Abstract
Pulmonary abnormalities, dysfunction or hyper-reactivity occurs in association with inflammatory bowel disease (IBD) more frequently than previously recognized. Emerging evidence suggests that subtle inflammation exists in the airways among IBD patients even in the absence of any bronchopulmonary symptoms, and with normal pulmonary functions. The pulmonary impairment is more pronounced in IBD patients with active disease than in those in remission. A growing number of case reports show that the IBD patients develop rapidly progressive respiratory symptoms after colectomy, with failure to isolate bacterial pathogens on repeated sputum culture, and often request oral corticosteroid therapy. All the above evidence indicates that the inflammatory changes in both the intestine and lung during IBD. Clinical or subclinical pulmonary inflammation accompanies the main inflammation of the bowel. Although there are clinical and epidemiological reports of chronic inflammation of the pulmonary and intestinal mucosa in IBD, the detailed mechanisms of pulmonary-intestinal crosstalk remain unknown. The lung has no anatomical connection with the main inflammatory site of the bowel. Why does the inflammatory process shift from the gastrointestinal tract to the airways? The clinical and subclinical pulmonary abnormalities, dysfunction, or hyper-reactivity among IBD patients need further evaluation. Here, we give an overview of the concordance between chronic inflammatory reactions in the airways and the gastrointestinal tract. A better understanding of the possible mechanism of the crosstalk among the distant organs will be beneficial in identifying therapeutic strategies for mucosal inflammatory diseases such as IBD and allergy.
Keywords: Inflammatory bowel disease, Pulmonary symptoms, Gut-lung crosstalk, Biao-Li relationship, Social manner
Core tip: According to traditional Chinese medicine, the lung and the intestine are a pair of related organ systems (Biao-Li). The lung has no anatomical connection with the main inflammatory site of the bowel. Why does the inflammatory process shift from the gastrointestinal tract to the airways? We hypothesize that each individual cell or molecule not only plays its local role in its own organ, but also plays a “social” role to contribute distal communication through the epithelia. Inflammatory bowel disease may be a good example to study crosstalk between the gut and the lungs.
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major forms of chronic relapsing and remitting inflammatory bowel diseases (IBDs)[1-3]. The incidence of UC and CD is increasing[4]. IBDs have become a major gastroenterological problem in developed countries[4-10], and there has been an alarming rise in the traditional low-incidence areas, such as East Asia[11,12], the Indian subcontinent[13], the Middle East[14], Latin America[15], and Eastern Europe[16,17]. Although the gastrointestinal tract is the main affected site, both UC and CD are systemic inflammatory disorders that often involve organs other than those of the gastrointestinal tract[18-23]. Systemic manifestations can present years after the onset of bowel disease and can affect almost all organs[18,19], including the musculoskeletal[18,24-26], mucocutaneous[18,19,27,28], hepatobiliary[18,29,30], cardiovascular[18,31-33], ocular[18,34,35], renal and genitourinary[18,36-38], pancreatic[18,29,39-42], nervous[18,43-45] and bronchopulmonary[18,19,46-48] Systems. The extraintestinal manifestations tend to follow the clinical course of IBD and have a high impact on quality of life, morbidity, and even mortality in these patients[18]. The reported frequency of extraintestinal syndromes in the patients with IBD varies from 6% to 47%[18,46-49].
Pulmonary involvement among IBD patients was first recognized by Kraft et al[50] about 40 years ago. Both UC and CD can affect any part of the respiratory system. The spectrum of respiratory disorders occurring among patients with IBD includes small and large airway dysfunction, as well as obstructive and interstitial pulmonary diseases[18,46-49]. Screening studies using respiratory symptoms, high-resolution computed tomography (HRCT), bronchoscopy, histological examination, and pulmonary function tests (PFTs) document some of the earliest changes in airways among the respiratory asymptomatic IBD patients[18,46-49]. These changes, especially the subclinical alteration of peripheral airways and parenchymal inflammation may not be detected by routine computed tomography (CT) scan and PFTs[18,46-49]. The pulmonary involvements trend to follow the clinical course of IBD[18,49,51]. Pulmonary impairment appears more pronounced in the patients with active disease than those with inactive disease[18,49,51,52]. The airway inflammation affects quality of life, morbidity, and even mortality among these patients[18,49,51,52]. This evidence suggests that local intestinal mucosal inflammation is responsible for the distant airways inflammation[53-57]. In a population-based cohort study, patients with chronic obstructive pulmonary disease (COPD) had significantly higher risk for both UC and CD[58]. IBD and COPD share many similarities in epidemiological and clinical characteristics, as well as in inflammatory pathology[59,60]. Exposure to air pollution may be an important environmental factor for IBD[61,62]. There appears to be a direct association between increased environmental pollution and hospitalization among adult IBD patients[63]. Inhalation injury is often accompanied by the alimentary tract response[64,65]. The local mucosal inflammation in airways may also be responsible for the intestine inflammation and vice versa. In view of the above phenomena, the lungs and intestine are a pair of interacting organ systems[53-57]. It is most likely that there are similar inflammatory and immune components of these organs that are the cause of the overlap in pathological changes in respiratory and intestinal mucosal diseases[59,60].
Why the inflammatory process shifts from the gastrointestinal tract to the airways or vice versa remains a mystery. Over the years many explanations for the intestine-lung communication have been proposed, however, no definite conclusions have been drawn. The purpose of this article is to update the plausible hypotheses of intestine-lung axis communication underlying the possible mechanism of pulmonary involvement during IBD pathogenesis.
PULMONARY INVOLVEMENT IN ASSOCIATION WITH IBD OCCURS MORE FREQUENTLY THAN PREVIOUSLY RECOGNIZED
Broad spectrum of respiratory disorders in patients with IBD
Kraft et al[50] in 1976 first observed that six patients developed severe, unexplained, chronic bronchopulmonary disease 3-13 years after the onset of nonspecific inflammatory disease of the colon. Since then, various pulmonary manifestations including small and large airway dysfunction or obstruction, inflammation of pulmonary parenchyma and vasculature, as well as bronchopulmonary hyper-reactivity have been reported in IBD[18,46-48,52,66,67]. The spectrum of respiratory disorders occurring among patients with IBD is broad[18,46-52,68]. Storch et al[69] recommend that primary care physicians must take a broader approach while treating patients with IBD and pulmonary diseases.
Epidemiological evidence to support high prevalence of pulmonary involvement in association with IBD
Lungs are not generally considered to be involved in IBD. In some studies only a few cases of pulmonary complications have been associated with IBD[59,67], but in others, there is a wide range of pulmonary complications associated with IBD[18,46-52,67,70]. Edwards et al[70] in a study of 624 IBD patients did not find any association with pulmonary complications. Rodgers et al[67] found that there were only three cases of pulmonary complications in their study of 1400 IBD cases. However, other epidemiological investigations and clinical case reports have documented that pulmonary complications occur more frequently in IBD patients than previously recognized[18,46,47,49,51,52]. Recently, a large population-based study from Canada indicated that pulmonary complications were the most common concomitant chronic disorder in patients with CD and the second most common in patients with UC[19]. The increase in incidence of IBD in the past few decades suggests that environmental factors such as air pollution could contribute to disease pathogenesis[51-57]. Association of IBD and polymorphism of many innate immunity genes has been identified[4,18,53,71,72]. The high concordance of extraintestinal manifestations in siblings and first-degree relatives with IBD suggests that genetic factors also contribute to the link between intestinal and extraintestinal manifestations[73]. The association of pulmonary involvement with IBD appears to be mediated by a complicated interaction of environmental and genetic factors, suggesting there are clear, but undefined, multiple centers of loss of homeostasis in cellular and molecular interactions between the environment and host in both respiratory and gastrointestinal systems.
Latent respiratory abnormalities or dysfunction in IBD patients discovered by HRCT, PFTs and bronchoscopy
HRCT, sensitive PFTs, bronchoscopy and histological examination have been widely used to discover the earliest pulmonary abnormalities or dysfunction among IBD patients. Many respiratory screening studies using HRCT and PFTs support a high prevalence of clinical and subclinical respiratory abnormalities or dysfunction among IBD patients[18,46-48,51,74]. Three studies of randomly selected IBD patients showed incidence rates of pulmonary involvement in 44%, 48%, and 50%, respectively[47,51,75]. Although overt pulmonary symptoms may be uncommon, abnormalities on PFT have been noted in > 50% of patients with UC and > 60% of those with CD[51,76,77].
Advances in imaging methods over the past decade have led to an increased understanding of anatomical and physiological pulmonary abnormalities in patients with IBD[77]. Even though the majority of patients with pulmonary abnormalities were asymptomatic, the incidence of at least one abnormality on HRCT of the chest was as high as 39% in UC and 92% in CD patients[51]. With the advent of HRCT, it has become obvious that the previously described rarity of pulmonary involvement in IBD is inaccurate[77]. HRCT showed changes like bronchectasis, mosaic perfusion and air trapping, suggestive of obliterative bronchiolitis, and patterns of centrilobular nodules and broncing linear opacities (“tree in bud” appearance), suggestive of either cellular bronchiolitis or bronchiolectasis with mucoid secretion[69,78]. HRCT images also indicate that there are multiple centers of airway responses and mucoid secretion. This suggests that airway epithelium (including goblet cells) has been activated. The response of airway epithelium may be a major contributor to IBD-related lung disease.
Munck et al[47] investigated 30 IBD patients (12 with CD, 18 with UC), and found dysfunction of small airways and parenchyma in all IBD patients despite their normal pulmonary physiology. It is likely that the earliest changes occur in the peripheral airways and pulmonary parenchyma, which result in reduced small airways and lung diffusion capacity in all the patients[49,51]. Increased disease activity in IBD patients has been shown to be associated with abnormal pulmonary function tests, and suggests a direct pathogenic link[49,51,79].
Bronchoscopy and histological examination from the lung biopsies show changes in the bronchial epithelium, consisting of basal cell hyperplasia, basement membrane thickening, submucosal nonspecific inflammation, small airway fibrosis, and an overall increase in epithelial thickness and granulomatous bronchiolitis[66,68,79]. Although the crosstalk mechanism between the bowel and lung remains unknown, the HRCT and histology evaluation show that there is a shift in the inflammatory process from the bowel to the lungs[49,51,79].
Respiratory hyper-responsiveness among IBD patients
Allergic symptoms, abnormal PFTs and positive skin prick test were more common among the IBD patients compared to the normal population[28]. The subclinical nonspecific bronchial hyper-responsiveness, independent of the presence of atopy, in IBD patients who have no other pulmonary symptoms and with normal baseline spirometry, has a high prevalence[48]. Colby et al[80] reported that the bronchial hyper-reactivity occurred in 48% of patients with UC and CD, even in the absence of any clinical, radiological and functional evidence of airway disease. Bronchial hyper-reactivity occurred in 71% of children and adolescents with CD[81]. Allergy is an inappropriate inflammatory reaction to antigen. The allergen triggers an exaggerated immune response, and can aggravate an immediately atopic allergic reaction[82-84]. Combination of HRCT and histological examination revealed multiple centers of pulmonary inflammatory responses, which may share many characteristics with atopic allergic reactions[48,80,81]. The lungs may duplicate the “social” inflammatory reaction of the intestine (Figure 1).
Figure 1.
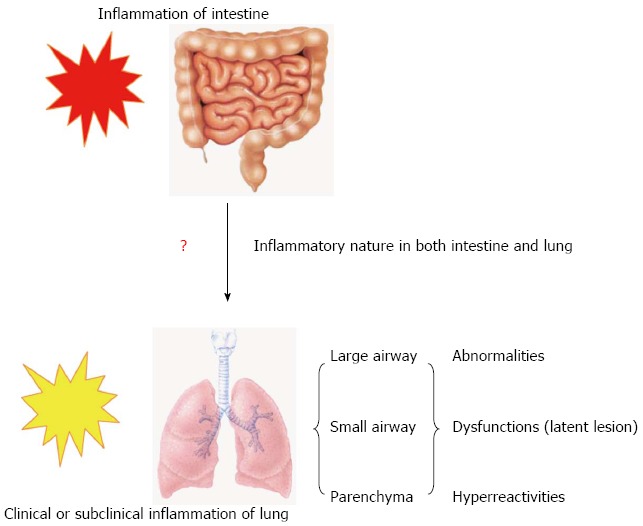
Model for inflammatory process in lung and intestine during inflammatory bowel disease. Clinical or subclinical inflammation in small and large airways and the lung parenchyma accompanies the main inflammation in the bowel during inflammatory bowel disease.
EPITHELIAL CELLS ARE THE INITIAL AND MAIN TARGET
The epithelial cells are considered not only the frontier sentinels and barriers, but also the central participants in innate and adaptive immune responses during mucosal inflammation[71,85-88]. In both the bowel and lungs, these cells provide an important physical barrier to the huge vulnerable biological surface, and are continuously exposed to the external environment. Upon activation, epithelial cells can release large quantities of proinflammatory cytokines, growth factors and chemokines that attract inflammatory cells to initiate and sustain the inflammatory reaction[71,85-88]. Direct or indirect interactions of epithelial cells with mast cells, T and B lymphocytes, dendritic cells (DCs), eosinophils, neutrophils, and basophils help to maintain the balance between the environment and the host[88]. The airway and gut epithelial cells are at the center of the inflammatory response and play the dominant role in initiation and sustaining the inflammatory reaction[88].
Intestinal epithelium is the center of cellular and molecular interaction between host and environment
The healthy mutual relationship between the microbiota and host depends on the balanced interaction between the microbiota and innate immune activation[60,71,87,89,90]. The intestinal barrier plays a crucial role in cellular and molecular interactions between the commensal microflora and possible harmful factors in the intestinal lumen and innate immune system[60,71,87,89-92]. The intestinal barrier is composed of a thick mucus layer containing host defense molecules, a monolayer of columnar intestinal epithelial cells, and underlying set of cells (mesenchymal cells, DCs, lymphocytes and macrophages)[60,71,87,89-92]. The intestinal epithelial cells are exactly at the center of the complex system called the intestinal barrier. The intestinal epithelial cells are a single layer of columnar cells consisting of four types of cells: the absorbent enterocytes, goblet cells, Paneth cells, and enteroendocrine cells. It is clear that these four types of epithelial cells present an anatomical and functional barrier to maintain the whole intestinal homeostasis. On the antigen-rich side of the lumen, they secrete and regulate the composition of the thick mucus layer. The intestinal epithelial cells serve as sentinels of innate immunity. The innate immune system is able to recognize bacterial and viral motifs through the toll-like receptors (TLRs) and the nucleotide-binding oligomerization domain families[93-95]. The intestinal epithelial cells express several members of the TLRs[93]. On the basolateral side, these cells interact and continuously crosstalk with inflammatory cell-rich lamina propria. The intestinal epithelial cells are nonprofessional antigen-presenting cells, which are able to process and present antigens directly to lymphocytes by a highly polarized system with apical antigen sorting, processing and exclusively basolateral presentation. They serve as the major participants in innate and adaptive immune responses[60,71,87,89-91]. The intestinal epithelium acts to maintain homeostasis during the steady state, restore the epithelium during insult or injury, and induce inflammatory responses.
Airway epithelium is also the center of cellular and molecular interaction between host and environment
Although initiation of pulmonary inflammation and its association with IBD remains unknown, it is well established that the airway epithelial cells play an important role in the initiation and maintenance of airway inflammation, as well as being the main target[60,85,88,96-98]. The airway epithelial cells are also at the center of the airway barrier system because of their anatomical and functional position. On the antigen-rich lumen side, they secrete and regulate the composition of the thick mucus layer, while on the basolateral side, they interact and crosstalk with the inflammatory cell-rich lamina propria[60,85,88,96-98]. Activated airway epithelial cells can induce synthesis and secretion of immune-cell-mediated host defense molecules such as antimicrobial and antiviral proteins that activate other mucosal innate immune cells[60,85,88,96-103]. Activated innate immune responses can secondarily induce the recruitment and activation of DCs and T and B lymphocytes that amplify antigen recognition and antibody production and other adaptive immune responses[60,96-103]. Many studies document that the IBD patients develop rapidly progressive respiratory symptoms after colectomy without any identified bacterial pathogens on repeated sputum culture and require oral corticosteroid therapy[18,66,99,104-106], which proves that pulmonary impairment in IBD is not a pathogen-induced inflammation. The pulmonary involvement in IBD may be due to aberrant immunity from the bowel that disrupts airway tissue homeostasis. This aberrant airway epithelial injury/repair may be the mechanism of IBD-related pulmonary involvement[53-57,107,108]. Lungs may duplicate atopic inflammation of the bowel. However, the connection between the primary site and the atopic site is unclear. Less attention has been paid to the mechanism of atopic inflammatory reaction that manifests at sites remote from the primary site. The mechanism should be either systemic-factor-mediated cellular and molecular events or some intrinsic connection between the primary and atopic sites. In future, detecting the earliest pulmonary epithelium activation signs or genetic responses during IBD pathogenesis would provide evidence of the linkage between the anatomically disconnected organ systems.
AIRWAY MAY DUPLICATE THE BOWEL INFLAMMATORY EVENT
Combined HRCT and histological examination have shown that there are multiple centers of airway epithelial (including goblet cells) responses and mucoid secretion in IBD[78-79,104,109]. The lung duplicates the inflammatory process of the bowel (Figures 1 and 2). The linkage between the lungs and intestine is hard to understand in the absence of anatomical connections or systemic neuroendocrine immune mediation. Pulmonary inflammation may intrinsically accompany the main inflammation in the bowel, and this distal intrinsic inflammatory response may be related to the “social manner” of cells and molecules, similar to the exaggerated allergen-induced multiple centers of allergy inflammatory response on the surface of the skin, conjunctivae, and airway and digestive tracts[83-85,88,99,107,108].
Figure 2.
Summary model of plausible mechanisms of lung-intestine communication underlying inflammatory bowel disease-associated pulmonary involvement. The lungs and intestines are a pair of mutually affected organs. The airways may intrinsically accompany inflammation of the bowel. This distal intrinsic inflammatory response may relate to the common features between the lung and intestine. According to Traditional Chinese Medicine, the lung and intestine have internal and external relationships (Biao-Li). This distal intrinsic inflammatory response may relate to the “social manner” of cells and molecules.
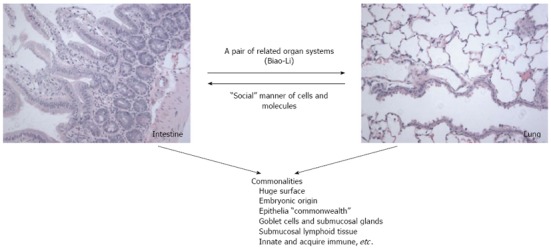
Commonalities between lung and intestine and crosstalk
There are many commonalities between the lungs and intestines[18,53,107,108,110,111]. The gastrointestinal tract and bronchial tree share a common embryological origin that arises from the primitive gut[10,74,75,77,78]. Both the lung and intestine possess goblet cells and submucosal glands as part of their luminal structure. In addition, both contain submucosal lymphoid tissue, host defense molecules, and play crucial roles in both innate and acquired immunity[18,107,108,110,111].
In IBD, inflamed bowel mucosa produces many inflammatory mediators and releases them to the circulating system[4,66,71,86,87,90,102,103]. The inflamed bowel mucosa also creates the prospect for systemic absorption of luminal contents, including dietary antigens, digestive enzymes, or specific bacterial products capable of inducing systemic inflammation[66,102,103]. Some investigators hypothesize that the pathogenesis of IBD-related airway disease may be associated with circulating inflammatory mediators, specific bacterial products, dietary antigens, or digestive enzymes[102,103]. Detectable levels of bacterial lipopolysaccharide and antibodies to bacterial lipid A and peptidoglycan in serum of IBD patients support this theory. However, it is less likely to be accurate in the case of IBD-associated lung disease, because it clearly occurs after colectomy in patients who do not have any ongoing bowel inflammation[4,66,105,106]. To the best of our knowledge, none of the circulating inflammatory mediators, dietary antigens, digestive enzymes, or specific bacterial products has been established as being responsible for extraintestinal syndromes during the intestine inflammatory process. In fact, circulating inflammatory mediators are not always enough to drive inflammation from the local mucosa to distal organs. Our previous work suggested that the skin, conjunctivae, airways and digestive tract may join together to fight allergens[107,108]. The atopic lesion may duplicate the primary contact site of cellular and molecular events. The atopic march may be due to the intrinsic “social” involvement of the positioned epithelial cells, but may not be totally controlled by the anatomic connection or the circulating systemic factors involved in allergy pathogenesis[53-57,107,108,112,113]. Thymic stromal lymphopoietin (TSLP), a general biomarker for skin-barrier defects[85,107,114] is an interleukin-7-like cytokine produced by epithelial cells, has emerged as a potential master regulator of both skin and airway inflammation[85,107,112-115]. TSLP signaling plays an important role in both airway and skin allergic inflammation. Recently, Zhang et al[114] found that increased TSLP expression in skin keratinocytes not only triggers atopic dermatitis-like lesions in skin locally, but also leads to a concomitant ovalbumin (OVA)-induced asthma-like lung inflammation in animals. Furthermore, they elucidated that increased epidermal keratinocyte TSLP expression and subsequent increase in circulating TSLP in a mouse model did not lead to any spontaneous lung inflammation in the absence of OVA sensitization and challenge. High levels of expression of TSLP mRNA in bronchial epithelial cells and submucosa of asthmatic patients[115], suggest that locally produced TSLP could be important for the development and maintenance of asthma[85,107,112-115]. TSLP appears to be a crucial factor in driving both skin and lung into inflammation, but solely increased epidermal keratinocyte TSLP expression and subsequent increase in blood circulating TSLP is not sufficient to drive both skin and lung into inflammation[85,107,112-115]. The lung inflammation is not triggered by circulating or epidermal TSLP, but requires local TSLP production by pulmonary epithelial cells[85,107,112-115]. OVA sensitization and challenge is extremely important to drive both skin and lung into inflammation[85,107,114]. The intrinsic connection of skin and lung is through locally produced TSLP after OVA sensitization and challenge[85,107,112-114].
The plausible mechanism for association of pulmonary involvement with IBD may be the intrinsic airway reactions that accompany the main inflammation in the bowel, as for allergen-triggered, enigmatic, multiple centers of allergic inflammation.
There is clearly communication among organs that share a common embryological origin, or similar cellular and molecular structure without any anatomical interaction[53-57,65,107,108,110,111]. Primitive gut has a shared common origin with the gastrointestinal tract, biliary tract, and respiratory system. Our previous work suggested that pulmonary surfactant protein A-like molecule is a frontier host defense protein and that its expression may be associated with some autoimmune diseases[53,65]. Latex-mediated allergy is another example of intrinsic activation of non anatomically related organs. During latex-mediated allergy, the skin allergic reaction occurs anywhere in the body and not necessarily at the site of the direct latex contact[74,75,83]. How does information transfer from the epidermal keratinocytes at latex-contacted sites to those at uncontacted sites[107,108,116]? It is possible that the epidermal keratinocytes at both sites intrinsically communicate because they are close relatives that share common features.
In fact, in IBD, the surface of skin, conjunctivae, and airways is involved in fighting inflammation in the gastrointestinal tract.
As above mentioned, OVA intrinsically drives skin and lung into allergic inflammation by locally produced TLSP[85,107,112-115]. Aberrant immune responses to the commensal microbiota in the bowel, which lead to pulmonary inflammation, may be related to some unknown TLSPS-like molecules. Detecting these molecules could provide the mechanism of lung-intestine crosstalk.
Pulmonary involvement associated with IBD may be a good example to understand the Biao-Li relationship between lung and intestine
According to Chinese Medicine, the lung and the intestine are a pair of related organ systems (Biao-Li). The interaction of lung and intestine is mutually affected by internal and external relationships[47,53-57,107,116-118]. It means that lung diseases often have colon syndromes and colon diseases may have lung syndromes. The mechanism is complicated, and immunological, environmental and genetic factors should trigger lots cellular and molecular events[107,108]. From the pathological findings, the microscopic hallmark of IBD in the intestine and lung is infiltration of chronic inflammatory cells and response of interstitial cells such as fibrocytes and epithelium. In our previous study, we investigated one type of frontier immune cell (CD68-positive macrophages) and one type of frontier host defense molecule [pulmonary surfactant protein (SP)-A], which reside in the lungs and colon. Both frontier immune cell and frontier host defense molecules are overexpressed in inflammatory areas of CD and UC, and in particular, there are many CD68-positive macrophages among the epithelia of lamina mucosa. The SP-A-positive macrophages are recruited by activated epithelial cells (Figure 3)[53]. The epithelium serves as the central participants in innate and adaptive immune responses as well as mucosal inflammation, laboratory evidence of activation of pulmonary epithelium would provide an insight into the “Biao-Li” relationship between lung and intestine during the pathogenesis of IBD associated pulmonary disorders. In view of this, association of pulmonary involvement with IBD may be a good example to understand the Biao-Li relationship between lung and intestine[53-57,107,116-118].
Figure 3.
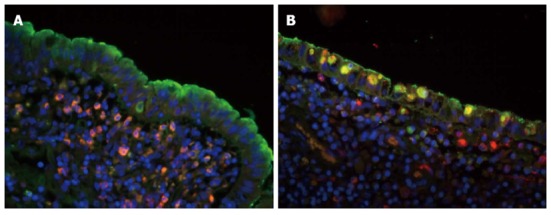
Co-localization of surfactant protein-A and CD68 in normal and inflammatory areas. A: Surfactant protein (SP)-A -positive signal was indicated by fluorescence microscopy (green) fluorescence; CD68-positive signal was identified by rhodamine red-X (red) fluorescence; and the cell nucleus was indicated by Hoechst dye (blue). In the normal area, SP-A was located in the surface of the villi, specific epithelium and submucosae, lamina muscularis, mucosae and lymphoid tissues. CD68-positive cells were mainly found in the submucosae, lamina muscularis, mucosae, and lymphoid tissues and in the epithelium; B: In the inflammatory area, CD68-positive cells were dramatically increased in all levels of the bowel wall; especially CD68-positive macrophages in the epithelia of lamina mucosa. The SP-A-positive macrophages were recruited by activated epithelial cells. Double labeled SP-A and CD68 shows that some CD68-positive macrophages expressed SP-A like molecule in the inflammatory bowel disease tissues (original magnification, × 200). Figures reproduced with permission from reference [53].
Lung-intestine connection may relate to the “social” property of cells and molecules
To date, there is a lack of evidence for the participation of any circulating inflammatory mediator, dietary antigens, digestive enzymes, or specific bacterial products as a linkage between the gastrointestinal tract and extraintestinal organs. Both gastrointestinal and airway lesions may be epithelium-mediated chronic inflammation. IBD-associated pulmonary involvement, such as epithelial disease with adoption of a chronic wound scenario, could also be a hypothesis about airway wall and gastrointestinal tract remodeling over the course of the disease[60,66,80]. The extraintestinal organs may duplicate “social” inflammation of the gastrointestinal tract. The inflammation of the gastrointestinal tract as a “social” factor drives inflammatory reaction to extra-intestinal organs. There is a clear but undefined communication among organs that share a common embryological origin, or similar cellular and molecular structure despite being located at a distance from each other without any anatomical interaction. The human body originates from a single cell, which makes the whole body a social system. A constant molecular and cellular interaction between the environment and host is required for the establishment and maintenance of homeostasis. Disrupted homeostasis in the gastrointestinal tract may also affect the airways. Pulmonary involvement associated with IBD as the “social” inflammatory reaction, is a plausible hypothesis. Sharing the common embryological origin or similar cellular and molecular structures may be “closer relatives” in our “social body”. The “social manner” of cell and molecules would also help to attain insight into the pathogenesis of enigmatic complications such as IBD, and the cellular and molecular mechanism of Chinese Traditional medicine.
CONCLUSION
The frequency of pulmonary abnormalities, dysfunction or hyper-reactivity that occurs in association with IBD is more than previously recognized. More evidence shows that latent inflammation exists in the airways of IBD patients, even without any bronchopulmonary symptoms and with normal pulmonary function. The pulmonary impairment is more pronounced in IBD patients with active disease than in those in remission, and the linkage between lung and intestine may not be easily supported by anatomic connection or systemic neuroendocrine immune mediation. The airways may intrinsically accompany the main inflammation in the bowel. Lung and intestine share a lot of common features. The interaction of lung and intestine is mutually affected by internal and external relationships (Biao-Li). The pulmonary abnormalities, dysfunction or hyper-reactivity in IBD patients may be related to the “social” manner of cells and molecules. Further investigation of epithelium-mediated cellular and molecular events and their interaction in lung and bowel may provide evidence of mutually affected internal and external relationships (Biao-Li) of the lung-intestine axis, and the “social property” of cells and molecules. A better understanding of the mechanism of crosstalk among the distant organs would be beneficial to the elucidation of the etiology and help in identifying therapeutic strategies for mucosal inflammatory diseases such as IBD and atopic allergic inflammation.
Footnotes
P- Reviewers Capasso R, Sipos F, Yamamoto T S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Mudter J, Neurath MF. Insight into Crohn’s disease pathomorphology. Abdom Imaging. 2012;37:921–926. doi: 10.1007/s00261-012-9885-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Patel NR, Walter L, Ingersoll S, Sitaraman SV, Garg P. Constitutive expression of MMP9 in intestinal epithelium worsens murine acute colitis and is associated with increased levels of proinflammatory cytokine Kc. Am J Physiol Gastrointest Liver Physiol. 2013;304:G793–G803. doi: 10.1152/ajpgi.00249.2012. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Qiao Y, Ran Z, Wang T, Xu J, Feng J. Intestinal protein expression profile identifies inflammatory bowel disease and predicts relapse. Int J Clin Exp Pathol. 2013;6:917–925. [PMC free article] [PubMed] [Google Scholar]
- 4.Latella G, Fiocchi C, Caprili R. News from the “5th International Meeting on Inflammatory Bowel Diseases” CAPRI 2010. J Crohns Colitis. 2010;4:690–702. doi: 10.1016/j.crohns.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein CN. Epidemiologic clues to inflammatory bowel disease. Curr Gastroenterol Rep. 2010;12:495–501. doi: 10.1007/s11894-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 7.Vernier G, Cortot A, Gower-Rousseau C, Salomez JL, Colombel JF. [Epidemiology and risk factors of inflammatory bowel diseases] Rev Prat. 2005;55:949–961. [PubMed] [Google Scholar]
- 8.Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 9.Zhulina Y, Hahn-Strömberg V, Shamikh A, Peterson CG, Gustavsson A, Nyhlin N, Wickbom A, Bohr J, Bodin L, Tysk C, et al. Subclinical inflammation with increased neutrophil activity in healthy twin siblings reflect environmental influence in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1725–1731. doi: 10.1097/MIB.0b013e318281f2d3. [DOI] [PubMed] [Google Scholar]
- 10.Ekbom A. The epidemiology of IBD: a lot of data but little knowledge. How shall we proceed? Inflamm Bowel Dis. 2004;10 Suppl 1:S32–S34. doi: 10.1097/00054725-200402001-00007. [DOI] [PubMed] [Google Scholar]
- 11.Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn’s disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11:161–166. doi: 10.1111/j.1751-2980.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- 13.Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian J Gastroenterol. 2007;26:285–289. [PubMed] [Google Scholar]
- 14.Al-Mofarreh MA, Al-Mofleh IA. Emerging inflammatory bowel disease in saudi outpatients: a report of 693 cases. Saudi J Gastroenterol. 2013;19:16–22. doi: 10.4103/1319-3767.105915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Muñoz JE. Emigration to western industrialized countries: A risk factor for developing inflammatory bowel disease. J Crohns Colitis. 2011;5:566–569. doi: 10.1016/j.crohns.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Lakatos L, Mester G, Erdelyi Z, Balogh M, Szipocs I, Kamaras G, Lakatos PL. Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977-2001. World J Gastroenterol. 2004;10:404–409. doi: 10.3748/wjg.v10.i3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819–4831. doi: 10.3748/wjg.v12.i30.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- 20.Patil SA, Cross RK. Update in the management of extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15:314. doi: 10.1007/s11894-013-0314-8. [DOI] [PubMed] [Google Scholar]
- 21.Aloi M, Cucchiara S. Extradigestive manifestations of IBD in pediatrics. Eur Rev Med Pharmacol Sci. 2009;13 Suppl 1:23–32. [PubMed] [Google Scholar]
- 22.Ricker JM, Harrison SA. Inflammatory bowel disease: thinking outside of the intestines (Part 2) J Miss State Med Assoc. 2008;49:365–369. [PubMed] [Google Scholar]
- 23.Jewell D. Do HLA antigens predict the occurrence of extraintestinal manifestations of IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S28. doi: 10.1002/ibd.20726. [DOI] [PubMed] [Google Scholar]
- 24.Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1915–1924. doi: 10.1002/ibd.20942. [DOI] [PubMed] [Google Scholar]
- 25.Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998;42:387–391. doi: 10.1136/gut.42.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vlam K, Mielants H, Cuvelier C, De Keyser F, Veys EM, De Vos M. Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol. 2000;27:2860–2865. [PubMed] [Google Scholar]
- 27.Huang BL, Chandra S, Shih DQ. Skin manifestations of inflammatory bowel disease. Front Physiol. 2012;3:13. doi: 10.3389/fphys.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1598–1619. doi: 10.1002/ibd.21219. [DOI] [PubMed] [Google Scholar]
- 30.Duerr RH, Targan SR, Landers CJ, LaRusso NF, Lindsay KL, Wiesner RH, Shanahan F. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991;100:1385–1391. [PubMed] [Google Scholar]
- 31.Tabibian JH, Streiff MB. Inflammatory bowel disease-associated thromboembolism: a systematic review of outcomes with anticoagulation versus catheter-directed thrombolysis. Inflamm Bowel Dis. 2012;18:161–171. doi: 10.1002/ibd.21307. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97–101. doi: 10.1046/j.1572-0241.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 34.Barabino AV, Gandullia P, Calvi A, Vignola S, Arrigo S, Marco RD. Sudden blindness in a child with Crohn’s disease. World J Gastroenterol. 2011;17:4344–4346. doi: 10.3748/wjg.v17.i38.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrelli EA, McKinley M, Troncale FJ. Ocular manifestations of inflammatory bowel disease. Ann Ophthalmol. 1982;14:356–360. [PubMed] [Google Scholar]
- 36.Tokuyama H, Wakino S, Konishi K, Hashiguchi A, Hayashi K, Itoh H. Acute interstitial nephritis associated with ulcerative colitis. Clin Exp Nephrol. 2010;14:483–486. doi: 10.1007/s10157-010-0294-z. [DOI] [PubMed] [Google Scholar]
- 37.Stokke KT, Teisberg PA, Myhre E, Hovig T, Flatmark A, Gjone E. Nephrotic syndrome in ulcerative colitis. Scand J Gastroenterol. 1976;11:571–576. [PubMed] [Google Scholar]
- 38.Glassman M, Kaplan M, Spivak W. Immune-complex glomerulonephritis in Crohn’s disease. J Pediatr Gastroenterol Nutr. 1986;5:966–969. doi: 10.1097/00005176-198611000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Broide E, Dotan I, Weiss B, Wilschanski M, Yerushalmi B, Klar A, Levine A. Idiopathic pancreatitis preceding the diagnosis of inflammatory bowel disease is more frequent in pediatric patients. J Pediatr Gastroenterol Nutr. 2011;52:714–717. doi: 10.1097/MPG.0b013e3182065cad. [DOI] [PubMed] [Google Scholar]
- 40.Weber P, Seibold F, Jenss H. Acute pancreatitis in Crohn’s disease. J Clin Gastroenterol. 1993;17:286–291. doi: 10.1097/00004836-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn’s disease. Gut. 1990;31:1076–1079. doi: 10.1136/gut.31.9.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31:517–523. doi: 10.3109/00365529609006775. [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Kumar N, Loftus EV, Kane SV. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflamm Bowel Dis. 2013;19:864–872. doi: 10.1002/ibd.23011. [DOI] [PubMed] [Google Scholar]
- 44.Lossos A, River Y, Eliakim A, Steiner I. Neurologic aspects of inflammatory bowel disease. Neurology. 1995;45:416–421. doi: 10.1212/wnl.45.3.416. [DOI] [PubMed] [Google Scholar]
- 45.Geissler A, Andus T, Roth M, Kullmann F, Caesar I, Held P, Gross V, Feuerbach S, Schölmerich J. Focal white-matter lesions in brain of patients with inflammatory bowel disease. Lancet. 1995;345:897–898. doi: 10.1016/s0140-6736(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 46.Mendoza JL, Lana R, Taxonera C, Alba C, Izquierdo S, Díaz-Rubio M. [Extraintestinal manifestations in inflammatory bowel disease: differences between Crohn’s disease and ulcerative colitis] Med Clin (Barc) 2005;125:297–300. doi: 10.1157/13078423. [DOI] [PubMed] [Google Scholar]
- 47.Munck A, Murciano D, Pariente R, Cezard JP, Navarro J. Latent pulmonary function abnormalities in children with Crohn’s disease. Eur Respir J. 1995;8:377–380. doi: 10.1183/09031936.95.08030377. [DOI] [PubMed] [Google Scholar]
- 48.Ceyhan BB, Karakurt S, Cevik H, Sungur M. Bronchial hyperreactivity and allergic status in inflammatory bowel disease. Respiration. 2003;70:60–66. doi: 10.1159/000068407. [DOI] [PubMed] [Google Scholar]
- 49.Tzanakis N, Samiou M, Bouros D, Mouzas J, Kouroumalis E, Siafakas NM. Small airways function in patients with inflammatory bowel disease. Am J Respir Crit Care Med. 1998;157:382–386. doi: 10.1164/ajrccm.157.2.97-04075. [DOI] [PubMed] [Google Scholar]
- 50.Kraft SC, Earle RH, Roesler M, Esterly JR. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976;136:454–459. [PubMed] [Google Scholar]
- 51.Songür N, Songür Y, Tüzün M, Doğan I, Tüzün D, Ensari A, Hekimoglu B. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37:292–298. doi: 10.1097/00004836-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 53.Luo JM, Liu ZQ, Eugene CY. Overexpression of pulmonary surfactant protein A like molecules in inflammatory bowel disease tissues. Zhongnan Daxue Xuebao Yixueban. 2008;33:979–986. [PubMed] [Google Scholar]
- 54.Liu Y, Wang XY, Yang X, Jing S, Zhu L, Gao SH. Lung and intestine: a specific link in an ulcerative colitis rat model. Gastroenterol Res Pract. 2013;2013:124530. doi: 10.1155/2013/124530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao L, Wang J, Li F, Gao S, Deng Y. Analysis on clinically drug-used law for lung-intestine related diseases. J Tradit Chin Med. 2012;32:523–528. doi: 10.1016/s0254-6272(13)60064-3. [DOI] [PubMed] [Google Scholar]
- 56.Liu P, Wang P, Tian D, Liu J, Chen G, Liu S. Study on traditional Chinese medicine theory of lung being connected with large intestine. J Tradit Chin Med. 2012;32:482–487. doi: 10.1016/s0254-6272(13)60059-x. [DOI] [PubMed] [Google Scholar]
- 57.Ni JX, Gao SH. Understanding the viscera-related theory that the lung and large intestine are exterior-interiorly related. J Tradit Chin Med. 2012;32:293–298. doi: 10.1016/s0254-6272(13)60028-x. [DOI] [PubMed] [Google Scholar]
- 58.Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 59.Mortaz E, Adcock IM, Folkerts G, Barnes PJ, Paul Vos A, Garssen J. Probiotics in the management of lung diseases. Mediators Inflamm. 2013;2013:751068. doi: 10.1155/2013/751068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. 2010;105:2412–2419. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. 2011;5:279–286. doi: 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis. 2011;17:1138–1145. doi: 10.1002/ibd.21455. [DOI] [PubMed] [Google Scholar]
- 64.Markell KW, Renz EM, White CE, Albrecht ME, Blackbourne LH, Park MS, Barillo DA, Chung KK, Kozar RA, Minei JP, et al. Abdominal complications after severe burns. J Am Coll Surg. 2009;208:940–947; discussion 940-947. doi: 10.1016/j.jamcollsurg.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 65.Luo JM, Wan YS, Liu ZQ, Wang GR, Floros J, Zhou HH. Regularity of distribution of immunoreactive pulmonary surfactant protein A in rat tissues. Int J Mol Med. 2004;14:343–351. [PubMed] [Google Scholar]
- 66.Spira A, Grossman R, Balter M. Large airway disease associated with inflammatory bowel disease. Chest. 1998;113:1723–1726. doi: 10.1378/chest.113.6.1723. [DOI] [PubMed] [Google Scholar]
- 67.Rogers BH, Clark LM, Kirsner JB. The epidemiologic and demographic characteristics of inflammatory bowel disease: an analysis of a computerized file of 1400 patients. J Chronic Dis. 1971;24:743–773. doi: 10.1016/0021-9681(71)90087-7. [DOI] [PubMed] [Google Scholar]
- 68.Vandenplas O, Casel S, Delos M, Trigaux JP, Melange M, Marchand E. Granulomatous bronchiolitis associated with Crohn’s disease. Am J Respir Crit Care Med. 1998;158:1676–1679. doi: 10.1164/ajrccm.158.5.9801070. [DOI] [PubMed] [Google Scholar]
- 69.Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:104–115. doi: 10.1097/00054725-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. III. complications. Gut. 1964;5:1–22. doi: 10.1136/gut.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison OJ, Maloy KJ. Innate immune activation in intestinal homeostasis. J Innate Immun. 2011;3:585–593. doi: 10.1159/000330913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Satsangi J, Grootscholten C, Holt H, Jewell DP. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741. doi: 10.1136/gut.38.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herrlinger KR, Noftz MK, Dalhoff K, Ludwig D, Stange EF, Fellermann K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol. 2002;97:377–381. doi: 10.1111/j.1572-0241.2002.05473.x. [DOI] [PubMed] [Google Scholar]
- 75.Mohamed-Hussein AA, Mohamed NA, Ibrahim ME. Changes in pulmonary function in patients with ulcerative colitis. Respir Med. 2007;101:977–982. doi: 10.1016/j.rmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Douglas JG, McDonald CF, Leslie MJ, Gillon J, Crompton GK, McHardy GJ. Respiratory impairment in inflammatory bowel disease: does it vary with disease activity? Respir Med. 1989;83:389–394. doi: 10.1016/s0954-6111(89)80070-8. [DOI] [PubMed] [Google Scholar]
- 77.Godet PG, Cowie R, Woodman RC, Sutherland LR. Pulmonary function abnormalities in patients with ulcerative colitis. Am J Gastroenterol. 1997;92:1154–1156. [PubMed] [Google Scholar]
- 78.Ozyilmaz E, Yildirim B, Erbas G, Akten S, Oguzulgen IK, Tunc B, Tuncer C, Turktas H. Value of fractional exhaled nitric oxide (FE NO) for the diagnosis of pulmonary involvement due to inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:670–676. doi: 10.1002/ibd.21085. [DOI] [PubMed] [Google Scholar]
- 79.Mahadeva R, Walsh G, Flower CD, Shneerson JM. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur Respir J. 2000;15:41–48. doi: 10.1183/09031936.00.15104100. [DOI] [PubMed] [Google Scholar]
- 80.Colby TV, Camus P. Pathology of pulmonary involvement in inflammatory bowel disease. ERSJ. 2007;39:199–207. [Google Scholar]
- 81.Louis E, Louis R, Drion V, Bonnet V, Lamproye A, Radermecker M, Belaiche J. Increased frequency of bronchial hyperresponsiveness in patients with inflammatory bowel disease. Allergy. 1995;50:729–733. doi: 10.1111/j.1398-9995.1995.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 82.Mansi A, Cucchiara S, Greco L, Sarnelli P, Pisanti C, Franco MT, Santamaria F. Bronchial hyperresponsiveness in children and adolescents with Crohn’s disease. Am J Respir Crit Care Med. 2000;161:1051–1054. doi: 10.1164/ajrccm.161.3.9906013. [DOI] [PubMed] [Google Scholar]
- 83.Yu LC. The epithelial gatekeeper against food allergy. Pediatr Neonatol. 2009;50:247–254. doi: 10.1016/S1875-9572(09)60072-3. [DOI] [PubMed] [Google Scholar]
- 84.Jaeger D, Kleinhans D, Czuppon AB, Baur X. Latex-specific proteins causing immediate-type cutaneous, nasal, bronchial, and systemic reactions. J Allergy Clin Immunol. 1992;89:759–768. doi: 10.1016/0091-6749(92)90385-f. [DOI] [PubMed] [Google Scholar]
- 85.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–659. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 86.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008;83:493–498. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 88.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cucchiara S, Stronati L, Aloi M. Interactions between intestinal microbiota and innate immune system in pediatric inflammatory bowel disease. J Clin Gastroenterol. 2012;46 Suppl:S64–S66. doi: 10.1097/MCG.0b013e31826a857f. [DOI] [PubMed] [Google Scholar]
- 90.Roda G, Sartini A, Zambon E, Calafiore A, Marocchi M, Caponi A, Belluzzi A, Roda E. Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol. 2010;16:4264–4271. doi: 10.3748/wjg.v16.i34.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Defendenti C, Sarzi-Puttini P, Grosso S, Croce A, Senesi O, Saibeni S, Bollani S, Almasio PL, Bruno S, Atzeni F. B lymphocyte intestinal homing in inflammatory bowel disease. BMC Immunol. 2011;12:71. doi: 10.1186/1471-2172-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao L, Kamalu O, Mayer L. Non-classical MHC class I molecules on intestinal epithelial cells: mediators of mucosal crosstalk. Immunol Rev. 2005;206:160–176. doi: 10.1111/j.0105-2896.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 94.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schröder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 96.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;13:646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 97.Oh K, Seo MW, Lee GY, Byoun OJ, Kang HR, Cho SH, Lee DS. Airway epithelial cells initiate the allergen response through transglutaminase 2 by inducing IL-33 expression and a subsequent Th2 response. Respir Res. 2013;14:35. doi: 10.1186/1465-9921-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin C, Frija J, Burgel PR. Dysfunctional lung anatomy and small airways degeneration in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:7–13. doi: 10.2147/COPD.S28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schleimer RP, Lane AP, Kim J. Innate and acquired immunity and epithelial cell function in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:51–78. [PubMed] [Google Scholar]
- 100.Upham JW, Stick SM. Interactions between airway epithelial cells and dendritic cells: implications for the regulation of airway inflammation. Curr Drug Targets. 2006;7:541–545. doi: 10.2174/138945006776818647. [DOI] [PubMed] [Google Scholar]
- 101.Yilmaz A, Yilmaz Demirci N, Hoşgün D, Uner E, Erdoğan Y, Gökçek A, Cağlar A. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol. 2010;16:4952–4957. doi: 10.3748/wjg.v16.i39.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sartor RB. Pathogenetic and clinical relevance of cytokines in inflammatory bowel disease. Immunol Res. 1991;10:465–471. doi: 10.1007/BF02919743. [DOI] [PubMed] [Google Scholar]
- 103.Pullman WE, Elsbury S, Kobayashi M, Hapel AJ, Doe WF. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992;102:529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- 104.D’Andrea N, Vigliarolo R, Sanguinetti CM. Respiratory involvement in inflammatory bowel diseases. Multidiscip Respir Med. 2010;5:173–182. doi: 10.1186/2049-6958-5-3-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eaton TE, Lambie N, Wells AU. Bronchiectasis following colectomy for Crohn’s disease. Thorax. 1998;53:529–531. doi: 10.1136/thx.53.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 107.Luo J, Li Y, Gong R. The mechanism of atopic march may be the ‘social’ event of cells and molecules (Review) Int J Mol Med. 2010;26:779–785. doi: 10.3892/ijmm_00000525. [DOI] [PubMed] [Google Scholar]
- 108.Luo J, Wan Y. Tightly regulated distribution of family members of proteins is related to social property in the open body system (Review) Int J Mol Med. 2006;17:411–418. [PubMed] [Google Scholar]
- 109.Desai D, Patil S, Udwadia Z, Maheshwari S, Abraham P, Joshi A. Pulmonary manifestations in inflammatory bowel disease: a prospective study. Indian J Gastroenterol. 2011;30:225–228. doi: 10.1007/s12664-011-0129-1. [DOI] [PubMed] [Google Scholar]
- 110.Bhagat S, Das KM. A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology. 1994;107:103–108. doi: 10.1016/0016-5085(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 111.Oshitani N, Watanabe K, Nakamura S, Higuchi K, Arakawa T. [Extraintestinal complications in patients with ulcerative colitis] Nihon Rinsho. 2005;63:874–878. [PubMed] [Google Scholar]
- 112.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 113.Zhu Z, Oh MH, Yu J, Liu YJ, Zheng T. The Role of TSLP in IL-13-induced atopic march. Sci Rep. 2011;1:23. doi: 10.1038/srep00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, Chambon P. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci USA. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 116.Nienhaus A, Kromark K, Raulf-Heimsoth M, van Kampen V, Merget R. Outcome of occupational latex allergy--work ability and quality of life. PLoS One. 2008;3:e3459. doi: 10.1371/journal.pone.0003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng X, Yang Y, Zheng X, Zhou X, Ding W, Liu W. Correlation between the lung and large intestine from the micro-ecological changes of lung and intestine flora. ZhongYi Zazhi. 2011;52:865–867. [Google Scholar]
- 118.Li D, Yang S, Chen R. Experimental study on the mechanism of protective effect of free Fu on gut-derived endotoxin-mediated lung damage. J Huazhong Univ Sci Technolog Med Sci. 2004;24:528–530. doi: 10.1007/BF02831128. [DOI] [PubMed] [Google Scholar]


