Abstract
Elevation of liver biochemistry has been reported with anti-tumor necrosis factor agents, but overt liver failure rarely reported. Autoimmune hepatitis has been more commonly reported with infliximab than adalimumab (ADA). Our case, however, describes the first reported case of ADA-associated severe cholestatic injury. A 39-year-old female with Crohn’s disease developed severe jaundice after initiation of ADA. All serologic tests and imaging studies were normal. Liver biopsy showed prominent pericentral canalicular cholestasis, without features of steatosis or sclerosing cholangitis, consistent with drug-induced cholestasis. The serum total bilirubin peaked at 280 μmol/L, and improvement was seen after 5 wk with eventual normalization of liver enzymes at 10 wk. Our case describes the first reported case of ADA-associated severe cholestatic liver disease and the first histopathologic examination of this adverse drug effect. Clinicians need to be aware of this potential drug-induced liver injury when prescribing this commonly used biologic medication.
Keywords: Crohn’s disease, Cholestasis, Adalimumab, Anti-tumor necrosis factor agents, Drug-induced liver injury
Core tip: Anti-tumor necrosis factor agents are commonly used in the treatment of inflammatory bowel disease and other inflammatory conditions. Drug-induced injury to the liver induced by the agents includes autoimmune hepatitis, direct hepatocellular necrosis and cholestasis. Our patient, a 39-year-old female with Crohn’s disease, developed severe jaundice after initiation of adalimumab. We present the first report of adalimumab-associated severe cholestatic injury and the first histopathologic examination of this adverse drug effect. Clinicians need to be aware of this potential severe drug-induced liver injury when prescribing this commonly used biologic medication.
INTRODUCTION
This case represents the first reported case of adalimumab-induced severe cholestatic liver disease, occurring in a patient treated for fistulizing Crohn’s disease (CD). All other causes for liver injury were ruled out with extensive investigations, with supporting evidence from a liver biopsy. Liver enzymes abnormalities normalized within 10 wk after drug cessation, with no recurrence. Anti-tumor necrosis factor (TNF) agents have been reported to cause a variety of liver-related abnormalities, but our case describes the first report of adalimumab (ADA)-associated severe cholestatic injury and the first histopathologic examination of this adverse drug effect. Clinicians need to be aware of this potential severe drug-induced liver injury when prescribing this commonly used biologic medication.
CASE REPORT
A 39-year-old female of East Indian descent with a history of CD, was treated with azathioprine (2 mg/kg) due to persistence of draining perianal fistulas and recurrent abscesses. Azathioprine led to marked improvement in her perianal fistulizing disease, without medication side effects or elevation of liver enzymes for over three years. Adalimumab (Humira, Abbott Laboratories Canada, St. Laurent, QC) was started, due to worsening perianal disease. She developed hives and arthralgias after with each injection of adalimumab, treated with antihistamines and Tylenol. Routine bloodwork [including liver biochemistry, international normalized ratio (INR)] performed monthly was consistently normal.
Seven months after commencing adalimumab, she developed worsening fatigue and arthralgias, along with jaundice, dark urine, pruritis and acholic stools. On presentation to hospital, she was found to be deeply jaundiced, without stigmata of chronic liver disease, encephalopathy or ascites. Initial bloodwork was as follows; total bilirubin 167 (normal < 22 μmol/L), direct bilirubin 129, alanine aminotransferase (ALT) 15, aspartate aminotransferase (AST) 16, gamma glutamyl aminotransferase 121, alkaline phosphatase 183, INR 1.8, platelets 276. Subsequent laboratory tests were obtained, with a normal alpha-1-antitrypsin level (2.31), normal ceruloplasmin (312), negative autoimmune serology (anti-mitochondrial antibody (AMA) negative, antinuclear antibody (ANA) negative, anti-smooth-muscle antibody (ASMA) negative, anti-tTG negative, IgA (0.47). Iron profile was normal, with negative serologies for hepatitis B and C.
Concomitant medications included azathioprine 200 mg/d (2 mg/kg), adalimumab 40 mg every 2 wk, hydromorphone contin, gabapentin, spironolactone, levothyroxine, and insulin. Adalimumab was discontinued on admission, with continuation of her other medications, except azathioprine. Further investigations including abdominal ultrasound with doppler and Magnetic resonance cholangiopancreatography did not show any abnormalities. endoscopic retrograde cholangiopancreatography showed no stones, strictures, beading or other features of primary sclerosing cholangitis. A liver biopsy showed marked cannalicular cholestasis, [predominantly in a pericentral (zone 2 and 3) distribution], associated with numerous large intracannicular bile thrombi throughout the parenchyma. This was accompanied by foamy degeneration, hepatocyte rosettes and patchy cytoplasmic condensation and eosinophilia. Large numbers of ceroid laden macrophages were identified on PASD stain in keeping with increased hepatocyte turnover. Only rare foci of lobular inflammation were present and no significant portal tract expansion or edema were identified. Furthermore, there was only minimal portal inflammation, composed predominantly of lymphocytes with very occasional eosinophils; plasma cells were not present, nor was there any evidence of interface hepatitis. Mild ductular reaction with occasional ductules containing intraluminal bile was present. Importantly, features of bile duct injury or loss were not present. There was no significant steatosis (< 2% of hepatocytes contain lipid droplets) and the connective tissue stains show mild patchy periportal fibrosis only, without any evidence of fibrous septa. Stains for iron did not demonstrate an increase in stainable iron and no alpha-1-antitrypsin like globules were detected on PASD stain (Figure 1).
Figure 1.
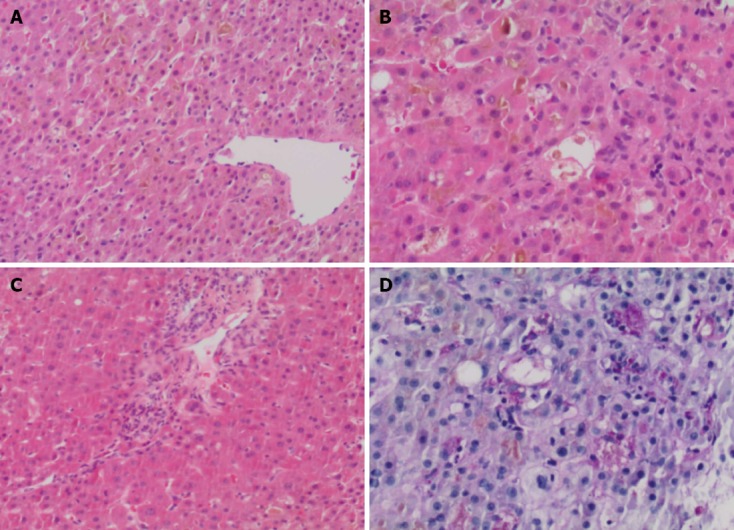
Histopathological changes. A: The liver biopsy showed marked cannalicular cholestasis, predominantly in a pericentral (zone 2 and 3) distribution; B: Numerous large intracannicular bile thrombi were present throughout the hepatic parenchyma, associated with early foamy degeneration, hepatocyte rosettes and patchy cytoplasmic condensation and eosinophilia. Lobular inflammation, as is the norm in cholestatic hepatitis, were minimal within the lobule; C: The portal tracts showed only minimal inflammation, without any evidence of bile duct injury or loss and there was no interface hepatitis present; D: Large numbers of ceroid laden macrophages were identified on PASD stain in keeping with increased hepatocyte turnover. Magnification: (A, C, D) x 40; (B) x 200.
In summary, the biopsy shows marked cholestasis without significant inflammation. From a histological perspective the possible causes included predominantly drugs/medications, total parenteral nutrition, sepsis and benign familial forms of cholestasis, the latter ones were considered to be unlikely in this clinical setting. There were no features to suggest large duct obstruction and recent radiology did not show any dilatation of the biliary tree. As such, it was felt that the biopsy findings represented the histological changes of a drug induced liver injury.
After initial worsening of her jaundice and INR, spontaneous improvement was seen after 5 wk, and eventual normalization of liver enzymes at 10 wk. The patient remains on azathioprine and currently has normal liver biochemistry, over 6 mo after her initial presentation with jaundice.
DISCUSSION
Elevation of liver enzymes has been reported during treatment with anti-TNF antibodies such as infliximab (IFX), but overt liver failure has been rarely reported[1]. However, cases of acute liver injury requiring liver transplantation (LT) have been reported in a review of post-marketing data and one of these cases occurred in a CD patient. Anti-TNF agents can lead to acute liver injury by at least three mechanisms: precipitation of de novo autoimmune hepatitis, cholestasis and direct hepatocellular necrosis[2]. The potential hepatotoxicity of IFX was addressed in a recent consensus statement[3]: IFX therapy may be considered in patients with clinically significant liver disease, but should be avoided in patients with aminotransferases (i.e., serum ALT and AST) over three times normal and that liver biochemistry should be obtained prior to IFX initiation. Autoimmune hepatitis due to anti-TNF agents for inflammatory bowel disease has more commonly been reported with infliximab, and the majority of patients responded to drug cessation and steroid treatment. Normalization of liver enzymes was typically rapid, occurring within 2 mo after infliximab discontinuation[4].
Cholestatic liver disease due to IFX has also been described, albeit much more seldom. One case report described a female patient with rheumatoid arthritis who developed severe cholestatic liver disease with hepatic failure necessitating LT[5]. Cholestatic liver injury has occurred after treatment with IFX for inflammatory bowel disease, with spontaneous resolution 6 wk after drug discontinuation[6]. Other cases of infliximab-related autoimmune hepatitis were subsequently treated with an alternate anti-TNF agent (etanercept, adalimumab), and was well tolerated[7].
The medical literature concerning the hepatotoxicity of ADA, however, is sparse and, unlike IFX, there are very few reports. Our case assumes importance as drug induced liver injury can be specific to the molecular structure of the drug in question. In terms of previously reported ADA liver injury, during controlled studies for rheumatoid arthritis, 1%-4% of ADA-treated patients developed liver enzyme elevation greater than twice the upper limit of normal. However, this was similar to the percentage of liver enzyme abnormalities found in placebo-treated patients. In clinical trials of patients with psoriatic arthritis and ankylosing spondylitis, elevation of ALT and AST in the range of 1.5-3 times the upper limit of normal was more common in patients receiving adalimumab than in controls, both when adalimumab was given as monotherapy and when it was used in combination with other immunosuppressive agents. ADA has been also been associated with development of autoimmune hepatitis in several case reports; one patient with rheumatoid arthritis was successfully switched to abatacept[8]. A case of subacute liver failure during therapy with adalimumab for psoriatic arthritis has been reported[9]. Another patient who had developed acute toxic hepatitis with necrosis due to infliximab for CD was successfully treated with adalimumab with no recurrence of liver enzyme abnormalities. We are unaware of any previous reports of cholestatic liver injury in association with ADA and similarly, the liver histopathology of ADA-associated cholestatic liver injury has not previously been reported.
Hepatotoxicity is a known side effect of azathioprine, occurring at a rate of hepatic abnormalities of 3%-10% after longterm treatment. Hepatic abnormalities are usually limited to abnormal liver function tests and minor change seen on liver biopsy specimens[10]. Azathioprine-induced hepatotoxicity can be grouped into three syndromes: hypersensitivity, idiosyncratic cholestatic reaction, and endothelial cell injury (with resultant raised portal pressures, veno-occlusive disease, or peliosis hepatis). Azathioprine has been associated with a wide variety of hepatic complications, including hepatocellular injury, veno-occlusive disease, peliosis hepatitis, hepatoportal sclerosis, and nodular regenerative hyperplasia[10]. In addition, bland cholestasis and cholestatic hepatitis with bile duct injury have been rarely reported in case report form. Even fewer cases of severe cholestatic hepatitis have been reported in the literature, developing in patients with inflammatory bowel disease, lupus or rheumatoid arthritis[11]. The latency from the initial exposure to azathioprine to the onset of jaundice ranges from 2 wk to 3 mo in previous reports.
Our patient had been maintained on azathioprine for 3 years until the development of jaundice, without previous elevations in liver enzymes, but developed jaundice within 7 mo after the initiation of adalimumab. Of importance, she is currently maintained on azathioprine (2 mg/kg) without any abnormalities in liver biochemistry which would almost certainly exclude azathioprine as the cause of her severe cholestasis. Our case describes the first reported case of ADA-associated severe cholestatic injury and the first histopathologic examination of this adverse drug effect. Clinicians need to be aware of this potential severe drug-induced liver injury when prescribing this commonly used biologic medication.
Footnotes
P- Reviewers Diamantis I, Tziomalos K S- Editor Zhai HH L- Editor A E- Editor Yan JL
References
- 1.Sokolove J, Strand V, Greenberg JD, Curtis JR, Kavanaugh A, Kremer JM, Anofrei A, Reed G, Calabrese L, Hooper M, et al. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1612–1617. doi: 10.1136/ard.2009.112136. [DOI] [PubMed] [Google Scholar]
- 2.Grasland A, Sterpu R, Boussoukaya S, Mahe I. Autoimmune hepatitis induced by adalimumab with successful switch to abatacept. Eur J Clin Pharmacol. 2012;68:895–898. doi: 10.1007/s00228-011-1191-4. [DOI] [PubMed] [Google Scholar]
- 3.Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P, Kaser A, Dejaco C, Petritsch W, Kapitan M, Maier H, et al. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4:221–256. doi: 10.1016/j.crohns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Doyle A, Forbes G, Kontorinis N. Autoimmune hepatitis during infliximab therapy for Crohn’s disease: a case report. J Crohns Colitis. 2011;5:253–255. doi: 10.1016/j.crohns.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Tobon GJ, Cañas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26:578–581. doi: 10.1007/s10067-005-0169-y. [DOI] [PubMed] [Google Scholar]
- 6.Menghini VV, Arora AS. Infliximab-associated reversible cholestatic liver disease. Mayo Clin Proc. 2001;76:84–86. doi: 10.4065/76.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Goldfeld DA, Verna EC, Lefkowitch J, Swaminath A. Infliximab-induced autoimmune hepatitis with successful switch to adalimumab in a patient with Crohn’s disease: the index case. Dig Dis Sci. 2011;56:3386–3388. doi: 10.1007/s10620-011-1748-1. [DOI] [PubMed] [Google Scholar]
- 8.Adar T, Mizrahi M, Pappo O, Scheiman-Elazary A, Shibolet O. Adalimumab-induced autoimmune hepatitis. J Clin Gastroenterol. 2010;44:e20–e22. doi: 10.1097/MCG.0b013e3181a745e7. [DOI] [PubMed] [Google Scholar]
- 9.Hagel S, Bruns T, Theis B, Herrmann A, Stallmach A. Subacute liver failure induced by adalimumab. Int J Clin Pharmacol Ther. 2011;49:38–40. doi: 10.5414/cpp49038. [DOI] [PubMed] [Google Scholar]
- 10.Mion F, Napoleon B, Berger F, Chevallier M, Bonvoisin S, Descos L. Azathioprine induced liver disease: nodular regenerative hyperplasia of the liver and perivenous fibrosis in a patient treated for multiple sclerosis. Gut. 1991;32:715–717. doi: 10.1136/gut.32.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romagnuolo J, Sadowski DC, Lalor E, Jewell L, Thomson AB. Cholestatic hepatocellular injury with azathioprine: a case report and review of the mechanisms of hepatotoxicity. Can J Gastroenterol. 1998;12:479–483. doi: 10.1155/1998/294752. [DOI] [PubMed] [Google Scholar]


