Abstract
Availability of the thyroid hormone triiodothyronine (T3) in the mediobasal hypothalamus plays a central role in seasonal reproductive responses to photoperiod. Across many vertebrates, Type 2 iodothyronine deiodinase (DIO2) is elevated under reproductively stimulatory long days (LD) and synthesizes the conversion of thyroxine to T3; type 3 iodothyronine deiodinase (DIO3) reduces T3 production and signaling, and is upregulated under reproductively-inhibitory short days (SD). In Siberian hamsters, regulation of hypothalamic T3 is dominated by dio3 expression, whereas dio2 expression is less-consistently affected by photoperiod. In adult hamsters, changes in deiodinase mRNA expression typically require several weeks to manifest, but it is not known whether or how quickly these mechanisms are engaged during the rapid responses to photoperiod observed in young, peri-pubertal hamsters. This experiment tested the hypotheses that (1) deiodinase responses to photoperiod are accelerated in juvenile hamsters and (2) photoperiodic downregulation of deiodinase expression occurs more rapidly than upregulation. Hypothalamic dio2 and dio3 mRNA expression was quantified in male and female Siberian hamsters that were weaned on postnatal day 18 (PND 18) into SD or remained in their natal LD, and on PND 31 were exposed to a single long or short day. In SD males and females, a single long day inhibited dio3 mRNA expression, but did not increase dio2 mRNA. In LD males, a single short day rapidly inhibited dio2 mRNA expression, but did not stimulate expression of dio3 mRNA. Downregulation of dio2 and dio3 mRNAs precedes gonadotrophin responses to day length. Rapid photoperiodic inhibition of deiodinase mRNAs may initiate changes in thyroid hormone signaling in advance of longer-term, melatonin-dependent, responses.
Keywords: photoperiodism, seasonality, dio2, dio3, thyroid, reproduction, hypothalamus, biological rhythms
1. Introduction
Day length provides temperate zone organisms a salient cue for coordinating physiology and behavior with seasonally-changing environmental conditions (Goldman, 2001). Reproduction in many species is affected by seasonal time, such that offspring are born during months when quality food is available (Bronson, 1985).
Among birds and mammals, thyroid hormone signaling provides a crucial step in inducing seasonal reproductive phenotypes. Infusions of exogenous triiodothyronine (T3) delivered under a winter-like short day (SD) photoperiod promote robust gonadal development in long day (LD)-breeding Japanese quail (Coturnix japonica; Yoshimura et al., 2003) and Siberian hamsters (Phodopus sungorus; Barrett et al., 2007; Freeman et al., 2007). Photoperiod manipulations have little effect on circulating plasma triiodothyronine (T3) or thyroxine (T4) concentrations (O’Jile & Bartness, 1992; Prendergast et al., 2002; Yoshimura et al., 2003). Rather, thyroid hormone availability and receptor-signaling are regulated locally through differential expression of iodothyronine deiodinase enzyme types II and III (DIO2 and DIO3) in the mediobasal hypothalamus (MBH; Yoshimura et al., 2003). Deiodinase enzymes have opposing effects on T3 production: DIO2 converts the prohormone T4 into biologically-active T3, whereas DIO3 both catabolizes T3 into diiodothryonine (T2) and converts T4 into biologically-inactive reverse-triiodothyronine (rT3; Lechan & Fekete, 2005; Yasuo et al., 2005). Changes in expression of either or both of these enzymes offer a potential mechanism for photoperiodic control of T3 availability in the MBH.
In Siberian hamsters, a canonical mammalian model for dissecting mechanisms of seasonal timekeeping, hypothalamic dio3 mRNA levels increase markedly following exposure to reproductively-inhibitory SD photoperiods (Barrett et al., 2007; Kampf-Lassin & Prendergast, 2013; Prendergast et al., 2013; Watanabe et al., 2007; Watanabe et al., 2004). In mammals, SD photoperiods are internalized by a lengthening of the duration of nocturnal pineal melatonin (MEL) secretion (Bartness & Goldman, 1988; Hoffman & Reiter, 1965; review, see Goldman, 2001). Artificially lengthening this signal in LD-housed hamsters, via exogenous MEL treatment, causes a rapid increase in hypothalamic dio3 mRNA expression (Prendergast et al., 2013), and abolishing the MEL signal, via pinealectomy, eliminates the dio3 mRNA response to SD (Barrett et al., 2007). Photoperiodically-induced changes in hypothalamic dio2 expression have been well-documented in Syrian hamsters (Mesocricetus auratus; Yasuo et al., 2009) and in Japanese quail (Watanabe et al., 2007), but dio2 responses to photoperiod appear to be of little reproductive consequence in European starlings (Bentley et al., 2013) and have yet to be consistently established in Siberian hamsters. Several studies using standard photoperiod treatments have failed to identify effects of photoperiod on dio2 expression (Barrett et al., 2007, Kampf-Lassin et al., 2013; Prendergast et al., 2013). Two earlier reports identified a robust increase in perihypothalamic dio2 expression after transfer from SD to LD for 2 weeks (Watanabe et al., 2007; Watanabe et al., 2004). However, no decrease in dio2 expression was evident after transfer from LD to SD for six weeks (Watanabe et al., 2007). In a recent study, exogenous SD-like MEL treatment rapidly inhibited dio2 mRNA expression in Siberian hamster MBH, and this inhibition was sustained for 4 weeks (Stevenson & Prendergast, in review).
When dio2 responses to photoperiod or MEL treatments are evident in adult hamsters, they occur rapidly, within intervals that range from < 24 h (Syrian hamsters; Yasuo et al., 2007) to ≤ 2 days (Siberian hamsters; Stevenson & Prendergast, in review). In contrast, dio3 responses to photoperiod in adults require between 2–6 weeks to manifest (Barrett et al., 2007). Several non-exclusive hypotheses may explain the difference in the latency of responsiveness to photoperiod between dio2 and dio3. First, whereas transfer from SD to LD shortens the duration of nocturnal MEL secretion abruptly (in the first short night; Yellon et al., 1985), when hamsters are transferred from LD to SD, MEL requires ≥2 weeks to fully expand to match the length of the dark phase (Gorman et al., 1997; Illnerová et al., 1984). Thus, according to this hypothesis (Hypothesis 1), dio2 and dio3 are capable of responding equally rapidly to changes in MEL, but the appropriate, inhibitory MEL signal is not instantaneously generated following transfer from LD to SD, and this accounts for the different latencies of dio mRNA responses to long versus short days. Alternatively, (Hypothesis 2), inhibition of dio mRNA expression may occur much more rapidly than induction. LDs stimulate dio2 and inhibit dio3; conversely, SDs stimulate dio3 and inhibit dio2. Rapid dio3 responses to SD photoperiod may not be evident in adulthood because the nature of adult dio3 responses is one of mRNA upregulation, which may require considerably more time, relative to mRNA downregulation. If this hypothesis is true, then one would predict that photoperiod transfers which induce dio3 downregulation (i.e., SD to LD transfers) would elicit rapid dio3 responses. Lastly, (Hypothesis 3) owing to as-yet-unspecified differences in the transcriptional regulation of these two genes, changes in dio3 expression -- upregulation and downregulation -- may simply require longer to manifest than changes in dio2 expression, even in the presence of the appropriate MEL signals. Recent work indicates that epigenetic mechanisms (specifically, DNA methylation) regulate dio3 responses to photoperiod and MEL (Stevenson & Prendergast, in review). At present, it is unknown if similar mechanisms operate in the photoperiodic control of dio2 expression, but changes in the methylation status of the dio3 proximal promoter may take several weeks to manifest, and this may place an upper limit on the rate of change in dio3 mRNA expression, independent of whether the change is an upregulation or a downregulation.
In an effort to discriminate among these hypotheses, this experiment evaluated dio2 and dio3 mRNA responses to a single day of exposure to long or short photoperiods. Photoperiod manipulations were applied during the late pubertal interval, when Siberian hamsters are acutely sensitive to photoperiod (Prendergast et al., 1996) and both dio2 and dio3 mRNA responses to photoperiod have been established to occur (Herwig et al., 2012).
2. Materials and Methods
2.1 Animals and housing
Male and female Siberian hamsters (Phodopus sungorus) were gestated and reared in a long-day (15L:9D, dark onset at 18:00 h; LD) light-dark cycle. Hamsters were weaned at 18 days of age (post natal day 18; PND 18), and singly housed thereafter in polypropylene cages (28 × 17 × 12 cm), with ad libitum access to food and filtered water. Ambient temperature and relative humidity were maintained at 20 ± 2°C and 50 ± 10%, respectively at all times. All animal treatments described in this experiment conformed to the USDA Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Chicago Institutional Animal Care and Use Committee.
2.2 Photoperiod treatments
At weaning (PND 18) hamsters either maintained in LD (n=38 [14 females, 24 males]) or were transferred to a short-day (9L:15D, light onset at 9:00 h; SD) photoperiod (n=35 [12 females, 23 males]). Hamsters were maintained in LD or SD for the next 13 days (until PND 31), at which time a subset of LD hamsters was transferred to SD (LD-SD: n=19 [6 females, 13 males]), and a subset of SD hamsters was transferred to LD (SD-LD: n=18 [7 females, 11 males]). Photoperiod transfers were performed via extension of the dark phase (Gorman et al., 1997); the timing of dark onset remained constant across all photoperiods. Hamsters that were not transferred between photoperiods on PND 31 remained in their immediate post-weaning photoperiod for the remainder of the study (LD-LD: n=19 [8 females, 11 males]; SD-SD: n=17 [5 females 12 males]). All photoperiod transfers were performed during the light phase. On the following day (PND 32) hamsters were weighed to ± 0.1g and killed via rapid decapitation during a 1 h interval that spanned the midpoint of the respective light phases (LD: 10:30 h; SD: 13:30 h).
2.3 Tissue collection
At autopsy, hypothalami were rapidly dissected and immediately frozen on dry ice for later determination of dio2 and dio3 mRNA expression (see below). The anatomical boundaries for hypothalamus dissection were the optic chiasm at the anterior border, the mammillary bodies at the posterior border, and laterally at the hypothalamic sulci. The tissue was cut dorsally 3–4 mm from the ventral surface. Trunk blood was collected at autopsy and stored on ice for 1 h. Blood was centrifuged for 20 min at 8000 × g; serum was extracted and stored at −80°C for later determination of FSH concentrations (see below). In males, paired testes and seminal vesicles were dissected and weighed; in females, uteri were dissected at the level of the cervix, cleaned of connective tissue and weighed, along with paired ovaries free of surrounding fat.
2.4 Quantitative PCR
dio2 and dio3 mRNAs were measured in whole hypothalamus samples via quantitative polymerase chain reaction (qPCR). Total RNA was extracted using RNeasy (Qiagen) according to the kit instructions. Extracted RNA was suspended in 30µl RNase-free water and RNA concentration and quality were determined by spectrophotometer. All RNA samples were stored at −70° C until further analysis. cDNA was created via reverse transcription (RT) of 2 µg RNA using MMLV RT enzyme (Invitrogen, Carlsbad, CA, USA).
To design primers and a probe for quantitative PCR with high specificity for this species, a portion of each gene of interest was sequenced via methods described in detail for this species elsewhere (Paul et al., 2009). Briefly, to sequence portions of these genes, semi-quantitative PCR was conducted on 1µl of pooled Siberian hamster hypothalamic cDNA with Taq DNA Polymerase enzyme (Invitrogen) according to the manufacturer’s protocol in a thermocycler for 40 cycles (Bio-Rad). Degenerate primers were designed based on conserved regions among multiple species with known gene sequences (GenBank) using PrimerExpress software (Applied Biosystems, Foster City, CA, USA). PCR gene product amplification was visualized on 2% TAE-agarose gels containing ethidium bromide using a CCD camera. To verify amplification of the correct gene, PCR products were purified (Centricon-100, Millipore, Billerica, MA, USA) and directly sequenced at the University of Chicago Cancer Research Center DNA Sequencing Facility. The resulting amplicon sequences for Siberian hamster dio2 and dio3 were >90% similar to published sequences for mouse and rat dio2 and dio3. Sequencing information was entered in the GENBANK database (dio2: EU812319; dio3: EU812320).
After confirmation of gene products, primers and probes for quantitative PCR were designed using PrimerExpress. Primers and probes were synthesized as follows, with probes labeled with 6-FAM and MGB (non-fluorescent quencher) at the 5′ and 3′ ends, respectively: dio2 forward 5’- ACCACCACCTTCCTTTGCAA-3’, dio2 reverse 5’- GCGGAAGGCTGGCAGTT-3’, dio2 probe 5’- AAGCAGAGTGCCCAGGA-3’; dio3 forward 5’- GTGCATCCGCAAGCATTTC-3’, dio3 reverse 5’- ACTTCAGGCTCGGGATGGT-3’, dio3 probe 5’- TGCGCCGTCGCCA-3’. A TaqMan 18S Ribosomal RNA primer and probe set (labeled with VIC; Applied Biosystems) was used as the control gene for relative quantification (Genbank: AY591918). Amplification was performed on an ABI 7900HT Sequencing Detection System by using Taqman® Universal PCR Master Mix. The universal two-step RT-PCR cycling conditions used were: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Relative gene expression of individual samples run in duplicate was calculated by comparison to relative standard curve consisting of serial dilutions of pooled P. sungorus hypothalamic cDNA (1:1, 1:10, 1:100, 1:1000, 1:10,000) followed by normalization to 18S rRNA gene expression. RNA quality for each sample was assessed via 260/280 ratio using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) before reverse transcription. All samples had acceptable ratios between 1.8 and 2.0.
2.5 FSH radioimmunoassay
Serum FSH was measured in duplicate 50-µl aliquots of serum by radioimmunoassay (RIA) using materials supplied by NIDDK (Rockville, MD). All assays were performed in the Ligand Assay Core of Northwestern University. The FSH standard was Rat FSH RP-3 and the antibody was rat FSH-S-11. The intraassay coefficient of variation was 5%, and the interassay coefficient of variance was 7%. The use of the heterologous (rat) reagents to measure FSH Siberian hamster serum has been validated previously by Milette & Turek (1986) and Yellon & Goldman (1984). The sensitivity of the assay was 1 ng/ml, as determined from the minimum detectable level on the standard curve. Due to a handling error, blood samples from male LD-LD and SD-SD groups were not available for RIA.
2.6 Statistics
All data are reported as mean ± S.E.M. Effects of photoperiod were assessed using factorial ANOVAs. To control for family-wise error rate, pairwise comparisons were made using Bonferroni corrected t-tests with α set to 0.05. All statistical calculations were performed using Statview 5 (SAS Institute; Cary, NC, USA).
3. Results
3.1 Somatic and reproductive responses to photoperiod manipulations
Overall, males weighed more than females on PND 32 (F1, 65 = 4.96, P < 0.05; data not illustrated). There was no main effect of photoperiod on body mass (F3,65=2.15, P>0.10), nor did photoperiod and sex interact (sex × photoperiod: F3, 65 = 0.94, P > 0.40). Within males, LD→LD hamsters did not differ in body mass from SD-SD hamsters on PND 32 (LD→LD: 20.6 ± 1.0 g; SD-SD: 18.5 ± 0.7 g; n.s.), and body mass did not differ across treatment groups (F3, 43 = 1.93, P > 0.10); a similar pattern of outcomes was obtained among females (LD-LD: 19.5 ± 0.7 g; SD-SD: 18.4 ± 0.4 g; n.s.; F3, 22 = 2.09, P > 0.10).
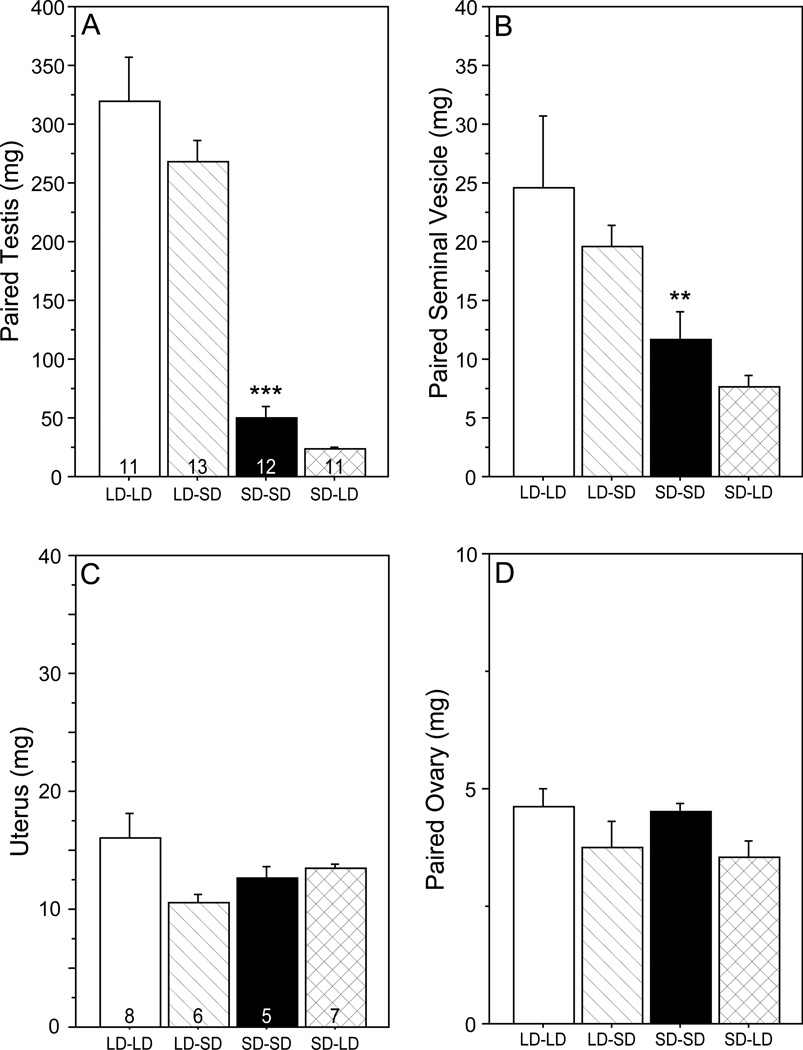
Photoperiod treatments significantly affected testis (F3, 43 = 49.1, P < 0.001; Fig. 1A) and seminal vesicle (F3, 43 = 5.26, P < 0.005; Fig. 1B) mass. Testes of LD-LD hamsters were larger than those of SD-SD hamsters (P<0.001). Transfer from LD to SD on PND 31 did not lead to significant changes in testis mass as measured 1 day later (LD-LD vs. LD-SD, n.s.), nor did transfer from SD to LD (SD-SD vs. SD-LD, n.s.). A similar pattern of pairwise comparisons was evident in seminal vesicle masses (LD-LD vs. SD-SD: P<0.0083; LD-LD vs. LD-SD, n.s.; SD-SD vs. SD-LD, n.s.). Among females, neither uterine mass (F3, 21 = 2.74, P > 0.05; Fig. 1C) nor ovarian mass (F3, 22 = 1.86, P > 0.10; Fig. 1D) was significantly affected by photoperiod treatments.
Figure 1.
Mean + SEM (A) testis mass, (B) seminal vesicle mass (C) uterus mass, and (D) paired ovary mass of male (A, B) and female (C, D) Siberian hamsters on PND 32. Hamsters were reared in LD (15L:9D) and on PND 18 were either transferred to SD (9L:15D; SD) or remained in LD. On PND 31, hamsters in SD were either transferred back to LD (SD-LD) or remained in SD (SD-SD); hamsters in LD on PND 31 were either transferred to SD (LD-SD group) or remained in LD (LD-LD group). **P<0.01, ***P<0.001 vs. LD-LD value.
3.2 Hypothalamic dio2 mRNA expression
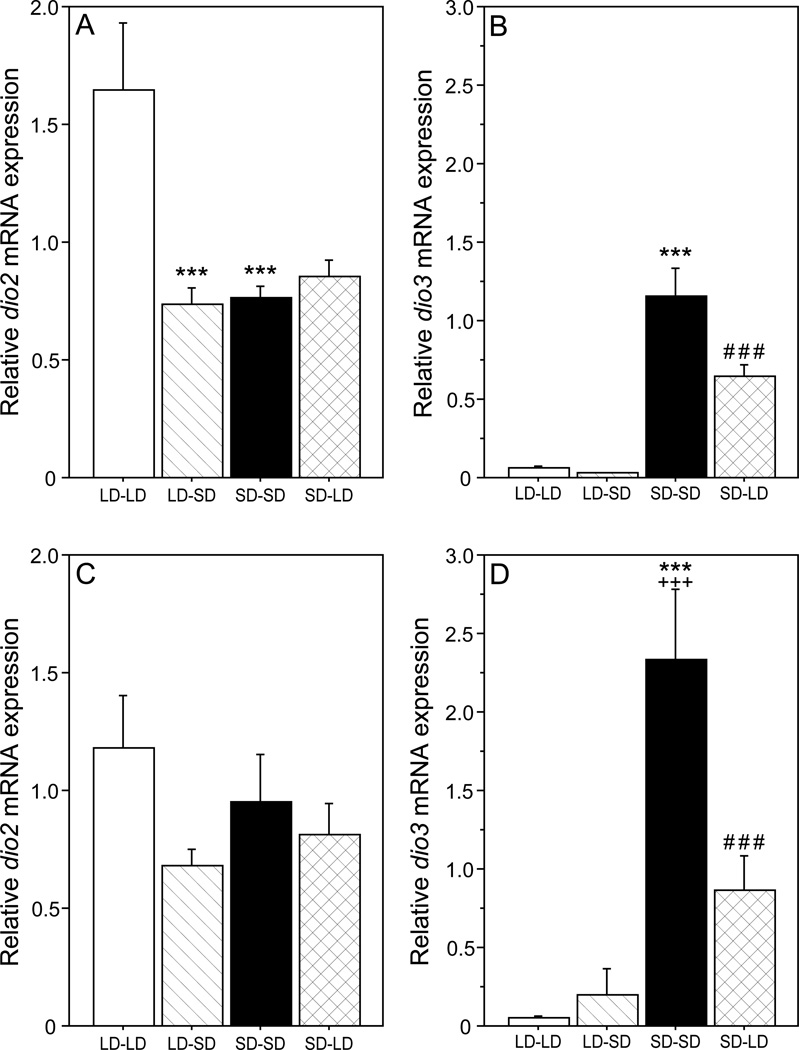
Photoperiod treatments significantly affected hypothalamic dio2 mRNA expression (F3,59 = 9.52, P < 0.001), with no omnibus difference between sexes (F1, 59 = 0.80, P > 0.30; Fig. 2). Among males, dio2 mRNA was >2-fold higher in LD-LD relative to SD-SD hamsters on PND 32 (P < 0.001; Fig. 2A). Transfer from SD to LD on PND 31 did not affect dio2 expression (SD-SD vs. SD-LD: P > 0.50), but transfer from LD to SD decreased dio2 mRNA by 55% (LD-LD vs. LD-SD: P < 0.001).
Figure 2.
Mean + SEM hypothalamic dio2 (A, C) and dio3 (B, D) mRNA expression (relative to 18S rRNA) on PND 32 of male (A, B) and female (C, D) hamsters that were weaned on PND 18 into LD or SD, and transferred to LD or SD on PND 31. Group abbreviations as in Figure 1. **P<0.01, ***P<0.001 vs. LD-LD value; ###P<0.001 vs. SD-SD value; +++P<0.001 vs. comparable value in males.
Among females, dio2 expression was comparable in LD-LD and SD-SD groups (P > 0.40), and transfer between photoperiods on PND 31 did not affect dio2 expression (P > 0.05, both comparisons; ; Fig. 2C).
3.3 Hypothalamic dio3 mRNA expression
Photoperiod (F3, 59 = 54.6, P < 0.001) and sex (F1, 59 = 14.9, P < 0.001) each significantly affected hypothalamic dio3 mRNA expression (Fig. 2). Hypothalamic dio3 expression in SD-SD hamsters was significantly (> 2-fold) higher in females relative to males (P < 0.001).
Among males, dio3 mRNA was ~20 fold higher in SD-SD relative to LD-LD hamsters on PND 32 (P < 0.001; Fig. 2B). Transfer from LD to SD on PND 31 did not significantly affect dio3 expression (LD-LD vs. LD-SD: P > 0.80), but transfer from SD to LD on PND 31 caused a 44% decrease in dio3 mRNA expression (P < 0.001).
A categorically similar effect of photoperiod was evident in females. Hypothalamic dio3 mRNA expression was ~48-fold higher in SD-SD relative to LD-LD females on PND 32 (P < 0.001; Fig. 2D). Transfer from LD to SD on PND 31 did not affect dio3 expression (LD-LD vs. LD-SD: P > 0.50), but transfer from SD to LD decreased dio3 expression by 63% (P < 0.001).
3.4 Serum FSH concentrations
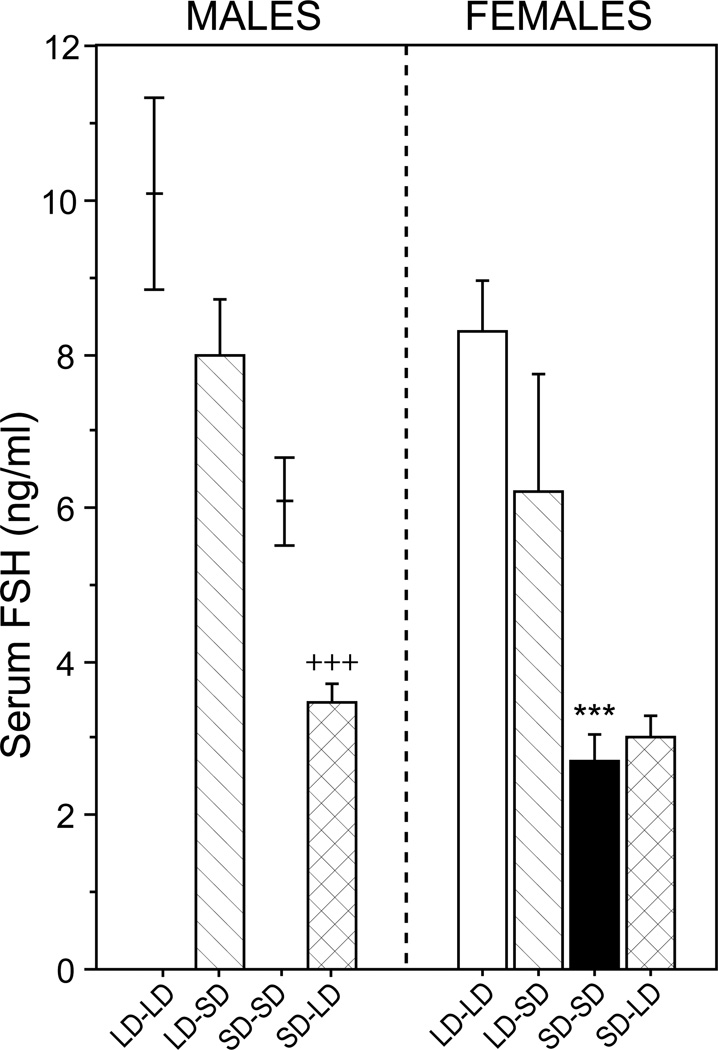
Among males, FSH data from LD-LD and SD-SD groups were not available (see Methods); however, for purposes of comparison, previously-published data (Prendergast et al., 2013) on serum FSH values on PND 32 of male hamsters gestated and raised in LD, and housed in LD or SD beginning on PND 18, are depicted in Fig. 3 (left panel). Serum FSH concentrations were significantly higher in LD-SD relative to SD-LD males (P < 0.001). Among females, there was a significant main effect of photoperiod on serum FSH concentrations (F3, 20 = 9.26, P < 0.001; Fig. 3; right panel). FSH concentrations were significantly higher in LD-LD relative to SD-SD females (P < 0.001). Transfer from LD to SD on PND 31 did not significantly reduce FSH concentrations (P > 0.10), and transfer from SD to LD on PND 31 did not significantly increase serum FSH (P > 0.80).
Figure 3.
Mean + SEM serum FSH concentrations on PND 32 of male (left) and female (right) hamsters that were weaned on PND 18 into LD or SD, and transferred to LD or SD on PND 31. Group abbreviations as in Figure 1. Due to a handling error, blood samples from male LD-LD and SD-SD groups were not available for assay; for comparison purposes only, previously-published data depicting serum FSH values on PND 32 of male hamsters gestated and raised in LD, and housed in LD or SD beginning on PND 18 are interpolated in the left panel (data from Prendergast et al., 2013). ***P<0.001 vs. LD-LD value; +++ P<0.001 vs. LD-SD value.
4. Discussion
Gating of hypothalamic T3 signaling via dio2 and dio3 expression is a key component of the pathway by which changes in photoperiod regulate reproductive physiology. In the present study, exposure to SD beginning at weaning (PND 18) prevented pubertal gonadal development in male hamsters, and in both sexes inhibited gonadotrophin secretion and upregulated hypothalamic dio3 mRNA expression. In addition, dio2 mRNA expression was significantly lower in males housed in SD relative to LD. In both males and females, a single long day delivered on PND 31 did not increase serum FSH concentrations or gonad size, but rapidly (within <24 h) inhibited hypothalamic dio3 mRNA expression. In addition, among males a single short day was sufficient to rapidly inhibit dio2 mRNA expression. The data are consistent with several prior reports documenting photoperiodic influences on reproduction and gonadotrophin secretion in both males and females of this species (Goldman et al., 1984; Whaling et al., 1993; Prendergast et al., 1996), and indicate that downregulation of both dio2 and dio3 mRNA expression occurs rapidly in response to abrupt changes in photoperiod.
Data from LD-LD and SD-SD groups in the present experiment are consistent with other reports of changes in hypothalamic dio3 mRNA expression occurring more quickly following a photoperiod manipulation in peripubertal hamsters than in adults (Herwig et al., 2012; Prendergast et al., 2013). In hamsters transferred from LD to SD on PND 21, increases in dio3 mRNA were evident within 16 days (Herwig et al., 2012), and in hamsters transferred from LD to SD on PND 18, dio3 upregulation was evident after 3 days (Prendergast et al., 2013). In the same report, no effect of photoperiod on hypothalamic dio2 expression was detected between PND 18–32; although mean levels of dio2 mRNA were quantitatively greater in both males and females housed in LD, these differences were not statistically different from those of SD hamsters (Prendergast et al., 2013). Several other reports have likewise failed to resolve effects of photoperiod on dio2 mRNA in the hamster hypothalamus (Barrett et al., 2007; Kampf-Lassin & Prendergast, 2013; Stevenson & Prendergast, in review; Watanabe et al., 2007). This is in marked contrast to the present study, in which dio2 mRNA was > 2-fold higher in LD relative to SD hamsters. The data in the present report are instead consistent with those ofHerwig et al. (2012), in which differences in dio2 mRNA expression were evident within 8 days of photoperiod transfer. Across many studies, the increase in hypothalamic dio2 mRNA expression in LD is not consistently reproducible, unlike the robust and widely-replicated effect of SD on dio3 expression.
In hamsters housed in SD, a single long day (SD-LD group) was sufficient to inhibit dio3 mRNA expression (Fig. 2). Photoperiodic control of hypothalamic dio3 mRNA is pineal-dependent (Barrett et al., 2007), and interruption of the dark phase with illumination rapidly terminates MEL secretion for the remainder of the night (Brainard et al., 1984; Lerchl, 1995; Rollag et al., 1980; Yellon et al., 1985; Yellon & Hilliker, 1994). In the present study, dio3 mRNA expression was inhibited within approximately 7.5 h of the onset of the additional light stimulus. Over this same interval, no upregulation of dio2 mRNA was evident. The magnitude of dio3 inhibition over this short interval was not maximal, however: dio3 mRNA expression was still markedly greater in the SD-LD relative to the LD-LD group. The rapid downregulation evident in SD-LD hamsters suggests that abrupt changes in photoperiod initiate molecular events that operate over very short latencies (< 24 h) to initiate inhibition of dio3 mRNA.
In hamsters housed in LD, a single short day (LD-SD group) inhibited dio2 mRNA expression (Fig. 2). This effect on dio2 was only significant in males, although data from females exhibited a similar, but non-significant, pattern. Due to smaller sample sizes in the female groups, decreases in statistical power may have decreased the ability to statistically resolve changes in dio2 mRNA expression. Upregulation of dio3 mRNA expression was not evident after a single short day, however. These data indicate that dio2 expression in LD-SD hamsters is inhibited within approximately 10.5 h of the onset of extended dark phase. Such a rapid response to a decrease in photoperiod is unlikely to be mediated by changes in the duration of nocturnal MEL secretion. In male Siberian hamsters transferred between photoperiods on PND 18, the duration of pineal MEL production is substantially (> 4 h) longer in SD relative to LD by PND 30 (Goldman et al., 1984), but several lines of evidence indicate that a single longer night is unlikely to result in a rapid decompression of MEL. Substantial expansion of the duration of pineal MEL content and the pineal N-acetyltransferase rhythm occurs within 2- 3 weeks after transfer from LD to SD (Hoffmann & Illnerová, 1986; Illnerová et al., 1984). Although the duration of MEL at earlier post-transfer intervals has not been investigated in this species, an acute extension of the scotophase by 4–8 h does not lengthen the duration of MEL production in Syrian hamsters (Tamarkin et al., 1980a, 1980b). Significant expansion of nocturnal locomotor activity is not evident 1 week after transfer from LD to SD, but only emerges after 2 or more weeks (Gorman et al., 1997). Given that the circadian locomotor activity rhythm reflects the activity of the same circadian pacemaker that drives nocturnal MEL secretion, and that the timing of activity is tightly correlated with the interval of elevated MEL secretion (Elliott & Tamarkin, 1994; Yellon et al., 1982), it is unlikely that MEL secretion expanded to a biologically-meaningful degree in LD-SD hamsters within the first week after transfer, let alone on the night of transfer. Thus, the present data are consistent with the conjecture that decreases in photoperiod acutely inhibit hypothalamic dio2 mRNA expression via MEL-independent mechanisms. Additional experimentation, for example, evaluation of short-term (< 24 h) dio2 responses to decreases in photoperiod in pinealectomized hamsters, is required to adequately assess this hypothesis, however.
Together, these data do not permit rejection of Hypothesis 1 or Hypothesis 2. According to Hypothesis 1, dio2 responses to LD occur more rapidly than dio3 responses to SD because LD-induced MEL signal compression occurs more rapidly than SD-associated decompression of MEL. In the present experiment, however, the rates of dio2 and dio3 responses to photoperiod did not differ. Significant inhibition of dio2 mRNA occurred within < 12 h of transfer to SD, and significant inhibition of dio3 mRNA was evident within < 12 h after transfer to LD.
dio2 mRNA responses to the LD-SD treatment were unexpected and are difficult to explain without conjecturing a MEL-independent effect of photoperiod on dio2 mRNA expression. One possible alternative explanation for the reduced dio2 mRNA in LD-SD hamsters is that the absence of light functioned as a dark pulse and induced a phase shift in the circadian system. If a shift in the circadian system were sufficiently rapid and large, this might allow the differences in dio2 mRNA expression between LD-LD and LD-SD hamsters to be interpreted as an effect of the time of tissue collection, potentially advanced by the dark pulse in LD-SD hamsters. However, this hypothesis is unlikely to explain the present data for several reasons. First, based on data from Syrian hamsters, long (3–6 h) dark pulses occurring during early subjective day vary widely in their effects on circadian phase. Boulos & Rusak (1982) reported shifts ranging from 4 h phase delays to 6 h phase advances, whereasEllis et al. (1982) reported minimal (<15 min) phase shifts during this interval. Importantly, circadian transients were ubiquitous in response to dark pulses, and ranged from 1–13 cycles (Boulos & Rusak, 1982). The circadian rhythm in hypothalamic dio2 mRNA expression in Syrian hamsters peaks in the midpoint of the light phase, but the waveform exhibits a broad peak, with values remaining above 75% of the daily maximum for a 6–7 h interval surrounding the peak (Yasuo et al., 2007). A large, systematic dark-induced phase shift, without transients, would be required to explain the <50% reduction in dio2 mRNA in terms of a circadian effect. Lastly, dio3 mRNA was entirely unaffected by transfer from LD to SD; if a rapid and systematic shift in the central pacemaker were indeed responsible for changes in hypothalamic dio2 mRNA expression, it would be parsimonious to expect the waveforms of other genes to be similarly affected, and this did not appear to be the case for dio3.
The present data do not permit elimination of Hypothesis 2. The only deiodinase response elicited by a single long day was the inhibition of dio3, and the only response elicited by a single short day was the inhibition of dio2. Transfer to LD failed to rapidly induce dio2, and transfer to SD failed to rapidly induce dio3. It is worth noting that neither dio2 responses to LD-SD nor dio3 responses to SD-LD were maximal (i.e., comparable in magnitude to those of hamsters held in SD-SD or LD-LD, respectively) in this acute-transfer paradigm. The hypothesis that deiodinase mRNA may be transcriptionally repressed or degraded more rapidly than it can be induced merits further investigation.
Lastly, the present data do permit rejection of Hypothesis 3, that due to differences in transcriptional control, broadly construed, changes in dio3 expression, both upregulation and downregulation, simply require longer to manifest than changes in dio2 expression. Rapid (<12 h) photoperiodic inhibition of both dio2 and dio3 mRNAs occurred in the hamster hypothalamus. These data offer no insights into the relative rates of upregulation of these two genes, however; SD-LD and LD-SD treatments failed to induce dio2 and dio3 mRNA expression, respectively. Both Hypotheses 1 and 2 remain viable explanations for the marked differences observed between the rates of dio2 induction by LD and dio3 induction by SD. Rapid decreases in dio2 and dio3 mRNA expression in response to photoperiod suggests that the latencies for inhibitory responses of these genes are comparable. Rapid photoperiodic inhibition of deiodinase mRNA may permit relatively faster initiation of changes in thyroid hormone signaling, in advance of the full effect of longer-term, MEL-dependent, systems.
Highlights.
Hypothalamic dio mRNA responses to photoperiod were measured in juvenile hamsters
A single long or short day was sufficient to downregulate dio3 and dio2, respectively.
Upregulation of dio2 and dio3 mRNAs was not evident after a single LD or SD.
Rapid dio responses to light may precede longer-term melatonin-dependent responses.
Acknowledgements
The authors thank Priyesh Patel, Dr. Leah M. Pyter, and Dr. Betty Theriault for expert technical assistance. This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant UL1 RR024999, Grant AI-67406 from the National Institute of Allergy and Infectious Diseases, and a seed grant from the Institute for Mind and Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, Archer ZA, Mercer JG, Morgan PJ. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD. Peak duration of serum melatonin and short-day responses in adult Siberian hamsters. Am. J. Physiol. 1988;255:812–822. doi: 10.1152/ajpregu.1988.255.5.R812. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Tucker S, Chou H, Hau M, Perfito N. Testicular growth and regression are not correlated with Dio2 expression in a wild male songbird, Sturnus vulgaris exposed to natural changes in photoperiod. Endocrinology. 2013;154:1813–1819. doi: 10.1210/en.2013-1093. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Rusak B. Circadian phase response curves for dark pulses in the hamster. J. Comp. Physiol. A. 1982;146:411–417. [Google Scholar]
- Brainard GC, Richardson BA, King TS, Reiter RJ. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res. 1984;294:333–339. doi: 10.1016/0006-8993(84)91045-x. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol. Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J. Comp. Physiol. A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Ellis GB, McKlveen RE, Turek FW. Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am. J. Physiol. 1982;242:R44–R50. doi: 10.1152/ajpregu.1982.242.1.R44. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Teubner BJ, Smith CD, Prendergast BJ. Exogenous T3 mimics long day lengths in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R2368–R2372. doi: 10.1152/ajpregu.00713.2006. [DOI] [PubMed] [Google Scholar]
- Goldman BD, Darrow JM, Yogev L. Effects of timed melatonin infusions on reproductive development in the Djungarian hamster (Phodopus sungorus) Endocrinology. 1984;114:2074–2083. doi: 10.1210/endo-114-6-2074. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms. 2001;16:283–380. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Freeman DA, Zucker I. Photoperiodism in hamsters: abrupt versus gradual changes in day length differentially entrain morning and evening circadian oscillators. J. Biol. Rhythms. 1997;12:122–135. doi: 10.1177/074873049701200204. [DOI] [PubMed] [Google Scholar]
- Herwig A, Petri I, Barrett P. Hypothalamic gene expression rapidly changes in response to photoperiod in juvenile Siberian hamsters (Phodopus sungorus) J. Neuroendocrinol. 2012;24:991–998. doi: 10.1111/j.1365-2826.2012.02324.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Illnerová H. Photoperiodic effects in the Djungarian hamster. Rate of testicular regression and extension of pineal melatonin pattern depend on the way of change from long to short photoperiods. Neuroendocrinology. 1986;43:317–321. doi: 10.1159/000124562. [DOI] [PubMed] [Google Scholar]
- Hoffman RA, Reiter RJ. Pineal gland: influence on gonads of male hamsters. Science. 1965;148:1609–1611. doi: 10.1126/science.148.3677.1609. [DOI] [PubMed] [Google Scholar]
- Illnerová H, Hoffmann K, Vanecek J. Adjustment of pineal melatonin and N-acetyltransferase rhythms to change from long to short photoperiod in the Djungarian hamsters Phodopus sungorus . Neuroendocrinology. 1984;38:226–231. doi: 10.1159/000123895. [DOI] [PubMed] [Google Scholar]
- Kampf-Lassin A, Prendergast BJ. Photoperiod history-dependent responses to intermediate day lengths engage hypothalamic iodothyronine deiodinase type III mRNA expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R628–R635. doi: 10.1152/ajpregu.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid. 2005;15:883–897. doi: 10.1089/thy.2005.15.883. [DOI] [PubMed] [Google Scholar]
- Lerchl A. Sustained response of pineal melatonin synthesis to a single one-minute light pulse during night in Djungarian hamsters (Phodopus sungorus) Neurosci. Lett. 1995;198:65–67. doi: 10.1016/0304-3940(95)11952-s. [DOI] [PubMed] [Google Scholar]
- Milette JJ, Turek FW. Circadian and photoperiodic effects of brief light pulses in male Djungarian hamsters. Biol. Reprod. 1986;35:327–335. doi: 10.1095/biolreprod35.2.327. [DOI] [PubMed] [Google Scholar]
- O'Jile JR, Bartness TJ. Effects of thyroxine on the photoperiodic control of energy balance and reproductive status in Siberian hamsters. Physiol, Behav. 1992;52:267–270. doi: 10.1016/0031-9384(92)90269-8. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. J. Neuroendocrinol. 2009;21:1007–1014. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Kelly KK, Zucker I, Gorman MR. Enhanced reproductive responses to melatonin in juvenile Siberian hamsters. Am. J. Physiol. 1996;271:R1041–R1046. doi: 10.1152/ajpregu.1996.271.4.R1041. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Mosinger B, Jr, Kolattukudy PE, Nelson RJ. Hypothalamic gene expression in reproductively photoresponsive and photorefractory Siberian hamsters. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16291–16296. doi: 10.1073/pnas.232490799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Pyter LM, Kampf-Lassin A, Patel PN, Stevenson TJ. Rapid induction of hypothalamic iodothyronine deiodinase expression by photoperiod and melatonin in juvenile Siberian hamsters. Endocrinology. 2013;154:831–841. doi: 10.1210/en.2012-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollag MD, Panke ES, Trakulrungsi W, Trakulrungsi C, Reiter RJ. Quantification of daily melatonin synthesis in the hamster pineal gland. Endocrinology. 1980;106:231–236. doi: 10.1210/endo-106-1-231. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Reppert SM, Orloff DJ, Klein DC, Yellon SM, Goldman BD. Ontogeny of the pineal melatonin rhythm in the Syrian (Mesocricetus auratus) and Siberian (Phodopus sungorus) hamsters and in the rat. Endocrinology. 1980a;107:1061–1064. doi: 10.1210/endo-107-4-1061. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Reppert SM, Klein DC, Pratt B, Goldman BD. Studies on the daily pattern of pineal melatonin in the Syrian hamster. Endocrinology. 1980b;107:1525–1529. doi: 10.1210/endo-107-5-1525. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, Yoshimura T. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145:1546–1549. doi: 10.1210/en.2003-1593. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, Ebihara S, Yoshimura T. Hypothalamic expression of thyroid hormone-activating and –inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R568–R572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- Whaling CS, Kelly KK, Finley CM, Spears N, Licht P, Zucker I. Sustained hormonal responses of Siberian hamsters (Phodopus sungorus) to a single longer day at weaning. Biol. Reprod. 1993;49:555–560. doi: 10.1095/biolreprod49.3.555. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Nakao N, Takagi T, Follett BK, Ebihara S, Yoshimura T. The reciprocal switching of two thyroid hormone-activating and –inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. doi: 10.1210/en.2005-0057. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology. 2007;148:4285–4392. doi: 10.1210/en.2007-0497. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW. Photoperiodic control of TSH-β expression in the mammalian pars tuberalis has different impacts on the induction and suppression of the hypothalamo-hypopysial gonadal axis. J. Neuroendocrinol. 2009;22:43–50. doi: 10.1111/j.1365-2826.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Tamarkin L, Pratt BL, Goldman BD. Pineal melatonin in the Djungarian hamster: photoperiodic regulation of a circadian rhythm. Endocrinology. 1982;111:488–492. doi: 10.1210/endo-111-2-488. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Goldman BD. Photoperiod control of reproductive development in the male Djungarian hamster (Phodous sungorus) Endocrinology. 1984;114:664–670. doi: 10.1210/endo-114-2-664. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Tamarkin L, Goldman BD. Maturation of the pineal melatonin rhythm in longand short-day reared Djungarian hamsters. Experientia. 1985;41:651–652. doi: 10.1007/BF02007704. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Hilliker S. Influence of acute melatonin treatment and light on the circadian melatonin rhythm in the Djungarian hamsterJBiol. Rhythms. 1994;9:71–81. doi: 10.1177/074873049400900107. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]