Abstract
There are sex differences in the rates of many stress-sensitive psychological disorders such as post traumatic stress disorder (PTSD). As medial prefrontal cortex and amygdala are implicated in many of these disorders, understanding differential stress effects in these regions may shed light on the mechanisms underlying sex-dependent expression of disorders like depression and anxiety. Prefrontal cortex and amygdala are key regions in the neural circuitry underlying fear conditioning and extinction, which thus has emerged as a useful model of stress influences on the neural circuitry underlying regulation of emotional behavior. This review outlines the current literature on sex differences and stress effects on dendritic morphology within medial prefrontal cortex and basolateral amygdala. Such structural differences and/or alterations can have important effects on fear conditioning and extinction, behaviors that are mediated by the basolateral amygdala and prefrontal cortex, respectively. Given the importance of extinction-based exposure therapy as a treatment for anxiety disorders such as PTSD, understanding the neural mechanisms by which stress differentially influences fear learning and extinction in males and females is an important goal for developing sex-appropriate interventions for stress-related disorders.
Keywords: Medial prefrontal cortex, basolateral amygdala, dendritic morphology, sex-dependent stress effects
1. Chronic stress, psychopathology, and corticolimbic structure and function
Women are more susceptible than men to stress-related mental illness and twice as likely to experience depression [1, 2]. There is also a greater incidence of most types of anxiety disorders, such as social anxiety, phobias, and post traumatic stress disorder (PTSD), among women compared to men [3]. However, after women experience menopause, a stage of life marked by a pronounced decline in ovarian hormones, this sex difference diminishes [4, 5]. In women, depression is also more likely to occur during periods of hormonal fluctuation, such as prior to menses, immediately after pregnancy, and during and after menopause [6, 7]. Thus, there may be a role for the cyclic release of ovarian hormones in exacerbating the high incidence of stress-sensitive psychological disorders in women.
Chronic stress is linked to cognitive and emotional dysfunction. For instance, stressful life events play a role in precipitating episodes of major depression, and can trigger posttraumatic stress disorder [8, 9]. Chronic stress also has detrimental effects on many behaviors. For instance, several studies have demonstrated stress-induced deficits on a variety of cognitive tasks, including fear conditioning and retrieval of extinction, attentional set-shifting, spatial learning and recognition, and working memory [reviewed in 10, 11–13]. However, it is not well understood how stress acts on the brain to contribute to the development of psychopathology.
Several stress-related disorders, including anxiety [14], depression [15], PTSD [16], and schizophrenia [17], have been associated with changes in the volume of prefrontal cortex and amygdala, implicating these regions as important targets for investigating stress effects in the brain. In fact, both prefrontal cortex and amygdala contain corticosteroid receptors and are involved in the regulation of hypothalamic-pituitary-adrenal axis activity [18–20]. Further, there is interconnectivity between prefrontal cortex and amygdala [21], allowing for prefrontal inhibition of amygdala activity [22–25]. Connections between these two regions are critical modulators of a useful model of the regulation of emotional behavior, fear conditioning and extinction.
Fear conditioning and extinction provides an excellent model system for examining how stress-induced changes in corticolimbic morphology are related to stress-induced changes in neural function and behavior, as the neural circuitry underlying this behavior is well characterized, and involves both medial prefrontal cortex and basolateral amygdala. During fear conditioning, an animal is placed in an operant chamber and acquires a learned fear response to a neutral stimulus, such as a tone, that is paired with an aversive unconditioned stimulus, such as a shock. Repeated pairings of the tone with the shock result in a conditioned fear response to the conditioned stimulus (CS) tone. In a rodent model, a common measure of the conditioned fear response (CR) is the animal’s freezing during the tone, which is defined as absence of all movement except that due to breathing. Time away from the operant chamber (typically ranging from 1 to 24 hr) is necessary for consolidation of the fear memory. The animal is then placed back in the operant chamber for subsequent presentations of the CS in the absence of the unconditioned stimulus. Multiple presentations of the CS will result in extinction of the fear response—the animal learns that the tone no longer predicts the shock, and no longer freezes in response to presentation of the tone [12, 26]. Memory for extinction of conditioned fear can be measured by presentation of the CS on subsequent days. High levels of freezing during the CS indicate poor retrieval of the extinction memory.
Variations in the ability to consolidate and/or retrieve the extinction memory could contribute to disorders such as PTSD [27, 28], which makes stress effects on retrieval of extinction an especially important topic of study. Patients with PTSD have impairments in both the ability to extinguish an aversive conditioned response [29, 30] and later retrieval of the extinction memory [27]. These extinction deficits could be responsible for the persistence of traumatic memories in the absence of the trauma-inducing stimulus, a hallmark of PTSD [28, 31].
Patients with PTSD have reduced ventral medial prefrontal cortex activity and increased activity in the amygdala [32–34], and alterations in prefrontal and amygdalar activity are associated with extinction deficits in these patients [27]. Indeed, connections between medial prefrontal cortex and amygdala are important for fear conditioning and extinction. Basolateral amygdala is a key site of convergence for unconditioned and conditioned stimuli, a critical requirement for the neuroplasticity necessary for learning of fear conditioning [35], and the acquisition of fear conditioning is mediated by amygdala [36, 37]. Basolateral amygdala is specifically involved in mediating the initial acquisition of extinction, as either temporary inactivation [38] or blockade of glutamatergic transmission [39, 40] in basolateral amygdala prevented or attenuated extinction.
Medial prefrontal cortex contributes to both acquisition and retrieval of extinction of conditioned fear [41]. Male rats with infralimbic cortex lesions showed normal acquisition of fear conditioning and initial extinction, but deficits in the ability to retrieve extinction memory [42]. Likewise, stimulation [43] or pharmacological activation [44] of infralimbic cortex facilitated extinction retrieval, while blockade of infralimbic cortex activity impaired extinction retrieval [45] . Further, neurons in infralimbic cortex showed an increase in firing in response to the CS during extinction retrieval [46]. This evidence suggests that infralimbic cortex is necessary for inhibiting fear responses during extinction [47].
Prelimbic cortex, on the other hand, seems to be involved in the expression of conditioned fear [47]. Temporary inactivation of prelimbic cortex during extinction disrupted conditioned fear expression [48], while stimulation of prelimbic cortex during extinction increased fear expression and slowed extinction of the fear memory [49]. Neural activity in prelimbic cortex is associated with freezing during extinction [50]. Thus, there is a regional specificity in the involvement of medial prefrontal cortex in the modulation of fear conditioning and extinction: prelimbic cortex facilitates fear expression while infralimbic cortex is involved in fear extinction.
The different output targets of infralimbic cortex and prelimbic cortex are key to the differential effects of each region on fear expression. Although a small percentage of infralimbic neurons innervate basolateral amygdala [51], infralimbic cortex neurons also heavily innervate the intercalated cells of the amygdala and the lateral division of the central nucleus of the amygdala [21, 52]. These regions contain GABAergic neurons that inhibit output neurons of the medial division of central amygdala to inhibit fear [53]. Lesion of intercalated cells impaired extinction [22], while activation of intercalated cells facilitated extinction [54]. On the other hand, prelimbic cortex targets basolateral amygdala. Basolateral amygdala connects via excitatory projections to output neurons of central amygdala [24, 55], and these neurons trigger midbrain and hypothalamic structures, resulting in the expression of fear [56]. Further, basolateral amygdala provides projections to both prelimbic cortex and infralimbic cortex [57, 58]. The presence of these reciprocal connections highlights the importance of investigating multiple structures within the circuit.
Not only is this corticolimbic circuit linked to psychopathology, it is involved in regulation of the stress response [20], and plays a role in emotion regulation [59]. Its involvement in fear conditioning and extinction is well documented, and thus provides a neural substrate to address questions of stress effects on behavior. However, despite the sex differences in the rates and expression of stress-related psychological disorders, most of the research on the neurobiological mechanisms underlying stress effects on emotional behavior focuses on males. Thus, research into the mechanisms underlying potential stress-induced plasticity of corticolimbic structures in females may provide the groundwork necessary to develop sex-specific treatment for stress-related psychopathology. In this review, we will focus on sex differences and potential differential effects of chronic stress on morphology of basolateral amygdala and medial prefrontal cortex and fear conditioning and extinction in rodents.
2. Chronic stress effects on neuronal morphology
As in primates, prefrontal cortex in rodents can be subdivided into several major subregions. Medial prefrontal cortex includes anterior cingulate, prelimbic, and infralimbic cortex. This region is functionally homologous to the primate dorsolateral and ventromedial prefrontal cortices, and plays a role in autonomic and HPA axis regulation, emotion regulation [e.g., prelimbic cortex plays a role in expression of conditioned fear, while infralimbic cortex plays a role in retrieval of extinction, see 60 and above], and working memory. Orbitofrontal cortex, which includes the medial, ventral, and lateral orbitofrontal subregions, is functionally homologous to primate orbitofrontal cortex and appears to play a role in modulating behavioral responses based on changing incentive values of reward-related stimuli [13].
Medial prefrontal cortex is involved in cognitive tasks that are influenced by chronic stress [as reviewed in 13], is a target for stress hormones like corticosterone [61], and helps regulate HPA axis activity [62]. In male rats, chronic restraint stress produced retraction of apical dendrites of pyramidal neurons in male prelimbic cortex [63–70], an effect that was mimicked with chronic corticosterone administration [71–73]. A similar pattern of stress-induced retraction was seen for apical dendritic branches of neurons within the infralimbic region of medial prefrontal cortex [74, 75]. Finally, even shorter, milder episodes of stress were sufficient to produce dendritic atrophy. Ten minutes of stress for 10 days [76] or 3 weeks of vehicle injection alone [71] reduced dendritic arborization within medial prefrontal cortex, again with retraction occurring only in distal portions of the apical arbor. Medial prefrontal cortex appears to be particularly sensitive to even acute stress, as a single episode of forced swimming produced reductions in infralimbic apical dendritic branching in mice [74].
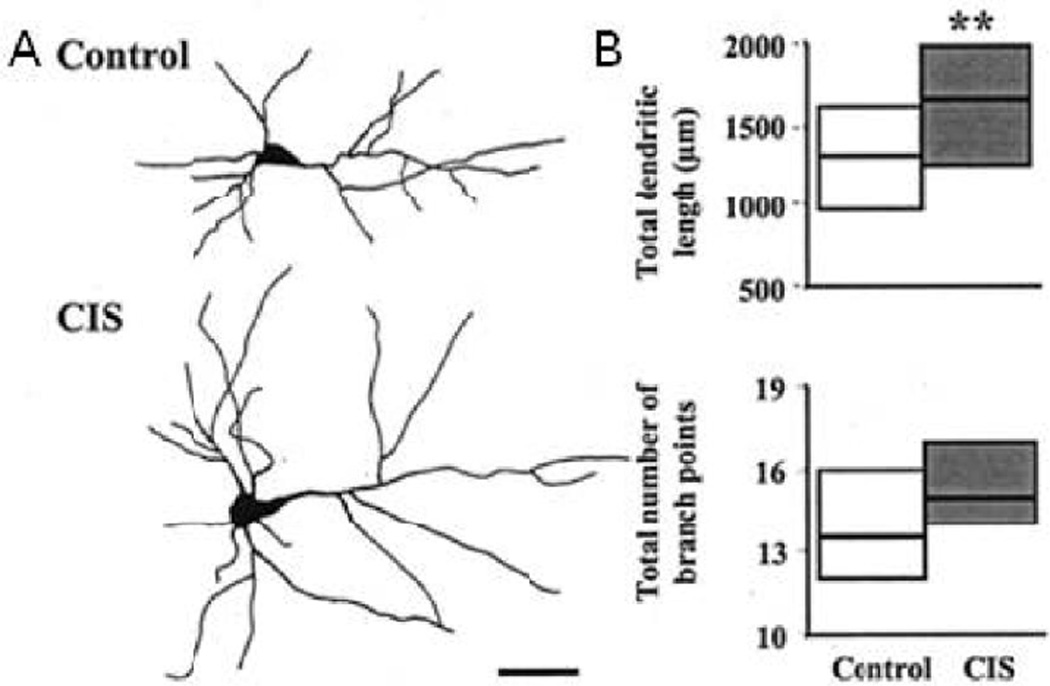
The amygdala is made up of separate subnuclei with disparate functions, including reproduction, aggression, and learning [as reviewed in 77]. Many studies have shown sex differences in medial amygdala [78–81], yet less is known about sex differences and stress effects in basolateral amygdala, a critical structure in the fear and extinction circuit (see above). The amygdala is rich in corticosteroid receptors [82] and is involved in the regulation of glucocorticoid activity [62], and thus is a likely target for stress. Indeed, like neurons within medial prefrontal cortex, basolateral amygdala neurons are affected by chronic stress. Long-term (10–21 days) immobilization stress increased dendritic length of pyramidal cells within the basolateral amygdala in male rats [83, 84] (see Figure 1). There is stressor sensitivity within the amygdala, as chronic immobilization stress but not chronic unpredictable stress induced dendritic remodeling in basolateral amygdala [85]. However, unlike recovery effects observed in medial prefrontal cortex [67], 21 days of stress-free recovery did not rescue the stress-induced hypertrophy of the amygdala [86].
Figure 1.
Chronic immobilization stress (CIS) resulted in dendritic hypertrophy in basolateral amygdale pyramidal neurons in male rats. A. Reconstructions of representative neurons from unstressed and stressed male rats. Scale bar, 50 µm. B. Medians and inter-quartile ranges for total dendritic length (top) and total number of branch points (bottom) for neurons from unstressed and stressed male rats. *p < 0.05, **p < 0.01. Modified from [83].
2.1 Chronic stress effects on behavior
Given that there is a relationship between dendritic structure, dendritic spines and synaptic input, and neural firing rates [87], stress-induced structural alterations may have important effects on neural function and behavior. Because chronic stress in males produces profound changes in dendritic morphology of medial prefrontal cortex and basolateral amygdala, it is not surprising that stress has important effects on fear conditioning and extinction. Several studies have shown that in males, the acquisition of either contextual or cued fear conditioning can be enhanced with chronic restraint stress, with stressed males freezing more than unstressed males [88–91]. Facilitated acquisition of conditioning is also seen in male rats with glucocorticoid exposure similar to that of chronic stress levels [92]. Such evidence suggests that chronic stress-induced changes in amygdala could be responsible for stress-induced changes in behavior; however, little research has been done to address this question.
Other studies have shown chronic stress effects on extinction in males. For instance, one week of chronic restraint stress impaired retrieval of extinction of conditioned fear [89, 93, 94]. Subsequent studies have shown that one week of unpredictable mild stress produced similarly specific changes in extinction retrieval [95], as does a longer-term stressor [6 hr of daily restraint stress for 3 weeks; 96]. In mice, one episode of swim stress occurring 24 hours before fear conditioning attenuated the rate of extinction, though the authors could not differentiate between extinction acquisition and extinction retrieval [74]. Further, stress-induced changes seen in medial prefrontal cortex were responsible for the stress-induced behavioral impairment in retrieval, as removal of infralimbic cortex before the chronic stressor occluded the stress-induced impairment of extinction retrieval [89]. Finally, in male rats, stress-induced alterations in fear conditioning and extinction were associated with stress-induced changes in physiology, as the stress-induced deficit in extinction retrieval was accompanied by alterations in activity of medial prefrontal cortex neurons [94]. In prelimbic cortex, stress prevented the tone-evoked inhibition of activity seen in unstressed rats. In infralimbic cortex, neurons in unstressed rats exhibited increased firing rate in response to the CS, whereas stressed rats showed inhibition of infralimbic cortex firing during the tone. Thus, the stress-induced dendritic changes seen in medial prefrontal cortex are consistent with the stress-induced impairment of a prefrontally mediated behavior, extinction retrieval, as well as alterations in neural activity in medial prefrontal cortex.
Differential dendritic remodeling patterns in medial prefrontal cortex and basolateral amygdala of male rats in response to chronic stress may contribute to the stress-induced impairment versus facilitation of medial prefrontal cortex- and amygdala-mediated behaviors. Dendritic retraction seen in medial prefrontal cortex may reflect less excitatory input and hypoactivity of the structure, resulting in impairment of extinction retrieval. On the other hand, dendritic proliferation seen in basolateral amygdala may reflect more excitatory input and hyperactivity of the structure, resulting in facilitation of the acquisition of fear conditioning. Whether or not this is the case remains to be determined, as one study found that stress-induced dendritic retraction within area CA3 of the hippocampus was associated with increased neuronal excitability [97]. Though work from our lab is consistent with the hypothesis that the stress-induced changes seen in male prefrontal cortex may be a neural mechanism for the stress-induced impairment in extinction retrieval [89], a direct demonstration that stress-induced dendritic changes in prefrontal cortex and basolateral amygdala are responsible for alterations in fear conditioning and extinction still awaits further testing. In addition, because medial prefrontal cortex and basolateral amygdala are interconnected, altering the structure of one region could alter function of the other region, thus influencing behaviors mediated by both. For example, if activity in infralimbic cortex is abnormally suppressed by chronic stress exposure, there may be an accompanying facilitation of fear expression from excessive activation in prelimbic cortex and amygdala. Thus, the balance of activity among regions in the circuit could be especially important, and disruption of that balance by chronic stress may have detrimental effects on behavior.
3. Sex differences in dendritic morphology
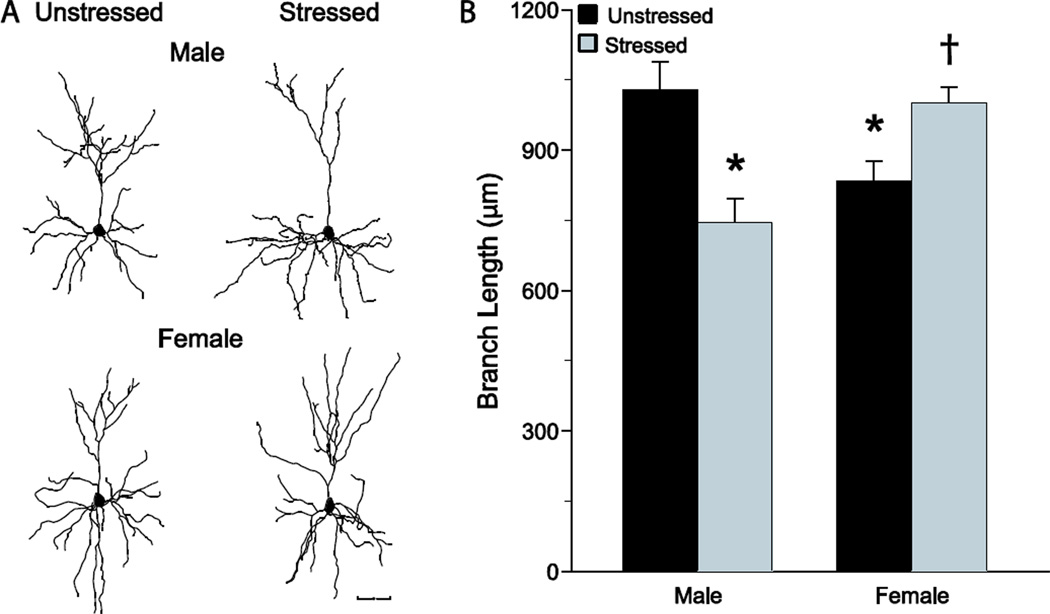
In rats, medial prefrontal cortex is sexually dimorphic. For instance, intact, unstressed females had smaller and less complex apical dendritic arbors in prelimbic cortex pyramidal neurons than intact, unstressed males [69, 98, 99] (see Figure 2). Given that medial prefrontal cortex contains estrogen and progesterone receptors [100], this sexual dimorphism could be mediated at least in part by gonadal hormones. In female rats, ovarian hormones fluctuate over a 4 to 5 day cycle characterized by elevated levels of estrogens and progesterone in proestrus compared to lower levels of ovarian hormones in estrus, metestrus, and diestrus [101].
Figure 2.
Chronic restraint stress produced apical dendritic retraction in pyramidal neurons in prelimbic cortex of males, and apical dendritic hypertrophy in females. A. Reconstructions of representative neurons from unstressed and stressed male and female rats. Scale bar, 50 µm. B. Mean (±SEM) length of apical dendrites for male and female unstressed versus stressed rats. *p < 0.05 relative to unstressed males, † p < 0.05 relative to unstressed females. Modified from [69].
Evidence from other limbic brain regions suggests that sexual dimorphism in dendritic structure is mediated by gonadal hormones. For instance, in the hippocampus, changes in circulating estrogens across the estrous cycle and manipulation of sex steroids have profound effects on dendritic morphology and spine density [reviewed in 102]. Estrogen modulates spine density in CA1 of the hippocampus, with a decrease in spine density in ovariectomized females compared to ovariectomized females with estradiol replacement [103–105]. Again, within CA1 of the hippocampus, intact females showed the largest spine densities while in proestrus and the smallest spine densities while in late estrus [106–108]. In comparison, little is known about the effect of sex steroids on dendritic morphology within medial prefrontal cortex. There was no effect of ovariectomy on dendritic morphology within prelimbic cortex [69] or in infralimbic cortex [109]; thus, exogenous manipulation of estradiol alone does not appear to have an effect on medial prefrontal cortex dendritic morphology. Nonetheless, because fluctuations in estradiol and progesterone can influence dendritic morphology, it is likely that potential remodeling of dendritic arbor in medial prefrontal cortex across the estrous cycle could occur, and should be assessed.
The amygdala has a high concentration of androgen and estrogen receptors [110, 111], suggesting that sex steroids may modulate the morphology or function of amygdalar neurons. Consistent with this hypothesis, estradiol treatment decreased the amplitude of excitatory postsynaptic potentials in basolateral amygdala neurons in vitro [112] and impaired performance on basolateral amygdala-dependent behavioral tasks such as the conditioned place preference task [113]. Though sex differences or estrous phase effects on dendritic morphology within basolateral amygdala have not been examined, sex differences in spine densities in basolateral amygdala have been reported. Male rats had greater spine density on pyramidal neurons than females [114]. Although this study failed to find differences in spine density across estrous phases, the authors only compared proestrous and estrous rats, leaving open the possibility of differences across proestrous and diestrous phases. Further, no study has explored the effect of experimental manipulations of gonadal hormones on basolateral amygdala dendritic morphology.
3.1 Sex differences in chronic stress effects on neuronal morphology and behavior
The relationship between sex and stress steroids in rodents is complex. There are sex differences in basal and stress-induced glucocorticoid levels, as males have lower levels of glucocorticoids than females and release less corticosterone during stress than females [115–118]. Further, corticosterone levels vary over the estrous cycle. Female rats in proestrus have higher basal and stress-induced plasma corticosterone levels compared to rats in other phases of the estrous cycle [115, 116, 118–121], and these fluctuations in estradiol and changes in glucocorticoid levels alter stress sensitivity in female rodents [119]. Given these sex- and gonadal-hormone-dependent differences in glucocorticoid levels, it is likely that stress differentially influences corticolimbic dendritic morphology in males and females. While several studies have demonstrated such sex differences in stress effects on hippocampal morphology [reviewed in 10], little is known about potential sex-dependent stress effects in amygdala and prefrontal cortex.
Whereas chronic stress produced dendritic retraction in male medial prefrontal cortex, female rats showed stress-induced dendritic proliferation in prelimbic cortex neurons (see Figure 2), and this effect was estradiol-dependent [69]. The stress-induced hypertrophy of apical dendrites was eliminated with removal of gonadal hormones by ovariectomy and rescued with ovariectomy plus estradiol treatment. Within infralimbic cortex of ovariectomized females implanted with estradiol, chronic stress-induced proliferation has been reported in a sub-population of neurons that project to basolateral amygdala [109]. This effect was absent in ovariectomized females without estradiol replacement, again suggesting a role for estradiol in the stress-induced dendritic proliferation in medial prefrontal cortex. Thus, there are sex differences in the morphology of pyramidal neurons in prelimbic and infralimbic cortex, and stress differentially affects structure of medial prefrontal cortex in male and female rats. However, it is unknown how chronic stress affects basolateral amygdala dendritic morphology in females, nor is it known how fluctuations in circulating hormones over the estrous cycle may interact with chronic stress to affect dendritic morphology within either medial prefrontal cortex or basolateral amygdala.
Sex differences in medial prefrontal cortex and basolateral amygdala morphology may contribute to sex differences in medial prefrontal cortex and amygdala-dependent behaviors. There are sex differences in prefrontal- and amygdala-mediated cognitive and emotional behaviors, for instance working memory [122–124] and anxiety [125, 126]. These sex differences may be mediated in part by gonadal hormones. Estrogen administration improved prefrontal cortex-dependent cognitive tasks (e.g., delayed nonmatching-to-sample recognition memory task) in primates [127], while removal of endogenous estrogens via ovariectomy impaired prefrontal cortex-dependent cognitive tasks (e.g. object recognition memory task) in rodents [128]. The effect of ovarian hormones may be task-specific; for example, estradiol administration impaired latent inhibition [129] and delayed spatial alternation [130], yet improved working memory [131]. Estrogens can also alter fear learning and fear inhibition [132], contributing to sex differences in fear expression. The first study of sex differences in fear conditioning showed that males and females differed in contextual but not cued fear conditioning, with males displaying more freezing to context than intact females [133]. Subsequent studies investigated how activational effects of ovarian hormones contribute to this sex difference. Ovariectomized females froze to the context CS at levels similar to those of males and more than those of intact females, and the ovariectomy-induced effect could be reversed with estradiol replacement [134], suggesting that removal of endogenous estrogens increased females’ freezing to the context CS. Thus, the activational effect of estrogens may contribute to the sexually dimorphic nature of acquisition of contextual fear conditioning. Contextual and cued fear conditioning involve different but overlapping neural circuits. Contextual fear conditioning is hippocampally-dependent [135–137], while cued conditioning is not. Thus, sex differences in the hippocampus [e.g. 106, 138] could be responsible for the sex difference in contextual conditioning.
Consistent with Maren and colleagues’ earlier report, Milad and colleagues [139] showed that during cued conditioning, when behavioral responses from females are collapsed across estrous cycle phase, there was no sex difference between males and females during any phase of fear conditioning or extinction. However, when estrous cycle phase was considered, females that were in diestrus during initial extinction showed levels of freezing 24 hours later during extinction retrieval that were similar to that of males. In contrast, females that were in proestrus during extinction displayed levels of freezing 24 hours later during extinction retrieval that were lower than those of diestrous females or males. Further, it seems that having high versus low ovarian hormone levels may be important only during extinction [139]. Females in proestrus during conditioning or extinction retrieval showed no differences in freezing from females in diestrus during conditioning or extinction retrieval. These data suggest that circulating levels of ovarian hormones over a three-day testing protocol can have important effects on behavior. Further, peak levels of circulating estrogens during initial extinction may be especially important for retrieval of the extinction memory 24 hours later.
However, there are conflicting findings. A study from Baran and colleagues found that, while they did not differ from males in acquisition of conditioned fear, intact females did not extinguish the conditioned fear [96]. One possible explanation for the differential findings is that the studies used somewhat different fear conditioning and extinction paradigms. Examples of variation in experimental paradigm included behavioral testing in light versus dark cycle, number of tone-shock pairings, intensity of shock, time period for extinction consolidation (1 hr vs. 24 hr), and whether or not rats were trained to bar press for food pellets during conditioning and extinction as a means of ensuring a baseline level of activity against which to measure freezing during massed extinction sessions [see 42]. Further, Baran and colleagues [96] examined females without regard to estrous phase, which could also contribute to the conflicting results. Alternatively, the difference between these studies could be due to handling effects. Baran and colleagues subsequently demonstrated that, unlike in their previous study, intact females that had been handled daily prior to fear conditioning acquired the fear response and subsequently were able to acquire extinction, but failed to retrieve the previous extinction session on the next day [140]. This pattern of results is reminiscent of that seen in Milad and colleagues’ [139] study, in which daily handling (vaginal lavage for characterization of estrous phase) occurred.
Changes in levels of circulating estrogens may mediate the effects of estrous phase on extinction retrieval. In nonovariectomized females, either immediate post-extinction injection of estradiol or estrogen receptor β activation via injection of the estrogen receptor β agonist diarylpropionitrile facilitated extinction retrieval [141]. Moreover, extinction retrieval testing following estradiol administration led to decreased c-Fos expression in basolateral amygdala and increased c-Fos expression in infralimbic cortex [141]. Thus, high levels of estradiol are associated with improvements in the retrieval of extinguished fear by modulating activity within infralimbic cortex and basolateral amygdala, likely through estrogen receptor β activation. However, there are some limitations to administering estradiol in intact, non-ovariectomized animals. Though all rats underwent extinction and estradiol or drug injection while in metestrus (when estrogens are low), administration of estradiol can alter subsequent release of ovarian hormones through feedback mechanisms [reviewed in 142].
Because females exhibit contrasting chronic stress-induced dendritic remodeling in medial prefrontal cortex compared to males, it is likely that stressed females also show a different behavioral pattern in fear learning and extinction. To date, only one study has addressed this issue. Baran and colleagues [96] demonstrated that, in contrast to males, chronically stressed female rats showed impaired acquisition of fear conditioning, but were not impaired in retrieval of fear extinction. However, handling again appears to influence this effect. A subsequent study by this group found that chronically stressed females that had been handled demonstrated facilitated acquisition of conditioning and more freezing to the tone during extinction than unstressed, handled control females, with no effect of estradiol treatment [143].
The paucity of evidence and inconsistencies in the existing literature currently prohibit development of a complete model to explain how stress-induced alterations in corticolimbic morphology might influence fear conditioning and extinction in females. However, it is tantalizing to speculate that the dendritic proliferation in prelimbic pyramidal neurons after stress in females [69] provides for hyperexcitability of prelimbic neurons. The resultant facilitation of expression of conditioned fear could account for Hoffman and colleagues’ [143] finding of facilitated acquisition of conditioning in handled, stressed females. This hypothesis could be tested with studies examining the activity of neurons in medial prefrontal cortex during fear conditioning and extinction in stressed and unstressed females.
Thus, at this point the fledgling literature on both sex differences and differential stress effects in prefrontal-amygdalar circuitry and fear conditioning and extinction has yielded inconsistent results, and further studies are required to fully characterize the nature of the sex differences and to clarify the role of gonadal hormones. An important first step is to determine whether or not chronic stress differentially influences dendritic morphology in basolateral amygdala in males versus females. Moreover, while gonadal hormonal status in adulthood has been implicated as being permissive of stress effects in medial prefrontal cortex in females, a similar examination of the potential influence of gonadal hormones on stress effects in males is absent. Further, a major unresolved question that must be addressed is the potential role of gonadal hormones acting early in development to organize sex differences in structure and function in prefrontal-amygdalar circuitry and the behaviors they mediate. For example, the sex differences in response to stress could reflect differences in circulating gonadal hormones (type, normal fluctuations) at adulthood or the consequences of the actions of those gonadal hormones during development, resulting in structural and/or functional differences that provide the substrate for the differential stress effects seen in adult males and females.
4. Summary and Conclusions
The current literature describes sex differences in dendritic morphology of pyramidal neurons in medial prefrontal cortex and spine density in basolateral amygdala, as well as sex differences in behavioral responses during fear conditioning and extinction. While chronic stress effects on these variables are well-documented in males, the data on females is sparse. Nonetheless, these data show that there are sex differences in dendritic morphology within the prelimbic and infralimbic regions of medial prefrontal cortex. Further, sex-dependent stress effects on dendritic morphology in medial prefrontal cortex have been documented. Overall, existing behavioral studies suggest that a) there are sex differences in fear conditioning and extinction, which may be estrogen-dependent; and b) stress effects on fear conditioning and extinction vary in males and females. However, methodological differences and limitations across published studies make it difficult to determine the exact nature of these differences and their dependence on estrogens. For instance, the literature on stress-induced changes in performance during fear conditioning and extinction in females often does not take into account gonadal hormonal status, or uses daily injections of estradiol (an additional stressor and possibly disruptive of the estrous cycle) to mimic natural variations in estradiol. No previous study examining fear conditioning and extinction has manipulated ovarian hormones via ovariectomy and estradiol replacement. In addition to the differences in using intact females versus females with gonadal hormone manipulation (with differences across studies in use of daily injections to mimic estrous cycle, dose and type of hormone used, duration of hormone treatment), differences in fear conditioning paradigms across previous studies further limit our ability to compare results across studies, and therefore potential differences across groups. Finally, there are differences across studies in handling (daily lavage or injection versus no handling) that may affect behavioral responses during fear conditioning and extinction. Nonetheless, collectively these studies provide our first evidence that stress may alter fear conditioning and extinction in a sex-dependent manner. It is now important to begin to understand the interaction between chronic stress, sex, and ovarian hormones as a first step in elucidating the mechanisms underlying both basic sex differences in this model of emotion regulation, and differential effects of stress in the model system. Given sex differences in rates of anxiety disorders, and deficits in extinction and dysfunction of prefrontal cortex and amygdala that characterize disorders such as PTSD, uncovering these mechanisms is an essential first step in developing sex-specific treatment strategies for stress-dependent psychopathology.
Highlights.
Expression and rates of stress-dependent psychopathology are sex-dependent.
Understanding how stress differentially affects circuits underlying emotional behaviors may elucidate mechanism of sex differences in psychopathology.
Recent data demonstrate sex differences in dendritic morphology within the prelimbic and infralimbic regions of medial prefrontal cortex and in basolateral amygdala, and differential stress effects in medial prefrontal cortex.
Recent data demonstrate sex differences in fear conditioning and extinction, and suggest that stress effects on fear conditioning and extinction vary in males and females.
Differential stress effects on dendritic morphology in medial prefrontal cortex and amygdala could contribute to the differential stress effects on fear conditioning and extinction.
Acknowledgements
Funding provided by National Institute of Mental Health MHR03087794 to CLW, Eunice Kennedy Shriver National Institute of Child Health & Human Development 5T32HD049336 to MRF, Indiana University Center for the Integrative Study of Animal Behavior to MRF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Solomon MB, Herman JP. Sex differences in psychopathology: of gonads adrenals mentalillness. Physiol. Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg CM, Beekman AT, Deeg DJ, van Tilburg W. Sex differences in late-life depression. Acta Psychiatr Scand. 2000;101:286–292. [PubMed] [Google Scholar]
- 5.Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estr0ogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37:434–441. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- 6.Young EA, Altemus M. Puberty, ovarian steroids, and stress. Annals of the New York Academy of Sciences. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- 7.Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13:175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- 8.Lechin F, Van der Dijs B, Benaim M. Stress versus depression. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:899–950. doi: 10.1016/0278-5846(96)00075-9. [DOI] [PubMed] [Google Scholar]
- 9.Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Archives of general psychiatry. 2004;61:481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin K, Baran S, Conrad C. Chronic Stress- and Sex-Specific Neuromorphological and Functional Changes in Limbic Structures. Molecular Neurobiology. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- 11.Graybeal C, Kiselycznyk C, Holmes A. Stress-induced impairments in prefrontal-mediated behaviors and the role of the N-methyl-d-aspartate receptor. Neurosci. 2012;211:28–38. doi: 10.1016/j.neuroscience.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2007;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord. 2006;94:219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Fossati P, Radtchenko A, Boyer P. Neuroplasticity: from MRI to depressive symptoms. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S503–S510. doi: 10.1016/j.euroneuro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Nohara S, Hagino H, Takahashi T, Kawasaki Y, Yamashita I, et al. Prefrontal abnormalities in patients with simple schizophrenia: structural and functional brain-imaging studies in five cases. Psychiatry Res. 2005;140:157–171. doi: 10.1016/j.pscychresns.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 19.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 22.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional Perseveration: An Update on Prefrontal-Amygdala Interactions in Fear Extinction. Learn Memory. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- 26.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and biobehavioral reviews. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatr. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends in Neurosciences. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiat. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 30.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 31.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 34.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 35.Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-06-j0001.2001. RC135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. The Journal of Neuroscience. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- 39.Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- 40.Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- 41.Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends in Neurosciences. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quirk GJ, Russo GK, Barron JK, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral neuroscience. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 44.Chang CH, Maren S. Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learn Memory. 2011;18:221–225. doi: 10.1101/lm.2070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 47.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal-Gonzalez I, Vidal-Gonzalez Bn, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Memory. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained Conditioned Responses in Prelimbic Prefrontal Neurons Are Correlated with Fear Expression and Extinction Failure. The Journal of Neuroscience. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. The Journal of Comparative Neurology. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 52.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 53.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pare D, Smith Y. GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. The Journal of Comparative Neurology. 1994;344:33–49. doi: 10.1002/cne.903440104. [DOI] [PubMed] [Google Scholar]
- 56.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoover W, Vertes R. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 58.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 59.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- 62.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 64.Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cerebral Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 67.Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 72.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cerebral Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- 74.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-Induced Dendritic Remodeling in the Prefrontal Cortex is Circuit Specific. Cerebral Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown S, Henning S, Wellman CL. Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 77.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 78.Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Rocha MI, Mestriner RG, Hermel EE, Xavier LL, Rasia-Filho AA, Achaval M. Neuronal somatic volume of posteroventral medial amygdala cells from males and across the estrous cycle of female rats. Neurosci Lett. 2007;420:110–115. doi: 10.1016/j.neulet.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 81.Hermel EE, Ilha J, Xavier LL, Rasia-Filho AA, Achaval M. Influence of sex and estrous cycle, but not laterality, on the neuronal somatic volume of the posterodorsal medial amygdala of rats. Neurosci Lett. 2006;405:153–158. doi: 10.1016/j.neulet.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 82.Reul J, De Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 83.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. The Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- 86.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Spruston N. Pyramidal neurons: dendritic structure synaptic integration Nature reviews. Neuroscience. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- 88.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 89.Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol Learn Mem. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Sanders MJ, Stevens S, Boeh H. Stress enhancement of fear learning in mice is dependent upon stressor type: Effects of sex and ovarian hormones. Neurobiology of Learning and Memory. 2010;94:254–262. doi: 10.1016/j.nlm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 91.Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress. 2001;4:305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conrad CD, MacMillan DD, 2nd, Tsekhanov S, Wright RL, Baran SE, Fuchs RA. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of Learning and Memory. 2004;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, et al. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiology of Learning and Memory. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 98.Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 99.Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. Journal of Neuroendocrinology. 1991;3:95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 100.Pilgrim C, Hutchison JB. Developmental regulation of sex differences in the brain: can the role of gonadal steroids be redefined? Neuroscience. 1994;60:843–855. doi: 10.1016/0306-4522(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 101.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 102.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and behavior. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 103.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M. Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci Lett. 2005;379:52–54. doi: 10.1016/j.neulet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 108.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen Promotes Stress Sensitivity in a Prefrontal CortexÄìAmygdala Pathway. Cerebral Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 111.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 112.Womble MD, Andrew JA, Crook JJ. 17beta-Estradiol reduces excitatory postsynaptic potential (EPSP) amplitude in rat basolateral amygdala neurons. Neurosci Lett. 2002;331:83–86. doi: 10.1016/s0304-3940(02)00871-6. [DOI] [PubMed] [Google Scholar]
- 113.Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioural Brain Research. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- 114.Rubinow MJ, Drogos LL, Juraska JM. Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiol Aging. 2009;30:137–146. doi: 10.1016/j.neurobiolaging.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 116.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. Journal of Endocrinology. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 117.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 118.Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- 119.Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- 120.Viau V, Meaney MJ. Variations in the Hypothalamic-Pituitary-Adrenal Response to Stress during the Estrous Cycle in the Rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 121.Pollard I, White BM, Bassett JR, Cairncross KD. Plasma glucocorticoid elevation and desynchronization of the estrous cycle following unpredictable stress in the rat. Behav Biol. 1975;14:103–108. doi: 10.1016/s0091-6773(75)90374-0. [DOI] [PubMed] [Google Scholar]
- 122.Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 123.Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- 125.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 126.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 127.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 129.Nofrey BS, Ben-Shahar OM, Brake WG. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn. 2008;66:156–160. doi: 10.1016/j.bandc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 130.Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, et al. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behavioral neuroscience. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Velazquez-Zamora DA, Garcia-Segura LM, Gonzalez-Burgos I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Hormones and Behavior. 2012;61:512–517. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 132.Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 134.Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- 135.Ross RT, Orr WB, Holland PC, Berger TW. Hippocampectomy disrupts acquisition and retention of learned conditional responding. Behav Neurosci. 1984;98:211–225. doi: 10.1037//0735-7044.98.2.211. [DOI] [PubMed] [Google Scholar]
- 136.Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behav Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- 137.Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav Neurosci. 1993;107:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- 138.Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Journal of Neurobiology. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- 139.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baran SE, Armstrong CE, Niren DC, Conrad CD. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn Memory. 2010;17:267–278. doi: 10.1101/lm.1778010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol Modulates Medial Prefrontal Cortex and Amygdala Activity During Fear Extinction in Women and Female Rats. Biol Psychiat. 2011 doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2009;21:327–333. doi: 10.1111/j.1365-2826.2009.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoffman AN, Armstrong CE, Hanna JJ, Conrad CD. Chronic stress, cyclic 17[beta]-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiology of Learning and Memory. 2010;94:422–433. doi: 10.1016/j.nlm.2010.08.010. [DOI] [PubMed] [Google Scholar]