Abstract
Fetal malformations are very frequent in industrialized countries. Although advanced maternal age may affect pregnancy outcome adversely, 80%-90% of fetal malformations occur in the absence of a specific risk factor for parents. The only effective approach for prenatal screening is currently represented by an ultrasound scan. However, ultrasound methods present two important limitations: the substantial absence of quantitative parameters and the dependence on the sonographer experience. In recent years, together with the improvement in transducer technology, quantitative and objective sonographic markers highly predictive of fetal malformations have been developed. These markers can be detected at early gestation (11-14 wk) and generally are not pathological in themselves but have an increased incidence in abnormal fetuses. Thus, prenatal ultrasonography during the second trimester of gestation provides a “genetic sonogram”, including, for instance, nuchal translucency, short humeral length, echogenic bowel, echogenic intracardiac focus and choroid plexus cyst, that is used to identify morphological features of fetal Down’s syndrome with a potential sensitivity of more than 90%. Other specific and sensitive markers can be seen in the case of cardiac defects and skeletal anomalies. In the future, sonographic markers could limit even more the use of invasive and dangerous techniques of prenatal diagnosis (amniocentesis, etc.).
Keywords: Prenatal diagnosis, Prenatal sonography, Chromosome abnormalities, Nuchal translucency, Fetal echocardiography, Skeletal dysplasia
Core tip: The aim of this paper is to review sonographic markers associated with the most frequent fetal abnormalities (chromosomal anomalies, cardiac defects, skeletal dysplasia) and their sensitivity in prenatal diagnosis. Fetal malformations are very frequent in industrialized countries and the only effective approach for prenatal screening is currently represented by an ultrasound scan. Early detection of abnormalities can optimize pregnancy management and childbirth timing, give the possibility of performing simpler procedures for termination of pregnancy in those patients in whom findings are abnormal, and plan therapeutic treatment of objectively selected diseased fetuses.
INTRODUCTION
Congenital malformations of different types and severity occur in 2%-3% of fetuses in industrialized countries, leading to perinatal death in 25%-30% of cases, and these percentages are increasing, mainly because of the marked increment of pregnancies in women older than 40 years. However, although advanced maternal age may affect pregnancy outcome adversely, with increased miscarriages, ectopic pregnancies, twinning, fetal and chromosomal abnormalities, low birth weight and prematurity, 80%-90% of fetal malformations occur in parents with no specific risk factor. A further cause of legal litigations starting in the delivery room is represented by the unexpected birth of a disabled child; in such cases, parents often denounce the gynecologist that did not provide a prenatal malformation diagnosis, but it is clear that ultrasound (US) potential in the field of obstetrics and gynecology is still far from being fully exploited. The only effective approach for prenatal screening is currently represented by prenatal US examinations[1] as the only possible way to reduce both child disability and perinatal mortality through early malformation diagnosis. US techniques definitely have the potential to provide accurate early diagnosis of fetal malformations, but their employment is significantly hindered by two main limitations: the substantial absence of fully objective approaches and the very low number of available quantitative parameters. It is also clear that pregnancy management needs new approaches and new guidelines to rely on, exploiting objective indications through suitable methods and technologies for standardized quantitative monitoring and appropriate medical decision taking[2]. Quantitative monitoring of pregnancy is improving by the use of sonographic markers that are objective and predictive of specific fetal malformations becoming a powerful tool of prenatal diagnosis.
Early detection of abnormalities can optimize pregnancy management and childbirth timing with timely referring of pregnant woman to specialized facilities for specific disease treatment.
The state of art
US monitoring of pregnancy consists of at least three scans, one for each trimester of gestation. The most important US examination for the study of fetal anatomy is the second trimester scan[3], whose sensitivity in different malformation detection is highly variable: 70%-90% for central nervous system malformations, 40%-50% for heart disease (e.g., aorta coarctation), 25%-70% for urinary tract, 46%-100% for abdomen and gastrointestinal abnormalities (e.g., obstructive anomalies, omphalocele or gastroschisis), 20%-50% for bone dysplasias (e.g., spina bifida, limb reduction defects) and 7%-55% for cleft lip and palate[4]. US examination in the second trimester may show markers of suspected chromosomopathies, with a detection rate of 16%-45%[5], but until now its actual clinical usefulness is limited to an indication for more accurate but also more invasive cytogenetic tests[6] (Figure 1). However, invasive testing with a detection rate of 95%, such as amniocentesis, chorionic villus sampling or cordocentesis, is associated with a risk of miscarriage of about 1%-2%. Moreover, the false positive rate of about 0.2% is calculated only on the 20% of women selected to receive DNA testing. If all pregnant women had DNA testing, the false-positive rate would be 30 times greater. Therefore, cytogenetic tests are carried out only in pregnancies considered to be at high-risk for chromosomal defects[7,8].
Figure 1.
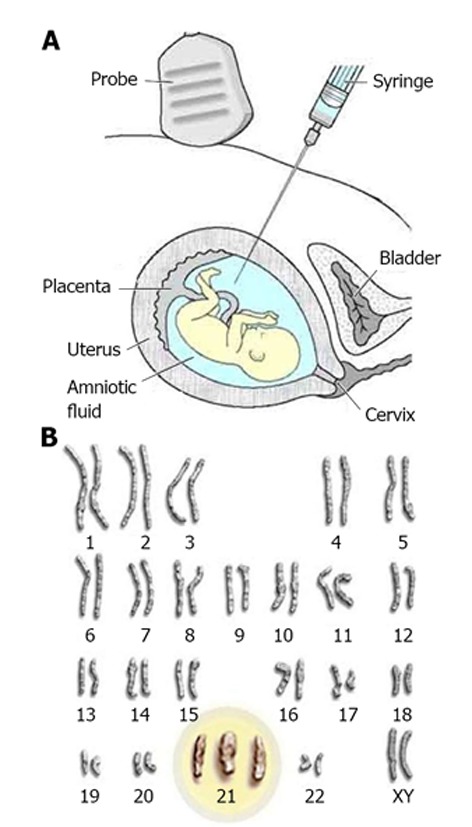
Prenatal cytogenetic tests. A: Amniocentesis: a small amount of amniotic fluid surrounding the baby during pregnancy is removed by a long needle for testing; B: Karyotype: the presence of an extra chromosome 21 is shown (diagnosis of Down’s syndrome)[111,112].
Then, on the one hand, the effectiveness of available US methods for prenatal malformation diagnosis is limited by a number of factors, such as period of gestation, variability of the fetal morphogenesis, natural history of the disease, thickness of maternal abdominal wall, possible unfavorable fetus position, but, above all, by the absence of objective parameters for most malformation types and by the strong dependence on operator experience[9]. On the other hand, US examinations are non-invasive and safe and there is a growing international interest in the development of new methods for US detection of malformations, driven also by recent improvements in transducer technology that through increased lateral resolutions and optimized transvaginal probes[2] has enabled examinations of first trimester fetus with an unprecedented level of detail. As a consequence, since the introduction of these technologies together with quantitative sonographic markers, there have been advances in first trimester prenatal diagnosis of major fetal anomalies, in particular concerning chromosomal abnormalities (80% detection rate), cardiac defects (57% detection rate) and skeletal anomalies (69% detection rate)[10,11].
Chromosomal abnormalities
The main part of fetal malformations are related to chromosomal abnormalities that occur in 0.1%-0.5% of live births[12,13]. The risk for many of the chromosomal defects increases with maternal age. Additionally, because fetuses with chromosomal defects are more likely to die in utero than normal fetuses, the risk decreases with gestation[7]. There is a great deal of interest in the US detection of aneuploidy and a second trimester US scan is able to detect, prior to karyotyping, two types of sonographic markers suggestive of aneuploidy[12]. The first group includes those that have a high rate of association with fetal chromosome abnormalities, whether in isolation or present with multiple other sonographic anomalies, and the second includes those that are much more likely to have associated chromosome abnormalities when seen in combination with other markers than in isolation[14].
Examples of data for the latter group are: ventriculomegaly, 2% (isolated) vs 17% (combined with other anomalies); holoprosencephaly, 4% vs 39%; choroid plexus cyst, 1% vs 48%; posterior fossa cyst, 0% vs 52%; facial clefting, 0% vs 51%; micrognathia, unknown percentage vs 62%; diaphragmatic hernia, 2% vs 49%; echogenic bowel, 7% vs 42%; renal abnormalities, 3% vs 24%; and exomphalos, 8% vs 46%. Examples of the former group are: cystic hygroma, 52% vs 71%; nuchal edema, 19% vs 45%; duodenal atresia, 38% vs 64%; and in a less pronounced way, for cardiac defects, 16% vs 66%[15].
Multiple anomaly associations, in particular cardiovascular anomalies, together with other markers are a more powerful indicator of aneuploidy[14]. Sonographically detectable aneuploidies include Down’s syndrome (trisomy 21), trisomy 13 and 18, Turner syndrome (monosomy X), and triploidy.
Sonographic markers of Down’s syndrome
The most common clinically significant aneuploidy among live-born infants is Down’s syndrome, with an estimated prevalence of 1.21 in 1000 live births[16]. Trisomy 21 is associated with a tendency for brachycephaly, mild ventriculomegaly, nasal hypoplasia, cardiac defects (mainly atrioventricular septal defects), duodenal atresia and echogenic bowel, mild hydronephrosis, shortening of the femur and, more so, of the humerus, sandal gap and clinodactyly or mid-phalanx hypoplasia of the fifth finger[7]. There are different screening methods to identify this ‘‘high risk group’’: advanced maternal age, maternal serum biochemical screening in the first and second trimester, and US screening in the first and second trimester[17]. US screening has recently been shown to decrease the prevalence of fetal Down’s syndrome in the second trimester to less than 85% by early identification of affected fetuses[18]. Sonographic findings in fetuses with Down’s syndrome or another detectable aneuploidy include both structural abnormalities and nonstructural abnormalities or “markers.” In themselves, these markers are not pathological but have an increased incidence in infants with chromosomal abnormalities and can be readily detected during the second-trimester US scan, although they are nonspecific and often transient.
The first reported marker associated with Down’s syndrome was the thickening of the neck area (nuchal fold)[19]: 40%-50% of affected fetuses have, in the second-trimester, a thickened nuchal fold measuring ≥ 6 mm, with a false-positive rate of 0.1%[20,21]. Nicolaides subsequently developed the measurement of the fetal nuchal soft tissues for use in the first-trimester, calling it the nuchal translucency (NT) and measuring it in the longitudinal midline plane of the fetus, using a very standardized technique (Figure 2A). NT, defined as the transient subcutaneous collection of fluid behind the fetal neck seen ultrasonographically at 11-14 wk, became a highly specific marker of Down’s syndrome. After the introduction of screening by NT, 83% of trisomy 21 pregnancies were identified in the first trimester[22]. Later, it was demonstrated that screening by a combination of maternal age, fetal NT and bi-test [pregnancy-associated plasma protein (PAPP-A) with second-trimester free chorionic gonadotropin (β-hCG)] or tri-test [alpha-fetoprotein (AFP), estriol and free β-hCG] has a potential sensitivity of 94% for a 5% false-positive rate[23].
Figure 2.
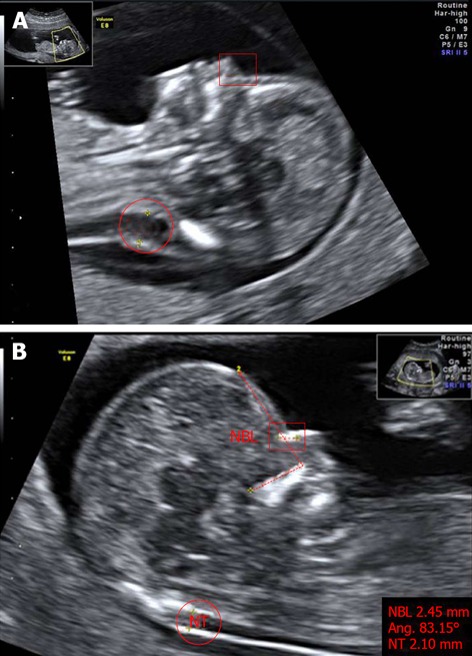
Markers of chromosomal defects. A: Fetus with Down’s syndrome: increased NT (red circle), and absent nasal bone (red square where nasal bone was expected) at 11 wk of pregnancy; B: Normal fetus: measurements of nuchal translucency (NT, red circle), facial angle (red dashed line) and nasal bone length (NBL, red square) at 13 wk of pregnancy. The image has been certified by the Fetal Medicine Foundation. Photos taken by Wolfgang Moroder. Creative Commons[113].
In addition to nuchal thickening, sonographic findings that are generally accepted as potential markers of trisomy 21 during the second trimester include shortened femur or humerus, renal pyelectasis, hyperechoic bowel, echogenic intracardiac focus, and, recently, nasal bone ossification[24]. The effectiveness of these markers are detailed in Table 1.
Table 1.
Sensitivity of sonographic markers of aneuploidies
| Markers of aneuploidy | DR (%) | FPR (%) |
| NT | 83 | |
| NT+ PAPP-A + β-hCG + AFP + estriol | 94 | 5 |
| Femoral length | 40-50 | 7 |
| Humeral length | 50-54 | 5-6 |
| Humeral length + NT | 75 | |
| Pyelectasis | 17-25 | 2-3 |
| Hyperechoic bowel | 3.3-27 | 1 |
| Absence of nasal bone | 73 | 0.5 |
| Nasal bone + NT + PAPP-A + β-hCG + AFP + estriol+ maternal age | 97 | |
| Aberrant right subclavian artery | 37.5 | 1.4 |
DR: Detection rate; FPR: False-positive rate; NT: Nuchal translucency; PAPP-A: Pregnancy-associated plasma protein; β-hCG: Chorionic gonadotropin; AFP: Alpha-fetoprotein.
Ultrasound studies have shown that fetuses with Down’s syndrome have a shorter femur and even shorter humerus when compared with normal fetuses[18]. The sensitivity for detecting trisomy 21 using the femoral length is 40%-50%, with a false-positive rate of up to 7%[25,26]. The humeral length is considered a slightly more efficient marker, with a sensitivity of 50%-54% and a false-positive rate of 5%-6%[27-29]. The combination of humeral length and nuchal fold increases the sensitivity to 75%, without substantially changing the false-positive rate based on humeral length alone[18,30].
Pyelectasis, defined as a diameter of the renal pelvis measuring ≥ 4 mm, is another second-trimester marker; in fact, renal dilatation has a higher incidence among fetuses with Down’s syndrome. However, pyelectasis remains a minor marker because the sensitivity is 17%-25%, with a false-positive rate of 2%-3%[31,32].
The sensitivity of hyperechoic bowel in Down’s syndrome ranges from 3.3% to 27.0% depending on the sonographer and of the frequency of the US transducer[33-36]. Its false-positive rate is less than 1%[33,37]. Hyperechoic bowel also conveys an increased risk of cystic fibrosis, cytomegalovirus and severe early growth issues[18].
The echogenic intracardiac focus is the least efficient marker among those used for detecting Down’s syndrome. It occurs in 16% of fetuses with Down’s syndrome, 29% of those with trisomy 13% and 2% of normal fetuses[38]. A recent study also confirms that the finding of an isolated echogenic intracardiac focus on prenatal sonography does not significantly increase the risk for fetal trisomy 21[39]. The false-positive rate is 17%[40].
In the literature, there are important examples of soft markers that have been successfully incorporated into fetal abnormality screening. The case of the nasal bone is the most recent and most powerful marker (Figure 2B). The absence of nasal bone in fetus at the 11-14 wk scan is related to Down’s syndrome (Figure 2A); this marker, initially, was found in 73% of trisomy 21 fetuses and in only 0.5% of chromosomally normal fetuses[41] and, subsequently, it was estimated that the combination of maternal age, NT, maternal serum biochemical screening (by bi-test or tri-test) and examination of nasal bone could increase the detection rate to 97%[42]. After the completion of further confirmation studies, it is generally accepted that fetal nasal bone is a very good sonographic marker, even if there are racial differences in the length of this bone[43,44].
Furthermore, there are minor markers of Down’s syndrome used less to screen the general population. These include iliac angle, specific cardiac features, choroid plexus cysts and others. The mean iliac wing angle is a useful marker in prenatal screening in fetuses with trisomy 21. A recent study demonstrates that in affected fetuses it is 90.32°, significantly higher than those seen in fetuses with normal karyotype in which the mean iliac wing angle was 63.72°[45]. The specific cardiac features associated with an increased risk of Down’s syndrome are primarily ventricular disproportion and the presence of septal defects[18]. In addition, the phenomenon of tricuspid regurgitation is highly associated and its prevalence increases with the NT[46]. Other findings suggest that the mitral valve-tricuspid valve distance could prove useful as an additional marker at the time of the second trimester sonogram. It increases with gestational age and is lower in fetuses with trisomy 21[47]. Fetal aberrant right subclavian artery is another potential marker because in a small group its prevalence in fetuses with Down’s syndrome is 37.5% vs 1.4% in a low-risk population[48]. Choroid plexus cysts were suggested as a possible minor marker but it has been demonstrated that the risk of Down’s syndrome is not raised in their presence[49,50]. Anyway, choroid plexus cysts are a well-known marker for trisomy 18. Finally, ear length at 11-14 wk of gestation has been evaluated in screening for chromosomal defects but the degree of deviation from normal is too small for this measurement to be useful as a marker for trisomy 21[51].
Other aneuploidies
Trisomy 18 is the second most common autosomal trisomy syndrome after trisomy 21. The estimated prevalence is 1 in 6000-8000 live births but the overall prevalence is higher (1 in 2500-2600) due to the high frequency of fetal loss and pregnancy termination after prenatal diagnosis. Currently, most cases of trisomy 18 are prenatally diagnosed, based on screening by maternal age, maternal serum marker screening or detection of sonographic abnormalities. The prenatal sonographic pattern of trisomy 18 is characterized by growth retardation, polyhydramnios, “strawberry-shaped” cranium, choroid plexus cyst, absent corpus callosum, enlarged cisterna magna, facial cleft, micrognathia, nuchal edema, heart defects, diaphragmatic hernia, esophageal atresia, overlapping of fingers, congenital heart defects, omphalocele, renal defects, echogenic bowel and single umbilical artery[52-55]. The prevalence of growth retardation and polyhydramnios increases with gestational age: 28% and 29% in the second trimester and 87% and 62% in the third trimester, respectively[56]. In particular, choroid plexus cyst is detected in about 50% of trisomy 18 fetuses, while hand abnormalities, exomphalos and single umbilical artery have been found in more than 30% of affected fetuses[55,56]. The most common soft sonographic markers detected in the early second trimester are, as in Down’s syndrome, the increased NT thickness and the absence or hypoplasia of the nasal bone[57,58]; the screening by assessment of nuchal fold and nasal bone identifies 66.7% of cases with trisomy 18 (and 13)[58]. Combining NT with bi-test or tri-test sensitivity is at least 78%[59,60]. Anyway, one or more sonographic anomalies are detected in over 90% of fetuses; two or more abnormalities are present in 55% of cases[61].
In trisomy 13, common defects include holoprosencephaly and associated facial abnormalities, microcephaly, cardiac and renal abnormalities with often enlarged and echogenic kidneys, exomphalos and postaxial polydactyly[7]. In particular, among craniofacial malformations detected by prenatal sonography, cleft deformities are found in 65.2% and ocular and orbital abnormalities were found in 28%[62]. Fetal tachycardia is observed in about two-thirds of cases and early-onset intrauterine growth restriction in about 30% of cases[63]. In trisomy 13, as well as in trisomy 18, maternal serum free β-hCG and PAPP-A are decreased[7,63-65].
Turner syndrome, usually due to loss of the paternal X chromosome, is the most common monosomy (45, X) in the fetus, with a prevalence of 25-55 cases per 100000 females and, unlike that of trisomies, is unrelated to maternal age[66]. Characteristic sonographic markers are highly predictive in early pregnancy: huge septated cystic hygroma, hydrops, subcutaneous edema, narrowed aortic arch, renal anomalies and short femur are detected in about 90% of affected fetuses. Some studies have reported that fetuses with Turner syndrome have cystic hygromas much larger than in trisomy 18 or trisomy 21[67]. The classic webbed neck in Turner syndrome is probably the end result of the huge fetal cystic hygroma. Coarctation of the aorta is observed in approximately 20% of the affected fetuses at 14 to 16 wk of gestation[68] and tachycardia observed in about 50% of cases.
Polyploidy is extremely rare and lethal and affects about 2% of recognized conceptions. In particular, triploidy is associated with molar placenta if the extra set of chromosomes is paternally derived. While in cases of double maternal chromosome contribution, the fetus demonstrates severe asymmetrical growth restriction. Ventriculomegaly, micrognathia, cardiac abnormalities, myelomeningocele and syndactyly are also common[7].
CONGENITAL HEART DISEASES
Congenital heart disease (CHD) is one of the most common congenital anomalies and incidence in different studies varies from about 0.4%-5.0% live births[69], while the prevalence rate in the general population is 0.8%-1.0%[70]. Approximately half of infant deaths are due to CHD and 3.0-4.4 per 1000 live births require intervention during the first year of life[71]. Most fetal CHDs occur in patients without any risk factors. Because of this, prenatal US screening of CHD is justified in the general low-risk population. Fetal echocardiography (echoCG) is considered to be an accurate diagnostic tool, reflecting postnatal outcomes well. Fetal echoCG is now widely used in pediatric cardiology and perinatology and even for fetal cardiac intervention, improving the preoperative condition, morbidity and mortality of patients with CHD[72].
The fetal heart is the organ that presents the most problems in diagnosis[73]. This is undoubtedly reflected in the low detection rate of cardiac abnormalities compared to those of most other organ systems in the fetus[74]. It is still a challenge, even for the most experienced ultrasonographer, to visualize the different cardiac structures at an early stage in gestation. All the changes producing the venous connections, the atrial and ventricular chambers, the arterial roots and the intrapericardial arterial trunks have been completed by 8 wk of gestation when the total length of the heart is no more than 8 mm and the distance between different structures is of the order of millimeters. At 12 wk of gestation, the fetal heart is positioned within the chest normally and, fortunately, between the 12th and 17th week, the heart doubles in size and triples in size by the 21st wk[75]. At this time, cardiac structures can be visualized and identified (Figure 3).
Figure 3.
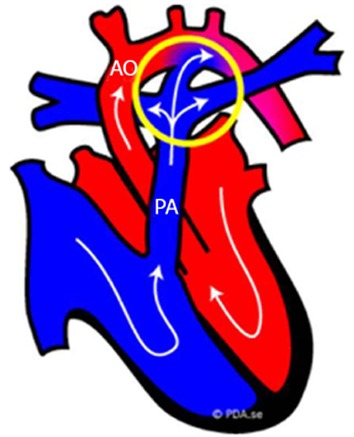
Fetal normal heart. Schematic representation of the blood circulation in fetus: the blood that comes into the right side of the fetal heart (blue part) is pumped into the pulmonary trunk (PA) and flows through the ductus arteriosus (circled in yellow) directly out into the aorta (AO). The ductus arteriosus is an extra blood vessel of the fetal heart that creates a bypass for the blood oxygenated not by the lungs, but through the placenta[114].
The application of an extended basic US cardiac examination improves the detection of CHD, in particular the conotruncal anomalies. The stepwise method suggested for fetal heart US screening during the mid-second trimester sonogram is based on 4 routine axial views of the heart and great vessels: (1) a transverse view of the superior abdomen (Figure 4A); (2) a 4-chamber view (Figure 4B); (3) a 3-vessel view (Figure 4C); and (4) a transverse view of the aortic arch (Figure 4D)[70].
Figure 4.
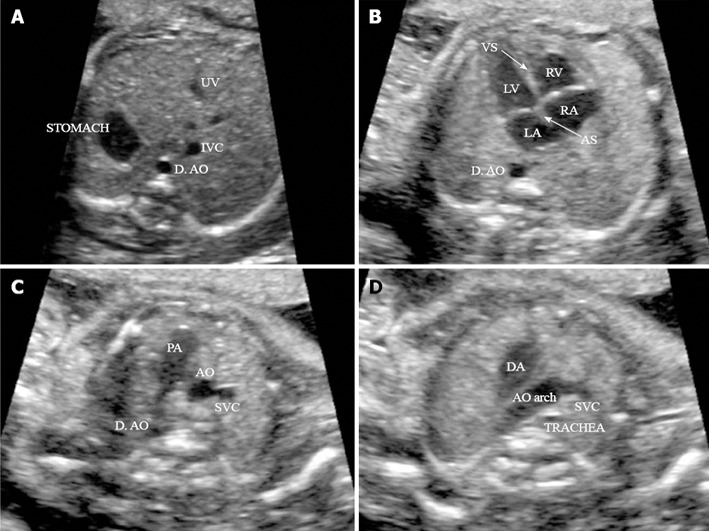
Four routine axial views of heart and great vessels. A: Transverse view of the superior abdomen: the stomach on the fetal left side; the descending aorta (D. AO) to the left side and inferior vena cava (IVC) to right side of the spine, respectively; B: Four-chamber view: in a normal fetal heart, approximately equal size of the right and left chambers, intact intact ventricular septum (VS) and normal offset of the two atrioventricular valve; C: Three-vessel view: pulmonary artery (PA), aorta (AO) and superior vena cava (SVC) in the correct position and alignment; PA, to the left, is the largest of the three and the most anterior, whereas the SVC is the smallest and most posterior; D: Transverse view of the aortic arch: in the normal heart, both the AO arch and the ductal arch (DA) are located to the left of the trachea, in a ‘V’-shaped configuration. (Adapted from ISUOG Practice Guidelines[115]). UV: umbilical vein; RV: right ventricle; LV: left ventricle; LA: left atrium; RA: right atrium; AS: Atrial septum.
Abnormalities of the right heart
Anatomically, the right atrium possesses all its morphological characteristics from at least 10 wk gestation and is usually a triangular structure, whereas the left atrium is more tubular and meandering.
In the structurally normal heart (Figure 3), the right atrium accepts the superior and inferior cava vein at its cranial and caudal ends. The third systemic veins to enter the right atrium are the coronary sinus[74]. Generally, abnormalities of the coronary sinus are rare but there can be fenestrations within its walls creating the second interatrial communication or else dilation when it drains the pulmonary venous return anomalously[76]. The most frequent anomaly, however, is the dilation of the coronary sinus (Figure 5A) in the setting of persistence of the left superior cava vein. Actually, this anomaly is present in up to one-thirtieth of the normal population but is many times more common when the heart is malformed[77].
Figure 5.
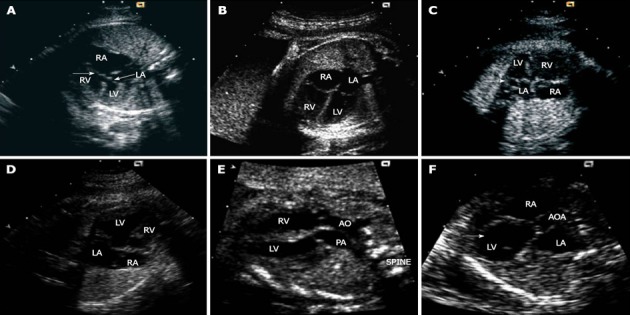
Markers of congenital heart disease. A: Atrioventricular septal defect with a common junction leading to loss of off-setting of the atrioventricular valves (arrows); B: Four-chamber echo view in a normal mid-trimester fetus; C: Enlarged coronary sinus seen as a circular structure (arrow) within the left atrium adjacent to the mitral valve; D: Pulmonary atresia with intact ventricular septum: apical muscle bundles are prominent in the apical portion of the right ventricle with trabeculations coarser than usual; E: Transposition of the great arteries (discordant ventriculoarterial connections): the arteries are parallel to one another with the aorta arising from the right ventricle and positioned to the right of the pulmonary trunk; F: Severe aortic stenosis with patent mitral valve: the left ventricle becomes bulb-shaped (arrow). LA: left atrium; RA: right atrium; RV: right ventricle; LV: left ventricle; AO and AOA: Aorta; PA: Pulmonary trunk. Adapted from Cook et al[74].
The right ventricle is usually easily identified on US by the coarse trabeculations found within its apex. One trabeculation is usually particularly prominent but it is the multiple prominent muscle bundles present within the apex of the right ventricle that give it a ‘filled-in’ appearance on US. In almost all structurally abnormal hearts, the trabeculated portion of the right ventricle is also present[78]. In some forms of cardiac disease, such as pulmonary atresia (PA) (Figure 5B) or stenosis with intact ventricular septum (IVS), the apical muscle bundles become even more prominent during fetal life, eventually obliterating completely the apical portion of the right ventricle. In others, such as tricuspid atresia or double inlet left ventricle, they are hypoplastic as well as the whole right ventricle[74]. The tricuspid valve is very important in recognizing the right ventricle. In this side of the heart, the tricuspid valve closes in trifoliate fashion, although a discrete septal leaflet is not seen prior to 12 wk gestation. When formed, the septal leaflet has multiple cordal and muscular attachments to the right side of the ventricular septum. On US, the septal leaflet appears to be hinged from the right side of the ventricular septum, more towards the ventricular apex than the mitral valve. The displacement of the tricuspid valve, seen in a four-chamber section, is largely caused by draping of the right atrium over the right side of the ventricular septum as a result of the atrioventricular canal closure, and the creation of discrete right and left inlets to the heart[79]. Consequently, in fetuses with atrioventricular septal (or canal) defects (Figure 5C), this draping cannot occur since there is no offsetting of the atrioventricular valves at the center of the four-chamber view. Similarly, in fetuses with a large ventricular septal defect (VSD), asymmetry of the atrioventricular valves is also lost, principally because there is no septum over which the tricuspid valve can be draped. The Ebstein’s defect is characterized by an apically displaced septal insertion of the tricuspid valve leaflet with an atrialized portion of the right ventricle. Closely related to this, is the tricuspid valve dysplasia. In this situation, the delamination of the leaflets of the tricuspid valve is normal, but there is a marked dysplasia of their leaflets and cords. Moreover, in some fetuses, the connection between the right atrium and ventricle is entirely lacking, creating classical tricuspid atresia. In others, a right-sided atrioventricular valve may be formed, but malalignment of the ventricular and atrial septums leads to double inlet left ventricle or straddling tricuspid valve[74].
Abnormalities of the left heart
The left ventricle is characterized by fine trabeculations and the leaflets of the mitral and aortic valves are in fibrous continuity in the roof of the left ventricle. The three or four pulmonary veins enter at the left atrium in the form of a cross and the lower ones can be seen in a standard four-chamber section of the heart (Figure 5D). A narrow junction of the pulmonary veins, or a wide separation between the pulmonary venous confluence and the left atrium, can indicate anomalous pulmonary venous connection. Usually, such anomalous veins drain either via an ascending channel to the superior vena cava, or through a descending channel to the hepatic portal venous system. They can also drain directly to the heart via a dilated coronary sinus. In ultrasonic four-chamber sections, the two unequal leaflets forming the mitral valve are also seen. In the structurally normal heart, the arterial leaflet is long and with an apron-shaped structure and the second leaflet is shallower than the first one but appreciably longer. Both the leaflets are supported by the same paired papillary muscle groups, leaving the left side of the ventricular septum smooth in contrast to its right-sided counterpart. Abnormalities of the mitral valve are very similar to those in the right heart. In mitral atresia, there is the complete atresia of the connection between the left atrium and ventricle or a malalignment of its orifice leading to double inlet right ventricle[74]. In some malformations, a complex and abnormal connection of the cardiac segments can be seen. Isolated dysplasia of the mitral valve leaflets is rare and is usually encountered in the setting of aortic valve stenosis or atresia[80].
Major abnormalities of the left ventricle usually involve the spectrum of left heart hypoplasia. On the one hand, the left ventricle is slitlike, with both the mitral and aortic valves being atretic. On the other hand, there is patency of the mitral valve, aortic valve stenosis or atresia and a thick-walled and calcific left ventricle. However, the first marker of abnormalities involving arterial malformations, such as discordant ventriculoarterial connections (“transposition”) or double outlet right ventricle, is often the lack of crossover of the arterial valves[74]. Finally, in assessment of the left ventricle, it is important to distinguish between real and false outflow in the region of the membranous septum. In fact, the ventricular septum is abnormal in many malformations of the outflow tract, including the tetralogy of Fallot, most forms of double outlet, common arterial trunk and the type of perimembranous ventricular defect seen in many chromosomal abnormalities[81]. Unfortunately, this region is filled by thin, fibrous tissue and is therefore prone to loss of signal when imaged in some orientations by US[74].
Abnormalities of the great arteries
The arrangement and branching of the distal great arteries is altered in many cardiac abnormalities. Normally, by an US oblique view of the fetal thorax, it is easy to see the aortic arch running to the left of the trachea and to the right of the arterial duct. Other inferior views show the branching pattern of the pulmonary trunk, as well as the linear relationship between the pulmonary trunk, aorta and superior cava vein. In case of discordant ventriculoarterial connections (Figure 5E) and in some forms of double outlet right ventricle, the pattern of branching that allows recognition of the pulmonary trunk from the aorta is the reverse of that seen in the normal fetal heart. Alterations in the size and shape of the distal arteries are seen when there is an abnormal balance of aortic to pulmonary blood flow. The size of the aortic or ductal arches also reflects the flow of blood through them. For instance, in fetuses with aortic coarctation (Figure 5F) or hypoplasia of the left heart, the aortic arch is small and enters into the side of a dominant ductal arch. In contrast, in fetuses with obstruction to the pulmonary outflow tract, the ductal arch is usually hypoplastic and enters into the underside of a dominant aortic arch.
Fetal echocardiography: Markers and diagnostic outcomes
Fetal echoCG becomes more consistently successful at a later gestational age and therefore early scanning does require justification. Cardiac examination is carried out in high-risk fetuses by a fetal cardiologist and/or gynecologist with particular experience in fetal echocardiography. Risk factors for a cardiac defect are increased NT ≥ 4 mm, a first-degree relative with a significant CHD and suspicion of a cardiac or extracardiac abnormality on the 10-14 wk scan. Occasionally, a full cardiac examination is performed at the parent’s request[82]. The NT measurement is only a modestly effective screening tool for all CHD when used alone; however, the combination of an increased NT, tricuspid regurgitation (TR) and an abnormal ductus venosus (DV) Doppler flow profile is a strong marker for CHD. Generally, an increased NT is considered a marker of fetal chromosomal aneuploidies, although the nuchal edema can be caused by the fluid accumulation due to a cardiac failure[83]. The phenomenon of TR is also associated with aneuploidy and its prevalence increases with the NT thickness and is substantially higher in fetuses with than in those without CHD. In chromosomally normal fetuses, the finding of TR at 11 to 13 + 6 wk gestation is associated with an eight-fold increase in the risk of CHD[84]. Moreover, an abnormal DV Doppler flow profile, where during late diastole, coincident with atrial contraction, reduced or reversed flow is seen, has been interpreted as indicative of cardiac failure as it was assumed to reflect increased central venous pressure[85]. The performance of a complete fetal echocardiogram at the end of the first trimester requires expertise. With modern equipment, it is usually possible, using the abdominal approach, to define the situs and cardiac connections, identify the cardiac chambers and their symmetry, the crossing of the great vessels, and to evaluate the flow in the chambers and the great vessels using Doppler and color flow mapping[46].
Fetal echoCG results have been ranked in 5 classes: normal, minor abnormalities, simple cardiac anomalies, moderate cardiac anomalies, and complex cardiac anomalies (Table 2). The criteria of this classification are as follows. Simple cardiac anomalies are defined as a defect able to be corrected by medical treatment or percutaneous cardiovascular interventions and only sometimes by surgery, such as VSD, atrial septal defect (ASD) and possible coarctation of the aorta (possible CoA). Moderate cardiac anomalies are defects able to be corrected surgically with a low risk for reoperation, such as tetralogy of Fallot, CoA, atrioventricular septal defect (AVSD) and complete transposition of the great arteries (TGA). Complex cardiac anomalies are defined as defects able to be corrected anatomically by surgery but with a high risk for sequelae or a Fontan operation candidate, such as double outlet right ventricle, TGA with pulmonary stenosis (PS), critical PS and Fontan candidates (PA with IVS), functional single ventricle, hypoplastic left heart syndrome][72].
Table 2.
Classes of fetal cardiac anomalies
| Complex CHD | Moderate CHD | Simple CHD |
| Transposition of the great arteries | Mild or moderate AS or aortic incompetence | Small VSD |
| Tetralogy of Fallot, Hypoplastic right heart | Moderate PS or incompetence | Small PDA |
| SV, DORV, Truncus arteriosus | Noncritical Coarc | Mild PS |
| Total anomalous pulmonary venous connection | Large ASD | BAV without AS or aortic incompetence |
| AVSD, Large VSD, Large PDA Critical or sever PS, Critical or severe AS, Critical Coarc | Complex forms of VSD | Small or spontaneously closed ASD |
CHD: Congenital heart disease; AS: Aortic stenosis; VSD: Ventricular septal defect; PS: Pulmonary stenosis; PDA: Patent ductus arteriosus; SV: Single ventricle; DORV: Double outlet right ventricle; ASD: Atrial septal defect; BAV: Bicuspid aortic valves; AVSD: Atrioventricular septal defect.
The relative frequency of different major forms of CHD differs greatly from study to study because of many reasons. Some studies are restricted to infancy and so miss patients who present later in life; others do not detect patients with a small ventricular or ASD, an abnormal patent ductus arteriosus (PDA) or many with CoA. Moreover, the increasing use of fetal echocardiography also leads to therapeutic abortion for complex heart diseases and can substantially reduce the incidence of specific lesions. However, isolated VSDs are the most common form of CHD and the incidence of these varies from 2% to 5%[86,87]. About 85%-90% of these defects close spontaneously by one year of age[88,89]. PDA is another common lesion, the incidence of which varies with the age at the time of study and the gestational age of the subject. Preterm infants have an increased incidence of PDA based on abnormal physiology rather than on a structural abnormality. In term infants, the normal ductus may stay open for some time after birth. Isolated partial anomalous pulmonary venous connection is a rare lesion clinically and it resembles an ASD in producing a right ventricular volume overload. Some studies, however, have shown an incidence of 0.6%-0.7%. AVSDs (endocardial cushion defects, common atrioventricular canal) have an incidence that varies with the age of the involved mothers. Trisomy 21 is much more common in mothers older than 34 years old and AVSDs are much more frequent in fetuses with trisomy 21 than with normal chromosomes. Bicuspid aortic valves (BAV) are important because of their frequency and their late complications. Most subjects with BAVs develop stenosis or incompetence after 40 years of age. There is some variation in incidence from about 0.40%-2.25%. Mitral incompetence as an isolated congenital lesion is rare in children; however, mitral valve prolapse is common, occurring in perhaps 4%-5% of the population[69,90,91].
With the recent advances in fetal echoCG on both technical and educational sides, the prenatal diagnosis of rare CHD, as well as the left isomerism, is also increasing. Left isomerism is associated with paired left-sided viscera, whereas right-sided viscera may be absent. Typical findings in left isomerism are bilateral morphological left atrial appendages, visceral-cardiac heterotaxy, multiple cardiac anomalies, congenital heart block, bilateral morphological left lungs with hyparterial bronchi, polysplenia, intestinal malrotation and interruption of the inferior vena cava with azygos continuation. By these markers, prenatal diagnosis of left isomerism is feasible with high accuracy[92].
Generally, the diagnostic accuracy of fetal echoCG is in the range 94.3%-99.0%[93-95]. Also, considering discrepancies between prenatal and postnatal echoCG results in the diagnostic accuracy of 98.6%[72]. Moreover, the diagnostic accuracy of fetal echoCG performed by a pediatric cardiologist is much higher than the reported diagnostic accuracy of sonographic screening performed by an obstetrician (59%)[95]. Anyway, paramount in the approach to the fetus with CHD is a multidisciplinary team composed of an obstetrician, geneticist, perinatologist, pediatrician, pediatric cardiologist and cardiac surgeon that review each case of fetal malformation, refining the diagnosis, establishing management plans and facilitating issues relating to the delivery of the patient[96]. The three main types of cardiovascular malformations in which prenatal diagnosis has been shown to be beneficial are coarctation of the aorta, hypoplastic left ventricle and TGA.
Recently, upgrades to commercially available equipment have improved image quality in order to use high-resolution real-time three-dimensional echocardiography in evaluation of fetal cardiac anatomy and function. With conventional imaging, the accuracy and reproducibility of quantifying ventricular size and function are limited by image plane positioning errors and geometric assumptions. Three-dimensional ultrasonography (3D-US) has been shown to provide more accurate measurements of volume and area than two-dimensional methods, possibly even for volumes encountered in the fetal heart. Moreover, by minimizing some of the time and imaging expertise demands compared to conventional fetal echocardiography, real-time 3D-imaging also may represent a more effective way for sonographers to screen for congenital heart disease in low risk pregnancies[97].
Early diagnosis of CHD gives the parents more time to make an informed decision regarding continuation of the pregnancy. Should they decide to interrupt the pregnancy, this can then be performed earlier, more safely and with less long-term psychological sequelae. If the pregnancy is continued, the timing, location (hospital with neonatal intensive care, pediatric cardiology and pediatric cardiac surgery facilities), mode of delivery and direct postnatal care can be planned. In this way, the postnatal outcome of these babies can be improved. Where the early fetal echocardiogram shows no evidence for a cardiac defect, it allows earlier reassurance of couples considered at high risk for CHD[46].
SKELETAL DYSPLASIAS
Skeletal dysplasias are a heterogeneous group of conditions associated with various anomalies in shape and size of the skeleton[98]. These conditions are caused by widespread disturbance of bone growth, beginning during the early stages of fetal development and evolving throughout life[99]. The prevalence of skeletal dysplasias is low, approximately 3.2 in 10000 live births[100], and the overall frequency of skeletal dysplasias among perinatal deaths is about 9 per 1000[98]. Based on postnatal radiological classification, more than 150 different conditions have been described[101]. Despite recent advances in imaging, fetal skeletal dysplasias are difficult to diagnose before birth (especially in the absence of a familial history) due to a number of factors, including their large number, their phenotypic variability with overlapping features, lack of precise molecular diagnosis for many disorders, lack of a systematic approach and variability in the time at which findings manifest the inability of US to provide an integrated view. It must be emphasized that the analysis of bone anomalies requires expertise and great knowledge of postnatal features. At present, accurate prenatal diagnosis of skeletal dysplasia remains a challenge, with only approximately 65% of cases being accurately diagnosed by conventional two-dimensional ultrasound (2D-US)[102]. US of suspected skeletal dysplasia involves systematic imaging of the long bones, thorax, hands and feet, skull, spine and pelvis[99].
Long bones
The bones should be assessed for the presence, curvature, fractures and degree of mineralization[99]. All the long bones of each limb and segment should be examined and measured. The shortened segments must be identified (rhizo-, meso- or acromelic shortening). A femur length-abdominal circumference ratio < 0.16 suggests lung hypoplasia and a femur length-foot length ratio < 1 is in favor of dysplasia[98]. In achondroplasia, femur length becomes abnormally short only in the third trimester. The presence of abnormal angulation (Figure 6A) suggests a fracture and joint deformities or disruption or incongruence are compatible with luxations. Anterior angulation of the tibia and the short fibula are pathognomonic features of campomelic dysplasia[103]. Abnormal epiphyseal calcification should also be looked for but bone mineralization is still very difficult to appreciate on US scans[98].
Figure 6.
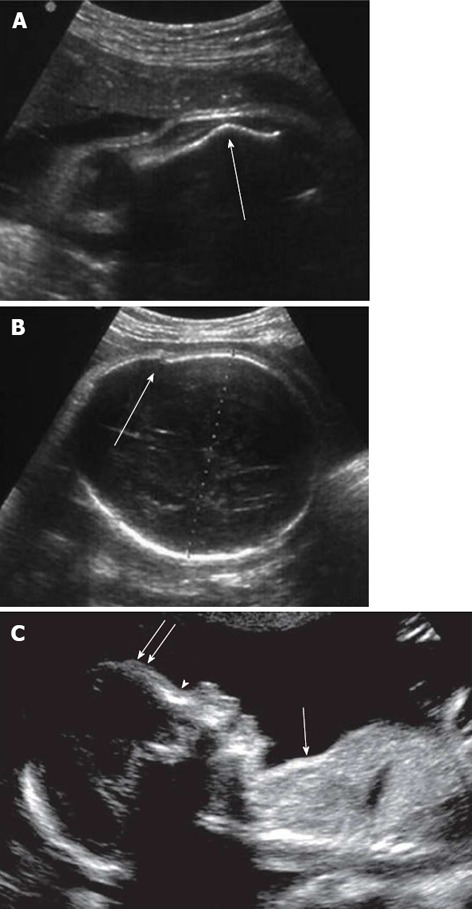
Marker of skeletal dysplasia by two-dimensional ultrasound. A: Abnormal angulated femur; B: Osteogenesis imperfecta: cranial vault distortion upon probe pressure. (Adapted from Cassart[98]); C: Features of thanatophoric dysplasia: depressed nasal bridge (arrowhead), prominent forehead (double arrows), and undersized thorax (single arrow) compared with the abdomen. Adapted from Dighe et al[99].
Thorax
The thorax must also be measured; in fact, a chest circumference less than the 5th percentile has been proposed as an indicator of pulmonary hypoplasia which is the main cause of neonatal death in many lethal skeletal dysplasias[104]. Hypoplastic thorax occurs in many skeletal dysplasias, such as thanatophoric dysplasia (Figure 6C), achondrogenesis, hypophosphatasia, camptomelic dysplasia, chondroectodermal dysplasia, osteogenesis imperfecta and short-rib polydactyly and may lead to pulmonary hypoplasia[99]. The shape and size of the ribs, clavicles and scapula should be analyzed, since the absence or hypoplasia of the clavicles is seen in cleidocranial dysplasia[105] and the absence of the scapula is a useful defining feature of camptomelic dysplasia[106].
Hands and feet
The hands and feet should be carefully analyzed to exclude the presence of pre- or postaxial polydactyly (the presence of more than five digits), syndactyly (soft-tissue or bone fusion of adjacent digits) and clinodactyly (deviation of a finger). Foot deformities, such as ‘hitchhiker’ thumb, rocker-bottom or clubbed feet, should also be evaluated[98]. Clubbing of the hand is suggestive of the spectrum of “radial ray” anomalies, which include an abnormal thumb (Holt-Oram syndrome), hypoplasia and absence of the thumb and sometimes, absence of the radius or of both the radius and the hand[99]. In diastrophic dysplasia, the fingers are short, with ulnar deviation and ‘hitchhiker’ thumbs. There are clubfeet and micrognathia. In achondroplasia, the phalanges are short; typical gaps between the fingers and digital deviation lead to the appearance of a “trident” hand[103].
Skull
Head circumference and biparietal diameter should be measured to exclude micro- or macrocephaly. The shape, mineralization and degree of ossification of the skull should be evaluated; osteogenesis imperfecta can be suspected in cases of cranial vault distortion upon probe pressure (Figure 6B). Interorbital distance should be measured to exclude hyper- or hypotelorism[99]. Other features, such as clover-leaf deformity, brachycephaly or scaphocephaly, should suggest craniosynostosis (premature fusion of the sutures). In thanatophoric dysplasia, the head circumference is large in half of cases, with the nasal bridge depressed, and an abnormal sonographic translucency can be detected[103]. Facial dysmorphisms may be better depicted by 3D-US[107-109].
Spine
The spine should be carefully imaged to assess the relative total length and the presence of curvature or incomplete closure of the neural tube. Mineralization of vertebral bodies and neural arches should be evaluated. Only marked platyspondyly (vertebral body height < disk height) which is typically seen in thanatophoric dysplasia can be diagnosed.
Pelvis
In the pelvis, the presence of the three ossification points (iliac, pubic and ischial bones) and the shape of the iliac bone (round, flat, lack of iliac flaring) can be important in certain dysplasias and dysostoses, such as limb-pelvic hypoplasia, femoral hypoplasia - unusual face syndrome and achondroplasia. Pelvic shape may be difficult to evaluate at routine US and 3D-US may be necessary[99].
Technical improvement in early diagnosis of skeletal dysplasias
In the absence of a previous history of skeletal dysplasia, fetal morphological examination by conventional 2D-US remains the screening test of choice[100]. As observed in different studies, most skeletal dysplasias are detected late in the second or third trimester of pregnancy[102]. Assessment of the fetus with 3D-US has been shown to improve diagnostic accuracy, since additional phenotypic features not detectable at 2D-US may be identified[107,108] (Table 3).
Table 3.
Comparison between two-dimensional ultrasound and three-dimensional ultrasound detection power of skeletal dysplasia markers
| Markers of skeletal dysplasia |
Power of detection |
|
| 2D-US | 3D-US | |
| Shortening of long bones | +++ | +++ |
| Increased thickness of femoral metaphysis | - | +++ |
| Bone fracture | ++ | +++ |
| Bowing of long bones | ++ | +++ |
| Decreased mineralization | - | ++ |
| Phalangeal hypoplasia | + | +++ |
| Point-calcified epiphysis | + | ++ |
| Macrocephaly | ++ | + |
| Frontal bossing | ++ | +++ |
| Facial dysmorphism | + | +++ |
| Narrow thorax | +++ | +++ |
| Increased intervertebral space | ++ | ++ |
| Deformation of the fetal pelvis | + | ++ |
+++: Height; ++: Medium; +: Low; -: Very low.
3D-US provides a global rather planar view of the anatomy by means of depth perception cues, rotation, surface-rendering techniques and multiplanar displays. In particular, multiplanar viewing capability allows to identify scapular anomalies, appreciate limb abnormalities, evaluate fetal facial profile and improve visualization of the spine[107]. 3D-US is superior in elucidating some of the features typical of each skeletal dysplasia. For example, brachydactyly, almost pathognomonic for achondroplasia, is under-appreciated by 2D-US, while 3D-US allows precise measurements of the phalanges, palms and feet and captures the trident configuration of the hands (Figure 7A); 3D-imaging also has the significant advantage of showing the relative disproportion of limb segment. Moreover, surface rendering of fetal facies allows the evaluation of the metopic prominence contour, bony structure, nasal contour and overall relationship of facial features. Facial dysmorphisms such as frontal bossing and mid-face hypoplasia are found by 3D-US in achondroplasia and thanatophoric dysplasia (Figure 7B). The Binder facies (depressed nasal bridge, mid-face hypoplasia, small nose with upturned alae) that is part of the spectrum of chondrodysplasia punctata is also visualized. The very rare case of laryngeal stippling found in some case of chondrodysplasia punctata is preferentially seen by 3D-imaging because of the ability to rotate the image 180°; multiple punctuate calcifications are visualized throughout the larynx[108]. The great advantage of 3D-US is the lower cost and the absence of fetal irradiation. However, this examination is more dependent on the amniotic fluid volume and fetal position[102].
Figure 7.
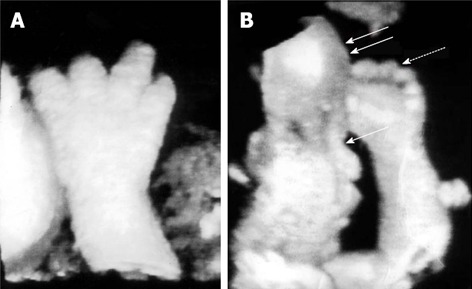
Skeletal anomalies detected by three-dimensional ultrasonography. A: Trident configuration of the digits and brachydactyly suggestive of achondroplasia; B: Facial dysmorphisms: frontal bossing (double arrows) and flattened mid-face (single arrow), disproportionate limb segments and brachydactyly (dotted arrow) typical of achondroplasia. Adapted from Krakow et al[108].
Sometimes US and genetic data are inconclusive to diagnose or exclude a suspected skeletal dysplasia[98]; then radiological findings are required in cases of a possible termination of pregnancy. For example, long bone deformities (curvature, fractures), cranial deformities (clover leaf skull) and spinal angulations (segmentation anomalies) are good indications for prenatal three-dimensional helical computer tomography (3D-HCT)[110]. Indeed, 3D-HCT can image the entire fetal skeleton, while 3D-US can image only limited and specific fetal parts[102] (Figure 8).
Figure 8.
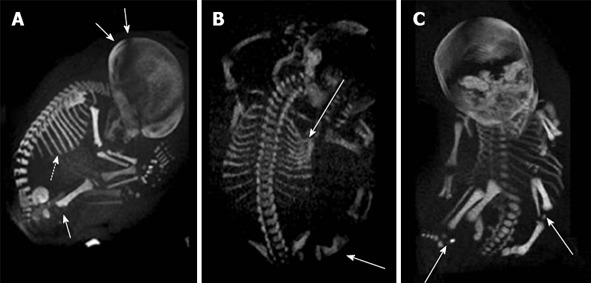
Prenatal diagnosis by three-dimensional helical computer tomography. A: Achondroplasia (sagittal view): macrocephaly (double arrows), short ribs (dotted arrow) and increased thickness of the femoral metaphysis (single arrow); B: Osteogenesis imperfecta (posterior view): fractures of ribs and femur (arrows); C: Chondrodysplasia punctata (frontal view): epiphyseal calcifications of long bones (arrows). Adapted from Ruano et al[102].
Nevertheless, this technique has important limitations since it requires ionizing radiation and additionally the image quality is dependent on bone mineralization (better after 30 wk gestation) and fetal immobility. CT is still often insufficient in the precise and complete visualization of the fetal extremities (hands and feet)[98].
CONCLUSION
Despite the advances in US technology, the diagnosis of multiple fetal structural defects and genetic syndromes currently still depends on the experience of physicians and sonographers. New diagnostic protocols are required to improve the accuracy of US detection of fetal abnormalities. In particular, an objective method based on quantitative parameters is of paramount importance to increase the use of US in prenatal diagnosis. In the last years, quantitative and objective sonographic markers highly predictive of specific fetal malformations have been studied and adopted in order to increase the sensitivity of sonography. US scan represents the most safe and non-invasive method for prenatal diagnosis. Then, innovative research approaches will need to allow the quantitative exploration of several new parameters and the identification of new sonographic markers for aneuploidy or other pathologies. In the future, highly innovative systems with respect to the state of the art of US-based prenatal diagnosis should be able to generate appropriate combinations of multiple sonographic markers with expected significant improvements in both sensitivity and specificity of the corresponding diagnoses. Moreover, these innovative methods should perform automatic comparisons among the values of the same parameter measured at different gestation ages, so automatically providing the temporal evolution of selected parameters, in order to easily check if fetal growth resembles an expected path and also if there are some “disproportions” between different anatomical regions. Therefore, research in prenatal diagnosis should introduce into clinical practice a number of new prenatal diagnostic parameters that must be quantitative, accurate and independent from operator experience. Early diagnosis of fetal malformations based on these new systems will be also useful for parental counseling and fetal treatment planning. In particular, main specific benefits will be related to optimized pregnancy management and childbirth timing, the possibility of performing simpler procedures for termination of pregnancy in those patients in whom findings are abnormal (reducing physical and psychological morbidity associated with late abortions), extension of malformations identifiable already in the first trimester of pregnancy and timely therapeutic treatment of objectively selected diseased fetuses.
Footnotes
Supported by FESR P.O. Apulia Region 2007-2013-Action 1.2.4 (grant number 3Q5AX31) and the National Council of Research Project AMOLAB
P- Reviewer Kavit AD S- Editor Ma YJ L- Editor Roemmele A E- Editor Yan JL
References
- 1.Kouamé N, N’goan-Domoua AM, Nikiéma Z, Konan AN, N’guessan KE, Sétchéou A, Tra-Bi ZO, N’gbesso RD, Kéita AK. Polyhydramnios: a warning sign in the prenatal ultrasound diagnosis of foetal malformation? Diagn Interv Imaging. 2013;94:433–437. doi: 10.1016/j.diii.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Casciaro S, Conversano F, Casciaro E, Soloperto G, Perrone E, Di Renzo GC, Perrone A. Automatic Evaluation of Progression Angle and Fetal Head Station Through Intrapartum Echographic Monitoring. Comput Math Methods Med. 2013:In press. doi: 10.1155/2013/278978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katorza E, Achiron R. Early pregnancy scanning for fetal anomalies--the new standard? Clin Obstet Gynecol. 2012;55:199–216. doi: 10.1097/GRF.0b013e3182446ae9. [DOI] [PubMed] [Google Scholar]
- 4.Fong KW, Toi A, Salem S, Hornberger LK, Chitayat D, Keating SJ, McAuliffe F, Johnson JA. Detection of fetal structural abnormalities with US during early pregnancy. Radiographics. 2004;24:157–174. doi: 10.1148/rg.241035027. [DOI] [PubMed] [Google Scholar]
- 5.Stoll C, Clementi M. Prenatal diagnosis of dysmorphic syndromes by routine fetal ultrasound examination across Europe. Ultrasound Obstet Gynecol. 2003;21:543–551. doi: 10.1002/uog.125. [DOI] [PubMed] [Google Scholar]
- 6.Agathokleous M, Chaveeva P, Poon LC, Kosinski P, Nicolaides KH. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstet Gynecol. 2013;41:247–261. doi: 10.1002/uog.12364. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaides KH. Screening for chromosomal defects. Ultrasound Obstet Gynecol. 2003;21:313–321. doi: 10.1002/uog.128. [DOI] [PubMed] [Google Scholar]
- 8.Wald NJ, Bestwick JP. Incorporating DNA sequencing into current prenatal screening practice for Down’s syndrome. PLoS One. 2013;8:e58732. doi: 10.1371/journal.pone.0058732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBrien A, Sands A, Craig B, Dornan J, Casey F. Impact of a regional training program in fetal echocardiography for sonographers on the antenatal detection of major congenital heart disease. Ultrasound Obstet Gynecol. 2010;36:279–284. doi: 10.1002/uog.7616. [DOI] [PubMed] [Google Scholar]
- 10.Grande M, Borrell A, Garcia-Posada R, Borobio V, Muñoz M, Creus M, Soler A, Sanchez A, Balasch J. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod. 2012;27:3109–3117. doi: 10.1093/humrep/des251. [DOI] [PubMed] [Google Scholar]
- 11.Boyd PA, Rounding C, Chamberlain P, Wellesley D, Kurinczuk JJ. The evolution of prenatal screening and diagnosis and its impact on an unselected population over an 18-year period. BJOG. 2012;119:1131–1140. doi: 10.1111/j.1471-0528.2012.03373.x. [DOI] [PubMed] [Google Scholar]
- 12.Raniga S, Desai PD, Parikh H. Ultrasonographic soft markers of aneuploidy in second trimester: are we lost? MedGenMed. 2006;8:9. [PMC free article] [PubMed] [Google Scholar]
- 13.Cerrillo Hinojosa M, Yerena de Vega MC, González Panzzi ME, Godoy H, Galicia J, Gutiérrez Nájar A. [Genetic amniocentesis in high-risk populations. Experience in 3081 cases] Ginecol Obstet Mex. 2009;77:173–182. [PubMed] [Google Scholar]
- 14.Daniel A, Athayde N, Ogle R, George AM, Michael J, Pertile MD, Bryan J, Jammu V, Trudinger BJ. Prospective ranking of the sonographic markers for aneuploidy: data of 2143 prenatal cytogenetic diagnoses referred for abnormalities on ultrasound. Aust N Z J Obstet Gynaecol. 2003;43:16–26. doi: 10.1046/j.0004-8666.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 15.Snijders RJ, Sebire NJ, Nicolaides KH. Assessment of risks. In: Snijders RJM, Nicolaides KH, editors. Ultrasound Markers for Fetal Chromosomal Defects. New York: Parthenon; 1996. pp. 62–120. [Google Scholar]
- 16.Snijders RJ, Sundberg K, Holzgreve W, Henry G, Nicolaides KH. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet Gynecol. 1999;13:167–170. doi: 10.1046/j.1469-0705.1999.13030167.x. [DOI] [PubMed] [Google Scholar]
- 17.Papp C, Bán Z, Szigeti Z, Csaba A, Lázár L, Nagy GR, Papp Z. Prenatal sonographic findings in 207 fetuses with trisomy 21. Eur J Obstet Gynecol Reprod Biol. 2007;133:186–190. doi: 10.1016/j.ejogrb.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Benacerraf BR. The history of the second-trimester sonographic markers for detecting fetal Down syndrome, and their current role in obstetric practice. Prenat Diagn. 2010;30:644–652. doi: 10.1002/pd.2531. [DOI] [PubMed] [Google Scholar]
- 19.Benacerraf BR, Barss VA, Laboda LA. A sonographic sign for the detection in the second trimester of the fetus with Down’s syndrome. Am J Obstet Gynecol. 1985;151:1078–1079. doi: 10.1016/0002-9378(85)90385-0. [DOI] [PubMed] [Google Scholar]
- 20.Benacerraf BR, Frigoletto FD. Soft tissue nuchal fold in the second-trimester fetus: standards for normal measurements compared with those in Down syndrome. Am J Obstet Gynecol. 1987;157:1146–1149. doi: 10.1016/s0002-9378(87)80279-x. [DOI] [PubMed] [Google Scholar]
- 21.Benacerraf BR, Cnann A, Gelman R, Laboda LA, Frigoletto FD. Can sonographers reliably identify anatomic features associated with Down syndrome in fetuses? Radiology. 1989;173:377–380. doi: 10.1148/radiology.173.2.2529580. [DOI] [PubMed] [Google Scholar]
- 22.Kadir RA, Economides DL. The effect of nuchal translucency measurement on second-trimester biochemical screening for Down’s syndrome. Ultrasound Obstet Gynecol. 1997;9:244–247. doi: 10.1046/j.1469-0705.1997.09040244.x. [DOI] [PubMed] [Google Scholar]
- 23.Hackshaw AK, Wald NJ. Assessment of the value of reporting partial screening results in prenatal screening for Down syndrome. Prenat Diagn. 2001;21:737–740. doi: 10.1002/pd.132. [DOI] [PubMed] [Google Scholar]
- 24.Nyberg DA, Souter VL, El-Bastawissi A, Young S, Luthhardt F, Luthy DA. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J Ultrasound Med. 2001;20:1053–1063. doi: 10.7863/jum.2001.20.10.1053. [DOI] [PubMed] [Google Scholar]
- 25.Benacerraf BR, Gelman R, Frigoletto FD. Sonographic identification of second-trimester fetuses with Down’s syndrome. N Engl J Med. 1987;317:1371–1376. doi: 10.1056/NEJM198711263172203. [DOI] [PubMed] [Google Scholar]
- 26.Lockwood C, Benacerraf B, Krinsky A, Blakemore K, Belanger K, Mahoney M, Hobbins J. A sonographic screening method for Down syndrome. Am J Obstet Gynecol. 1987;157:803–808. doi: 10.1016/s0002-9378(87)80059-5. [DOI] [PubMed] [Google Scholar]
- 27.FitzSimmons J, Droste S, Shepard TH, Pascoe-Mason J, Chinn A, Mack LA. Long-bone growth in fetuses with Down syndrome. Am J Obstet Gynecol. 1989;161:1174–1177. doi: 10.1016/0002-9378(89)90658-3. [DOI] [PubMed] [Google Scholar]
- 28.Benacerraf BR, Neuberg D, Frigoletto FD. Humeral shortening in second-trimester fetuses with Down syndrome. Obstet Gynecol. 1991;77:223–227. doi: 10.1097/00006250-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Nyberg DA, Luthy DA, Resta RG, Nyberg BC, Williams MA. Age-adjusted ultrasound risk assessment for fetal Down’s syndrome during the second trimester: description of the method and analysis of 142 cases. Ultrasound Obstet Gynecol. 1998;12:8–14. doi: 10.1046/j.1469-0705.1998.12010008.x. [DOI] [PubMed] [Google Scholar]
- 30.Bahado-Singh RO, Mendilcioglu I, Copel J. Ultrasound markers of fetal Down syndrome. JAMA. 2001;285:2857–2858. [PubMed] [Google Scholar]
- 31.Benacerraf BR, Mandell J, Estroff JA, Harlow BL, Frigoletto FD. Fetal pyelectasis: a possible association with Down syndrome. Obstet Gynecol. 1990;76:58–60. [PubMed] [Google Scholar]
- 32.Corteville JE, Dicke JM, Crane JP. Fetal pyelectasis and Down syndrome: is genetic amniocentesis warranted? Obstet Gynecol. 1992;79:770–772. [PubMed] [Google Scholar]
- 33.Nyberg DA, Resta RG, Luthy DA, Hickok DE, Mahony BS, Hirsch JH. Prenatal sonographic findings of Down syndrome: review of 94 cases. Obstet Gynecol. 1990;76:370–377. [PubMed] [Google Scholar]
- 34.Nyberg DA, Resta RG, Mahony BS, Dubinsky T, Luthy DA, Hickok DE, Luthardt FW. Fetal hyperechogenic bowel and Down’s syndrome. Ultrasound Obstet Gynecol. 1993;3:330–333. doi: 10.1046/j.1469-0705.1993.03050330.x. [DOI] [PubMed] [Google Scholar]
- 35.Dicke JM, Crane JP. Sonographically detected hyperechoic fetal bowel: significance and implications for pregnancy management. Obstet Gynecol. 1992;80:778–782. [PubMed] [Google Scholar]
- 36.Sepulveda W, Sebire NJ. Fetal echogenic bowel: a complex scenario. Ultrasound Obstet Gynecol. 2000;16:510–514. doi: 10.1046/j.1469-0705.2000.00322.x. [DOI] [PubMed] [Google Scholar]
- 37.Bromley B, Doubilet P, Frigoletto FD, Krauss C, Estroff JA, Benacerraf BR. Is fetal hyperechoic bowel on second-trimester sonogram an indication for amniocentesis? Obstet Gynecol. 1994;83:647–651. [PubMed] [Google Scholar]
- 38.Roberts DJ, Genest D. Cardiac histologic pathology characteristic of trisomies 13 and 21. Hum Pathol. 1992;23:1130–1140. doi: 10.1016/0046-8177(92)90031-w. [DOI] [PubMed] [Google Scholar]
- 39.Shanks AL, Odibo AO, Gray DL. Echogenic intracardiac foci: associated with increased risk for fetal trisomy 21 or not? J Ultrasound Med. 2009;28:1639–1643. doi: 10.7863/jum.2009.28.12.1639. [DOI] [PubMed] [Google Scholar]
- 40.Winn VD, Sonson J, Filly RA. Echogenic intracardiac focus: potential for misdiagnosis. J Ultrasound Med. 2003;22:1207–1214; quiz 1216-1217. doi: 10.7863/jum.2003.22.11.1207. [DOI] [PubMed] [Google Scholar]
- 41.Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides K. Absence of nasal bone in fetuses with trisomy 21 at 11-14 weeks of gestation: an observational study. Lancet. 2001;358:1665–1667. doi: 10.1016/S0140-6736(01)06709-5. [DOI] [PubMed] [Google Scholar]
- 42.Cicero S, Bindra R, Rembouskos G, Spencer K, Nicolaides KH. Integrated ultrasound and biochemical screening for trisomy 21 using fetal nuchal translucency, absent fetal nasal bone, free beta-hCG and PAPP-A at 11 to 14 weeks. Prenat Diagn. 2003;23:306–310. doi: 10.1002/pd.588. [DOI] [PubMed] [Google Scholar]
- 43.Bromley B, Lieberman E, Shipp TD, Benacerraf BR. Fetal nose bone length: a marker for Down syndrome in the second trimester. J Ultrasound Med. 2002;21:1387–1394. doi: 10.7863/jum.2002.21.12.1387. [DOI] [PubMed] [Google Scholar]
- 44.Suwanrath C, Pruksanusak N, Kor-Anantakul O, Suntharasaj T, Hanprasertpong T, Pranpanus S. Reliability of fetal nasal bone length measurement at 11-14 weeks of gestation. BMC Pregnancy Childbirth. 2013;13:7. doi: 10.1186/1471-2393-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belics Z, Fekete T, Beke A, Szabó I. Prenatal ultrasonographic measurement of the fetal iliac angle during the first and second trimester of pregnancy. Prenat Diagn. 2011;31:351–355. doi: 10.1002/pd.2690. [DOI] [PubMed] [Google Scholar]
- 46.Clur SA, Ottenkamp J, Bilardo CM. The nuchal translucency and the fetal heart: a literature review. Prenat Diagn. 2009;29:739–748. doi: 10.1002/pd.2281. [DOI] [PubMed] [Google Scholar]
- 47.Grace D, Eggers P, Glantz JC, Ozcan T. Mitral valve-tricuspid valve distance as a sonographic marker of trisomy 21. Ultrasound Obstet Gynecol. 2010;35:172–177. doi: 10.1002/uog.7538. [DOI] [PubMed] [Google Scholar]
- 48.Zalel Y, Achiron R, Yagel S, Kivilevitch Z. Fetal aberrant right subclavian artery in normal and Down syndrome fetuses. Ultrasound Obstet Gynecol. 2008;31:25–29. doi: 10.1002/uog.5230. [DOI] [PubMed] [Google Scholar]
- 49.Gupta JK, Cave M, Lilford RJ, Farrell TA, Irving HC, Mason G, Hau CM. Clinical significance of fetal choroid plexus cysts. Lancet. 1995;346:724–729. doi: 10.1016/s0140-6736(95)91502-8. [DOI] [PubMed] [Google Scholar]
- 50.Bromley B, Lieberman R, Benacerraf BR. Choroid plexus cysts: not associated with Down syndrome. Ultrasound Obstet Gynecol. 1996;8:232–235. doi: 10.1046/j.1469-0705.1996.08040232.x. [DOI] [PubMed] [Google Scholar]
- 51.Sacchini C, El-Sheikhah A, Cicero S, Rembouskos G, Nicolaides KH. Ear length in trisomy 21 fetuses at 11-14 weeks of gestation. Ultrasound Obstet Gynecol. 2003;22:460–463. doi: 10.1002/uog.903. [DOI] [PubMed] [Google Scholar]
- 52.Cereda A, Carey JC. The trisomy 18 syndrome. Orphanet J Rare Dis. 2012;7:81. doi: 10.1186/1750-1172-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamanaka M, Setoyama T, Igarashi Y, Kurosawa K, Itani Y, Hashimoto S, Saitoh K, Takei M, Hirabuki T. Pregnancy outcome of fetuses with trisomy 18 identified by prenatal sonography and chromosomal analysis in a perinatal center. Am J Med Genet A. 2006;140:1177–1182. doi: 10.1002/ajmg.a.31241. [DOI] [PubMed] [Google Scholar]
- 54.Sepulveda W, Wong AE, Dezerega V. First-trimester sonographic findings in trisomy 18: a review of 53 cases. Prenat Diagn. 2010;30:256–259. doi: 10.1002/pd.2462. [DOI] [PubMed] [Google Scholar]
- 55.Cho RC, Chu P, Smith-Bindman R. Second trimester prenatal ultrasound for the detection of pregnancies at increased risk of Trisomy 18 based on serum screening. Prenat Diagn. 2009;29:129–139. doi: 10.1002/pd.2166. [DOI] [PubMed] [Google Scholar]
- 56.Hill LM. The sonographic detection of trisomies 13, 18, and 21. Clin Obstet Gynecol. 1996;39:831–850. doi: 10.1097/00003081-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Sherod C, Sebire NJ, Soares W, Snijders RJ, Nicolaides KH. Prenatal diagnosis of trisomy 18 at the 10-14-week ultrasound scan. Ultrasound Obstet Gynecol. 1997;10:387–390. doi: 10.1046/j.1469-0705.1997.10060387.x. [DOI] [PubMed] [Google Scholar]
- 58.Geipel A, Willruth A, Vieten J, Gembruch U, Berg C. Nuchal fold thickness, nasal bone absence or hypoplasia, ductus venosus reversed flow and tricuspid valve regurgitation in screening for trisomies 21, 18 and 13 in the early second trimester. Ultrasound Obstet Gynecol. 2010;35:535–539. doi: 10.1002/uog.7597. [DOI] [PubMed] [Google Scholar]
- 59.Perni SC, Predanic M, Kalish RB, Chervenak FA, Chasen ST. Clinical use of first-trimester aneuploidy screening in a United States population can replicate data from clinical trials. Am J Obstet Gynecol. 2006;194:127–130. doi: 10.1016/j.ajog.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 60.Breathnach FM, Malone FD, Lambert-Messerlian G, Cuckle HS, Porter TF, Nyberg DA, Comstock CH, Saade GR, Berkowitz RL, Klugman S, et al. First- and second-trimester screening: detection of aneuploidies other than Down syndrome. Obstet Gynecol. 2007;110:651–657. doi: 10.1097/01.AOG.0000278570.76392.a6. [DOI] [PubMed] [Google Scholar]
- 61.Viora E, Zamboni C, Mortara G, Stillavato S, Bastonero S, Errante G, Sciarrone A, Campogrande M. Trisomy 18: Fetal ultrasound findings at different gestational ages. Am J Med Genet A. 2007;143:553–557. doi: 10.1002/ajmg.a.31615. [DOI] [PubMed] [Google Scholar]
- 62.Ettema AM, Wenghoefer M, Hansmann M, Carels CE, Borstlap WA, Bergé SJ. Prenatal diagnosis of craniomaxillofacial malformations: a characterization of phenotypes in trisomies 13, 18, and 21 by ultrasound and pathology. Cleft Palate Craniofac J. 2010;47:189–196. doi: 10.1597/08-285_1. [DOI] [PubMed] [Google Scholar]
- 63.Snijders RJ, Sebire NJ, Nayar R, Souka A, Nicolaides KH. Increased nuchal translucency in trisomy 13 fetuses at 10-14 weeks of gestation. Am J Med Genet. 1999;86:205–207. [PubMed] [Google Scholar]
- 64.Tul N, Spencer K, Noble P, Chan C, Nicolaides K. Screening for trisomy 18 by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn. 1999;19:1035–1042. doi: 10.1002/(sici)1097-0223(199911)19:11<1035::aid-pd694>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 65.Spencer K, Ong C, Skentou H, Liao AW, H Nicolaides K. Screening for trisomy 13 by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn. 2000;20:411–416. [PubMed] [Google Scholar]
- 66.Snijders RJ, Sebire NJ, Nicolaides KH. Maternal age and gestational age-specific risk for chromosomal defects. Fetal Diagn Ther. 1995;10:356–367. doi: 10.1159/000264259. [DOI] [PubMed] [Google Scholar]
- 67.Nadel A, Bromley B, Benacerraf BR. Nuchal thickening or cystic hygromas in first- and early second-trimester fetuses: prognosis and outcome. Obstet Gynecol. 1993;82:43–48. [PubMed] [Google Scholar]
- 68.Bronshtein M, Zimmer EZ, Blazer S. A characteristic cluster of fetal sonographic markers that are predictive of fetal Turner syndrome in early pregnancy. Am J Obstet Gynecol. 2003;188:1016–1020. doi: 10.1067/mob.2003.230. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 70.Lapierre C, Rypens F, Grignon A, Dubois J, Déry J, Garel L. Prenatal ultrasound screening of congenital heart disease in the general population: general concepts, guidelines, differential diagnoses. Ultrasound Q. 2013;29:111–124. doi: 10.1097/RUQ.0b013e3182915867. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Riehle-Colarusso T, Correa A, Li S, Feng X, Gindler J, Lin H, Webb C, Li W, Trines J, et al. Observed prevalence of congenital heart defects from a surveillance study in China. J Ultrasound Med. 2011;30:989–995. doi: 10.7863/jum.2011.30.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cha S, Kim GB, Kwon BS, Bae EJ, Noh CI, Lim HG, Kim WH, Lee JR, Kim YJ, Choi JY. Recent trends in indications of fetal echocardiography and postnatal outcomes in fetuses diagnosed as congenital heart disease. Korean Circ J. 2012;42:839–844. doi: 10.4070/kcj.2012.42.12.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grandjean H, Larroque D, Levi S. The performance of routine ultrasonographic screening of pregnancies in the Eurofetus Study. Am J Obstet Gynecol. 1999;181:446–454. doi: 10.1016/s0002-9378(99)70577-6. [DOI] [PubMed] [Google Scholar]
- 74.Cook AC, Yates RW, Anderson RH. Normal and abnormal fetal cardiac anatomy. Prenat Diagn. 2004;24:1032–1048. doi: 10.1002/pd.1061. [DOI] [PubMed] [Google Scholar]
- 75.Cook AC. The spectrum of fetal cardiac malformations. Cardiol Young. 2001;11:97–110. doi: 10.1017/s1047951100012518. [DOI] [PubMed] [Google Scholar]
- 76.Knauth A, McCarthy KP, Webb S, Ho SY, Allwork SP, Cook AC, Anderson RH. Interatrial communication through the mouth of the coronary sinus. Cardiol Young. 2002;12:364–372. doi: 10.1017/s104795110001297x. [DOI] [PubMed] [Google Scholar]
- 77.Freedom RM, Benson LN. Anomalies of systemic venous connections, persistence of the right venous valve and silent cardiovascular causes of cyanosis. In: Freedom RM, Benson LN, Smallhorn JF, editors. Neonatal Heart Disease. Springer-Verlag: New York; 1992. p. 486. [Google Scholar]
- 78.Anderson RH, Cook AC. Morphology of the functionally univentricular heart. Cardiol Young. 2004;14 Suppl 1:3–12. doi: 10.1017/s1047951104006237. [DOI] [PubMed] [Google Scholar]
- 79.Anderson RH, Ho SY, Falcao S, Daliento L, Rigby ML. The diagnostic features of atrioventricular septal defect with common atrioventricular junction. Cardiol Young. 1998;8:33–49. doi: 10.1017/s1047951100004613. [DOI] [PubMed] [Google Scholar]
- 80.Hornberger LK. Mitral valve abnormalities in the fetus. In: Allan LD, Sharland G, Hornberger L, editors. Textbook of Fetal Cardiology. London: Greenwich Media Publishing; 2000. pp. 151–152. [Google Scholar]
- 81.Allan LD, Sharland GK, Milburn A, Lockhart SM, Groves AM, Anderson RH, Cook AC, Fagg NL. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23:1452–1458. doi: 10.1016/0735-1097(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 82.Huggon IC, Ghi T, Cook AC, Zosmer N, Allan LD, Nicolaides KH. Fetal cardiac abnormalities identified prior to 14 weeks’ gestation. Ultrasound Obstet Gynecol. 2002;20:22–29. doi: 10.1046/j.1469-0705.2002.00733.x. [DOI] [PubMed] [Google Scholar]
- 83.Berger A. What is fetal nuchal translucency? BMJ. 1999;318:85. doi: 10.1136/bmj.318.7176.81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faiola S, Tsoi E, Huggon IC, Allan LD, Nicolaides KH. Likelihood ratio for trisomy 21 in fetuses with tricuspid regurgitation at the 11 to 13 + 6-week scan. Ultrasound Obstet Gynecol. 2005;26:22–27. doi: 10.1002/uog.1922. [DOI] [PubMed] [Google Scholar]
- 85.Matias A, Montenegro N, Areias JC, Brandão O. Anomalous fetal venous return associated with major chromosomopathies in the late first trimester of pregnancy. Ultrasound Obstet Gynecol. 1998;11:209–213. doi: 10.1046/j.1469-0705.1998.11030209.x. [DOI] [PubMed] [Google Scholar]
- 86.Roguin N, Du ZD, Barak M, Nasser N, Hershkowitz S, Milgram E. High prevalence of muscular ventricular septal defect in neonates. J Am Coll Cardiol. 1995;26:1545–1548. doi: 10.1016/0735-1097(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 87.Sands AJ, Casey FA, Craig BG, Dornan JC, Rogers J, Mulholland HC. Incidence and risk factors for ventricular septal defect in “low risk” neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81:F61–F63. doi: 10.1136/fn.81.1.f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hiraishi S, Agata Y, Nowatari M, Oguchi K, Misawa H, Hirota H, Fujino N, Horiguchi Y, Yashiro K, Nakae S. Incidence and natural course of trabecular ventricular septal defect: two-dimensional echocardiography and color Doppler flow imaging study. J Pediatr. 1992;120:409–415. doi: 10.1016/s0022-3476(05)80906-0. [DOI] [PubMed] [Google Scholar]
- 89.Du ZD, Roguin N, Wu XJ. Spontaneous closure of muscular ventricular septal defect identified by echocardiography in neonates. Cardiol Young. 1998;8:500–505. doi: 10.1017/s1047951100007174. [DOI] [PubMed] [Google Scholar]
- 90.Dhuper S, Ehlers KH, Fatica NS, Myridakis DJ, Klein AA, Friedman DM, Levine DB. Incidence and risk factors for mitral valve prolapse in severe adolescent idiopathic scoliosis. Pediatr Cardiol. 1997;18:425–428. doi: 10.1007/s002469900220. [DOI] [PubMed] [Google Scholar]
- 91.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 92.Berg C, Geipel A, Kamil D, Knüppel M, Breuer J, Krapp M, Baschat A, Germer U, Hansmann M, Gembruch U. The syndrome of left isomerism: sonographic findings and outcome in prenatally diagnosed cases. J Ultrasound Med. 2005;24:921–931. doi: 10.7863/jum.2005.24.7.921. [DOI] [PubMed] [Google Scholar]
- 93.Berkley EM, Goens MB, Karr S, Rappaport V. Utility of fetal echocardiography in postnatal management of infants with prenatally diagnosed congenital heart disease. Prenat Diagn. 2009;29:654–658. doi: 10.1002/pd.2260. [DOI] [PubMed] [Google Scholar]
- 94.Plesinac S, Terzic M, Stimec B, Plecas D. Value of fetal echocardiography in diagnosis of congenital heart disease in a Serbian university hospital. Int J Fertil Womens Med. 2007;52:89–92. [PubMed] [Google Scholar]
- 95.Meyer-Wittkopf M, Cooper S, Sholler G. Correlation between fetal cardiac diagnosis by obstetric and pediatric cardiologist sonographers and comparison with postnatal findings. Ultrasound Obstet Gynecol. 2001;17:392–397. doi: 10.1046/j.1469-0705.2001.00381.x. [DOI] [PubMed] [Google Scholar]
- 96.Friedman AH, Kleinman CS, Copel JA. Diagnosis of cardiac defects: where we’ve been, where we are and where we’re going. Prenat Diagn. 2002;22:280–284. doi: 10.1002/pd.305. [DOI] [PubMed] [Google Scholar]
- 97.Sklansky MS, Nelson T, Strachan M, Pretorius D. Real-time three-dimensional fetal echocardiography: initial feasibility study. J Ultrasound Med. 1999;18:745–752. doi: 10.7863/jum.1999.18.11.745. [DOI] [PubMed] [Google Scholar]
- 98.Cassart M. Suspected fetal skeletal malformations or bone diseases: how to explore. Pediatr Radiol. 2010;40:1046–1051. doi: 10.1007/s00247-010-1598-6. [DOI] [PubMed] [Google Scholar]
- 99.Dighe M, Fligner C, Cheng E, Warren B, Dubinsky T. Fetal skeletal dysplasia: an approach to diagnosis with illustrative cases. Radiographics. 2008;28:1061–1077. doi: 10.1148/rg.284075122. [DOI] [PubMed] [Google Scholar]
- 100.Dugoff L, Thieme G, Hobbins JC. Skeletal anomalies. Clin Perinatol. 2000;27:979–1005. doi: 10.1016/s0095-5108(05)70060-9. [DOI] [PubMed] [Google Scholar]
- 101.Azouz EM, Teebi AS, Eydoux P, Chen MF, Fassier F. Bone dysplasias: an introduction. Can Assoc Radiol J. 1998;49:105–109. [PubMed] [Google Scholar]
- 102.Ruano R, Molho M, Roume J, Ville Y. Prenatal diagnosis of fetal skeletal dysplasias by combining two-dimensional and three-dimensional ultrasound and intrauterine three-dimensional helical computer tomography. Ultrasound Obstet Gynecol. 2004;24:134–140. doi: 10.1002/uog.1113. [DOI] [PubMed] [Google Scholar]
- 103.Schramm T, Gloning KP, Minderer S, Daumer-Haas C, Hörtnagel K, Nerlich A, Tutschek B. Prenatal sonographic diagnosis of skeletal dysplasias. Ultrasound Obstet Gynecol. 2009;34:160–170. doi: 10.1002/uog.6359. [DOI] [PubMed] [Google Scholar]
- 104.Nimrod C, Davies D, Iwanicki S, Harder J, Persaud D, Nicholson S. Ultrasound prediction of pulmonary hypoplasia. Obstet Gynecol. 1986;68:495–498. [PubMed] [Google Scholar]
- 105.Jones KL. Cleidocranial dysostosis. 6th ed. Smith’s recognizable patterns of human malformation. Philadelphia, Pa: Saunders; 2006. p. 462–465. [Google Scholar]
- 106.Mortier GR, Rimoin DL, Lachman RS. The scapula as a window to the diagnosis of skeletal dysplasias. Pediatr Radiol. 1997;27:447–451. doi: 10.1007/s002470050166. [DOI] [PubMed] [Google Scholar]
- 107.Garjian KV, Pretorius DH, Budorick NE, Cantrell CJ, Johnson DD, Nelson TR. Fetal skeletal dysplasia: three-dimensional US--initial experience. Radiology. 2000;214:717–723. doi: 10.1148/radiology.214.3.r00mr23717. [DOI] [PubMed] [Google Scholar]
- 108.Krakow D, Williams J, Poehl M, Rimoin DL, Platt LD. Use of three-dimensional ultrasound imaging in the diagnosis of prenatal-onset skeletal dysplasias. Ultrasound Obstet Gynecol. 2003;21:467–472. doi: 10.1002/uog.111. [DOI] [PubMed] [Google Scholar]
- 109.Roelfsema NM, Hop WC, van Adrichem LN, Wladimiroff JW. Craniofacial variability index determined by three-dimensional ultrasound in isolated vs. syndromal fetal cleft lip/palate. Ultrasound Obstet Gynecol. 2007;29:265–270. doi: 10.1002/uog.3921. [DOI] [PubMed] [Google Scholar]
- 110.Tsutsumi S, Sawai H, Nishimura G, Hayasaka K, Kurachi H. Prenatal diagnosis of thanatophoric dysplasia by 3-D helical computed tomography and genetic analysis. Fetal Diagn Ther. 2008;24:420–424. doi: 10.1159/000170092. [DOI] [PubMed] [Google Scholar]
- 111. Available from: http: //www.medicinamaternofetale.it/medicina-fetale/screening-per-la-sindrome-di-down/amniocentesi-villocentesi.
- 112. Available from: http: //www.vividown.org/sindro-me-di-down/
- 113. Available from: http: //it.wikipedia.org/wiki/Translucenza_nucale. Creative Commons.
- 114. Available from: http: //neonatologyforparents.org/parents/italian/page5/page5.html.
- 115.The International Society of Ultrasound in Obstetrics and Gynecology. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41:348–359. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]


