Abstract
Objective
To comparatively examine the effects of adiposity on the levels of plasma renin activity (PRA), plasma aldosterone concentrations (PAC), and aldosterone-renin ratios (ARR) in young black and white children.
Study design
We prospectively assessed 248 black and 345 white children and adolescents. A novel analytical technique was used to assess the concurrent influences of age and body mass index (BMI) on PRA, PAC, and ARR. The estimated effects were depicted by colored contour plots.
Results
In contrast to whites, blacks had lower PRA (2.76 vs 3.36 ng/ml/h; p<0.001) and lower PAC (9.01 vs 14.59 ng/dl; p<0.001). In blacks, BMI was negatively associated with PRA (p=0.001), consistent with an association with a more expanded plasma volume; there was no association with PAC. In whites, BMI was positively associated with PAC (p=0.005); we did not detect a BMI-PRA association. The effects of BMI on ARR were directionally similar in the two race groups but more pronounced in blacks. Mean systolic BP was greater in blacks with lower PRA (p<0.01), higher PAC (p=0.015), and higher ARR (p=0.49).
Conclusions
An increase in adiposity was associated with a suppressed PRA in blacks and an increase in PAC in whites. The unique relationship between adiposity and renin-aldosterone axis in blacks suggests the possible existence of a population-specific mechanism characterized by volume expansion, which could in turn enhance the influences of adiposity on BP in black children and adolescents.
Keywords: Adolescent, hypertension, obesity
Although blood pressures and rates of onset of hypertension are typically much lower in children and adolescents than in adults, recent epidemiological data indicate a shift of the disease toward young people, possibly a result of the marked increase in childhood obesity.1, 2 Indeed, most cases of hypertension in children and adolescents are accompanied by obesity, defined as body mass index or BMI greater than 30 kg/M2.3 The mechanism for how obesity raises the level of BP is not fully understood. Rocchini et al, in a study of obese and non-obese adolescents, found that the higher BP in obese subjects was characterized as being salt-sensitive.4 They also found that the PAC was higher in obese hypertensive adolescents than in non-obese adolescents, whereas the level of PRA was unrelated to BMI. Other investigative groups have also found that PAC was increased when BMI was increased.5–7
Compared with whites, blacks are more prone to develop hypertension regardless of age.8 Even before there is any increase in BP, blacks show evidence for increased sodium reabsorption to the extent that PRA and PAC are often lower.9, 10 This raises the question of whether influences of BMI on BP regulation are different in blacks and whites. Previous studies on the effects of BMI have for the most part not provided information on race groups. In the present study, we address the question of whether the renin-aldosterone axis in relationship to adiposity is different in blacks and whites, by analyzing data from a biracial cohort of children and adolescents.
METHODS
The study design and data collection process were previously described.11, 12 Briefly, subjects were healthy black and white young people recruited from 33 participating schools in Indianapolis. The schools were selected to provide a range in socioeconomic status. Those with a history of cardiac and kidney diseases, hypertension, diabetes mellitus, and those taking medications that can affect BP were excluded. Although subjects were followed prospectively with measurements of blood pressure, height, and weight at six month intervals, analyses were limited to data collected during visits when blood samples were obtained for measurement of PRA and PAC. Aldosterone-renin activity ratio (ARR) was calculated as PAC/PRA. BMI was calculated from the height and weight (BMI=weight / height2). Other adiposity measures such as waist and hip circumferences, tricep and subscapular skinfold were ascertained at the time of visit. Overnight urine samples were collected and sodium (Na) and potassium (K) excretion rates were calculated per unit of creatinine. The study protocol was approved by the Indiana University Institutional Review Board. All subjects (and a parent) provided written informed consent or assent as appropriate.
PRA was measured using a radioimmunoassay for generated angiotensin I (Clinical Assays, GammaCount radioimmunoassay kit from Diasorin), and PAC was measured using a radioimmunoassay (Coat-A-Count kit from Diagnostic Products Corporation).
Statistical analyses
Subjects’ race was self-reported. As part of the analysis, we examined the data distributions of continuous variables; skewed data were transformed using logarithmic transformation before analysis. Log-transformed values were then compared between the races using generalized estimating equation models (GEE). Binary variables were compared using Chi-square tests. To accommodate the potentially nonlinear influences of adiposity on PRA, PAC, and ARR, we used semiparametric regression models to examine the BMI effects on these outcomes. Semiparametric regression is a class of statistical methods that are most useful for the exploration of nonlinear relationships and it does not restrict the depicted relation to a linear form.13 To simultaneously account for the age-related changes in PRA, PAC, and ARR, we expressed each of these outcomes as a smooth bivariate function of age and BMI, eg. log(PRA) = f(age, BMI). The predicted levels of the response variable at different age-BMI combinations were presented as colored contour plots, in which the color scheme and contour lines indicate the mean response levels. A main advantage of this analytical approach is that it allows for the examination of adiposity effect on the outcome variable at any given age in the study population. Separate models were fitted for black and white subjects. The analysis was implemented using mgcv package in R software (Version 2.11).14 To test the individual effects of age and BMI on the response variable, we further fitted linear models. For each of the outcomes, the estimated effects of BMI, and BMI-race interaction were reported in a tabular form along with their corresponding p-values. Other adiposity measures were analyzed in a similar manner. As a sensitivity analysis, we also compared the levels of PRA, PAC, and ARR in normal weight and overweight/obese children using age and sex adjusted BMI percentile values.15 Finally, we dichotomized PRA, PAC, and ARR levels at their sample median values and examined mean and 95% confidence intervals of systolic BP corresponding to the dichotomized categories. In all analysis, p values less than 0.05 were considered statistically significant.
RESULTS
Five hundred ninety-three subjects contributed a total of 728 visits where blood samples were collected. Of these, 134 subjects contributed multiple blood samples during the course of follow-up. Demographical and clinical characteristics of the study subjects are presented in Table I. On average, blacks were slightly younger than whites (14.21 vs. 15.10 years; p < 0.001), and had greater BMI (24.41 vs. 22, p < 0.001), greater tricep skinfold (18.3 vs 16.3mm; p=0.002) and great subscapular skinfold (16.9 vs 12.9mm; p<0.0001). There was no significant difference in systolic BP between blacks and whites (108.79 vs 108.41 mmHg; p-value = 0.682). Mean diastolic BP was higher in blacks than in whites (67.10 vs. 65.28 mmHg, p=0.025). Compared with whites, blacks had a lower PRA (2.76 vs. 3.36 ng/ml/h, p-value < 0.001), lower PAC (9.01 vs. 14.59 ng/dl, p-value < 0.001), and slightly lower ARR (5.00 vs. 5.57 ng/dl per ng/ml/h), but the difference in ARR did not reach the level of statistical significance (p-value = 0.249). We did not detect a sex difference in BMI when age and race were adjusted (BMI=22.89 in males and 23.14 in females; adjusted p value = 0.8049), nor did we detect significance sex difference in PRA and PAC levels (p=0.9872 and 0.4376, respectively). There were no sex differences in BMI, PRA, and PAC within individual race groups. Urinary excretion rates of Na and K were not significantly different between the races.
Table 1.
Characteristics of study subjects.
| Black (n=248) | White (n=345) | p-value | |
|---|---|---|---|
| Sex(male) | 113(45.6%) | 186 (53.9%) | 0.054 |
| Age(year) | 14.21 (13.95, 14.46) | 15.10 (14.84, 15.35) | <0.001 |
| BMI(kg/m2) | 24.41(23.58, 25.23) | 21.06 (21.61, 22.52) | <0.001 |
| SBP(mmHg) | 108.79 (107.40, 110.18) | 108.41 (107.22, 109.59) | 0.682 |
| DBP(mmHg) | 67.10 (65.90, 68.28) | 65.28 (64.23, 66.32) | <0.025 |
| PRA(ng/ml/h) | 2.76 (2.51, 3.01) | 3.36 (3.12, 3.60) | <0.001 |
| PAC (ng/dl) | 9.01 (8.21, 9.82) | 14.59 (13.66, 15.51) | <0.001 |
| ARR (ng/dl per ng/ml/h) | 5.00 (4.11, 5.89) | 5.57 (5.17, 5.97) | 0.249 |
| Urine Na excretion (mmol/gm) | 90.4 (83.2, 97.7) | 96.7 (90.2, 103.4) | 0.208 |
| Urine K excretion (mmol/gm) | 23 (21, 25) | 25 (22.8, 26.8) | 0.147 |
| Waist circumference (cm) | 76.199(74.358,78.040) | 74.332(73.143,75.521) | 0.095 |
| Hip circumference (cm) | 96.022(94.301,97.744) | 93.088(92.039, 94.136) | 0.004 |
| Triceps skinfold (mm) | 18.348(17.268,19.429) | 16.305(15.543,17.068) | 0.002 |
| Subscapular skinfold (mm) | 16.884(15.778,17.989) | 12.918(12.144,13.692) | <0.001 |
Notes: BMI – Body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, PRA – plasma renin activity, PAC – plasma aldosterone concentration, ARR – aldosterone-renin ratio.
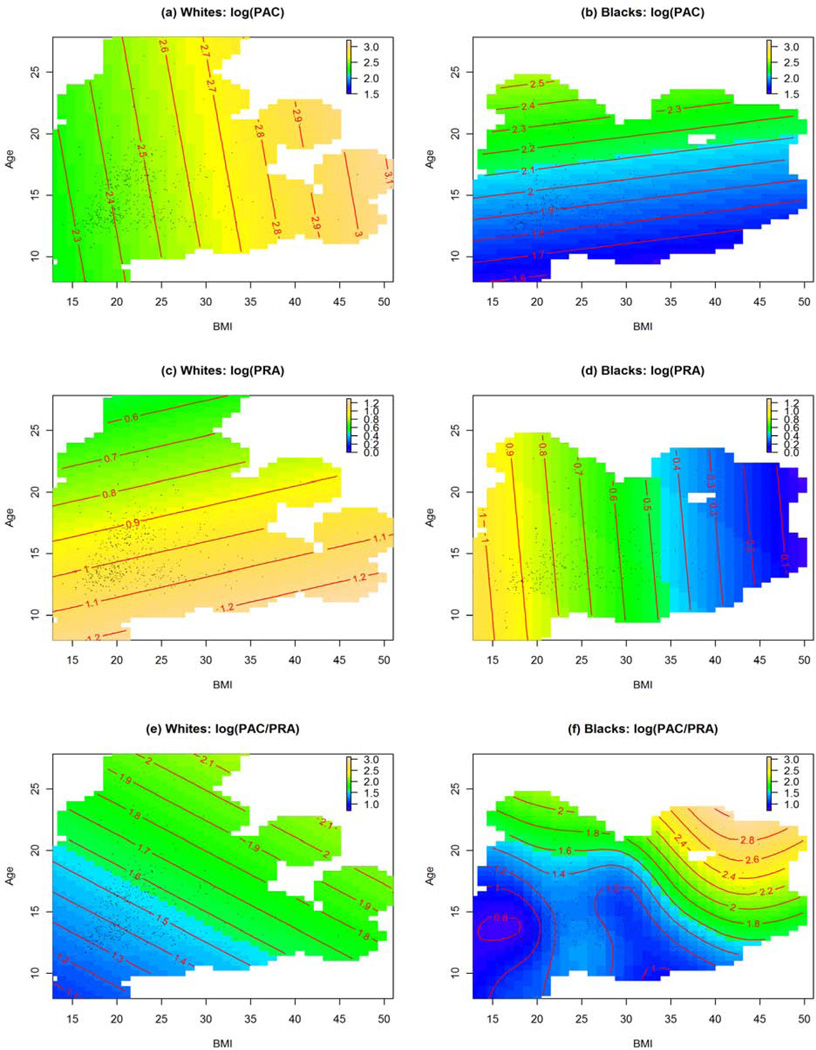
Smooth surface estimates of age and BMI effects on PRA, PAC, and ARR in black and white subjects are presented in Figure 1. In each plot, we presented mean values of the response variable at all age-BMI combination. The magnitude of the response was indicated by colors and numbers on the contour lines. Race differences were evident in the effects of BMI on PAC, PRA, and ARR. For example, blacks had on average lower PAC than whites, and PAC increased with BMI at all ages in whites, but not in blacks (Figure 1, A and B). Similarly, PRA was on average lower in blacks, especially for individuals with higher BMI values. PRA decreased with BMI at all ages in blacks, but not in whites (Figure 1, C and D). ARR increased with BMI and age in both races, but the magnitude of the increase was greater in blacks, especially for older adolescents with greater BMI (Figure 1, F).
Figure 1.
Estimated effect contours of BMI and age on A and B, PAC, C and D, PRA, and E and F, ARR in blacks and whites. In each plot, the mean levels of the response variables were represented by colors with colder colors (blue) indicating lower values and warmer color indicating higher values. The mean response levels, at all age-BMI combinations were indicated by the numbers on the contour lines.
Figure 1 showed that the BMI effects on PAC, PRA, and ARR were largely linear. To formally test the individual effects of BMI on these outcomes, we repeated the analysis using linear models. The linear model fitting results were reported in Table II. These results essentially confirmed what were observed in Figure 1. In whites, BMI was positively associated with PAC after controlling for the effects of age and sex (β=0.020, p= 0.005). In blacks, BMI was not significantly associated with PAC (p = 0.793). BMI effects on PRA were different between black and white subjects (p = 0.005). In whites, BMI was not significantly associated with PRA (β = 0.004, p = 0.551). In blacks, PRA decreased with increasing BMI (β = − 0.026, p = 0.001). BMI was positively associated with ARR in both races, but the BMI effect on ARR was much more pronounced in blacks (β = 0.024, p = 0.010) than in whites (β = 0.015, p = 0.046). We similarly examined the relationships between other adiposity measures (waist and hip circumferences, subscapular and tricep skinfolds) and PRA, PAC, and ARR. The results essentially mirrored what we observed for BMI (Table II).
Table 2.
Effects of adiposity measures on renin-angiotensin axis in black and white subjects.
| Dependent Variable | Black (n=248) | White (n=345) | BMI-Race Interaction |
|---|---|---|---|
| Coefficient of BMI | Coefficient of BMI | P value | |
| PRA* (ng/ml/h) | −0.026 (p=0.001) | 0.004 (p=0.551) | 0.005 |
| PAC* (ng/dl) | −0.002 (p=0.793) | 0.020 (p=0.005) | 0.040 |
| ARR* | 0.024 (p=0.010) | 0.015 (p=0.046) | 0.413 |
| Coefficient of Waist Circumference | Coefficient of Waist Circumference | Waist Circumference-Race Interaction; P value | |
| PRA* (ng/ml/h) | −0.011(0.003) | 0.003(0.229) | 0.0018 |
| PAC* (ng/dl) | 0.003(0.417) | 0.008 (0.008) | 0.304 |
| ARR* | 0.014(0.001) | 0.004(0.144) | 0.072 |
| Coefficient of hip circumference | Coefficient of hip circumference | Hip-Race Interaction; P value | |
| PRA* (ng/ml/h) | −0.012(0.001) | −0.0002(0.943) | 0.016 |
| PAC* (ng/dl) | 0.001(0.787) | 0.010(0.003) | 0.112 |
| ARR* | 0.013(0.003) | 0.010(0.004) | 0.539 |
| Coefficient of triceps skinfold | Coefficient of triceps skinfold | Triceps-Race Interaction; P value | |
| PRA* (ng/ml/h) | −0.006(0.288) | 0.009 (0.040) | 0.039 |
| PAC* (ng/dl) | −0.0001(0.977) | 0.016(0.0002) | 0.021 |
| ARR* | 0.006(0.350) | 0.007(0.143) | 0.947 |
| Coefficient of subscapular skinfold | Coefficient of subscapular skinfold | Subscapular skinfold-Race interaction; P value | |
| PRA* (ng/ml/h) | −0.016(0.004) | 0.006(0.152) | 0.002 |
| PAC* (ng/dl) | −0.003(0.659) | 0.020(<0.001) | 0.002 |
| ARR* | 0.013(0.026) | 0.014(0.002) | 0.982 |
Notes:
Logarithmic transformation was performed on PRA, PAC, and ARR before analysis due to strong skewness in the data. In this table, we reported regression coefficients of BMI, waist and hip circumferences, and triceps and subscapular skinfold on each dependent variable as well as the corresponding p-values (in parentheses) in each race group (column). We also reported p values for the adiposity-race interaction, which tests the difference of coefficients between blacks and whites.
We additionally examined the interactions between BMI percentile values and renin-aldosterone axis by comparing the PRA, PAC, and ARR levels in normal weight and overweight/obese children. The results remained consistent (Table III: available at www.jpeds.com).
Table 3.
Plasma renin activity (PRA), plasma aldosterone concentration (PAC), and aldosterone-renin ratio (ARR) in overweight and normal weight children; Subjects were considered overweight or obese if their age and sex adjusted BMI percentile values were 850025; or greater.
| Dependent Variable(log) |
Black | White | ||||
|---|---|---|---|---|---|---|
| Overweight & Obese |
Normal weight |
p-value | Overweight & Obese |
Normal weight |
p-value | |
| PRA (ng/ml/h) | 0.61(0.88) | 0.84(0.74) | 0.01816 | 1.08(0.67) | 0.95(0.68) | 0.078 |
| PAC (pg/ml) | 1.94(0.84) | 1.88(0.79) | 0.5148 | 2.6(0.66) | 2.41(0.65) | 0.0168 |
| ARR | 1.33(0.92) | 1.04(0.86) | 0.0060 | 1.51(0.72) | 1.47(0.68) | 0.5938 |
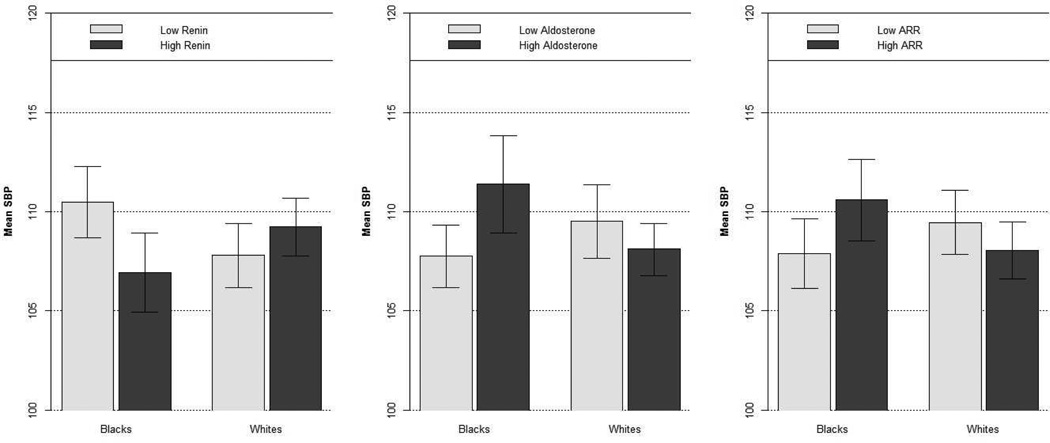
Finally, mean systolic BP values corresponding to dichotomized PRA, PAC, and ARR were reported in Figure 2. Analysis showed that systolic BP was higher in blacks with lower PRA (110 mmHg in individuals with lower PRA vs 106 mmHg in individuals with higher PRA; p<0.01), higher PAC (111 mmHg in individuals with higher PAC vs 107 mmHg in individuals with lower PAC; p=0.015), and higher ARR (111 mmHg in individuals with higher ARR vs 108 mmHg in individuals with lower ARR; p=0.049). No significance differences were detected in whites.
Figure 2.
Mean systolic BP with 95% confidence intervals in black and white subjects with PRA, PAC, and ARR below and above sample median values.
DISCUSSION
Obesity, which has taken on an alarmingly high prevalence and more so in blacks than in whites,16, 17 is a major contributor to the risk for hypertension.18, 19 Previous studies in adolescents as well as in adults have shown that levels of adiposity can be accompanied by increases in aldosterone production.20, 21,22, 23 In the present study, we also found that PAC increased with BMI, but only in white subjects (Fig 1, A). In black subjects on the other hand, there was little evidence that aldosterone production was related to BMI; instead, BMI was inversely related to the level of PRA (Figure 1, B and D). Similar relationships were consistently demonstrated in other adiposity measures where significant race differences remained (Table II). A lower PRA is often regarded as an indication of expanded extracellular fluid volume (ECFV) and PRA levels in blacks are known to be lower than that of whites.24, 25 Our data showed that an increase in adiposity was accompanied by a suppressed level of PRA in blacks whereas BMI and PRA were unrelated in whites (aldosterone production in whites appeared to be predominantly driven by non-angiotensin II stimuli; Figure 1, A and C).
Interestingly, in a recent study Visser et al measured ECFV in a group of white subjects and found it to be proportional to BMI, but only after ECFV had first been expanded by a high salt intake.26 Although we did not measure ECFV in the current study, we did observe a relationship between adiposity and renin in blacks that is essentially parallel to the observation of Visser et al on volume-expanded individuals. If blacks on average have a more expanded volume than whites as some have suggested,27 this research would raise logical questions of whether and how preexisting volume expansion, a result of sustained increases in Na reabsorption (equivalent to the high salt intake used in the aforementioned study) could sensitize volume to the influence of adiposity.
In the present study, ARR (PAC relative to the corresponding level of PRA) increased with BMI in both race groups, although more so in blacks (Figure 1, E and F). The observation supports the notion that a suppressed level of PRA could allow the lower PAC in blacks to be physiologically relevant for increasing BP. It also offers an explanation for why in whites BP did not increase as PAC increased in response to adiposity. The plasma volume may not have been sufficiently expanded in whites, a certain threshold may not have been reached to allow the aldosterone to raise BP.
Despite the observational nature of the study, the findings may have clinical implication: If blacks indeed retain more sodium than whites, being obese could certainly aggravate the risk for hypertension. Considering the higher rates of both obesity and hypertension in blacks of all ages,8, 17 the current research showed a synergistic adiposity effect that compounds blacks’ susceptibility to hypertension through increased salt sensitivity. As our data showed, the risk could be in existence even before the clinical manifestation of hypertension. Therefore, early interventions directed at reducing obesity while simultaneously reducing sodium intake may serve the health needs of blacks, possibly more than would be true for whites. Furthermore, a racial disparity in blood pressure control could be based as much on physiological differences as those related to socio-economics. Indeed, there may be a need to develop population-specific treatment strategies for better blood pressure management in children, especially when overweight or obese.
Methodologically, the current research highlights the utility of new analytical tools for data exploration, such as the semiparametric regression techniques and related visualization tools employed. These new analytical techniques assisted in revealing the subtle features of the relationships that otherwise might have been overlooked.
We recognize several limitations to the current study. First, the focus on healthy young people precluded the possibility of directly generalizing the findings to adult populations where hypertension is more common. On the other hand, the current sample also presents an opportunity for mechanistic exploration without interference that can occur when there is hypertension including residual effects of treatment of hypertension. Second, as an observational study, we did not control for or measure the dietary intake of Na and K in our study subjects. But there is no indication that blacks consumed greater amount of Na than whites in our study, as evidenced by the similar urinary Na and K excretion rates based on overnight urine samples. Third, the lack of data on population groups other than blacks and whites also limits the generalizability of the findings. Finally, as previous reviews have noted, renin-aldosterone axis activation may just be one of the mechanisms that have the potential to mediate the adiposity effect on blood pressure.28–30
Acknowledgments
Funded by the National Institutes of Health (RO1 HL095086) and the VA (<>).
ABBREVIATIONS
- ARR
aldosterone-renin ratios
- BMI
body mass index
- BP
blood pressure
- ECFV
extracellular fluid volume
- GEE
generalized estimating equation models
- PAC
plasma aldosterone concentrations
- PRA
plasma renin activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 2.Falkner B. Hypertension in children and adolescents: epidemiology and natural history. Pediatr Nephrol. 2010;25:1219–1224. doi: 10.1007/s00467-009-1200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn JT, Falkner BE. Obesity hypertension in adolescents: epidemiology, evaluation, and management. J Clin Hypertens (Greenwich) 2011;13:323–331. doi: 10.1111/j.1751-7176.2011.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. New England Journal Of Medicine. 1989;321:580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 5.Goodfriend TL, Egan BM, Kelley DE. Aldosterone in obesity. EndocrRes. 1998;24:789–796. doi: 10.3109/07435809809032689. [DOI] [PubMed] [Google Scholar]
- 6.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocr Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun DA, Sharma K. The Role of Aldosteronism in Causing Obesity-Related Cardiovascular Risk. Cardiology Clinics. 2010;28 doi: 10.1016/j.ccl.2010.04.001. 517-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 9.Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. New England Journal Of Medicine. 1989;321:1152–1157. doi: 10.1056/NEJM198910263211703. [DOI] [PubMed] [Google Scholar]
- Tu W, Eckert GJ, Pratt JH, Jan Danser AH. Plasma levels of prorenin and renin in blacks and whites: their relative abundance and associations with plasma aldosterone concentration. American journal of hypertension. 2012;25:1030–1034. doi: 10.1038/ajh.2012.83. [DOI] [PubMed] [Google Scholar]
- 11.Tu W, Eckert GJ, Saha C, Pratt JH. Synchronization of adolescent blood pressure and pubertal somatic growth. The Journal of clinical endocrinology and metabolism. 2009;94:5019–5022. doi: 10.1210/jc.2009-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu W, Eckert GJ, DiMeglio LA, Yu Z, Jung J, Pratt JH. Intensified effect of adiposity on blood pressure in overweight and obese children. Hypertension. 2011;58:818–824. doi: 10.1161/HYPERTENSIONAHA.111.175695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruppert D, Wand MP, Carroll RJ. Semiparametric Regression. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- 14.Wood SN. Generalized Additive Models: An Introduction with {R} Boca Raton: Chapman & Hall/CRC. 2006 [Google Scholar]
- 15.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. The Journal of pediatrics. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 18.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obesity research. 1999;7:355–362. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. The Journal of clinical endocrinology and metabolism. 2008;93:2566–2571. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 20.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28:517–527. doi: 10.1016/j.ccl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma AM. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension. 2004;44:12–19. doi: 10.1161/01.HYP.0000132568.71409.a2. [DOI] [PubMed] [Google Scholar]
- 22.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, et al. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. American journal of hypertension. 2005;18:657–665. doi: 10.1016/j.amjhyper.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Fasola AF, Martz BL, Helmer OM. Plasma renin activity during supine exercise in offspring of hypertensive parents. Journal of Applied Physiology. 1968;25:410–415. doi: 10.1152/jappl.1966.21.6.1709. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(Suppl II):II-127–II-134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 26.Visser FW, Krikken JA, Muntinga JH, Dierckx RA, Navis GJ. Rise in extracellular fluid volume during high sodium depends on BMI in healthy men. Obesity (Silver Spring) 2009;17:1684–1688. doi: 10.1038/oby.2009.61. [DOI] [PubMed] [Google Scholar]
- 27.Chrysant SG, Danisa K, Dem DC, Dillard BL, Smith WJ, Frolich ED. Racial differences in pressure, volume and renin interrelationship in essential hypertension. Hypertension. 1979;1:136–141. doi: 10.1161/01.hyp.1.2.136. [DOI] [PubMed] [Google Scholar]
- Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–R813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 29.Torrance B, McGuire KA, Lewanczuk R, McGavock J. Overweight, physical activity and high blood pressure in children: a review of the literature. Vascular health and risk management. 2007;3:139–149. [PMC free article] [PubMed] [Google Scholar]
- 30.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]