Abstract
Background
Calcium plays an essential role in nearly all cellular processes. As such, cellular and systemic calcium concentrations are tightly regulated. During sepsis derangements in such tight regulation frequently occur, and treating hypocalcemia with parenteral calcium administration remains the current practice guideline.
Objective
We investigated whether calcium administration worsens mortality and organ dysfunction using an experimental murine model of sepsis and explored the mechanistic role of the family of calcium/calmodulin-dependent protein kinases in mediating these physiologic effects. To highlight the biological relevance of these observations, we conducted a translational study of the association between calcium administration, organ dysfunction and mortality among a cohort of critically ill septic ICU patients
Design
Prospective, randomized controlled experimental murine study. Observational clinical cohort analysis.
Setting
University research laboratory. Eight ICUs at a tertiary care center.
Patients
870 septic ICU patients.
Subjects
C57BL/6 and CaMKK−/− mice.
Interventions
Mice underwent cecal ligation and puncture polymicrobial sepsis and were administered calcium chloride (0.25 or 0.25 mg/kg) or normal saline.
Measurements and Main Results
Administering calcium chloride to septic C57BL/6 mice heightened systemic inflammation and vascular leak, exacerbated hepatic and renal dysfunction, and increased mortality. These events were significantly attenuated in CaMKK−/− mice. In a risk–adjusted analysis of septic patients, calcium administration was associated with an increased risk of death, OR 1.92 (95% CI 1.00–3.68, p=0.049), a significant increase in the risk of renal dysfunction, OR 4.74 (95% CI 2.48–9.08, p<0.001), and a significant reduction in ventilator free days, mean decrease 3.29 days (0.50–6.08 days, p=0.02).
Conclusions
Derangements in calcium homeostasis occur during sepsis that are sensitive to calcium administration. This altered calcium signaling, transduced by the CaMKK cascade, mediates heightened inflammation and vascular leak that culminates in elevated organ dysfunction and mortality. In the clinical management of septic patients calcium supplementation provides no benefit and may impose harm.
Keywords: calcium, sepsis, infection, inflammation, calcium/calmodulin-dependent protein kinase, mortality, organ failure
Introduction
Calcium plays an essential role in nearly all biological processes. As such, ionized calcium concentration [Ca2+]i is tightly regulated at both the cellular and systemic level. However, during critical illness, derangements in calcium homeostasis occur with considerable frequency. Indeed, hypocalcemia is prevalent in up to 88% of critically ill patients.[1–4] Prior studies suggest that the development of hypocalcemia is an independent risk factor for increased mortality.[1–3] However a causal relationship between hypocalcemia and either organ dysfunction or mortality has not been elucidated.[5]
These observations led to the current practice guideline of treating low [Ca2+]i with the parenteral administration of calcium.[2, 6–10] However, evidence has emerged to challenge this common practice. A recent systematic review concluded that there is no evidence to support treating hypocalcemia of critical illness.[11] Further, animal models of sepsis and trauma have demonstrated that calcium supplementation actually worsens mortality and organ dysfunction[12–16], whereas calcium antagonism may improve cellular function.[17–20]
Currently, it is hypothesized that dysregulated intracellular calcium handling underlies the potential detriment in parenteral calcium supplementation to address hypocalcemia of critical illness.[15, 21] Though systemic [Ca2+]i is low, intracellular [Ca2+]i is high in the setting of sepsis and trauma. These alterations in intracellular [Ca2+]i are thought to contribute to a heightened inflammatory response, cellular death and subsequent organ dysfunction.[16, 21–29]
Though derangements in calcium handling have been implicated in critical illness, the specific mechanisms underlying this process remain unknown. The calcium/calmodulin-dependent protein kinases (CaMK) are a family of multifunctional kinases, sensitive to changes in intracellular [Ca2+] and, thus, may act as receivers of these abnormal calcium signals. The CaMKs coordinate a variety of cellular functions including gene expression, cell cycle progression, and apoptosis, and recent data suggest that specific CaMK members play a functional role in innate immunity.[30] [31–35]
In this study we propose that 1) the administration of parenteral calcium worsens mortality and organ dysfunction in a murine model of intraabdominal sepsis, 2) the CaMK cascade plays a mechanistic role in mediating these physiologic and clinical effects, and 3) a T1 translational clinical study will demonstrate an association between calcium administration, organ dysfunction and mortality among a cohort of critically ill septic ICU patients, thus highlighting the relevance of our observations to human biology.
Materials and Methods
Reagents
Evan’s Blue dye (Sigma, St. Louis, MO) was reconstituted in PBS. Imipenem (Merck, Whitehouse Station, NJ) was reconstituted in normal saline (NS). Calcium chloride (CaCl2) 10% w/v was purchased from American Regent (Shirley, NY). The broad CaMK inhibitor, KN93 (Calbiochem), and its less functional analogue KN92, were dissolved in sterile PBS.
Animal experimentation
We performed all animal experiments in accordance with the National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. We randomly assigned 6–8 week old male mice of C57BL/6J and B6.129X1-Camkk1tm1Tch/J (CaMKK−/−) strains to a specific experiment. The CaMKK−/− strain is derived from a C57BL/6J background; these mice are viable, fertile, and do not display any gross physical abnormalities.
We performed cecal ligation and puncture (CLP) as previously described, using a single 21-gauge puncture.[31,32] Imipenem/Cilastin (0.75 mg/kg, SQ) was administered 12 hours postoperatively. Treatment groups consisted of CaCl2 (0.25 mg/kg or 2.5 mg/kg) or 0.9% NS isovolemic control administered 12 hours after CLP by IP injection. For animals assigned to biochemical CaMK manipulation, KN93 or KN92 (6 mg/kg, IP) was administered 12- and 1- hours prior to CLP.
For all studies, one investigator performed the surgical experimentation and collected the samples. A separate individual administered the NS and CaCl2 without prior knowledge of the surgical treatment. The data were then analyzed by an investigator blinded to the specific treatment.
Evans Blue Permeability
Mice were anesthetized, and a 1% w/v solution of Evans Blue was administered by lateral tail vein injection.[36, 37] After 30 minutes, the intravascular compartment was flushed by right ventricular injection of 1mL of NS. The lungs, liver, and kidneys were then collected and stored overnight in 1 ml of formamide at 55°C. The Evans Blue content of the extracts was measured by spectrometry (OD620-OD500).
Organ Physiology
Serum concentrations of alanine aminotransferase (ALT) and blood urea nitrogen (BUN) were quantitatively determined using a HESKA DRI-CHEM Analyzer.
ELISA
IL-10, TNFα, and IL-6 concentrations were quantified by an enzyme immunoassay kit (R&D Systems, Minneapolis, MN).
Myeloperoxidase activity
Tissue myeloperoxidase (MPO) determination assay was modified from previously published sources.[38, 39]° Snap frozen tissues were weighed and homogenized in MPO buffer (6.8 g KH2PO4 and 8.7 g K2HPO4 in 1 L H20) containing 0.5% hexadecyltrimethylammonium bromide at a ratio of 50 mg tissue:1 mL MPO buffer. The homogenate was centrifuged (20,000 g) for 4 minutes at 4°C. 7 μL of supernatant was placed into a 96 well plate with 200 μL of O-dianisidine hydrochloride solution. Each sample was measured in triplicate, and the average used to calculate the MPO activity by dividing the Δ Absorbance450 nm (between 0 and 60 sec)/time (min)/mL by the extinction coefficient, 1.13 × 10−2.
Statistical analysis
Statistical analyses were performed using Stata 12 SE software (College Station, TX). Values are expressed as means ± SEM. Groups are compared by non-parametric Wilcoxon Rank-Sum test. A p<0.05 was considered statistically significant.
Clinical study
Study design
We conducted a retrospective cohort study to determine the association between calcium administration and mortality or organ dysfunction among septic ICU patients. We used the University of Pittsburgh Medical Center (UPMC) APACHE III dataset, an ongoing database that randomly calculates APACHE III scores for one third of patients.[40, 41] De-identified data were linked to electronic demographic, clinical, and pharmacological data from the HiDenIC (High Density Intensive Care) database, which retrospectively collects information on patients admitted to 8 ICUs at UPMC Presbyterian and Montefiore hospitals.[40, 41] This study was approved by the Institutional Review Board at the University of Pittsburgh.
Patients
Demographic and physiological variables were available for 1276 patients admitted between June 30, 1999 to July 31, 2004 with a primary diagnosis of sepsis. Of these, 870 (82.6%) possessed calculated APACHEIII scores and formed the cohort for analysis. We focused our analysis on patients requiring more than 24 hours of ICU care.
Variables and risk adjustment
Our primary exposure of interest was parenteral calcium administration: total ampules of calcium chloride or calcium gluconate administered parenterally. The primary outcome was hospital mortality during the index admission. We examined cumulative calcium administration both as a continuous variable (0, 1, 2, ≥ 3 doses) and as a binary outcome (calcium vs. none). Secondary outcomes included acute renal dysfunction (creatinine > 2.0 mg/dL) and highest BUN concentration. Pulmonary dysfunction was quantified using ventilator-free days (VFD).[42]
We addressed potential confounding by controlling for the severity of illness, quartiles of lowest [Ca2+]i, and additional variables related to the outcome. Hypocalcemia was defined as a serum [Ca2+]i < 1.15 mEq/L.[2] The severity of illness was determined according to the APACHE III score (range, 0 to 299).[43] Other risk-adjustment variables included age, sex, race, the season and year of admission, and the type of ICU.
Statistical analyses
We performed a random effects multivariate logistic regression to assess the association between the parenteral administration of calcium and the risk of mortality, calculating crude and adjusted odds ratios (aOR) with 95% confidence intervals (95% CI), accounting for the correlation within ICUs and covariates specified a priori as potential confounders: age, sex, race, season and year, ICU location, APACHE III, and quartiles of lowest [Ca2+]i. We explored whether the association between calcium supplementation and mortality varied with the degree of hypocalcemia by including an interaction term between calcium administration and the hypocalcemia covariate. A similar analysis was performed to assess the association between calcium administration and the development of renal dysfunction and pulmonary dysfunction. A p value <0.05 was considered significant.
Results
Calcium supplementation during sepsis worsens organ function and mortality in septic ICU patients
A total of 526 of 870 (60.4%) septic ICU patients had at least 1 measurement of total calcium or [Ca2+]i. The mean nadir [Ca2+]i was 1.09 ± 0.14 mEq/L, and 377 (71.7%) were hypocalcemic. Of these 526, 93 (17.7%) received parenteral calcium and a median of 1 [IQR: 0 to 2] ampules of calcium. Those receiving calcium had higher APACHEIII scores 80.0 vs. 66.4, p<0.001. Those patients for whom [Ca2+]i was not measured were less ill with a mean APACHEIII score of 58.9, and only 7 (2.0%) received calcium.
In multivariate analysis the magnitude of hypocalcemia was not associated with an increased risk of death: [Ca2+]i 1.00–1.15 mEq/L, aOR 1.01 (95% CI 0.56 to 1.79, p=0.57); [Ca2+]i 0.85–0.99 mEq/L, aOR 1.27 (95% CI 0.56 to 2.86; p=0.97); [Ca2+]i <0.85 mEq/L, aOR 1.02 (95% CI 0.27 to 3.92; p=0.97). However, the administration of any calcium was associated with an increased risk of death, aOR 1.92 (95% CI 1.00 to 3.68, p=0.049) (Figure 1). With calcium as a continuous variable, increasing doses of calcium were associated with increased mortality, aOR 1.53 (95% CI 1.10 to 2.13, p=0.01). The association was similar for calcium chloride and calcium gluconate. An adjusted analysis incorporating those patients without [Ca2+]i measurements yielded similar results, aOR 2.11 (95% CI 1.18 to 3.78, p=0.01). There was no significant association between the magnitude of hypocalcemia and renal dysfunction (data not shown). Yet, parenteral calcium was associated with a significant increase in BUN, adjusted mean increase 15.5 mg/dL (95% CI 6.18 to 24.9, p<0.001) and risk of renal dysfunction, aOR 4.74 (95% CI 2.48 to 9.08, p<0.001). Increasing doses of calcium were associated with an increased risk of renal dysfunction (aOR 2.08, 95% CI 1.45 to 2.98, p<0.001) and BUN (8.44 mg/dL, 95% CI 3.48 to 13.4, p=0.001). There was no significant association between the magnitude of hypocalcemia and VFD (data not shown). However, parenteral calcium was associated with reduced VFD, adjusted mean decrease 3.29 days (0.50 to 6.08 days, p=0.02).
Figure 1. Calcium supplementation is associated with increased mortality in septic patients: Cox proportional hazards survival estimates.
Risk-adjusted Cox proportional hazards estimates of the association between calcium administration and mortality in a human cohort of septic patients.
Calcium supplementation during sepsis induces organ failure and increases mortality
We explored whether physiologic doses of CaCl2 altered mortality in a murine model of sepsis. We observed an apparent dose-response relationship between CaCl2 and increased death (Figure 2). By comparison to NS, CaCl2 (0.25 mg/kg) increased mortality: 8.3% vs. 50% (p=0.01). At a CaCl2 dose of 2.5 mg/kg mortality was further increased to 66.7% (p=0.001). This increased mortality appeared to be mediated by an exacerbation in organ injury/dysfunction. As shown in Figure 3, CaCl2 (2.5 mg/kg) worsened both liver injury and renal dysfunction: serum ALT (30±2.4 vs. 155±53.9 mg/dL, p=0.009) and BUN (19±4.6 vs. 90.7±31.0 mg/dL, p=0.049).
Figure 2. Calcium administration during sepsis increases mortality via calcium/calmodulin-dependent protein kinase kinase (CaMKK) signaling.
C57Bl/6 and CaMKK−/− mice were subjected to cecal ligation and puncture, followed by normal saline (3 mL/100 gm) and imipenem/cilastatin (0.5 mg/kg) administration. After 12 hours, mice were administered CaCl2 (0.25 mg/kg or 2.5 mg/kg) or equivolume normal saline by intraperitoneal injection. Survival was assessed every 6 hours for 7 days. *compared to C57Bl/6 receiving normal saline. †compared to C57Bl/6 receiving 2.5 mg/kg CaCl2. (n=12 animals per group)
Figure 3. Calcium administration during sepsis induces liver injury and renal dysfunction via calcium/calmodulin-dependent protein kinase kinase (CaMKK) signaling.

C57Bl/6 and CaMKK−/− mice were subjected to cecal ligation and puncture, followed by normal saline (3 mL/100 gm) and imipenem/cilastatin (0.5 mg/kg) administration. After 12 hours, mice were administered CaCl2 (2.5 mg/kg) or equivolume normal saline by intraperitoneal injection. After 24 hours, mice were euthanized, and blood was isolated by cardiac puncture, and the serum analyzed for alanine aminotransferase (ALT) concentrations and blood urea nitrogen (BUN). (n=12 animals per group)
CaMKK mediates calcium signaling and the increased risk of organ failure and mortality
Our laboratory has previously shown that members of the CaMK mediate the inflammatory response during sepsis.[31, 32] As shown in Figure 2, the increased mortality with CaCl2 (2.5 mg/kg) was attenuated in CaMKK−/− mice: 66.7% vs. 33.3%, p=0.03. Similarly, organ injury/dysfunction induced by CaCl2 was reduced in CaMKK−/− mice: ALT (51.8±6.5 vs. 155±53.9, p=0.06); BUN (23.5±1.0 vs. 90.7±31.0, p=0.04) (Figure 3).
Calcium administration during sepsis heightens and prolongs systemic inflammation via CaMKK signaling
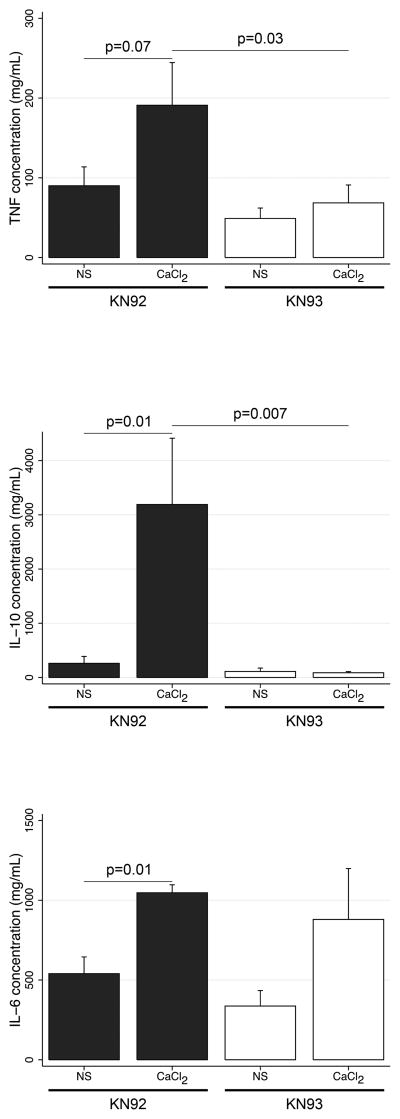
We observed that LPS elevates Mφ cytosolic [Ca2+] (data not shown). We hypothesized that calcium administration heightens inflammation. As shown in Figure 4, CaCl2 (2.5 mg/kg) elevated and prolonged heightened systemic concentrations of TNFα, IL-10 and IL-6 in WT mice, which was not observed in CaMKK−/− mice. Pharmacological blockade of CaMK using KN93, by comparison to its inactive analogue KN92, similarly attenuated the increase in TNFα and IL-10 induced with CaCl2 (Figure 5). Twelve hours following CaCl2, the spleen exhibited a marked increase in MPO activity that was attenuated in CaMKK−/− mice (103.8±13.4 vs. 66.1±3.3, p=0.05) (data not shown). Thus, our data suggest that calcium supplementation heightens the inflammatory response during sepsis.
Figure 4. Calcium administration during sepsis heightens and prolongs systemic inflammation via calcium/calmodulin-dependent protein kinase kinase (CaMKK) signaling.
C57Bl/6 and CaMKK−/− mice were subjected to cecal ligation and puncture, followed by normal saline (3 mL/100 gm) and imipenem/cilastatin (0.5 mg/kg) administration. After 12 hours, mice were administered CaCl2 (2.5 mg/kg) or equivolume normal saline by intraperitoneal injection. At the timepoints indicated, blood was isolated by cardiac puncture, and the serum analyzed for cytokine concentrations. *compared to C57Bl/6 receiving 2.5 mg/kg CaCl2. (n=6-8 animals per group)
Figure 5. Calcium administration during sepsis heightens systemic inflammation via calcium/calmodulin-dependent protein kinase (CaMK) signaling.
C57Bl/6 were intraperitoneally administered the broad CaMK inhibiter KN93 (6 mg/kg) or its inactive analogue, KN92 (6 mg/kg), 12- and 1-hour prior to experimentation. Mice were then subjected to cecal ligation and puncture, followed by normal saline (3 mL/100 gm) and imipenem/cilastatin (0.5 mg/kg) administration. After 12 hours, mice were administered CaCl2 (2.5 mg/kg) or equivolume normal saline by lateral tail vein injection. After 16 hours, blood was isolated by cardiac puncture, and the serum analyzed for cytokine concentrations. (n=6-8 animals per group)
Calcium supplementation during sepsis increases vascular permeability via CaMKK signaling
In light of the systemic alterations in inflammation and in organ function, we hypothesized that a common theme of increased vascular leak might underlie our observations. Indeed, CaCl2 significantly worsened vascular leak (Figure 6). However, this was attenuated in CaMKK−/− by comparison to WT mice: liver (0.09 vs. 0.25, p=0.11), kidney (0.16 vs. 0.25, p=0.08), and lung (0.08 vs. 0.33, p=0.003). Using hematoxylin and eosin staining, we did not observe any histologic differences in cellular or organ architecture at 12 hours after CaCl2 or NS (data not shown).
Figure 6. Calcium administration during sepsis increases vascular permeability via calcium/calmodulin-dependent protein kinase kinase (CaMK) signaling.
C57Bl/6 and CaMKK−/− mice were subjected to cecal ligation and puncture, followed by normal saline (3 mL/100 gm) and imipenem/cilastatin (0.5mg/kg) administration. After 12 hours, mice were administered CaCl2 (2.5 mg/kg) or equivolume normal saline by intraperitoneal injection. After 12 hours, each organ was harvested and vascular permeability assessed by Evans Blue dye as detailed in the Methods (n=6 animals per group). Top panel represents data from liver, middle panel represents data from kidney, and bottom panel represents data from lung.
Discussion
During critical illness perturbations in systemic electrolyte concentrations are commonly observed.[2, 5, 45] These deviations have classically been considered harmful, and thus necessitate monitoring and medical treatment.[45–47] Studies do support a benefit in maintaining certain threshold concentrations of potassium and magnesium.[48–50] However, evidence for a similar approach to address hypocalcemia is sparse.[5, 11] In a murine model of severe sepsis, we observed an apparent dose-response between a single pharmacological dose of calcium and an increased risk of organ dysfunction and death. These untoward ramifications appear to be mediated by a CaMK-dependent exacerbation in vascular leak and shock. The clinical relevance of these biological observations is highlighted by a risk adjusted clinical analysis demonstrating an association between parenteral calcium administration and increased pulmonary and renal dysfunction and death.
The high prevalence of hypocalcemia of critical illness and its association with elevated mortality was first described decades ago and subsequently reported in several independent analyses of both medical and surgical populations.[1–3, 7] Cognizant that calcium homeostasis is essential for normal cell function and organ physiology, the medical community has viewed this relationship as causal. However, contemporary critics propose that hypocalcemia is merely a surrogate marker of severity of illness, a confounding characteristic not incorporated into these prior associative studies.[5] In our analysis, we did not identify a significant association between the magnitude of hypocalcemia and renal dysfunction, VFD or death. Other recent studies either confirm our observations or identify elevated mortality only in the setting of severe hypocalcemia (i.e. [Ca2+]<0.80 mEq/L).[5, 51] Thus, the existing observational evidence strongly supports that mild to moderate hypocalcemia is not causally associated with worse outcome.
Numerous studies have shown that despite hypocalcemia, intracellular [Ca2+] is elevated in a variety of models of severe illness.[52–54] High intracellular [Ca2+] is toxic, and all cells require an effective homeostatic system to maintain intracellular [Ca2+] at 100 nM, about 20,000 times lower than the extracellular mileau.[55, 56] These mechanisms are altered during sepsis and not addressed through the parenteral administration of calcium. In fact, they may be exacerbated by such interventions.
Collectively, these data call for a reassessment of our current perspective of hypocalcemia of critical illness.[6–10] A recent systematic review concluded that there are no data to support parenteral calcium supplementation among critically ill patients.[11] We observed that a single pharmacological dose of calcium heightened inflammation and vascular leak that correlated with worse organ dysfunction and mortality in mice. Other animal studies, albeit administering much higher doses of calcium, have observed similar increases in death with calcium supplementation, though causal mechanisms were not explored.[17, 19] Intracellular [Ca2+] is elevated in immune cells of septic patients.[52] In vitro studies have shown that elevating intracellular [Ca2+] heightens LPS-induced monocyte/macrophage cytokine production.[44] Thus, we hypothesized that calcium administration heightens inflammation, which culminates in increased vascular leak and shock, and greater organ dysfunction and mortality.
This perspective is supported by our in vivo studies, wherein CaMKK−/− mice exhibited resistance to calcium-induced elevations in systemic inflammation and an attenuation in vascular leak that reduced organ dysfunction/injury and mortality. Others and we have described the dependency of septic inflammation on CaMK signaling. In an in vivo model of intraabdominal sepsis, a CaMKK-CaMKI signaling pathway regulated systemic HMGB1 and IL-10 release that was causally related to the development of renal dysfunction.[31] More recently, the function of this CaMKK-CaMKI cascade has been extended to include alveolar cytokine concentrations during LPS-induced acute lung injury (ALI). CaMKIV serves as a component of a signaling pathway that regulates bcl-2 dependent dendritic cell survival and LPS-induced macrophage HMGB1 production.[32, 57, 58] Both effector kinases are regulated by the upstream CaMKK studied herein.[30] Thus, we hypothesize that the physiologic perturbations induced by calcium supplementation are consequential to CaMKK dependent inflammation. Of note, though broad biochemical CaMK inhibition attenuated IL-6, CaMKK inhibition did not, suggesting that an alternate CaMK, such as CaMKII, I or IV, regulates calcium-induced elevations in IL-6 during sepsis. We recognize that alternate mechanisms, including endothelial and parenchymal CaMKK signaling, may underlie the alterations in vascular leak and organ physiology/injury.
We recognize that limitations exist that may explain some of our observations. A significant proportion of patients did not have [Ca2+] measurements. However, the associations between calcium administration and each outcome for the entire cohort were similar to those of the final analyzed cohort possessing [Ca2+] measurements. Patients receiving calcium were more ill and exhibited lower nadirs of [Ca2+]. However, after including a host of validated covariates, including APACHE III scores, the associations persisted. Furthermore, our murine studies, which were performed in a randomized, blinded and controlled fashion, yielded similar results. We acknowledge that the magnitude of the associations in the clinical cohort was less than that observed in the murine model. Many additional interventions are pursued to achieve survival in the context of human disease: volume resuscitation, vasopressors, and cardiopulmonary resuscitation. Furthermore, the clinical study also represents a heterogeneous spectrum of disease (e.g. pneumonia, bacteremia) and host susceptibility that are not represented in the murine model.
Conclusions
In conclusion, the combined data of our murine and translational human studies suggest that calcium supplementation provides no benefit in the management of severe sepsis and may impose harm. Recognizing that hypocalcemia and critical illness are both continuums, we propose that further studies are needed to determine if a threshold [Ca2+] concentration exists, below which, such therapy is warranted. The collective evidence, to which these new data are added, highlights that a derangement in homeostatic calcium regulation evolves during sepsis that is sensitive to manipulation by calcium administration. This altered calcium signaling, transduced by the CaMKK cascade, mediates a heightened state of inflammation and vascular leak that culminates in elevated organ dysfunction and mortality. Further investigations to determine the exact location and mechanisms of CaMKK signaling that mediate the physiologic perturbations observed will contribute to the understanding, and hopefully management, of sepsis and critical illness.
Table 1.
Characteristics of septic subjects according to the parenteral administration of calcium
| Variable | None (n = 431) | Calcium (n = 95) | P value |
|---|---|---|---|
| Age (years) | 62.7 ± 0.9 | 60.1 ± 1.6 | 0.20 |
| Sex (%) | |||
| Male | 54.9 | 55.7 | 0.89 |
| Race (%) | |||
| Caucasian | 74.8 | 80.5 | 0.52 |
| African-American | 16.9 | 12.6 | |
| Other | 8.3 | 6.9 | |
| APACHE III | 66.4 ± 1.3 | 80.0 ± 3.8 | <0.001 |
| Type of ICU (%) | |||
| Medical | 64.3 | 64.3 | 0.64 |
| Cardiothoracic | 14.5 | 11.9 | |
| SICU | 9.1 | 7.2 | |
| Coronary | 5.7 | 9.5 | |
| Neuro/Multidisciplinary | 6.4 | 7.1 | |
| Lowest calcium | 1.11 ± 0.01 | 1.00 ± 0.02 | <0.001 |
| Length of ICU stay (days) | 7.9 ± 0.4 | 9.9 ± 1.2 | 0.04 |
| Mortality (%) | 21.7 | 35.2 | 0.007 |
Acknowledgments
Source of Funding: This work supported by National Institutes of Health grants R01 GM082852 (MRR) and HL086884 (JSL).
We thank Dr. Gilles Clermont for his assistance in generating and providing the HiDenIC human cohort database.
Footnotes
No reprints will be ordered.
Conflicts of Interest: The authors have no conflicts of interest.
References
- 1.Zaloga GP, Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann Intern Med. 1987;107(1):36–41. doi: 10.7326/0003-4819-107-1-36. [DOI] [PubMed] [Google Scholar]
- 2.Zivin JR, Gooley T, Zager RA, Ryan MJ. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am J Kidney Dis. 2001;37(4):689–698. doi: 10.1016/s0272-6386(01)80116-5. [DOI] [PubMed] [Google Scholar]
- 3.Desai TK, Carlson RW, Geheb MA. Prevalence and clinical implications of hypocalcemia in acutely ill patients in a medical intensive care setting. Am J Med. 1988;84(2):209–214. doi: 10.1016/0002-9343(88)90415-9. [DOI] [PubMed] [Google Scholar]
- 4.Zaloga GP. Hypocalcemia in critically ill patients. Crit Care Med. 1992;20(2):251–262. doi: 10.1097/00003246-199202000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Hastbacka J, Pettila V. Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anaesthesiol Scand. 2003;47(10):1264–1269. doi: 10.1046/j.1399-6576.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 6.Aslam S, Llack F. Hypercalcemia, Hypocalcemia, and other Divalent Cation Disorders. 3. Philadelphia: WB Saunders; 2003. [Google Scholar]
- 7.Burchard KW, Gann DS, Colliton J, Forster J. Ionized calcium, parathormone, and mortality in critically ill surgical patients. Ann Surg. 1990;212(4):543–549. doi: 10.1097/00000658-199010000-00016. discussion 549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman DT, Lorenzo L. Ionized calcium: its significance and clinical usefulness. Ann Clin Lab Sci. 1991;21(5):297–304. [PubMed] [Google Scholar]
- 9.Hammer M, Brennan S, Lederer E. Severe Electrolyte Disorders. 2. New York: McGraw Hill; 1998. [Google Scholar]
- 10.Marino PL. Calcium and Phosphorus. 2. Baltimore: Williams & Wilkins; 1998. [Google Scholar]
- 11.Forsythe RM, Wessel CB, Billiar TR, Angus DC, Rosengart MR. Parenteral calcium for intensive care unit patients. Cochrane Database Syst Rev. 2008;(4):CD006163. doi: 10.1002/14651858.CD006163.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Bosson S, Kuenzig M, Schwartz SI. Verapamil improves cardiac function and increases survival in canine E. coli endotoxin shock. Circ Shock. 1985;16(3):307–316. [PubMed] [Google Scholar]
- 13.Bosson S, Kuenzig M, Schwartz SI. Increased survival with calcium antagonists in antibiotic-treated bacteremia. Circ Shock. 1986;19(1):69–74. [PubMed] [Google Scholar]
- 14.Deakin CD, Fagan EA, Williams R. Cytoprotective effects of calcium channel blockers. Mechanisms and potential applications in hepatocellular injury. J Hepatol. 1991;12(2):251–255. doi: 10.1016/0168-8278(91)90947-a. [DOI] [PubMed] [Google Scholar]
- 15.Sayeed MM. Signaling mechanisms of altered cellular responses in trauma, burn, and sepsis: role of Ca2+ Arch Surg. 2000;135(12):1432–1442. doi: 10.1001/archsurg.135.12.1432. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Karl IE. Dantrolene ameliorates the metabolic hallmarks of sepsis in rats and improves survival in a mouse model of endotoxemia. Proc Natl Acad Sci U S A. 1994;91(8):3039–3043. doi: 10.1073/pnas.91.8.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlstedt F, Eriksson M, Kiiski R, Larsson A, Lind L. Hypocalcemia during porcine endotoxemic shock: effects of calcium administration. Crit Care Med. 2000;28(8):2909–2914. doi: 10.1097/00003246-200008000-00037. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Karl IE. Calcium: a regulator of the inflammatory response in endotoxemia and sepsis. New Horiz. 1996;4(1):58–71. [PubMed] [Google Scholar]
- 19.Malcolm DS, Zaloga GP, Holaday JW. Calcium administration increases the mortality of endotoxic shock in rats. Crit Care Med. 1989;17(9):900–903. doi: 10.1097/00003246-198909000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Zaloga GP, Sager A, Black KW, Prielipp R. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock. 1992;37(3):226–229. [PubMed] [Google Scholar]
- 21.Sayeed MM. Alterations in calcium signaling and cellular responses in septic injury. New Horiz. 1996;4(1):72–86. [PubMed] [Google Scholar]
- 22.Fekete Z, Hauser CJ, Adams JM, Adams CA, Jr, Forsythe RM, Hasko G, Xu DZ, Livingston DH, Deitch EA. Injury-enhanced calcium mobilization in circulating rat neutrophils models human PMN responses. Shock. 2001;16(1):15–20. doi: 10.1097/00024382-200116010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hauser CJ, Fekete Z, Livingston DH, Adams J, Garced M, Deitch EA. Major trauma enhances store-operated calcium influx in human neutrophils. J Trauma. 2000;48(4):592–597. doi: 10.1097/00005373-200004000-00003. discussion 597–598. [DOI] [PubMed] [Google Scholar]
- 24.Tomera JF, Friend KD, Kukulka SP, Lilford K. Modification of calcium flux of twitch skeletal muscle in mice subjected to 20% body surface area burn. J Burn Care Rehabil. 1992;13(5):546–555. doi: 10.1097/00004630-199209000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Fujita J, Tsuda K, Takeda T, Yu L, Fujimoto S, Kajikawa M, Nishimura M, Mizuno N, Hamamoto Y, Mukai E, et al. Nisoldipine improves the impaired erythrocyte deformability correlating with elevated intracellular free calcium-ion concentration and poor glycaemic control in NIDDM. Br J Clin Pharmacol. 1999;47(5):499–506. doi: 10.1046/j.1365-2125.1999.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sowemimo-Coker SO, Debbas NM, Kovacs IB, Turner P. Ex vivo effects of nifedipine, nisoldipine and nitrendipine on filterability of red blood cells from healthy volunteers. Br J Clin Pharmacol. 1985;20(2):152–154. doi: 10.1111/j.1365-2125.1985.tb05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowemimo-Coker SO, Kovacs IB, Kirby JD, Turner P. Effects of verapamil on calcium-induced rigidity and on filterability of red blood cells from healthy volunteers and patients with progressive systemic sclerosis. Br J Clin Pharmacol. 1985;19(6):731–737. doi: 10.1111/j.1365-2125.1985.tb02707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer JA, Deaciuc IV. Effect of endotoxicosis and sepsis on intracellular calcium homeostasis in rat liver. Mitochondrial and microsomal calcium uptake. Circ Shock. 1986;18(2):81–93. [PubMed] [Google Scholar]
- 29.Maier RV. Pathogenesis of multiple organ dysfunction syndrome--endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. Surg Infect (Larchmt) 2000;1(3):197–204. doi: 10.1089/109629600750018123. discussion 204–195. [DOI] [PubMed] [Google Scholar]
- 30.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24(6):232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Guo L, Collage RD, Stripay JL, Tsung A, Lee JS, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) Ialpha mediates the macrophage inflammatory response to sepsis. J Leukoc Biol. 2011;90(2):249–261. doi: 10.1189/jlb.0510286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181(7):5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. The Journal of experimental medicine. 2007;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends Immunol. 2008;29(12):600–607. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Cuschieri J, Gourlay D, Garcia I, Jelacic S, Maier RV. Modulation of endotoxin-induced endothelial function by calcium/calmodulin-dependent protein kinase. Shock. 2003;20(2):176–182. doi: 10.1097/01.shk.0000074789.29800.a5. [DOI] [PubMed] [Google Scholar]
- 36.Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983;8(1):41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- 37.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. The Journal of clinical investigation. 2002;109(3):383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infection and immunity. 2004;72(12):7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113(5):1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro RA, Angus DC, Hong SY, Lee C, Weissfeld LA, Clermont G, Rosengart MR. Light and the outcome of the critically ill: an observational cohort study. Critical care. 2012;16(4):R132. doi: 10.1186/cc11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visweswaran S, Mezger J, Clermont G, Hauskrecht M, Cooper GF. Identifying Deviations from Usual Medical Care using a Statistical Approach. AMIA Annu Symp Proc. 2010;2010:827–831. [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Critical care medicine. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 44.Lo CJ, Garcia I, Cryer HG, Maier RV. Calcium and calmodulin regulate lipopolysaccharide-induced alveolar macrophage production of tumor necrosis factor and procoagulant activity. Arch Surg. 1996;131(1):44–50. doi: 10.1001/archsurg.1996.01430130046008. [DOI] [PubMed] [Google Scholar]
- 45.Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158(8):917–924. doi: 10.1001/archinte.158.8.917. [DOI] [PubMed] [Google Scholar]
- 46.Paltiel O, Salakhov E, Ronen I, Berg D, Israeli A. Management of severe hypokalemia in hospitalized patients: a study of quality of care based on computerized databases. Arch Intern Med. 2001;161(8):1089–1095. doi: 10.1001/archinte.161.8.1089. [DOI] [PubMed] [Google Scholar]
- 47.Weisberg LS, Szerlip HM, Cox M. Disorders of potassium homeostasis in critically ill patients. Crit Care Clin. 1987;3(4):835–854. [PubMed] [Google Scholar]
- 48.Gennari FJ. Disorders of potassium homeostasis. Hypokalemia and hyperkalemia. Crit Care Clin. 2002;18(2):273–288. vi. doi: 10.1016/s0749-0704(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 49.Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med. 2000;160(16):2429–2436. doi: 10.1001/archinte.160.16.2429. [DOI] [PubMed] [Google Scholar]
- 50.Hamill RJ, Robinson LM, Wexler HR, Moote C. Efficacy and safety of potassium infusion therapy in hypokalemic critically ill patients. Critical care medicine. 1991;19(5):694–699. doi: 10.1097/00003246-199105000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Egi M, Kim I, Nichol A, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M, Bellomo R. Ionized calcium concentration and outcome in critical illness. Critical care medicine. 2011;39(2):314–321. doi: 10.1097/CCM.0b013e3181ffe23e. [DOI] [PubMed] [Google Scholar]
- 52.Zaloga GP, Washburn D, Black KW, Prielipp R. Human sepsis increases lymphocyte intracellular calcium. Crit Care Med. 1993;21(2):196–202. doi: 10.1097/00003246-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Thompson M, Kliewer A, Maass D, Becker L, White DJ, Bryant D, Arteaga G, Horton J, Giroir BP. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatr Res. 2000;47(5):669–676. doi: 10.1203/00006450-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Song SK, Karl IE, Ackerman JJ, Hotchkiss RS. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis? Proc Natl Acad Sci U S A. 1993;90(9):3933–3937. doi: 10.1073/pnas.90.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews Molecular cell biology. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 56.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42(4–5):345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Illario M, Giardino-Torchia ML, Sankar U, Ribar TJ, Galgani M, Vitiello L, Masci AM, Bertani FR, Ciaglia E, Astone D, et al. Calmodulin-dependent kinase IV links Toll-like receptor 4 signaling with survival pathway of activated dendritic cells. Blood. 2008;111(2):723–731. doi: 10.1182/blood-2007-05-091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lobo FM, Zanjani R, Ho N, Chatila TA, Fuleihan RL. Calcium-dependent activation of TNF family gene expression by Ca2+/calmodulin kinase type IV/Gr and calcineurin. J Immunol. 1999;162(4):2057–2063. [PubMed] [Google Scholar]