Abstract
Gastric metastasis of pulmonary carcinoma has been reported to range from 0.19%-5.1%. An autopsy review of cancer disclosed 1.7%-29.6% of gastric metastases, primarily from breast cancer, lung cancer and melanoma. A 71-year-old man was referred to our department because of persistent cough, sputum and sweating for 20 d. Chest posteroanterior view and chest computed tomography scan demonstrated an irregular tumor mass measuring 5.8 cm with central necrosis at the right lower lung. Bronchoscopic biopsy revealed pulmonary squamous carcinoma. Esophagogastroduodenoscopy revealed a huge bleeding ulcer at the body of the stomach and a biopsy diagnosed a metastatic lesion. We performed a palliative total gastrectomy, splenectomy and distal pancreatectomy. The patient did not receive any adjuvant chemotherapy due to his refusal. He was controlled conservatively and survived for 11 mo after surgery. Surgical resection may provide an option for safe palliative treatment. Although gastric metastasis from lung cancer is associated with dismal outcomes, a longer survival or more favorable outcome has been demonstrated in patients undergoing palliative surgical resection of the metastatic site. Considerable improvements in the understanding of metastatic diseases and therapeutic strategies are needed to improve the clinical outcome.
Keywords: Squamous cell carcinoma, Lung cancer, Gastric metastasis
Core tip: The common gastrointestinal metastatic site of lung cancer is the small bowel, with sporadic case reports involving the stomach. Most patients with gastric metastasis are asymptomatic. The survival and standard treatment of gastric metastasis from the lung are not satisfactory. Although gastric metastasis from lung cancer is associated with dismal outcomes, a longer survival or more favorable outcome has been demonstrated in patients undergoing palliative surgical resection of the metastatic site.
INTRODUCTION
Gastrointestinal tract involvement by lung cancer is considered a late stage of the disease because of diffuse hematogenous tumor spread. It is generally detected in patients with a documented previous history of a primary lung malignancy. In contrast, the finding of lung cancer initially manifesting as gastrointestinal tract involvement is exceedingly rare and has been cited in isolated case reports. Gastric metastasis from lung carcinoma is rare and its incidence has been reported to range from 0.19%-5.1%[1-5]. Metastatic disease involving the stomach is an unusual and difficult clinical problem. An autopsy review of cancer disclosed 1.7%-29.6% of gastric metastases related to breast cancer, lung cancer and melanoma as the most frequent primaries[1,6]. It is rarely found in clinical situations because only a few patients are symptomatic. The common clinical presentations of gastrointestinal metastases are abdominal pain, gastrointestinal bleeding, obstruction and perforation associated with small bowel metastasis[7].
The survival and standard treatment of gastric metastasis from the lung are not satisfactory. Therefore, gastric metastasis from lung cancer is associated with dismal outcomes. The aim of this study was to report the clinical characteristics and outcomes of our patient with special emphasis on treatment after a review of the literature.
CASE REPORT
A 71-year-old man was referred to our department because of a 20 d history of persistent cough, sputum and sweating. The patient was diagnosed with pneumonia and suspicious lung cancer of the right lower lobe at a community hospital. He had a history of smoking 50 packs a year and stable pulmonary tuberculosis on both upper lung fields. Recently, the patient had experienced easy fatigue and had a weight loss of about 7 kg/mo. Chest radiography showed a solitary mass with solid opacity in the right lower lung (RLL) (Figure 1A, arrow). Chest computed tomography (CT) scan demonstrated an irregular tumor mass measuring 5.8 cm in size with central necrosis at RLL and a biopsy was performed (Figure 1B, arrow). The biopsy showed syncytial growth of polygonal shaped poorly differentiated cells which were strongly positive for p63 and consistent with squamous cell carcinoma (Figure 2). A complete blood cell count revealed hemoglobin 8.6 g/dL, hematocrit 25.3%, platelets 341000/μL and white blood corpuscles 6000/μL. Tumor marker study revealed that alpha fetoprotein was 1.8 ng/mL, carcinoembryonic antigen was 4.9 ng/mL, and carbohydrate antigen 19-9 was 5.5 U/mL. A gastroduodenoscopy was performed for evaluation of anemia. On gastroduodenoscopy, there was a huge ulcer with easy bleeding from the mid to lower body of the stomach and a biopsy was performed. The patient had no symptoms associated with an ulcer and no history of Helicobacter pylori infection.
Figure 1.
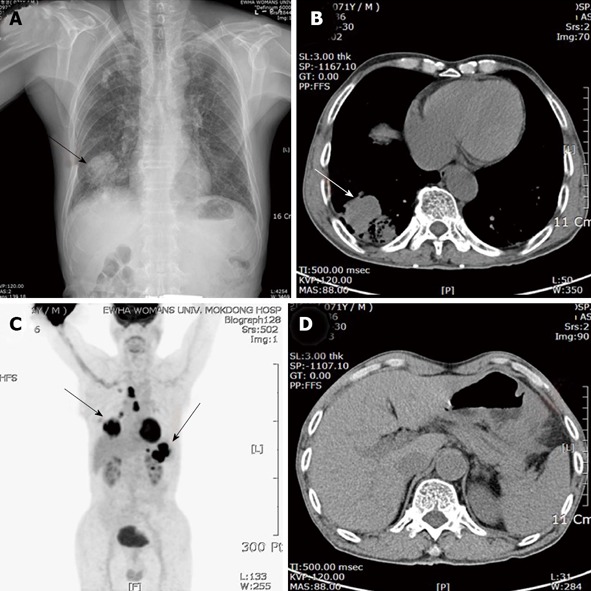
Chest posteroanterior view, computed tomography scan and (18F) fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography. A: A solitary mass with solid opacity in the right lower lung (RLL) (arrow); B: Chest computed tomography (CT) scan demonstrated an irregular mass measuring 5.8 cm in size with central necrosis at RLL (arrow); C: [18F] fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET)/CT showed a FDG-avid mass on the right lower lobe, mediastinum, stomach and splenic hilum; D: Abdominal CT showed an encircling mass at the body of the stomach with multiple enlarged perigastric lymph nodes and splenic hilar invasion.
Figure 2.
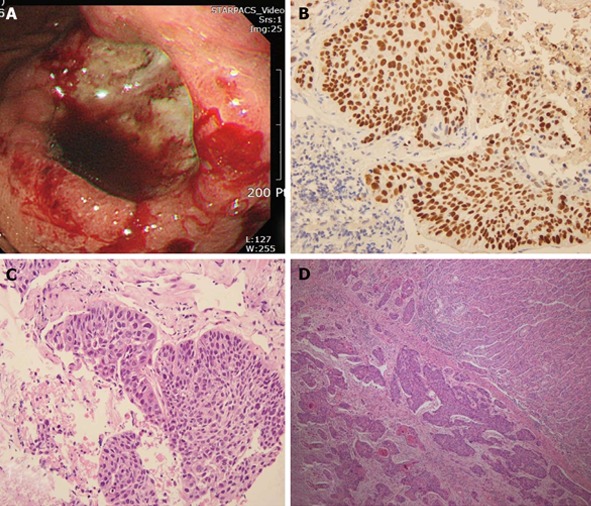
The biopsy showed syncytial growth of polygonal shaped poorly differentiated cells. A: Macroscopically, there was an ulcerofungating 4.0 cm × 3.5 cm lesion on the body; B, C: The bronchoscopic biopsy showed growth of polygonal shaped cells which were strongly positive for p63 and consistent with squamous cell carcinoma; D: Gastric ulcer specimen revealed multiple lymphatic invasion of carcinoma and chronic gastritis.
Initial gastric ulcer biopsy specimen revealed multiple lymphatic invasion of carcinoma and chronic gastritis (Figure 2D). In additional immunohistochemical stains, cells were positive for p63, CK5/6 and carcinoembryonic antigen, which were indicative of metastasis of pulmonary carcinoma. Therefore, the gastric lesion was diagnosed as a squamous cell carcinoma consistent with metastasis from primary lung carcinoma.
Simultaneously performed abdominal CT showed an encircling mass at the body of the stomach with multiple enlarged perigastric lymph nodes, exophytic mass of the pancreatic tail, and splenic hilar invasion (Figure 1D). Positron emission tomography using [18F]-fluoro-2-deoxy-D-glucose (FDG-PET)/CT showed a FDG-avid mass on the right lower lobe, mediastinum, stomach and splenic hilum (Figure 1C).
We performed a palliative total gastrectomy, splenectomy and distal pancreatectomy due to bleeding and dysphagia. Macroscopically, there was an ulcerovegetative 4.0 cm × 3.5 cm lesion on the body and its side (Figure 2A). In 22 lymph nodes, carcinoma metastases were detected in seven nodes. Metastatic carcinoma invasion was noted in the spleen as well as the pancreas. The patient made an uneventful postoperative recovery and did not receive any adjuvant chemotherapy due to his refusal. The patient was controlled conservatively and survived for 11 mo after surgery.
DISCUSSION
Lung carcinoma is the leading cause of cancer death and about 50% have distant metastasis at the time of diagnosis. The brain, liver, adrenal glands and bone are the most likely sites of extra-thoracic metastasis[8]. The common metastatic site of the gastrointestinal tract is the small bowel with sporadic case reports involving the stomach[9-16]. Gastrointestinal metastases were detected in 10 (0.19%) of 5239 lung cancer patients. The prognosis of gastrointestinal metastasis was poor, with a median survival of 96.5 d after diagnosis[3]. Hematogenous metastasis to the stomach is a rarer event. The most frequent tumors involved in secondary gastric sites are melanoma, breast and lung cancer, with its incidence being reported as 1.7%-29.6%[6,8]. However, the actual incidence of lung cancers metastasizing to the gastrointestinal tract is expected to be higher. The reasons are: (1) the increased incidence of lung cancer in women; (2) the recent increase in the number of endoscopic examinations performed in general hospitals; and (3) the use of immunostains by pathologists in neoplasms showing an undifferentiated morphology[17]. Furthermore, gastrointestinal metastasis has probably been underdiagnosed in living patients because it is frequently regarded as part of generalized metastatic disease or the lesions are considered side effects of chemotherapy, such as ulcers, enteritis or colitis[17].
Most patients with gastric metastasis are asymptomatic. Only a few symptomatic cases have presented with non-specific symptoms of epigastralgia, chronic bleeding, anemia and hematemesis after a considerable growth of the metastatic tumor. Therefore, we should pay more attention to gastrointestinal signs since they are commonly related to an advanced metastatic tumor.
The bull’s eye sign (volcano-like or umbilicated on the tip) is a well known characteristic of the radiographic findings of metastatic lesions[6]. However, it is not always the typical configuration. Therefore, some cases resemble early or advanced gastric cancer. Also, the differential diagnosis of lymphoma, ectopic pancreas, carcinoid tumor, eosinophilic granuloma, Kaposi’s sarcoma and gastric ulcer should be considered[2].
Although gastric metastasis from lung cancer is associated with dismal outcomes, a longer survival or more favorable outcome has been demonstrated in patients undergoing palliative surgical resection of the metastatic site[17-20]. Our patient also made an uneventful postoperative recovery and survived for 11 mo after surgery without any adjuvant chemotherapy.
In conclusion, surgical resection may provide an option of safe palliative treatment and gives longer survival with improved quality of life. However, considerable improvements in the understanding and therapeutic strategies for metastatic disease are needed to improve the clinical outcome.
Footnotes
P- Reviewers Kir G, Temiz P S- Editor Gou SX L- Editor Roemmele A E- Editor Yan JL
References
- 1.Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975;20:903–913. doi: 10.1007/BF01070875. [DOI] [PubMed] [Google Scholar]
- 2.Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65:1596–1600. doi: 10.1002/1097-0142(19900401)65:7<1596::aid-cncr2820650724>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH, Han MS, Kim CH, Lee JC. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J Cancer Res Clin Oncol. 2009;135:297–301. doi: 10.1007/s00432-008-0424-0. [DOI] [PubMed] [Google Scholar]
- 4.Kim SY, Ha HK, Park SW, Kang J, Kim KW, Lee SS, Park SH, Kim AY. Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol. 2009;193:W197–W201. doi: 10.2214/AJR.08.1907. [DOI] [PubMed] [Google Scholar]
- 5.Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, Tsai JR, Huang MS. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54:319–323. doi: 10.1016/j.lungcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Oda H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, Yoshida S, Shimoda T. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33:507–510. doi: 10.1055/s-2001-14960. [DOI] [PubMed] [Google Scholar]
- 7.Berger A, Cellier C, Daniel C, Kron C, Riquet M, Barbier JP, Cugnenc PH, Landi B. Small bowel metastases from primary carcinoma of the lung: clinical findings and outcome. Am J Gastroenterol. 1999;94:1884–1887. doi: 10.1111/j.1572-0241.1999.01224.x. [DOI] [PubMed] [Google Scholar]
- 8.Hillers TK, Sauve MD, Guyatt GH. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non-small cell lung cancer. Thorax. 1994;49:14–19. doi: 10.1136/thx.49.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Matsuzaki K, Kusumoto H, Uchida H, Mine H, Kabashima A, Maehara Y, Sugimachi K. Gastric metastasis from lung carcinoma. Case report. Hepatogastroenterology. 2002;49:363–365. [PubMed] [Google Scholar]
- 10.Alpar S, Kurt OK, Ucar N, Orsel O, Aydog G, Kurt B. A case of squamous cell lung carcinoma with gastric metastasis. South Med J. 2006;99:1313–1314. doi: 10.1097/01.smj.0000240697.56774.45. [DOI] [PubMed] [Google Scholar]
- 11.De Palma GD, Masone S, Rega M, Simeoli I, Donisi M, Addeo P, Iannone L, Pilone V, Persico G. Metastatic tumors to the stomach: clinical and endoscopic features. World J Gastroenterol. 2006;12:7326–7328. doi: 10.3748/wjg.v12.i45.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benghalia K, Colardelle P, Chochon M, Bretagne SR, Doll J. [Gastric metastasis revealing lung cancer] Gastroenterol Clin Biol. 2009;33:494–495. doi: 10.1016/j.gcb.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 13.Koch B, Tannapfel A, Vieth M, Grün R. [Gastric metastasis from small cell lung cancer] Pneumologie. 2009;63:585–587. doi: 10.1055/s-0029-1214905. [DOI] [PubMed] [Google Scholar]
- 14.Lee MH, Kim SR, Soh JS, Chung MJ, Lee YC. A solitary gastric metastasis from pulmonary adenocarcinoma: a case report. Thorax. 2010;65:661–662. doi: 10.1136/thx.2009.122382. [DOI] [PubMed] [Google Scholar]
- 15.Shomura H, Nakano S, Funai T, Akabane H, Inagaki M, Yanagida N, Kudo T, Orimo T, Oikawa F, Emoto S, et al. [A case of metastasis to the stomach from primary adenocarcinoma of the lung cancer] Gan To Kagaku Ryoho. 2010;37:2481–2483. [PubMed] [Google Scholar]
- 16.Sileri P, D’Ugo S, Del Vecchio Blanco G, Lolli E, Franceschilli L, Formica V, Anemona L, De Luca C, Gaspari AL. Solitary metachronous gastric metastasis from pulmonary adenocarcinoma: Report of a case. Int J Surg Case Rep. 2012;3:385–388. doi: 10.1016/j.ijscr.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi G, Marchioni A, Romagnani E, Bertolini F, Longo L, Cavazza A, Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2:115–120. [PubMed] [Google Scholar]
- 18.Hishida T, Nagai K, Yoshida J, Nishimura M, Ishii G, Iwasaki M, Nishiwaki Y. Is surgical resection indicated for a solitary non-small cell lung cancer recurrence? J Thorac Cardiovasc Surg. 2006;131:838–842. doi: 10.1016/j.jtcvs.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Garwood RA, Sawyer MD, Ledesma EJ, Foley E, Claridge JA. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg. 2005;71:110–116. [PubMed] [Google Scholar]
- 20.Aokage K, Yoshida J, Ishii G, Takahashi S, Sugito M, Nishimura M, Ochiai A, Nagai K. Long-term survival in two cases of resected gastric metastasis of pulmonary pleomorphic carcinoma. J Thorac Oncol. 2008;3:796–799. doi: 10.1097/JTO.0b013e31817c925c. [DOI] [PubMed] [Google Scholar]


