Abstract
Objective
The hippocampus has been implicated in the pathogenesis of schizophrenia, and hippocampal volume deficits have been a consistently reported abnormality, but the subregional specificity of the deficits remains unknown. The authors explored the nature and developmental trajectory of subregional shape abnormalities of the hippocampus in patients with childhood-onset schizophrenia (COS), their healthy siblings, and healthy volunteers.
Method
Two hundred twenty-five anatomic brain magnetic resonance images were obtained from 103 patients with COS, 169 from their 79 healthy siblings, and 255 from 101 age- and sex-matched healthy volunteers (age range = 9–29 years). The hippocampus was segmented using Free-Surfer automated image analysis software, and hippocampal shape was evaluated by comparing subjects at more than 6,000 vertices on the left and right hippocampal surfaces. Longitudinal data were examined using mixed model regression analysis.
Results
Patients with COS showed significant bilateral inward deformation in the anterior hippocampus. Healthy siblings also showed a trend for anterior inward deformation. However, the trajectory of shape change did not differ significantly between the groups. Inward deformations in the anterior hippocampus were positively related to positive symptom severity, whereas outward surface displacement was positively related to overall functioning.
Conclusion
This is the first and largest longitudinal three-way analysis of subregional hippocampal shape abnormalities in patients with COS and their healthy siblings compared with healthy controls. The anterior hippocampal abnormalities in COS suggest the pathophysiologic importance of this subregion in schizophrenia. The trend level and overlapping shape abnormalities in the healthy siblings suggest a more subtle, subregionally specific neuroanatomic endophenotype.
Keywords: hippocampus, longitudinal, magnetic resonance imaging, neurodevelopment, schizophrenia
Decreased hippocampal volume is one of the most consistently reported findings in schizophrenia in postmortem1 and anatomic imaging2–4 studies, with a recent meta-analysis of 44 anatomic magnetic resonance imaging (MRI) studies showing patients to have significantly decreased bilateral hippocampal volume.5 These volume decreases have been correlated with illness severity and specific cognitive deficits such as verbal memory deficits.6–8 Disruptions of hippocampal circuitry also have been thought to contribute to the clinical presentation of schizophrenia, namely positive symptoms, negative symptoms, and other cognitive deficits.9–11
The hippocampus is not a uniform structure, but rather a structure consisting of at least two structurally and functionally separate regions.12,13 The posterior region appears to be preferentially involved in spatial learning and memory, whereas the anterior region is involved in anxiety-related behaviors, emotional processing, and associative memory.12,13 Thus, regional specificity of volume deficits can indicate specific neurocircuitry alterations in schizophrenia.
Hippocampal shape analysis can reveal subtle alterations at a subregional level.14 Prior studies of hippocampal shape in adult patients with schizophrenia have shown deformations to be most pronounced in the anterior hippocampus,3,14–17 although deformations have been seen in the posterior region18 and in the anterior and posterior regions.19
Examining the shape and trajectory of the hippocampus in nonpsychotic full siblings could help address whether abnormalities of the hippocampus are trait markers or disease related. Neurocognitive studies have shown that first-degree relatives share deficits with patients with schizophrenia in cognitive functions related to the hippocampus, such as verbal memory recall, executive functioning, and attentional functioning.20 Evidence from anatomic studies has been inconsistent, although a recent meta-analysis of 25 volume studies found that first-degree relatives of adult patients shared hippocampal volume deficits, but to a lesser degree than the probands.6 Few studies have examined hippocampal shape in siblings, although two prior cross-sectional studies found hippocampal shape abnormalities in first-degree relatives.16,17 Thus, there is some evidence, mostly from anatomic volumetric studies, that the structural and functional deficits in the hippocampus in schizophrenia may be trait markers.
The authors explored this question further by examining the longitudinal development of the hippocampus at a subregional level by carrying out shape analysis in a large sample of patients with childhood-onset schizophrenia (COS), their unaffected siblings, and matched healthy comparison subjects. COS, defined by the onset of psychosis before 13 years of age, is clinically and neurobiologically continuous with later-onset schizophrenia21–24 but provides a unique cohort to address this question from a neurodevelopmental perspective in pediatric populations. The authors’ previous study of hippocampal volume in COS found a fixed 6% to 7% bilateral hippocampal volume deficit,25 and a previous smaller study in 29 probands using dynamic brain mapping showed anterior and posterior hippocampal deficits.26 Based on results of previous adult studies of hippocampal shape showing anterior deformations,3,14–17 the authors hypothesized patients with COS would show significant shape abnormalities compared with healthy controls, which would most likely be inward deformations and most pronounced in the anterior hippocampus. They also hypothesized healthy siblings would show hippocampal deformations in similar regions, but to a lesser magnitude. Based on evidence from several previous studies showing a correlation for mesiotemporal volume and functional deficits with positive but not with negative symptoms,27–29 the authors hypothesized that localization of hippocampal deficits in patients with COS would be more correlated with positive than with negative symptom severity. Studies of the developmental trajectory of hippocampal shape were carried out on an exploratory basis.
METHOD
Subjects
Patients with COS were recruited nationally and were diagnosed after an inpatient observation that in most cases included a medication-free observation. Patients were enrolled before 18 years of age and diagnosed using the DSM-IV criteria for schizophrenia with onset of psychosis before 13 years of age. Exclusionary criteria were medical or neurobiological illness, history of head trauma, or premorbid IQ below 70. Subjects also were excluded for any lifetime history of substance abuse, which was assessed during the clinical interview at admission and follow-up using the Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version. All patients and their full siblings were followed prospectively with anatomic rescans at 2-year intervals. The study group of 103 subjects with COS consisted of 45 girls and 58 boys, for whom at least one scan was available, and included a total of 255 scans. Thirty-one subjects with COS had only one scan. Patients with COS were assessed for symptom severity and overall functioning using the Scale for the Assessment of Positive Symptoms (SAPS), the Scale for the Assessment of Negative Symptoms (SANS), and the Children’s Global Assessment Scale (CGAS) during the period of medication washout during the inpatient observation.30
Seventy-nine healthy full siblings of patients with COS for whom at least one scan was available were included in the study, including 43 girls and 36 boys. This included a total of 169 scans, with 35 siblings having only a single scan. Siblings were interviewed using the Structured Clinical Interview for DSM-IV Axis I Disorders and the Structured Interview for DSM-IV Personality Disorders for Axis II diagnoses at each scan time point. Siblings were included only if they were free of any schizophrenia spectrum diagnoses, including schizophrenia, schizoaffective disorder, or any psychotic illness on Axis I or paranoid, schizotypal, schizoid, or avoidant personality disorders on Axis II. Nonschizophrenia spectrum diagnoses of healthy siblings are listed in Table 1. For the 79 healthy siblings, there were a total of 52 families represented. Thirty-three families contributed 1 sibling; 15 families contributed 2 siblings; 2 families contributed 3 siblings; and 2 families contributed 5 siblings.
TABLE 1.
Psychiatric Diagnoses in Healthy Siblings of Patients With Child-Onset Schizophrenia
| Psychiatric Diagnoses | Healthy Siblings, n |
|---|---|
| None | 41 |
| Affective disorder (nonbipolar) | 8 |
| Affective disorder and anxiety disorder | 7 |
| ADHD, ODD, or CDO | 5 |
| Anxiety disorder | 4 |
| Affective disorder, anxiety disorder, and substance abuse | 3 |
| Substance abuse | 3 |
| Affective disorder, anxiety disorder, and ADHD/ODD/CDO | 1 |
| Affective disorder, anxiety disorder, substance abuse, and ADHD/ODD/CDO | 1 |
| Affective disorder and ADHD/ODD/CDO | 1 |
| Affective disorder and substance abuse | 1 |
| Unknown (data not collected) | 4 |
Note: ADHD = attention-deficit/hyperactivity disorder; CDO = conduct disorder; ODD = oppositional-defiant disorder.
One hundred one healthy comparison subjects, including 41 girls and 60 boys, were selected from a larger prospective study of normal brain development and were matched for age, sex, and scan interval to patients with COS and their healthy siblings. A total of 255 scans from the healthy volunteers were used, with 28 control subjects having only a single scan. Comparison subjects were free of lifetime medical or psychiatric disorders, determined through clinical examination and standardized interview using the Structured Clinical Interview for DSM-IV Axis I Disorders and the Structured Interview for DSM-IV Personality Disorders. Psychiatric illness in a first-degree relative also was exclusionary. Further details have been described previously.31
The research protocol was approved by the National Institute of Mental Health institutional review board. Written informed consent was obtained from parents and subjects older than 18 years, and written informed assent was obtained from minors.
Imaging Processing and Hippocampal Analysis
All scans were obtained on the same MRI scanner using a previously published sequence.25,32 T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using a three-dimensional spoiled gradient recalled echo sequence in the steady state. Imaging parameters were as follows: echo time = 5 ms, repetition time = 24 ms, flip angle = 45°, acquisition matrix = 256 × 192, number of excitations = 1, and field of view = 24 cm. Head placement was standardized as previously described.32
Before processing, raw scans were evaluated for motion artifact by a trained rater (L.C.) and placed into one of four categories, as described previously33: 1 = no motion; 2 = mild motion; 3 = moderate motion; and 4 = severe motion. Those with moderate or severe motion were excluded from analyses. Hippocampal segmentations were created automatically for each scan (i.e., each subject at each time point) using the FreeSurfer 5.1 image analysis suite, which is documented and available online (http://surfer.nmr.mgh.harvard.edu/). This procedure automatically provides segmentations for subcortical brain structures including the hippocampus on MRI scans based on probabilistic information estimated automatically from a manually labeled training set.34,35 Briefly, this process includes motion correction and removal of nonbrain tissue using a hybrid watershed/surface deformation procedure,36 automated Talairach transformation, and segmentation of gray matter volumetric structures including the hippocampus.34,35 The segmentation uses the following data: prior probability of a given tissue class at a specific atlas location, likelihood of the image intensity given the tissue class, and probability of the local spatial configuration of labels given the tissue class. This technique has been shown to be comparable in accuracy to manual labeling.34 FreeSurfer output, overlaid with the color-coded segmentation, was visually reviewed by an expert rater for a general determination of the accuracy of the segmentation, and nine scans were eliminated at this stage.
However, the FreeSurfer segmentations are noisy and therefore not suitable for shape analysis, which requires smoothness of data. To overcome this, smooth surfaces were created for the FreeSurfer segmentations by incorporating Large Deformation Diffeomorphic Metric Mapping37 into the FreeSurfer pipeline. In this procedure, previously developed, smooth binary template hippocampal segmentations (left and right)14,15 were injected into the FreeSurfer segmentations to produce “de-noised” hippocampal segmentations.38 Template surfaces (left and right, 6,611 vertices on each side) were transferred onto the now smooth target segmentations using the underlying Large Deformation Diffeomorphic Metric Mapping maps to create hippocampal surfaces with corresponding vertices in each scan. Final segmentations were visually assessed for quality by a single rater (S.J.), and 5 scans were eliminated from the sample for failing Large Deformation Diffeomorphic Metric Mapping processing.
All hippocampal surfaces were then rotated and aligned to the template surfaces to account for differences in native orientation. An average surface was generated from scan 1 of the entire sample by averaging coordinates at each vertex index.39 The distance from each vertex on every scan to the average point for that vertex with direction (e.g., positive for pointing outward and negative for pointing inward relative to the average surface) was calculated.
Of 303 possible scans of patients with COS acquired in the target age range of 9 to 29 years, 42 (13.86%) were deemed unusable owing to moderate or severe motion detected on the raw image. Three additional scans were excluded after FreeSurfer output examination, and another 3 scans were excluded owing to hippocampus pipeline failure, yielding a total of 15.84% that had failed at any of the 3 stages. Healthy siblings of patients with COS in the target age range and selected for overall age and sex comparability to the patient group yielded a pool of 177 scans. Of these 177, 6 were excluded at the raw image stage, one was excluded at the FreeSurfer output stage, and one additional scan was excluded at the hippocampus pipeline stage, resulting in exclusion rates of 3.39% at the raw scan stage and 4.52% at any of the three stages. Healthy controls were selected from a larger pool so that just one person per family was included and they were comparable to the COS and sibling groups in age and sex. Based on the larger, nonindependent pool of healthy controls 9 to 29 years old, 45 (4.91%) were excluded at the raw scan stage, 5 were excluded at the FreeSurfer output stage, and 1 was excluded at the hippocampus pipeline stage, with a total of 5.56% excluded at any of the 3 stages.
Statistical Analysis
Age and sex differences between groups were tested using analysis of variance for age and χ2 tests of independence for sex.
Mixed effect regression models were used to examine group differences in vertex distance at every vertex. False discovery rate (FDR), which controls for the expected proportion of false positives in a statistical test, was applied to adjust for multiple comparisons with q = 0.05. Fixed effects included age (centered at sample average age [17.4 years]), group, group by age, intracranial volume, and sex. Random effects included an intercept per family (to account for within-family dependence) and an intercept for a person nested within a family (to account for within-person within-family dependence). Group differences in intercept (at the average age) and slope for each vertex were tested with t tests. The authors’ primary hypotheses were tested by the group term (which tests if there are differences at the average age) and the group-by-age term (which tests if patients with COS, healthy siblings, and controls develop differently from one another). The mixed effect regression40,41 was used to model group trajectories because these models allow estimation of group developmental parameters using unbalanced data (e.g., participants measured at different time points and different intervals) while accounting for within-subject correlation. Patterns of group differences were visualized by projecting results onto template surfaces. Analyses were run in SAS using Proc Mixed (SAS Institute, Cary, NC).
Using all COS scans, mixed effect regression was applied to explore relations between the vertex distance at each surface vertex and scores on clinical measurements (CGAS, SAPS, and SANS) while controlling for age and sex. Results were projected onto template surfaces.
RESULTS
Demographic Information
The 3 study groups were well matched for age and sex. The age range was 9.02 to 29.9 years. Demographic characteristics are listed in Table 2.
TABLE 2.
Demographic Characteristics Among Probands With Childhood-Onset Schizophrenia, Healthy Controls, and Healthy Siblings
| COS (n = 103) | Healthy Controls (n = 101) | Siblings (n = 79) | Statistic (df) | p | |
|---|---|---|---|---|---|
| F/M | 45/58 | 41/60 | 43/36 | χ2 = 3.66 | .161 |
| Scan 1 | |||||
| Age, mean (SD) | 14.9 (2.7) | 14.5 (4.4) | 15.8 (5.3) | F = 2.19 (280) | .114 |
| n (F/M) | 103 (45/58) | 101 (41/60) | 79 (43/36) | ||
| Scan 2 | |||||
| Age, mean (SD) | 16.9 (2.7) | 16.8 (3.7) | 17.5 (4.6) | F = 0.557 (186) | .574 |
| n (F/M) | 72 (27/45) | 73 (26/47) | 44 (22/22) | ||
| Scan 3 | |||||
| Age, mean (SD) | 19.7 (3.1) | 19.3 (3.6) | 20.2 (4.5) | F = 0.621 (121) | .539 |
| n (F/M) | 48 (16/32) | 44 (14/30) | 32 (16/16) | ||
| Scan 4 | |||||
| Age, mean (SD) | 22.4 (3.3) | 22.1 (2.9) | 22.1 (2.5) | F = 0.053 (52) | .948 |
| n (F/M) | 20 (5/15) | 25 (8/17) | 10 (7/3) | ||
| Scan 5 | |||||
| Age, mean (SD) | 24.2 (2.1) | 24.0 (2.2) | 23.6 (2.6) | F = 0.078 (17) | .925 |
| n (F/M) | 7 (1/6) | 9 (6/3) | 4 (3/1) | ||
| Scan 6 | |||||
| Age, mean (SD) | 26.5 (1.9) | 26.0 (1.6) | — | F = 0.102 (6) | .76 |
| n (F/M) | 5 (1/4) | 3 (2/1) | |||
| Overall age, mean (SD) [n] | 17.4 (4.0) [255] | 17.2 (4.8) [255] | 17.6 (5.3) [169] | F = 0.423 (676) | .655 |
Note: COS = childhood-onset schizophrenia; F = female; M = male.
Hippocampal Shape of Patients With COS (at Average Age)
Figure 1 shows statistically significant differences in deformation (at the 6,000 hippocampal vertices) in the COS group compared with the healthy volunteers at the average age (17.4 years). COS probands showed significant bilateral inward deformation that was most pronounced in the anterior hippocampus, medial and lateral surfaces, and the body. Outward deformation was seen in the inferior anterior hippocampus bilaterally, larger on the right side, and in smaller regions throughout the body and tail of the hippocampus. A similar pattern was seen in patients with COS compared with their healthy siblings (Figure S1, available online). There were no sex differences.
FIGURE 1.
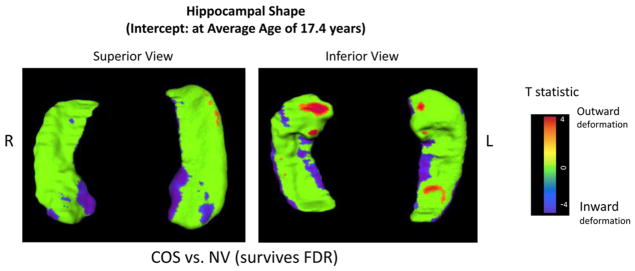
Hippocampal shape of patients with childhood-onset schizophrenia (COS). Note: Statistical maps show significant differences in vertex distances between patients with COS and healthy volunteers (NV) at the average age (17.4 years). The color bar shows t values obtained at each hippocampal surface location, masked to show a color other than green only at vertices with a two-tailed probability value that survives false discovery rate (FDR) correction. Positive t values indicate significant outward deformation in the hippocampal surface of patients with COS compared with healthy volunteers, whereas negative t values indicate inward surface deformation. In the superior view, the anterior hippocampus is toward the bottom of the panel; in the inferior view, the anterior hippocampus is toward the top of the panel.
Hippocampal Shape of Healthy Siblings (at Average Age)
Figure 2 shows the difference in deformation between healthy siblings and controls atthe average age (17.4 years). As seen in the probands, healthy siblings showed bilateral areas of inward deformation in the central anterior hippocampus, larger on the left and on the medial edge of the anterior hippocampus, which overlapped with that seen for COS. The siblings also showed outward deformation in the lateral anterior hippocampal surface compared with controls. However, none of these areas of deformation survived FDR correction. There were no sex differences in sibling findings.
FIGURE 2.
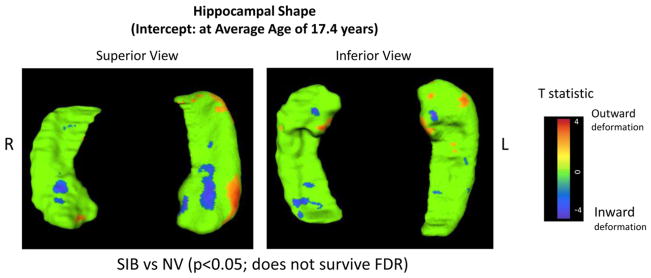
Hippocampal shape of healthy siblings. Note: Statistical maps show significant differences in vertex distances from the results of t tests at each vertex between the groups at the average age (17.4 years). The color bar shows t values obtained at each hippocampal surface location, masked to show a color other than green only at vertices with a two-tailed probability value with p < .05; these results did not survive false discovery rate (FDR) correction. Positive t values indicate significant outward deformation in the hippocampal surface of healthy siblings of patients with childhood-onset schizophrenia (SIB) compared with healthy volunteers (NV), whereas negative t values indicate inward surface deformation.
Longitudinal Changes in Hippocampal Shape (Developmental Trajectory)
In general, neither patients with COS nor their healthy siblings showed significant change in shape over time. There was some change in inward and outward deformation areas across the age range in patients and siblings compared with controls; however, none of the findings were robust and none survived the FDR correction (Figure S2, available online).
Relation to Symptom Scores (Patients With COS)
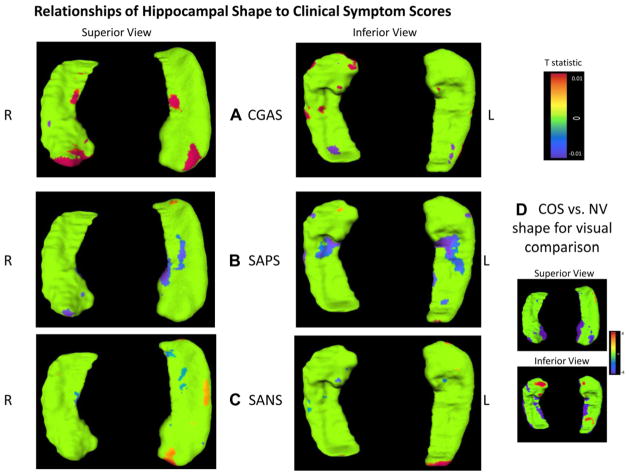
Figure 3 shows the relations between symptom scores and hippocampal displacement at each vertex in patients with COS. In subjects with COS, clinical symptom scores on all three measurements (CGAS, SANS, and SAPS) were related to hippocampal surface deformation (p < .05), but the relations did not survive FDR correction for multiple analyses. For the CGAS, higher scores of patients with COS (indicating better overall functioning) were related to more outward deformation in the anterior and body of the hippocampus, overlapping an area of deformation in patients with COS compared with controls. Higher scores on the SANS (more severe negative symptoms) were related to small areas of inward and outward deformation. Higher scores on the SAPS (more severe positive symptoms) had the strongest relation with inward displacement of the anterior hippocampus bilaterally, overlapping the areas of inward deformity in patients with COS compared with controls. Scatter plots of the relations are shown in Figure S3 (available online).
FIGURE 3.
Relations of clinical symptoms to hippocampal surface deformations in patients with childhood-onset schizophrenia (COS). Note: Statistical maps show vertices with positive relations with (A) Children’s Global Assessment Scale (CGAS), (B) Scale for the Assessment of Positive Symptoms (SAPS), and (C) Scale for the Assessment of Negative Symptoms (SANS) clinical measurement scores. The color bar shows t values obtained at each surface vertex location, masked to show a color other than green only at surface locations with two-tailed probability values with p < .05. None of these correlations survived false discovery rate (FDR) correction for multiple analyses. Positive t values (warm colors) indicate a higher score correlated with outward deformation in the hippocampal surface of patients with COS, whereas negative t values (cool colors) indicate a higher score correlated with inward surface deformation. (D) Statistical maps showing significant differences in vertex distances between patients with COS and healthy volunteers (NV) at the average age are shown for reference.
DISCUSSION
In this study, the largest longitudinal hippocampal shape analysis of patients with schizophrenia and the first to include healthy siblings, patients with COS showed significant bilateral inward deformations of the anterior hippocampus compared with controls and healthy siblings, whereas siblings showed a mild/trend level deformity in overlapping anterior regions compared with healthy controls that did not reach significance.
The deficits in COS were located mainly in the anterior hippocampus, which is consistent with prior cross-sectional studies of adult patients with schizophrenia, which found bilateral anterior hippocampal deficits.3,14,16,39 The anterior hippocampus contains a preponderance of hippocampal CA1 neurons and abnormalities in these neurons could be crucial in the development of schizophrenia. Functional MRI studies have shown abnormal cerebral blood flow in the CA1 subfield in patients with schizophrenia, which correlated with clinical progression to psychosis in prodromal patients.42
Although the mechanism of anterior hippocampal damage is unknown, several mechanisms have been proposed. For example, Disc1, a gene implicated in schizophrenia pathophysiology, is highly expressed in the hippocampus especially during prenatal development43 and has been shown to be necessary for the migration and layer formation of CA1 pyramidal neurons during hippocampal development.44 Decreased migration of CA1 neurons to the anterior hippocampus could thus explain the bilateral inward deformations. Similarly, α-7 nicotinic acetylcholine receptors are expressed at particularly high levels in CA1 neurons near birth, and activation of these receptors with choline, which is present in amniotic fluid, is essential for normal hippocampal development. A decrease in these receptors during gestation has been linked to abnormal hippocampal development.45 If substantiated, these could provide targets for treatment interventions such as prenatal supplementation with dietary choline.45
The present finding of anterior hippocampal deficits lends further support to the “early fixed injury to anterior hippocampus model” of schizophrenia.43,46 Rats with neonatal ventral (anterior) hippocampal lesions display many behavioral traits analogous to the negative, positive, and cognitive symptoms seen in schizophrenia.43 In this model, a neonatal ventral hippocampal lesion causes improper maturation and functional deficits in prefrontal cortex inter-neurons during adolescence.47 The CA1 neurons in the anterior hippocampus in humans project to the medial prefrontal cortex,48 suggesting a potential disturbance in the hippocampal-prefrontal neurocircuitry. The cortical gray matter deficits in COS indeed localize to prefrontal (and temporal) regions with age,49 supporting the involvement of hippocampal-prefrontal connections in schizophrenia. A whole brain diffusion tensor imaging study of white matter tracts and regional connectivity in these specific regions in patients with COS could address this and is currently underway by the authors’ group.
The authors further investigated the relation of surface deformations at each vertex to severity of clinical symptoms in patients with COS. Overall, each clinical measurement correlated with shape abnormalities of the hippocampal vertices in the expected direction. Better overall functioning, as measured by the CGAS, was related to outward hippocampal deformation, and worse positive and negative symptoms were mainly related to inward hippocampal deformation. There was also a subregional overlap of deformities with regions that correlated with clinical measurements, particularly in the body and anterior regions (Figure 3). The strongest association was seen for the SAPS score, which localized mostly to the head of the hippocampus and to areas of significant deformation in patients with COS. Prior functional MRI studies have shown correlations between positive symptoms and decreased cerebral blood flow in the hippocampus50 and decreased left anterior hippocampal volume.10 However, these findings must be interpreted with caution, because they did not survive correction for multiple analyses.
Nonpsychotic siblings also showed areas of deformation in the anterior hippocampus compared with controls, although these did not survive FDR correction, suggesting that deformities are at a trend level at best. This has been observed in prior smaller cross-sectional studies in relatives of patients with adult-onset schizophrenia, which showed inward deformation in the head of the hippocampus.16,17 Nevertheless, the sibling findings are interesting, because in the authors’ prior volumetric study, healthy siblings did not show a hippocampal volume deficit different from controls,25 and early prefrontal cortical deficits in siblings normalize with age. Thus, the deficits seen in siblings are likely to be a subtle, subregionally specific endophenotype that may be detectable with more sensitive imaging modalities (e.g., functional MRI) or, alternatively, the subtle deficits could be the result of overall normalization of prefrontal-hippocampal circuitry with age.
The authors explored the hippocampal developmental trajectory at the subregional level, which has not been done previously. Overall, the shape deformities remained static with age even at a subregional level, as was seen for total hippocampal volume. There is indirect evidence to support this from adult studies, which have shown that hippocampal volume deficits were similarly present in patients with a first episode and patients with long-term schizophrenia,5 suggesting static deficits. Taken together, these observations could further support an early fixed deficit model of hippocampal injury in schizophrenia.
Despite the large sample, inclusion of siblings, and longitudinal design, there are several limitations to this study. First, patients with COS are on antipsychotic medications and the effects of these medications cannot be ruled out. However, by identifying a trend toward similar shape abnormalities in healthy siblings who have not been exposed to any psychiatric medications, the authors suspect that the hippocampal abnormalities are unlikely to be solely due to medication effects. Second, there were four subjects included in the sample with 22q11 deletions, which have been shown to be associated with hippocampal abnormalities.51 However, it is unlikely that the significant differences found in patients with COS would be due solely to the inclusion of these subjects, because they account for only 18 MRI scans of the 255 total COS scans. Third, although the present findings were in line with previous adult studies on schizophrenia, hippocampal deficits have been seen in other psychiatric disorders, such as depression, bipolar disorder, posttraumatic stress disorder, and borderline personality disorder.52–55 This suggests that the hippocampal abnormalities the authors found could be due to a more general pathologic process potentially associated with a multitude of psychiatric disorders. Further, studies have shown that attention-deficit/hyperactivity disorder, oppositional-defiant disorder, and generalized anxiety disorder are prevalent in siblings of patients with schizophrenia.56 Because of the variance in the comorbid disorders present in this sample, the authors were unable to control for sibling psychopathology. Therefore, the authors cannot rule out the possibility that the non-schizophrenia spectrum psychiatric diagnoses may have contributed to the subtle hippocampal shape abnormalities in the healthy siblings.
Also, at current resolution, surface deformities still fail to provide definitive localization to specific hippocampal internal subfields. Similarly, it is difficult to comment on the significance of inward versus outward deformation at the current resolution, and histopathologic studies will be needed to understand the significance of shape deformities in schizophrenia. Moreover, the relation between symptom measurements and deformation was not exactly overlapping with deformations comparing COS with healthy volunteers except for the SAPS. This is probably due to the fact that correlations between clinical symptoms and deformation were statistically weaker in general and probably would need additional sampling. Further studies are also needed to explore deficits in hippocampal-prefrontal connections and hippocampal internal subfields using higher-resolution imaging, which are currently underway at the National Institute of Mental Health and other centers.
Supplementary Material
Acknowledgments
This research was funded by the Intramural Division of the National Institute of Health. Ms. Johnson’s participation was made possible through the Clinical Research Training Program, a public-private partnership supported jointly by the National Institute of Health and Pfizer, Inc. (by a grant to the Foundation for the National Institute of Health from Pfizer, Inc.).
Dr. Greenstein served as the statistical expert for this research.
Footnotes
Disclosure: Drs. Wang, Greenstein, Clasen, Lalonde, Miller, Rapoport, and Gogtay, Ms. Johnson, and Ms. Alpert report no biomedical financial interests or potential confiicts of interest.
Contributor Information
Ms. Sarah L.M. Johnson, Child Psychiatry Branch of the National Institute of Mental Health
Dr. Lei Wang, Northwestern University Feinburg School of Medicine
Ms. Kathryn I. Alpert, Northwestern University Feinburg School of Medicine
Dr. Deanna Greenstein, Child Psychiatry Branch of the National Institute of Mental Health
Dr. Liv Clasen, Child Psychiatry Branch of the National Institute of Mental Health
Dr. Francois Lalonde, Child Psychiatry Branch of the National Institute of Mental Health
Dr. Rachel Miller, Child Psychiatry Branch of the National Institute of Mental Health
Dr. Judith Rapoport, Child Psychiatry Branch of the National Institute of Mental Health
Dr. Nitin Gogtay, Child Psychiatry Branch of the National Institute of Mental Health
References
- 1.Bogerts B, Falkai P, Haupts M, et al. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res. 1990;3:295–301. doi: 10.1016/0920-9964(90)90013-w. [DOI] [PubMed] [Google Scholar]
- 2.Davatzikos C, Shen D, Gur RC, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 3.Narr KL, Thompson PM, Szeszko P, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- 6.Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A, Wobrock T, Falkai P, et al. Hippocampal integrity and neurocognition in first-episode schizophrenia: a multidimensional study [published online November 2, 2011] World J Biol Psychiatry. doi: 10.3109/15622975.2011.620002. http://dx.doi.org/10.3109/15622975.2011.620002. [DOI] [PubMed]
- 8.van Erp TG, Therman S, Pirkola T, et al. Verbal recall and recognition in twins discordant for schizophrenia. Psychiatry Res. 2008 Jun 30;159:271–280. doi: 10.1016/j.psychres.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 10.Rajarethinam R, DeQuardo JR, Miedler J, et al. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res. 2001;108:79–87. doi: 10.1016/s0925-4927(01)00120-2. [DOI] [PubMed] [Google Scholar]
- 11.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 12.Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannerman DM, Rawlins JN, McHugh SB, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 15.Csernansky JG, Joshi S, Wang L, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho BC, Magnotta V. Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. Neuroimage. 2010;49:3385–3393. doi: 10.1016/j.neuroimage.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–1240. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- 18.Styner M, Lieberman JA, Pantazis D, Gerig G. Boundary and medial shape analysis of the hippocampus in schizophrenia. Med Image Anal. 2004;8:197–203. doi: 10.1016/j.media.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Kim SH, Jang DP, et al. Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. Neuroimage. 2004;22:831–840. doi: 10.1016/j.neuroimage.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Frazier JA, Giedd JN, Hamburger SD, et al. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen LK, Rapoport JL. Research update: childhood-onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry. 1998;39:101–113. [PubMed] [Google Scholar]
- 23.Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr Psychiatry Rep. 2005;7:81–82. doi: 10.1007/s11920-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 24.Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34:30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattai A, Hosanagar A, Weisinger B, et al. Hippocampal volume development in healthy siblings of childhood-onset schizophrenia patients. Am J Psychiatry. 2011;168:427–435. doi: 10.1176/appi.ajp.2010.10050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nugent TF, 3rd, Herman DH, Ordonez A, et al. Dynamic mapping of hippocampal development in childhood onset schizophrenia. Schizophr Res. 2007;90:62–70. doi: 10.1016/j.schres.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Bogerts B, Lieberman JA, Ashtari M, et al. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- 28.Goghari VM, Sponheim SR, MacDonald AW., III The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zierhut K, Bogerts B, Schott B, et al. The role of hippocampus dysfunction in deficient memory encoding and positive symptoms in schizophrenia. Psychiatry Res. 2010;183:187–194. doi: 10.1016/j.pscychresns.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Shaw P, Sporn A, Gogtay N, et al. Childhood-onset schizophrenia: a double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63:721–730. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- 31.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 32.Castellanos FX, Giedd JN, Berquin PC, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal JD, Zijdenbos A, Molloy E, Giedd JN. Motion artifact in magnetic resonance imaging: implications for automated analysis. Neuroimage. 2002;16:89–92. doi: 10.1006/nimg.2002.1076. [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Beg MF, Miller MI, Trouve A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vis. 2005;61:139–157. [Google Scholar]
- 38.Qiu A, Miller MI. Multi-structure network shape analysis via normal surface momentum maps. Neuroimage. 2008;42:1430–1438. doi: 10.1016/j.neuroimage.2008.04.257. [DOI] [PubMed] [Google Scholar]
- 39.Csernansky JG, Wang L, Joshi SC, Ratnanather JT, Miller MI. Computational anatomy and neuropsychiatric disease: probabilistic assessment of variation and statistical inference of group difference, hemispheric asymmetry, and time-dependent change. Neuroimage. 2004;23(suppl 1):S56–S68. doi: 10.1016/j.neuroimage.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 41.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data. Vol. 25. New York: Oxford University Press; 2002. [Google Scholar]
- 42.Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita K, Kubo K, Ishii K, Nakajima K. Disrupted-in-Schizophrenia-1 (Disc1) is necessary for migration of the pyramidal neurons during mouse hippocampal development. Hum Mol Genet. 2011;20:2834–2845. doi: 10.1093/hmg/ddr194. [DOI] [PubMed] [Google Scholar]
- 45.Freedman R, Goldowitz D. Studies on the hippocampal formation: from basic development to clinical applications: studies on schizophrenia. Prog Neurobiol. 2010;90:263–275. doi: 10.1016/j.pneurobio.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 47.Tseng KY, Lewis BL, Hashimoto T, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 50.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 51.Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome) Schizophr Res. 2009;115:173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci. 2011;13:453–461. doi: 10.31887/DCNS.2011.13.4/kholzschneider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruocco AC, Amirthavasagam S, Zakzanis KK. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: a meta-analysis of magnetic resonance imaging studies. Psychiatry Res. 2012;201:245–252. doi: 10.1016/j.pscychresns.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 56.de la Serna E, Baeza I, Andrés S, et al. Comparison between young siblings and offspring of subjects with schizophrenia: clinical and neuropsychological characteristics. Schizophr Res. 2011;131:35–42. doi: 10.1016/j.schres.2011.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.