] [PubMed] [Google Scholar]
- 45.Ono T, et al. Suppressive mechanisms of Sairei-to on mesangial matrix expansion in rat mesangioproliferative glomerulonephritis. Nephron Exp Nephrol. 2005;100:e132–142. doi: 10.1159/000085059. [DOI] [PubMed] [Google Scholar]
- 46.Kimura K, et al. Effects of a Japanese medicinal plant on the rat subtotal nephrectomy model: evaluation of its effect by microvascular casts. Am J Chin Med. 1990;18:167–174. doi: 10.1142/S0192415X90000216. [DOI] [PubMed] [Google Scholar]
- 47.Kawachi H, Takashima N, Orikasa M, Oite T, Shimizu F. Effect of traditional Chinese medicine (sairei-to) on monoclonal antibody-induced proteinuria in rats. Pathol Int. 1994;44:339–344. doi: 10.1111/j.1440-1827.1994.tb02933.x. [DOI] [PubMed] [Google Scholar]
- 48.Satoh S, et al. The effect of enalapril and sairei-to on survival-time for the rat with subtotal nephrectomy. Nihon Jinzo Gakkai Shi. 1995;37:112–118. [PubMed] [Google Scholar]
- 49.Ohno I, et al. Effect of Sairei-to on gentamicin nephrotoxicity in rats. Arch Toxicol. 1993;67:145–147. doi: 10.1007/BF01973686. [DOI] [PubMed] [Google Scholar]
- 50.Ito T, et al. Unique therapeutic effects of the Japanese-Chinese herbal medicine, Sairei-to, on Th1/Th2 cytokines balance of the autoimmunity of MRL/lpr mice. J Dermatol Sci. 2002;28:198–210. doi: 10.1016/s0923-1811(01)00161-x. [DOI] [PubMed] [Google Scholar]
- 51.Li P, et al. Therapeutic mechanism of Saikosaponin-d in anti-Thy1 mAb 1-22-3-induced rat model of glomerulonephritis. Nephron Exp Nephrol. 2005;101:e111–118. doi: 10.1159/000087437. [DOI] [PubMed] [Google Scholar]
- 52.Abe H, Orita M, Konishi H, Arichi S, Odashima S. Effects of saikosaponin-d on aminonucleoside nephrosis in rats. Eur J Pharmacol. 1986;120:171–178. doi: 10.1016/0014-2999(86)90537-6. [DOI] [PubMed] [Google Scholar]
- 53.Yoshikawa N, et al. A prospective controlled study of sairei-to in childhood IgA nephropathy with focal/minimal mesangial proliferation. Japanese Pediatric IgA Nephropathy Treatment Study Group. Nihon Jinzo Gakkai Shi. 1997;39:503–506. [PubMed] [Google Scholar]
- 54.Lu P.a.W., Chiuhong Discussion on Xiao Chai Hu Tang clinical application and its side effects. Chinese Primary Health Care. 2008;22:80–82. [Google Scholar]
- 55.Asano T, Fujii Y, Numao N, Kageyama Y, Kihara K. The efficiency of Sairei-to for retroperitoneal fibrosis: two case reports. Hinyokika Kiyo. 2006;52:543–547. [PubMed] [Google Scholar]
- 56.Miyagawa T, et al. A case of drug-induced pneumonitis due to Sai-rei-to. Nihon Kokyuki Gakkai Zasshi. 2009;47:47–51. [PubMed] [Google Scholar]
- 57.Aiba T, et al. Liver injury induced by a Japanese herbal medicine, sairei-to (TJ-114, Bupleurum and Hoelen Combination, Chai-Ling-Tang) R1. J Gastroenterol Hepatol. 2007;22:762–763. doi: 10.1111/j.1440-1746.2006.03373.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: part II. J Altern Complement Med. 1998;4:429–457. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 59.Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69:1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi Y, Kagota S, Nakamura K, Shinozuka K, Kunitomo M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis. Phytother Res. 2000;14:647–649. doi: 10.1002/1099-1573(200012)14:8<647::aid-ptr670>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 61.Lin CY, et al. Inhibition of activated human mesangial cell proliferation by the natural product of Cordyceps sinensis (H1-A): an implication for treatment of IgA mesangial nephropathy. J Lab Clin Med. 1999;133:55–63. doi: 10.1053/lc.1999.v133.a94239. [DOI] [PubMed] [Google Scholar]
- 62.Yang LY, Huang WJ, Hsieh HG, Lin CY. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J Lab Clin Med. 2003;141:74–83. doi: 10.1067/mlc.2003.6. [DOI] [PubMed] [Google Scholar]
- 63.Li SP, et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 64.Shahed AR, Kim SI, Shoskes DA. Down-regulation of apoptotic and inflammatory genes by Cordyceps sinensis extract in rat kidney following ischemia/reperfusion. Transplant Proc. 2001;33:2986–2987. doi: 10.1016/s0041-1345(01)02282-5. [DOI] [PubMed] [Google Scholar]
- 65.Yang LY, Chen A, Kuo YC, Lin CY. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin Med. 1999;134:492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- 66.Zhong F, et al. 1H NMR spectroscopy analysis of metabolites in the kidneys provides new insight into pathophysiological mechanisms: applications for treatment with Cordyceps sinensis. Nephrol Dial Transplant. 2012;27:556–565. doi: 10.1093/ndt/gfr368. [DOI] [PubMed] [Google Scholar]
- 67.Xu F, Huang JB, Jiang L, Xu J, Mi J. Amelioration of cyclosporin nephrotoxicity by Cordyceps sinensis in kidney-transplanted recipients. Nephrol Dial Transplant. 1995;10:142–143. [PubMed] [Google Scholar]
- 68.Li Y, et al. Clinical application of Cordyceps sinensis on immunosuppressive therapy in renal transplantation. Transplant Proc. 2009;41:1565–1569. doi: 10.1016/j.transproceed.2009.02.085. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Z, Wang X, Zhang Y, Ye G. Effect of Cordyceps sinensis on renal function of patients with chronic allograft nephropathy. Urol Int. 2011;86:298–301. doi: 10.1159/000323655. [DOI] [PubMed] [Google Scholar]
- 70.Shao G. Treatment of hyperlipidemia with cultivated Cordyceps--a double-blind, randomized placebo control trial. Zhong Xi Yi Jie He Za Zhi. 1985;5:652–654. 6–2. [PubMed] [Google Scholar]
- 71.Xu RH, Peng XE, Chen GZ, Chen GL. Effects of cordyceps sinensis on natural killer activity and colony formation of B16 melanoma. Chin Med J (Engl) 1992;105:97–101. [PubMed] [Google Scholar]
- 72.Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH. Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29:457–475. doi: 10.1039/c2np00088a. [DOI] [PubMed] [Google Scholar]
- 73.Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74:424–436. doi: 10.1111/j.1365-2125.2012.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11:377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 76.Zheng CX, et al. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int. 2008;74:596–612. doi: 10.1038/ki.2008.203. [DOI] [PubMed] [Google Scholar]
- 77.Chen ZH, et al. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int. 2010;77:974–988. doi: 10.1038/ki.2010.41. [DOI] [PubMed] [Google Scholar]
- 78.Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol. 2008;19:1659–1662. doi: 10.1681/ASN.2008030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leuenroth SJ, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao Q, et al. Treatment of db/db diabetic mice with triptolide: a novel therapy for diabetic nephropathy. Nephrol Dial Transplant. 2010;25:3539–3547. doi: 10.1093/ndt/gfq245. [DOI] [PubMed] [Google Scholar]
- 81.Tao X, et al. Effective therapy for nephritis in (NZB × NZW)F1 mice with triptolide and tripdiolide, the principal active components of the Chinese herbal remedy Tripterygium wilfordii Hook F. Arthritis Rheum. 2008;58:1774–1783. doi: 10.1002/art.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, et al. Triptolide induces adverse effect on reproductive parameters of female Sprague-Dawley rats. Drug Chem Toxicol. 2011;34:1–7. doi: 10.3109/01480541003774358. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Liu Z, Tolosa E, Yang J, Li L. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology. 1998;40:139–149. doi: 10.1016/s0162-3109(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 84.Li J, et al. Role of Nrf2 in protection against triptolide-induced toxicity in rat kidney cells. Toxicol Lett. 2012;213:194–202. doi: 10.1016/j.toxlet.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51:199–211. doi: 10.1053/j.ajkd.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 86.Isnard Bagnis C, Deray G, Baumelou A, Le Quintrec M, Vanherweghem JL. Herbs and the kidney. Am J Kidney Dis. 2004;44:1–11. doi: 10.1053/j.ajkd.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 87.Colson CR, De Broe ME. Kidney injury from alternative medicines. Adv Chronic Kidney Dis. 2005;12:261–275. doi: 10.1016/j.ackd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Wojcikowski K, Johnson DW, Gobe G. Medicinal herbal extracts -- renal friend or foe? Part one: the toxicities of medicinal herbs. Nephrology (Carlton) 2004;9:313–318. doi: 10.1111/j.1440-1797.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 89.Lin Wu, F. L, et al. Does Chinese Herb Nephropathy Account for the High Incidence of End-Stage Renal Disease in Taiwan? Nephron Clin Pract. 2012;120:c215–c222. doi: 10.1159/000341120. [DOI] [PubMed] [Google Scholar]
- 90.Yang L, et al. Aristolochic acid nephropathy: variation in presentation and prognosis. Nephrol Dial Transplant. 2012;27:292–298. doi: 10.1093/ndt/gfr291. [DOI] [PubMed] [Google Scholar]
- 91.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 92.Chen D, Tang Z, Luo C, Chen H, Liu Z. Clinical and pathological spectrums of aristolochic acid nephropathy. Clin Nephrol. 2012;78:54–60. doi: 10.5414/cn107414. [DOI] [PubMed] [Google Scholar]
- 93.HU YY, H. F. Chinese herb and drug-induced liver injury. Zhonghua Gan Zang Bing Za Zhi. 2012;20:173–175. [PubMed] [Google Scholar]
- 94.Li QE, Z. Y. Meta-analysis of Chinese herbs on the damage of hematopoietic system. Zhongguo Yi Yuan Yong Yao Ping Jia Yu Fen Xi. 2002;2:40–42. [Google Scholar]
- 95.Bailey CJ, Flatt PR, Marks V. Drugs inducing hypoglycemia. Pharmacol Ther. 1989;42:361–384. doi: 10.1016/0163-7258(89)90031-4. [DOI] [PubMed] [Google Scholar]
- 96.Yu CM, Chan JC, Sanderson JE. Chinese herbs and warfarin potentiation by ‘danshen’. J Intern Med. 1997;241:337–339. doi: 10.1046/j.1365-2796.1997.134137000.x. [DOI] [PubMed] [Google Scholar]
- 97.Page RL, 2nd, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy. 1999;19:870–876. doi: 10.1592/phco.19.10.870.31558. [DOI] [PubMed] [Google Scholar]
- 98.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 99.Fugh-Berman A, Ernst E. Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–595. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel) 2010;2:2428–2466. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ashour ML, et al. Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae) J Pharm Pharmacol. 2009;61:1079–1087. doi: 10.1211/jpp/61.08.0012. [DOI] [PubMed] [Google Scholar]
- 102.Chugh R, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gagnier JJ, et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364–367. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 104.Wang C, et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med. 2011;155:217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 105.Wang YJ, et al. Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol. 2012;139:757–764. doi: 10.1016/j.jep.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 106.Berger SI, Ma’ayan A, Iyengar R. Systems pharmacology of arrhythmias. Sci Signal. 2010;3:ra30. doi: 10.1126/scisignal.2000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang L, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]