Abstract
Objective
Distressed marriages enhance risk for a variety of health problems. Immune dysregulation is one potential mechanism; cross-sectional studies have demonstrated that marital distress is linked to maladaptive immune alterations. The current study filled an important gap in the literature by examining the ability of marital distress to prospectively predict immune alterations over a two-year period.
Method
Participants were 90 couples (N =180 individuals; Mage =25.67) married less than a year at the time of their first study visit. Both members of a couple completed a baseline assessment of marital quality and provided blood samples at baseline and two years later. 63 couples (N = 123 individuals) completed the follow-up assessment
Results
Spouses in more distressed marriages had larger declines in cellular immune function over time than spouses in less distressed marriages. Furthermore, the results were highly consistent across two different indices, proliferative responses to two mitogens, concanavalin A (Con A) and phytohemagglutinin (PHA).
Conclusions
Marital distress has a variety of negative health consequences. The current study provided important evidence that marital distress has longer-term immune consequences. Accordingly, the present results provide a glimpse into the pathways through which marital distress may impact health over time.
Keywords: psychoneuroimmunology, marriage, marital distress, romantic relationships, longitudinal, cellular immunity
Being married confers health benefits. For example, married people had lower premature all-cause mortality rates, higher 5-year cancer survival rates, and fewer chronic health conditions than their non-married counterparts (Johnson et al., 2000; Schoenborn, 2004; Sprehn et al., 2009). On the other hand, distressed marriages enhance risk for a variety of health problems, such as coronary heart disease, delayed wound healing, metabolic syndrome, and premature all-cause mortality (Orth-Gomér et al., 2000; Kiecolt-Glaser and Newton, 2001; Kiecolt-Glaser et al., 2005; Troxel et al., 2005; Holt-Lunstad et al., 2010). Importantly, the links between distressed marriages and health remain after controlling for sociodemographic and health-relevant risk factors. Furthermore, a recent meta-analysis concluded that the link between distressed marriages and health was on par with the negative effects of health behaviors like a poor diet (Robles et al., in press).
Because marital distress is a chronic interpersonal stressor, stress-relevant physiological mechanisms may provide insight into the link between marital distress and health. Immune dysregulation may be one mechanistic pathway because stress alters immune function and the immune system is essential to health (Robles et al., in press; Robles and Kiecolt-Glaser, 2003). For example, inflammation and other forms of immune dysregulation increase risk for premature all-cause mortality and a variety of diseases including cardiovascular disease, cancer, and metabolic syndrome (Ershler and Keller, 2000; Hansson, 2005; Hotamisligil, 2006; Nabipour et al., 2006; Parkin, 2006).
A growing body of works demonstrates cross-sectional links between distressed marriages and immune dysregulation. For example, people in more distressed marriages had smaller antibody responses to an influenza virus vaccine than people in less distressed marriages (Phillips et al., 2006). Compared to more happily married people, spouses in more distressed marriages had poorer cellular immune function, as indexed by lower proliferative responses to two mitogens, concanavalin A (Con A) and phytohemagglutinin (PHA; Kiecolt-Glaser et al., 1987). Furthermore, individuals in more distressed marriages had higher Epstein-Barr virus (EBV) antibody titers than those in less distressed marriages (Kiecolt-Glaser et al., 1987, 1988). Because herpesviruses such as EBV are better able to reactivate and replicate when the cellular immune system is compromised, higher antibody titers to a latent herpesvirus reflect poorer cellular immune system control over viral latency (Glaser and Jones, 1994).
Observational studies of marital conflict discussions provide another window into marital distress; behavioral coding systems assess relationship behaviors between romantic partners as an index of relationship quality. A provocative study using this paradigm demonstrated that wound healing, an immune-mediated event, was slower after a marital disagreement than a socially supportive discussion (Kiecolt-Glaser et al., 2005). In addition, production of inflammatory cytokines at the wound site was lower following the conflict than the support discussion. In contrast to heightened systemic inflammation, which is linked to a variety of age-related diseases (Harris et al., 1999; Ershler and Keller, 2000; Hansson, 2005; Hotamisligil, 2006), local inflammation at the wound site is adaptive and critical to effective wound healing.
Negative and hostile behaviors during a conflict discussion, such as blaming or interrupting the partner, appear to be particularly detrimental. A conflict discussion led to slower wound healing among couples displaying more hostile behaviors compared with their less hostile counterparts (Kiecolt-Glaser et al., 2005). Furthermore, whereas hostile couples had higher systemic inflammation following a conflict discussion compared to a social support discussion, low hostile couples had similar levels of inflammation across both discussions (Kiecolt-Glaser et al., 2005). Other studies using the marital disagreement paradigm provide evidence for additional immune alterations. For example, spouses whose discussions were more hostile had higher serum antibody titers to latent EBV than spouses whose discussions were less hostile (Kiecolt-Glaser et al., 1993). The cellular immune response plays an important role in controlling the expression of latent EBV, and thus these data are consistent with greater EBV reactivation among distressed individuals (Glaser et al., 1994). Compared to their less hostile counterparts, more hostile couples also had larger declines in two different indices of cellular immune function during the 24 hours following the disagreement (Kiecolt-Glaser et al., 1993).
The immunological consequences of marital distress may be particularly strong for women compared with men (Kiecolt-Glaser and Newton, 2001). For example, marital stress was associated with heightened systemic inflammation in young women but not young men (Whisman and Sbarra, 2012). A marital conflict discussion led to greater immune dysregulation among women compared with men (Kiecolt-Glaser et al., 1993; Mayne et al., 1997).
The Current Study
Taken together, prior research suggests a reliable link between marital distress and concurrent immune dysregulation, particularly cellular immunity. To date, little to no work has addressed whether marital distress alters the trajectory of immune function over time (Robles et al., in press). Longitudinal studies are important to understanding the directional nature of the link between marriage and immune function in a naturalistic context. Accordingly, the current study, which followed newlywed married couples over a two-year period, fills an important gap in the literature.
We selected three measures of cellular immune function, EBV antibody titers and T-cell proliferation in response to Con A and PHA, because previous studies have shown that cellular immunity is modulated by interpersonal stress (Kiecolt-Glaser et al., 1987, 1993). In addition, proliferative responses to Con A and PHA may index the immune system's response to bacterial and viral challenges. Indeed, the cellular immune response is critical to the effective resolution of viral and bacterial infections, among other functions. Proliferative responses also decrease with age (Antel et al., 1980; Wayne et al., 1990); decreased T-cell proliferation is part of immunosenesence, the aging of the immune system that is linked to age-related diseases (Malaguarnera et al., 2001).
We hypothesized that, compared to spouses in less distressed marriages, those who were more distressed would exhibit greater immune dysregulation over time, as evidenced by higher EBV antibody titers and decreased proliferative responses. Based on prior research suggesting possible immune-related gender differences, we also explored whether gender moderated the relationship between marital distress and immune function over time.
Method
Participant Selection Procedure
We used a stringent 3-stage selection process to recruit newlywed couples. First, we sent letters to couples who obtained their first marriage license within the previous 4-6 months. Couples who responded then completed two sets of phone interviews assessing their mental and physical health.
Individuals were ineligible for the study if they were pregnant, drank excessive amounts of alcohol or caffeine, smoked, used any type of medication other than birth control, had health problems that had immunological consequences (e.g., cancer, diabetes), or if they were not within 20% of their ideal weight for their height. We also excluded people who met DSM-III-R criteria for any psychotic diagnosis, a depressive or anxiety disorder other than simple phobia, or a substance abuse disorder, which eliminated individuals whose psychopathology might produce marital discord or immunological alterations (Johnson and Jacob, 1997). Couples who told us they were planning to move or have children within the next two years were also ineligible, since they might be lost to follow-up. Aside from our stringent mental and physical health criteria, couples were eliminated if they reported any needle or hospital phobias or if they could not be scheduled for their first study visit within 14 months of their marriage. Anyone who developed an immunological disorder prior to the follow-up visit was excluded from the study (n = 0). The project was approved by The Ohio State University Institutional Review Board; participants provided written informed consent before participating.
Study Procedure
Couples completed 2 study visits that were 2 years apart. At both visits, couples were admitted to the Clinical Research Center (CRC), a hospital research facility, at 7:00 a.m. for a 24-hour visit. Participants provided their first blood sample shortly after arrival and their second sample around the same time the following morning. During the remainder of the visit, couples engaged in a marital problem solving task which is discussed elsewhere and is not the focus of the current study (Kiecolt-Glaser et al., 1993, 1996).
Questionnaires
The Marital Adjustment Test (MAT) is a widely used measure of marital quality. The MAT can discriminate between satisfied and dissatisfied couples and demonstrates adequate reliability and good criterion validity (Locke and Wallace, 1959; Freeston and Plechaty, 1997). Participants completed the MAT assessing their baseline marital distress during their first telephone screening interview. Lower scores on the MAT indicate more marital distress.
The Structured Clinical Interview for DSM-IIIR Axis I disorders - Nonpatient version (SCID-NP) measured current mood, anxiety, substance abuse, and psychotic disorders. The SCID-NP is a reliable and valid method for evaluating psychopathology (First et al., 1997). Interviews were completed by advanced clinical psychology graduate students or clinically supervised staff. At 3 least three trained personnel then arrived at a diagnosis during a consensus meeting. The SCID-NP was used to screen people with a mental disorder from the study. We also re-administered the SCID-NP at the follow-up to account for potential changes in mental health status over time.
Immunological Assays
The EBV VCA serum antibody titers (IgG) were measured by the indirect immunofluorescence (IF) test using HR-1 cells by previously described procedures (Kiecolt-Glaser et al., 1991). Proliferative responses to Con A and PHA provided data on functional in vitro cellular immune changes. The concentrations for PHA and Con A were 2.5, 5.0, 10.0, and 20.0 μg/ml. Cells, 1 × 105/well, were incubated for 92 hours at 37°C in 96-well round-bottom plates, then pulsed for 4 hours with 3H TdR (0.5 μ Ci/well, specific activity 1 mCi/ml). All samples were run in triplicate (Kiecolt-Glaser et al., 1991).
Data Analytic Strategy
The immune assay scores were highly skewed. Accordingly, each measure was log10 transformed prior to analyses. Because the Con A and PHA data had 5 levels (i.e., couple, subject, visit, sample time, and dilution) and only 63 couples completed the follow-up visit, scores were averaged across dilutions, simplifying the complex model. The EBV data only had 3 levels; the assay does not require different dilutions and we only measured IgG EBV antibody titers once at each visit (because the half-life of IgG antibodies is at least one week).
Mixed models were utilized to account for the correlations within couples and subjects. All models were analyzed with SPSS version 19.0 (IBM, New York) using random effects for couple and subject. The random couple effect accounted for dependency between husbands and wives and the random subject statement accounted for the repeated effects of visit (first vs. second) and sample time (CRC entry vs. CRC exit, when applicable). An identity variance-covariance matrix was fitted to estimate the error variance.
To test the hypothesis that marital distress predicts the trajectory of immune function over time, we tested whether marital distress at the first study visit predicted changes in immune function between the first and second visit, two-years later. Specifically, we were interested in whether the marital distress X visit interaction predicted EBV antibody titer levels, Con A responses, and PHA responses, separately. Although our hypothesis was about the marital distress and visit interaction, we included fixed effects for the 3-way marital distress X visit X sample time interaction predicting Con A and PHA responses, and all corresponding 2-way interactions. This allowed us to test whether sample time (CRC entry vs. CRC exit) affected our results. Significant interactions were decomposed using simple slopes tests that examined the hypotheses of interest.
Potential confounds were selected based on their theoretical and empirical relationships to marital distress and cellular immune function. Every model adjusted for age, gender, and body mass index (BMI: kg/m2) using time-varying covariates. We also conducted a series of ancillary analyses to test whether the effects held when controlling for health behaviors. Specifically, we adjusted for participants' typical number of alcoholic drinks per week, cups of caffeinated drinks per day, and hours of exercise per week. We conducted a second set of auxiliary analyses that excluded anyone who had a mental disorder (n = 3) or was pregnant (n = 4) at the time of the follow-up visit.
We initially included all possible gender interactions in each model. None of the interactions involving gender were significant and thus the gender interaction terms were omitted from all analyses.
Results
Participant Information
Participants were 90 newlywed couples (N = 180 individuals) married an average of 10.45 months (SD = 2.08, range 6-15) prior to their first study visit. Husbands' and wives' average age was 25.67 years (SD = 3.06, range 20-37) and the majority of participants were White (N = 171 individuals). All couples were in their first marriage and had no children at the time of their first study visit. Additional sample characteristics are listed in Table 1.
Table 1.
Study sample characteristics.
| Characteristic | Category | Number(%) or Mean(SD) |
|---|---|---|
| Race | White | 171(95) |
| Non-White | 9(5) | |
|
| ||
| Gender | Female | 90(50) |
| Male | 90(50) | |
|
| ||
| Education | High school or below | 11(6.1) |
| Some college or college graduate | 138(76.6) | |
| Graduate or professional training | 31(17.2) | |
|
| ||
| Age (years) | N/A – numbers are mean (SD) | 25.67(3.06) |
|
| ||
| Body mass index (kg/m2) | N/A – numbers are mean (SD) | 22.64(2.44) |
|
| ||
| Time dated before marriage (months) | N/A – numbers are mean (SD) | 36.56(25.32) |
|
| ||
| Length of marriage (months) | N/A – numbers are mean (SD) | 10.45(2.08) |
A total of 63 couples (N = 126 individuals) completed the two-year follow-up visit, consistent with the average 31% attrition rate in longitudinal marital studies (Karney and Bradbury, 1995). Of the 27 couples who did not return for the follow-up, 8 separated or divorced in the interim, 10 either moved out of town or could not be contacted, and 9 declined to participate. In addition, couples who returned for the follow-up had higher marital quality at baseline (M = 129.81, SD = 13.67) than couples who did not return (M = 124.72, SD = 16.15), t(178)=2.16, p = .032. All reported coefficients are unstandardized.
Primary Analyses
Contrary to expectations, the interaction between marital distress and visit predicting EBV antibody titers was non-significant (b = .002, F(1,121)= 1.826, p = .179). However, there was a significant main effect of visit (b = -.10, F(1,364)= 21.29, p < .001), and a significant marital distress X visit interaction (b = .003, F(1,346) = 5.60, p = .019) predicting Con A responses. Specifically, proliferative responses to Con A decreased on average from the first to the second visit. This relationship was stronger for spouses in more distressed marriages (see Figure 1). None of the interactions with sample time were significant, indicating that the link between visit and Con A, marital distress and Con A, or the combination of the two did not differ whether the serum sample was collected at the beginning of the CRC visit or 24-hours later.
Figure 1.
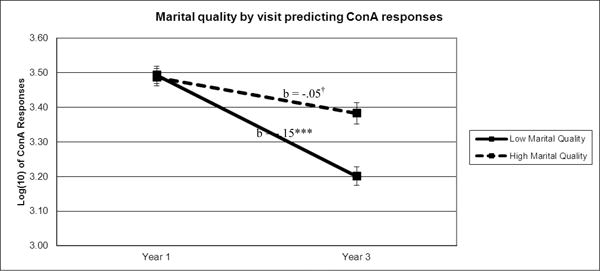
Note. To illustrate the relationship between marital quality and Con A responses over time, Con Aaverage values were graphed for participants 1 standard deviation above (high marital quality) and below (low marital quality) the mean of marital quality. Sample means were estimated using a model adjusted for BMI, age, and gender. †p≤.10, ***p≤.001
Next, we examined functional immune responses to PHA. There was a significant main effect of visit (b = -.11, F(1,368)= 61.60, p < .001), and a significant marital distress X visit interaction (b = .002, F(1,348)= 4.87, p = .028). Specifically, proliferative responses to PHA decreased on average from the first to the second visit. Furthermore, this link was stronger for spouses in more distressed marriages (see Figure 2). None of the interactions with sample time were significant, indicating that the link between visit and PHA, marital distress and PHA, or the combination of the two did not differ whether the serum sample was collected at the beginning of the CRC visit or 24-hours later.
Figure 2.
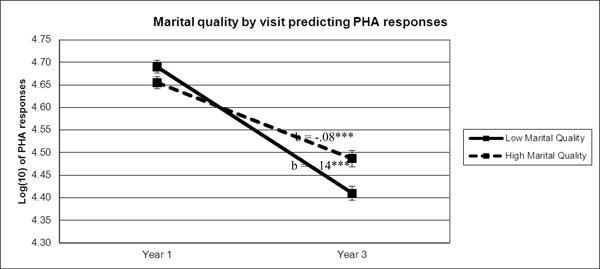
Note. To illustrate the relationship between marital quality and PHA responses over time, PHA average values were graphed for participants 1 standard deviation above (high marital quality) and below (low marital quality) the mean of marital quality. Sample means were estimated using a model adjusted for BMI, age, and gender. ***p≤.001
In accord with prior work, we were also interested in testing potential differences between spouses who showed patterns of higher versus lower immune responses across our three immunological measures (Kiecolt-Glaser et al., 1997). Accordingly, participants whose values were above the median for their gender on at least two of the three assays were designated “high responders” and remaining participants were deemed “low responders.” A logistic generalized estimating equations analysis revealed a a significant main effect of visit (b = -2.45, χ2(1, N = 180) = 4.71, p = .030), and a marginally significant marital distress X visit interaction (b = .01, χ2(1, N = 180) = 2.75, p = .097). Specifically, the likelihood of being a high responder decreased on average from the first to the second visit. Follow-up tests demonstrated that this relationship was stronger for spouses in more distressed marriages.
Ancillary Analyses
Ancillary analyses tested whether the Con A and PHA effects held when controlling for health behaviors. The results were unchanged after adjusting for participants' typical number of alcoholic drinks per week, cups of caffeinated drinks per day, and hours of exercise per week. The results also remained the same when we excluded participants who had a mental disorder (n = 3) or were pregnant (n = 4) at the time of the follow-up visit.
Discussion
The current study demonstrated that spouses in more distressed marriages had larger declines in cellular immune function over a two-year period than spouses in less distressed marriages. Contrary to expectations, latent EBV reactivation was unrelated to marital quality. However, the results were highly consistent across two different indices of cellular immune function, proliferative responses to Con A and PHA. In addition, spouses in more distressed marriages had relatively larger immunological declines across our three immune measures than spouses who were more happily married.
A growing body of cross-sectional studies have demonstrated that poorer marital quality is related to greater immune dysregulation (e.g., Kiecolt-Glaser et al., 1987, 1988, 2005; Phillips et al., 2006). The present results extend these findings in an important new direction by investigating immune alterations over a two-year period, an important first step in establishing a causal pathway. Furthermore, the stringent mental and physical health criteria used in this study helped rule out potential confounding factors that might explain the link between marital quality and immune function. Accordingly, the present findings provide robust evidence that marital quality is related to changes in cellular immune function over time.
The current results suggest that marital quality may influence health through long-term alterations in cellular immunity; the cellular immune response is critical to the effective resolution of viral and bacterial infections, among other functions. Furthermore, proliferative responses decrease with age, one characteristic of immunosenesence (Antel et al., 1980; Wayne et al., 1990). In the current study, age-related proliferative declines were accelerated among spouses in more distressed marriages.
Other physiological mechanisms may work independently or in tandem with changes in cellular immunity to influence health (Robles et al., in press; Robles and Kiecolt-Glaser, 2003). For example, hostile marital interactions were related to maladaptive alterations in cardiovascular activity (Smith et al., 2009) and increased catecholamine levels among newlywed and older adult couples (Malarkey et al., 1994; Kiecolt-Glaser et al., 1997). Accordingly, research incorporating the autonomic, neuroendocrine, and immune consequences of marital distress would help provide a more complete picture about the ways that these physiological systems influence each other to affect health.
Mental health and health behaviors may also contribute to the link between marital distress and health. For instance, recurrent depressive episodes have been linked to chronic interpersonal troubles; depression enhances risk for immune dysregulation (Herbert and Cohen, 1993; Monroe et al., 2007). In addition, people in more distressed marriages have a greater risk of developing alcohol problems than people in less distressed marriages (Whisman, Uebelacker, & Bruce, 2006); alcohol abuse has deleterious effects on immune function (Cook, 1998). The current study used stringent selection criteria that excluded people with mental or physical health problems; as a result, our participants had exceptional health habits (e.g., commitment to regular exercise). By admitting couples to the CRC for 24 hours, we were able to control factors like physical activity, diet, and caffeine intake that could influence immune function (Kiecolt-Glaser et al., 2010), while simultaneously providing a uniform environment across couples. In addition, ancillary analyses demonstrated that our results were unchanged after accounting for participants' typical number of alcoholic drinks per week, cups of caffeinated drinks per day, and hours of exercise per week. Accordingly, the current data suggest that marital distress affects cellular immune function independent of spouses' health behaviors and mental and physical comorbidities.
In sum, spouses in more distressed marriages had larger declines in cellular immune function over a two-year period than spouses in less distressed marriages. These results are particularly notable in light of the sample under investigation; only healthy couples were allowed to participate and the marital distress scores were on the lower end of the spectrum. Thus, the cellular immune alterations evident in this sample likely underestimate the true effects. Accordingly, the current study demonstrates that marital distress has long-term physiological costs and provides a glimpse into the pathways through which social relationships can impact health.
Table 2.
Raw means and standard errors of the mean for the outcome variables broken down by marital quality.
| Outcome | Marital Quality | T1 Mean (SE) |
T2 Mean (SE) |
b | p |
|---|---|---|---|---|---|
| Con A responses | Low marital quality (-1 SD) | 6291(931) | 3273(1135) | -.15 | <.001 |
| High marital quality (+1 SD) | 6491(932) | 44381049 | -.05 | .052 | |
|
| |||||
| PHA responses | Low marital quality (-1 SD) | 56130(2156) | 301062802 | -.14 | <.001 |
| High marital quality (+1 SD) | 50753(2163) | 37255(2519) | -.08 | <.001 | |
|
| |||||
| EBV antibody titers | Low marital quality (-1 SD) | 405(36) | 267(46) | --- | --- |
| High marital quality (+1 SD) | 359(37) | 335(42) | --- | --- | |
Note. Raw sample means and standard deviations were estimated using a model adjusted for BMI, age, and gender. b refers the the unstandardized slope of the outcome from T1 to T2 either for the lower or higher marital quality group. Simple slopes tests were calculated using log transformed data. The simple slopes for the analysis predicting EBV antibody titers are not reported because the marital quality by visit interaction was not significant.
Acknowledgments
Funding Sources: Work on this project was supported in part by NIH grants MH44660, M01-RR0034, and CA-16058-09 as well as American Cancer Society Postdoctoral Fellowship Grant 121911-PF-12-040-01-CPPB and a Pelotonia Postdoctoral Fellowship from the Ohio State University Comprehensive Cancer Center.
Footnotes
Conflicts of Interest: All authors declare that there are no financial conflicts of interest.
Contributors: Lisa M. Jaremka: substantial contributions to the analysis and interpretation of data, primary person responsible for writing and revising the article, final approval of the version to be published
Ronald Glaser: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
William B. Malarkey: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
Janice K. Kiecolt-Glaser: primary person responsible for designing the study, substantial contributions to the analysis and interpretation of data, helped revise the article for important intellectual content, final approval of the version to be published
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antel JP, Oger JJF, Dropcho E, Richman DP, Kuo HH, Arnason BGW. Reduced T-lymphocyte cell reactivity as a function of human aging. Cellular Immunology. 1980;54:184–192. doi: 10.1016/0008-8749(80)90200-2. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system - A review. Alcoholism: Clinical and Experimental Research. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased Interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Freeston MH, Plechaty M. Reconsideration of the Locke-Wallace marital adjustment test: Is it still relevant for the 1990s? Psychol Rep. 1997;81:419–434. doi: 10.2466/pr0.1997.81.2.419. [DOI] [PubMed] [Google Scholar]
- Glaser R, Jones J. Human herpesvirus infections. New York, NY: Dekker; 1994. [Google Scholar]
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: A meta-analytic review. Psychological Bulletin. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: The National Longitudinal Mortality Study. Annals of Epidemiology. 2000;10:224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Jacob T. Marital interactions of depressed men and women. J Consult Clin Psychol. 1997;65:15–23. doi: 10.1037//0022-006x.65.1.15. [DOI] [PubMed] [Google Scholar]
- Karney BR, Bradbury T. The longitudinal course of marital quality and stability: A review of theory, method, and research. Psychol Bull. 1995;118:3–34. doi: 10.1037/0033-2909.118.1.3. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosom Med. 1987;49:13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, Malarkey WB. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosom Med. 1997;59:339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Kennedy S, Malkoff S, Fisher L, Speicher CE, Glaser R. Marital discord and immunity in males. Psychosom Med. 1988;50:213–229. doi: 10.1097/00006842-198805000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Malarkey W, Chee M, Newton T, Cacioppo J, Mao H, Glaser R. Negative behavior during marital conflict is associated with immunological down-regulation. Psychosom Med. 1993;55:395–409. doi: 10.1097/00006842-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB. Marital conflict and endocrine function: Are men really more physiologically affected than women? J Consult Clin Psychol. 1996;64:324–332. doi: 10.1037//0022-006x.64.2.324. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Locke H, Wallace K. Short marital-adjustment and prediction tests: Their reliability and validity. Marriage and Family Living. 1959;21:251–255. [Google Scholar]
- Malaguarnera L, Ferlito L, Imbesi RM, Gulizia GS, Di Mauro S, Maugeri D, Malaguarnera M, Messina A. Immunosenescence: A review. Archives of Gerontology and Geriatrics. 2001;32:1–14. doi: 10.1016/s0167-4943(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosom Med. 1994;56:41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Mayne TJ, O'leary A, McCrady B, Contrada R, Labouvie E. The differential effects of acute marital distress on emotional, physiological and immune functions in maritally distressed men and women. Psychology and Health. 1997;12:277–288. [Google Scholar]
- Monroe SM, Slavich GM, Torres LD, Gotlib IH. Major life events and major chronic difficulties are differentially associated with history of major depressive episodes. J Abnorm Psychol. 2007;116:116–124. doi: 10.1037/0021-843X.116.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabipour I, Vahdat K, Jafari SM, Pazoki R, Sanjdideh Z. The association of metabolic syndrome and Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, and herpes simplex virus type 1: The Persian Gulf Healthy Heart Study. Cardiovasc Diabetol. 2006;5 doi: 10.1186/1475-2840-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth-Gomér K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection -associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Carroll D, Bums VE, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun. 2006;20:279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiology & Behavior. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychological Bulletin. doi: 10.1037/a0031859. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA. Marital status and health: United States, 1999-2002. Adv Data. 2004;351:1–32. [PubMed] [Google Scholar]
- Smith TW, Uchino BN, Berg CA, Florsheim P, Pearce G, Hawkins M, Henry NJM, Beveridge RM, Skinner MA, Ko KJ, et al. Conflict and collaboration in middle-aged and older couples: II. Cardiovascular reactivity during marital interaction. Psychology and Aging. 2009;24:274–286. doi: 10.1037/a0016067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprehn GC, Chambers JE, Saykin AJ, Konski A, Johnstone PAS. Decreased cancer survival in individuals separated at time of diagnosis: Critical period for cancer pathophysiology? Cancer. 2009;115:5108–5116. doi: 10.1002/cncr.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Gallo LC, Kuller LH. Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med. 2005;165:1022–1027. doi: 10.1001/archinte.165.9.1022. [DOI] [PubMed] [Google Scholar]
- Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990;45:M45–M48. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Sbarra DA. Marital adjustment and interleukin-6 (IL-6) J Fam Psychol. 2012;26:290–295. doi: 10.1037/a0026902. [DOI] [PMC free article] [PubMed] [Google Scholar]


