Abstract
Female mice emit a low-frequency harmonic (LFH) call in association with distinct behavioral contexts: mating and physical threat or pain. Here we report the results of acoustic, behavioral, and neurophysiological studies of the contextual analysis of these calls in CBA/CaJ mice. We first show that the acoustical features of the LFH call do not differ between contexts. We then show that male mice avoid the LFH call in the presence of a predator cue (cat fur) but are more attracted to the same exemplar of the call in the presence of a mating cue (female urine). The males thus use nonauditory cues to determine the meaning of the LFH call, but these cues do not generalize to noncommunication sounds, such as noise bursts. We then characterized neural correlates of contextual meaning of the LFH call in responses of basolateral amygdala (BLA) neurons from awake, freely moving mice. There were two major findings. First, BLA neurons typically displayed early excitation to all tested behaviorally aversive stimuli. Second, the nonauditory context modulates the BLA population response to the LFH call but not to the noncommunication sound. These results suggest that the meaning of communication calls is reflected in the spike discharge patterns of BLA neurons.
Introduction
Animals use acoustic communication to pass information from a sender to a listener (Endler, 1993; Seyfarth and Cheney, 2003; Grimsley et al., 2011). When interpreting the meaning of these sounds, researchers investigating the relationship between the behavioral state of the caller and the physical characteristics of the sound have focused mainly on the caller (Berryman, 1976; Gadziola et al., 2012a). What happens when the same sound is emitted when the caller is in different behavioral states? The listener needs to evaluate the contextual cues surrounding the vocalization to accurately interpret its message. For example, female mice give the same low-frequency harmonic (LFH) call during mating (Wang et al., 2008) as mice of both sexes give in response to fear or pain (Williams et al., 2008). Although the female “caller” may be in some distress in both contexts, a male “listener” may interpret these calls differently. This provides a paradigm to explore how contextual cues modulate the meaning of communication calls. We show that male mice use nonauditory cues to determine whether to approach or avoid the LFH call. This finding enabled us to investigate how the meaning of sounds is coded in the brain.
We hypothesized that neurons of the amygdala perform a contextual analysis of the LFH call that provides the basis for the male mouse behavior. We focused on the amygdala for several reasons. Amygdalar neurons assess the salience of sensory stimuli and mediate many features of the emotional response to these stimuli (Cardinal et al., 2002; Price, 2003; Sah et al., 2003; Phelps and LeDoux, 2005). In humans, salient speech sounds lead to increased fMRI activation of the amygdala and can involve speech of negative or positive emotion (Fecteau et al., 2007) or from familiar individuals (Andics et al., 2010). Further, the amygdalar response to speech sounds can be modified by context; for example, visual attention modulates fMRI activation by angry voices (Mothes-Lasch et al., 2011). Since contextual modulation often depends on input from multiple sensory systems, it is noteworthy that the majority of auditory responsive neurons tested in the rat amygdala are multimodal, with subpopulations responding to one or often more of the other modalities (Uwano et al., 1995).
The experiments described here investigate the contextual analysis of sounds by the amygdala in normal-hearing, CBA/CaJ mice. We first show that LFH calls emitted during different behaviors are acoustically indistinguishable, then demonstrate in behavioral tests that the attractiveness of these sounds change with the presentation of nonauditory cues. Next, in neurophysiological tests from awake, unrestrained animals, we use the previous finding to investigate whether amygdalar neurons code the context in which the LFH call is presented. We show that across the basolateral amygdala, responses to the LFH call are systematically modified by nonauditory contextual cues in a manner that does not occur in responses to BBN. These findings suggest that amygdalar neurons encode the meaning of the LFH call rather than solely respond to the acoustic structure.
Materials and Methods
Subjects
All procedures were approved by the Institutional Animal Care and Use Committee at the Northeast Ohio Medical University (approval ID number 10-001). Adult CBA/CaJ mice ranging between ages postnatal day (P) 80 and P150 were used for this study (68 males and 37 females). CBA/CaJ mice maintain good hearing thresholds until at least 10 months of age (Zheng et al., 1999). Behavioral and neural data were obtained from male mice. To ensure that these animals had prior experience with social vocalizations, males were each pair-housed with a female for 3 consecutive days in the week before their use.
Vocalization recording
Recordings of mouse vocalizations were obtained in a single-walled acoustic chamber (Industrial Acoustics) lined with anechoic foam. LFH calls were recorded from 13 pairs of animals (26 mice total) during male–female interactions and from an additional 12 females during mild distress. For vocal recordings during mating, mice were placed within an open topped chamber (width, 15 cm; length, 15 cm; height, 30 cm) for which the lower third of one side wall was constructed of wire mesh (for microphone placement). Vocalizations were recorded using two ultrasonic condenser microphones (CM16/CMPA, Avisoft Bioacoustics); one microphone was situated 8 cm above the center of the chamber pointing downward, and the other was situated outside of the wire mesh wall pointing inward. The microphone that picked up the best signal for each vocalization varied depending on the location of the animal. The signals were digitized at 500 kHz with a 16-bit depth (UltraSoundGate, Avisoft Bioacoustics). Gain was adjusted online to prevent signal saturation, while maintaining a good signal-to-noise ratio. Mild distress was induced by hand-restraining the mice and gently poking their whiskers with a cotton applicator. Vocalizations were recorded from a microphone positioned 8 cm in front of the mouse. To determine the number of LFH calls typically heard by a male during sexual experience, the first hour of a male–female mating interaction was recorded from 12 mouse pairs situated within the female's home cage.
Playback experiment
The attractiveness of the LFH call or broadband noise (BBN) was assessed in a Y-maze (arm dimensions: length, 35 cm; width, 5 cm; height, 12 cm) under three different contexts or conditions: neutral, predator, and mating. In the neutral condition, an empty weigh boat was positioned outside the mesh at the end of each arm. In the predator condition, a 1.5 cm ball of cat fur was placed in weigh boats at the end of each arm. Fur was collected weekly from four spayed or neutered cats and mixed to create a generic blend (female calico, 17 years old; female tortoise shell, 5 years old; male orange tabby, 8 years old; female gray and white, 7 years old). Fur was collected by brushing and was stored in an air-tight container. As presented, cat fur provides visual and olfactory cues. Cat fur was chosen as the predator cue because it is an effective aversive stimulus in mice (Garbe et al., 1993). Moreover, fur-derived odors have superior potency as predator cues when compared with urine or feces (Staples et al., 2008). For example, cat fur but not cat urine or feces has been used as a rapid aversive contextual conditioning cue (for review, see Apfelbach et al., 2005).
In the mating condition, 0.3 ml of freshly collected female urine (mixed from at least six gonadally intact females from three cages to control for estrus) was placed on a cotton ball in a fresh weigh boat outside the end of each arm. Urine was collected by holding mice over a clean glass dish; most mice spontaneously expressed urine within 30 s. This was collected from the dish using a syringe and mixed with urine from other mice in an aliquot. Fresh urine was collected for each mouse and was used within 15 min of collection.
The bottom 4 cm of the distal end of all arms of the Y-maze was covered in wire mesh to allow sound to enter the arms. Sounds were presented from speakers situated 5 cm beyond the end of the two mesh-covered arms, Arms 1 and 2. The system response had a gradual roll-off of 3 dB/10 kHz. Harmonic distortion components were not detectable at 55 dB below the maximum signal level. Digital acoustic stimuli were converted to analog signals at 250 kHz and 16-bit depth using SciWorks (DataWave Technologies), antialias filtered, attenuated, amplified, and sent to a loudspeaker (Infinity EMIT-B, Harman International). Sounds were either a 30 ms burst of BBN or an LFH vocalization. The LFH call exemplar was chosen because it was within 1 SD of the mean for both contexts, it was recorded with high signal-to-noise ratio, and it had no overlapping high-frequency song (often described as ultrasonic vocalizations). Each sound was presented with peak level of ∼80 db SPL (15 cm from speaker), a level that approximately corresponds to observed levels of LFH calls in our laboratory.
From a total of 20 mice, 10 were presented with BBN and 10 were presented with the LFH call. Animals were placed in the center of the Y-maze and left to acclimate for 15 min. After the acclimation period, the sound stimulus (either BBN or LFH) was presented at a rate of 1/s for 60 s from the speaker at the end of Arm 1. The same sound then alternated between the two arms for a total of four playbacks, always with a 60 s interval of silence between arm presentations. This sequence of sound was presented during the three contextual conditions in the following order: neutral, predator, mating. At the beginning of each subsequent contextual condition, the mice were allowed to acclimate for 5 min to the new contextual cue before sounds were presented. The animals' location within the arena was recorded using a security camera (VD21W, VideoSec) and tracked using VideoBench software (DataWave Technologies).
Approach behavior in response to the contextual cues was assessed for 11 mice. Animals were introduced to the same Y-maze described above and allowed to acclimate for 15 min. After the acclimation period, a contextual cue (either cat fur or female urine) was presented in a weigh boat at the end of one arm of the Y-maze, while empty weigh boats were placed at the end of the two other arms. The animals' location in the maze was video-tracked for 10 min, then the weigh boat was replaced with a weigh boat holding the other contextual cue. New empty weigh boats were placed at the end of the other two arms of the maze in both contexts. Cat fur was presented first for six mice and female urine was presented first for the remaining five mice. The arm with the contextual cue varied among animals in a clockwise rotation to control for any potential arm biases.
Electrophysiological experiment
Surgical procedures.
Twelve male mice were anesthetized to effect with isoflurane (2–4%; Abbott Laboratories). Depilatory lotion was used to remove fur on the skin overlying the skull. A midline incision was made to bilaterally expose the posterior portion of the nasal plate and the frontal and parietal plates. A tungsten ground electrode was inserted through a small hole in the right nasal plate and cemented into place. A craniotomy, ∼0.5 mm in diameter, was made using stereotaxic coordinates overlying the basolateral amygdala (bregma, −1.75 mm; midline. +3.4 mm) and the underlying dura was removed. An electrode bundle was advanced through a small guide cannula, which was inserted through the skull surface. These were secured in place using dental cement. Custom-made electrode bundles, containing 4–16 electrodes, were either fixed implants or attached to a screw-driven microdrive (dimensions, 14 × 5 × 5 mm; weight, 0.8 g). Electrodes were fabricated from PFA-coated platinum-iridium wire with a diameter of 0.001 inch when bare and 0.0015 inch when coated (AM Systems). Subsequently, topical local anesthetic (lidocaine) and antibiotic cream (Neosporin) were applied to the surgical area. For further pain relief, animals were given 3–5 mg/kg carprofen by subcutaneous injection. Animals were placed in a clean, heated cage to recover. Animals with screw-driven electrode bundles were anesthetized briefly with isoflurane during the next several days to advance the bundle through the brain. After postsurgery day 3, an acrylic training weight was attached to the implant to allow animals to acclimate to the 4.8 g of total weight of the recording system (implant plus wireless headstage/transmitter). Even on the first day, animals carried the weight of the wireless transmitter well. Implanted animals showed normal mating behaviors including vocalizations when placed in the presence of a female.
Acoustic stimulation and electrophysiological recording.
Electrophysiological recordings were made within a single-walled acoustic chamber lined with both radio frequency absorbing (ETS Lindgren) and anechoic foam (Sonex Acoustical). Animals were lightly anesthetized with isoflorane for <1 min and a wireless headstage (Triangle Biosystems) was attached to the implant. Animals were awake and moving within seconds. Mice were placed within a spherical recording arena (circumference, 25 cm) and positioned on a raised platform that was padded with absorbent bedding. The raised floor and outward sloped edges of the arena reduced contact between the wireless headstage and the side walls. When such contact occurred, the resulting high-amplitude noise transient could be removed via spike sorting. An enclosed chamber was used for this study because the animals would not reliably remain on an open, raised platform during presentation of predator cues and communication calls. The glass recording chamber created detectable reverberation lasting <10 ms. Animals were highly active throughout the recording session, as monitored by a webcam. Recordings were terminated if animals showed signs of fatigue.
Physiological signals were recorded continuously at a 36 kHz sampling rate (DataWave Sciworks) and filtered (300–5000 Hz) for spiking activity. Spikes were extracted post hoc using a threshold line at least 4 SDs above the noise floor. To ensure that a unit was only sampled once across the different electrodes, signals were spike-sorted using multielectrode methodology (DataWave Sciworks). Data were collected in the following sequence: background spiking activity (30 s), BBN presentation (1 repetition/s for 100 repetitions at 80 db SPL), background (60 s), LFH call presentation (1 repetition/s for 100 repetitions at 80 db SPL), background (30 s). After this sequence, a predator cue (cat fur) was added to the arena, and after a 5 min rest interval the data collection sequence was repeated. To ensure that there was maximal opportunity for the contextual cues to modulate responses to the control (BBN) stimulus, BBN was always presented before the LFH call. If the mice habituated to the contextual cues, responses to the LFH call would be less affected by the sound than the control BBN sound. For a subset of neurons (42 of 72), recovery was tested by transferring the mouse back to a clean cage and presenting the acoustic stimuli again.
Histology
Two procedures aided visualization of recording location. First, electrodes were coated with a thin, dried layer of India ink before implantation. Second, small electrolytic lesions were made on the last day of recording (10 mA for 5 s) to mark recording sites. After lesions were made, animals were perfused with 4% paraformaldehyde. Sections were Nissl-stained to aid with histological identification of the basolateral amygdala (BLA).
Data analysis
Vocal behavior.
Syllables were detected offline using SASLab Pro 5.1 (Avisoft Bioacoustics). Because LFH calls often overlapped with purely ultrasonic calls produced by the male in the cage, automated thresholds were not used to detect sounds. Instead, call start and end times were manually tagged onto the sound file and used to compute call duration. The fundamental frequency (Fo) was measured automatically (SASLab Pro 5.1) at nine evenly spaced points within each LFH. All recorded LFH calls were included in the analysis so long as they were not distorted by saturation. Statistical analysis was undertaken on the first 10 LFH calls emitted during each recording session. Interactions between context and either Fo or call duration were assessed using ANOVA. Across an additional 12 mouse pairs, the number of LFH calls emitted during the first of two 1-h-long male–female encounters was calculated, and a mean computed.
Playback experiment.
Each 60 s period of sound presentation was treated as a single trial, for which we computed the percentage of time an animal spent in the arm where sound was being presented. The attractiveness rating for each context was calculated as the average time spent in the sound arm for four trials. We then tested the hypothesis that attractiveness is context-dependent and sound-dependent using a two-way ANOVA. Post hoc analyses used Fisher's least-significant difference test. Approach behavior in response to the contextual cues alone was compared using a paired samples t test. The test compared the amount of time animals spent in the arm with each contextual cue during the second half of the 10 min sound presentation period.
Neural response analyses.
For each neuron, an average spike waveform was computed and the peak-to-peak duration was measured. The distribution of the spike durations was examined for evidence that the population could be split into presumptive interneuron or projection neuron groups. The presence of subpopulations was determined using the coefficient of bimodality (b); a value exceeding 0.55 indicates a multimodal distribution (García-Cano et al., 2008). Mean background firing rate was computed from 10 ms bins of the 30 s prior of BBN presentation in neutral and predator contexts. For each neuron, average firing rate was assessed in 10 ms intervals during the 1 s recording window for each neuron. Because t tests revealed that 78% of neurons showed significant modulations in background firing with the introduction of a predator cue, the 200 ms before the onset of each sound was used to compute background discharge for measures of auditory-evoked responses. A neuron was considered sound-responsive if it fulfilled one or more of the following criteria: (1) a spike rate during at least one 10 ms bin from the first 150 ms that was 2 SDs above the background; (2) a spike rate that was 2 SDs above background for two consecutive 10 ms bins anywhere within the 1 s recording window; (3) a spike rate that was 2 SDs below background for two consecutive 10 ms bins. The number of excitatory or inhibitory phases was computed using a 10 ms hold time at the end of each phase. If the response continued after this hold time, it was considered part of the preceding phase. The start of the first phase and the end of the last phase were used to compute response duration.
To compare response magnitudes, a rate modulation index (RMI) was computed: [RMI = (evoked rate − background rate)/(evoked rate + background rate)]. The RMI was calculated for two poststimulus windows, 0–50 and 60–200 ms, after sound onset. Positive RMI values represent excitatory responses, values close to zero represent no response, and negative values represent inhibitory responses (Eliades and Wang, 2008). RMI represents the detectability of the sound stimulus (Eliades and Wang, 2008); this test is appropriate to use when background rates differ between conditions within neurons. To test for systematic shifts in amygdala responses, RMI values were compared between contexts using Wilcoxon signal ranks. Absolute RMI values were used as a measure of signal detectability regardless of inhibition or excitation. To examine the time course of contextual information, mutual information was computed in 50 ms bins using the direct method of the “information breakdown toolbox” (Magri et al., 2009) and using Panzeri and Treves's bias correction (Panzeri et al., 2007).
Results
The acoustic features of the LFH call are context-independent
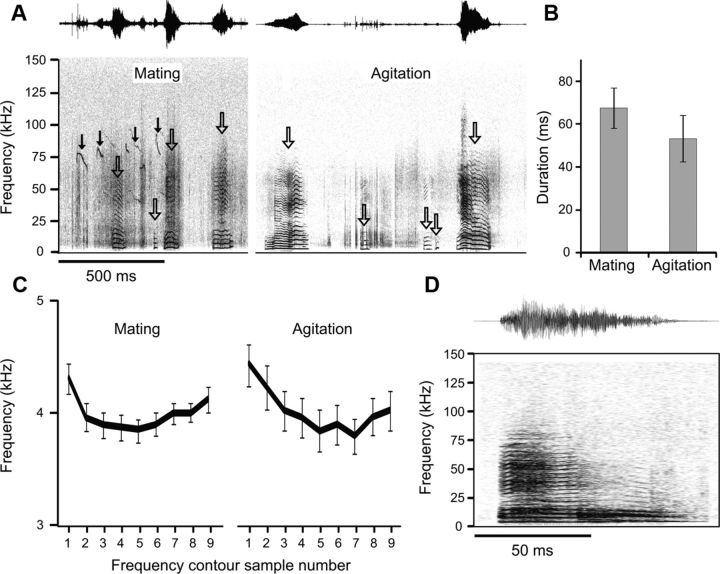
We first obtained recordings of LFH calls that were emitted under different behavioral contexts. From 13 male–female pairs of reproductively mature mice, we recorded LFH calls emitted by females (Fig. 1A) across a 1 h period. While we cannot rule out the possibility that some of the LFH calls may have been produced by male mice, this seems unlikely as bouts of LFH calls often co-occurred with bouts of mating song (Fig. 1A). In a second experiment, we recorded experimenter-induced distress vocalizations (“agitation”) from a different set of female mice (Fig. 1A). Even within the same context, consecutively emitted LFH calls showed a high degree of variability in measures of call duration and bandwidth. Comparisons of the spectrotemporal characteristics of the LFH call revealed no contextual differences. The mean call duration for LFH calls did not differ between the two contexts (Fig. 1B; independent samples t test, t(1,249) = 1.71, p = 0.088), nor was there a difference in the fundamental frequency contour for the LFH calls emitted in the two behavioral contexts (Fig. 1C; t test minimum p value = 0.096). A comparison of the first 10 calls and the last 10 calls emitted in the mating context revealed no significant difference indicative of changes in the behavioral state of the females over time (duration independent samples t test, t(1,319) = 1.6, p = 0.114; mean fundamental frequency independent samples t test, t(1,319) = 0.026, p = 0.980). These findings indicate that the LFH call itself does not carry the contextual information about threats or mating to the male listener. We therefore used a single exemplar of the LFH call (Fig. 1D) to test for contextual effects on behavioral and neural responses.
Figure 1.
LFH calls emitted by female mice do not differ with context. A, Spectrograms and amplitude envelopes of two series of LFH calls emitted during mating and agitation. Open arrows indicate LFH calls. Both long and short LFH calls were emitted in both contexts. During the mating context, ultrasonic mating vocalizations likely produced by the male mouse are also observed (solid arrows). B, Mean and 95% confidence intervals for duration of LFH calls in the two contexts. There were no significant contextual differences. C, Mean and 95% confidence intervals of the frequency contours of LFH calls emitted during mating and agitation. D, Spectrogram and amplitude envelope of the exemplar used for the study of approach behavior and for amygdalar electrophysiology.
Approach behavior of male mice to the LFH call is modulated by nonauditory context
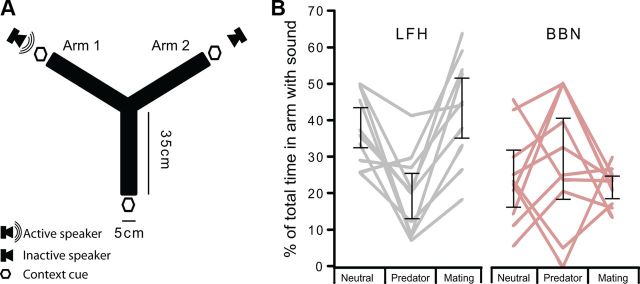
The attractiveness of LFH calls and BBN was assessed in sexually experienced male mice in a behavioral approach test. All males were familiar with the LFH call in a mating context; during the first hour of their sexual experience period, females emitted an average of 210 ± 97 LFH calls (mean ± SD). To assess how multisensory, nonauditory cues might modulate the attractiveness of the LFH call, we placed sexually experienced male mice in the center of a Y-maze (Fig. 2A). Sounds were played from one arm of the maze, while contextual cues (clean weigh boat, cat fur, or female urine) were presented from the ends of all three arms, and were therefore considered omnidirectional. We then recorded the amount of time the mice spent in each arm during 60 s trials. The mouse spent significantly more time in the arm from which the LFH call was presented when in the presence of female urine compared with cat fur. This pattern was consistent across mice (Fig. 2B). A two-way ANOVA revealed that the sound stimulus (BBN or LFH) had a main effect on the percentage of time mice spent in the arm where sound was being presented (F(5,54) = 6.8, p = 0.012); this accounted for 11% of the variance in the attractiveness of the sound. Overall, the LFH call was significantly more attractive than BBN (LFH mean percentage time in sound arm: 33.7%, SE 2.3; BBN mean: 25.2%, SE 2.3; LSD post hoc p = 0.012), and BBN was generally aversive. There was no significant main effect of nonauditory context on the proportion of time mice spent in the arm where sound was being presented (p = 0.113). There was a significant interaction between stimulus and context (F(2,54) = 8.9, p < 0.001); 24% of the variance in attractiveness was explained by an interaction between sound type and the nonauditory context. Post hoc LSD analyses on the interaction between sound type and nonauditory context on the attractiveness of the sound stimulus revealed the following: in the neutral context, the LFH call was significantly more attractive than BBN (mean difference, 14%, p = 0.016); in the predator context they were aversive to a similar extent (p = 0.078); and in the mating context the LFH call was significantly more attractive than BBN (mean difference, 22%, p < 0.001).
Figure 2.
Approach behavior of male mice to the female LFH call, but not to BBN, is context-dependent. A, Schematic of the Y-maze. Contextual cues were presented simultaneously from the end of each arm, while sound was presented from either Arm 1 or Arm 2 during a given trial. B, Attractiveness of acoustic stimuli under different contexts. Gray or pink lines indicate, for individual mice, the percentage of total time the animal spent in the arm of the Y-maze from which the sound originated. Error bars represent 95% confidence intervals of the mean. The LFH call was significantly more attractive to male mice in the neutral and mating conditions than it was in the predator condition. The approach behavior for BBN was not significantly modulated by nonauditory cues.
The univariate test showed that there were significant contextual differences in the attractiveness of the LFH call (F(2,54) = 9.9, p < 0.001), but not BBN (p = 0.363). The LFH call was attractive in the mating context and mildly aversive in the predator context (23% difference, p < 0.001). Further, the LFH was more attractive in the neutral context than in the predator context (18% more attractive, p = 0.002). There was no significant difference in the attractiveness of the LFH call between the neutral context and the mating context (p = 0.342). The attractiveness of the LFH call in the neutral context may be due to the males' recent prior experience with the LFH call in a mating context. These findings indicate that BBN is aversive to male mice regardless of the surrounding context, but that the attractiveness of the LFH call can be modulated from attractive to aversive by nonauditory contextual cues.
Contextual cues alone significantly affected the behavior of male mice within the Y-maze. Mice avoided the arm from which cat fur was presented, but were attracted to the same arm of the maze when female urine was presented. Mice spent an average of 22.5% (SD, 17%) of their time in the arm with cat fur, compared with 54.0% (SD, 35%) of their time in the arm with female urine. A comparison of approach behavior to the contextual cues alone revealed that mice were significantly more attracted to female urine than they were to cat fur (t(10) = 2.758, p = 0.020).
Neural recordings in the basolateral amygdala
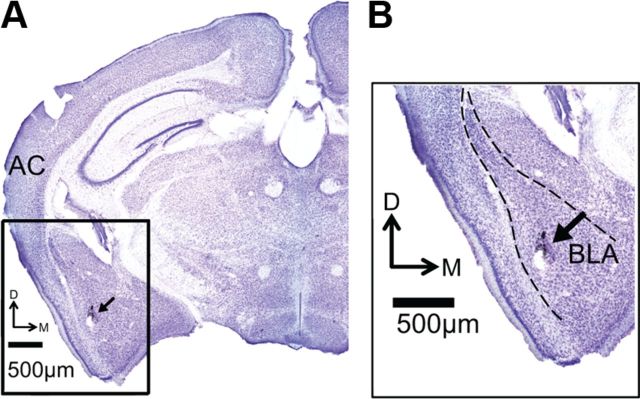
Using multielectrode arrays, we recorded spiking activity from 72 single neurons in the basolateral amygdala of awake, unrestrained, sexually experienced male mice. Background firing, assessed during the 30 s before presentation of the first acoustic stimulus, was bimodally distributed (b = 0.75; Peak 1: median, 1.2 Hz; Peak 2: median, 6.0 Hz). These firing rates are consistent with established firing rates of neurons in the BLA (Buffalari and Grace, 2009; Gadziola et al., 2012b), and localization in the BLA was histologically confirmed for each experiment (Fig. 3). Although previous work has shown that amygdalar interneurons generally have higher spontaneous firing rates and narrower spike widths than do projection neurons (Bienvenu et al., 2012), we found only a unimodal distribution of peak-to-peak spike width (b = 0.40) and no association between spike width and background firing rate (Pearson's r = 0.38, p = 0.783). As a result, our subsequent analyses considered the neurons as part of a single population.
Figure 3.
Histological verification of electrode placement. A, Nissl-stained coronal section shows electrode track marked by India ink deposit (black arrow) and small lesion at the end of the electrode track. B, View of amygdala at higher magnification. Dashed line marks borders of the BLA. AC, Auditory cortex.
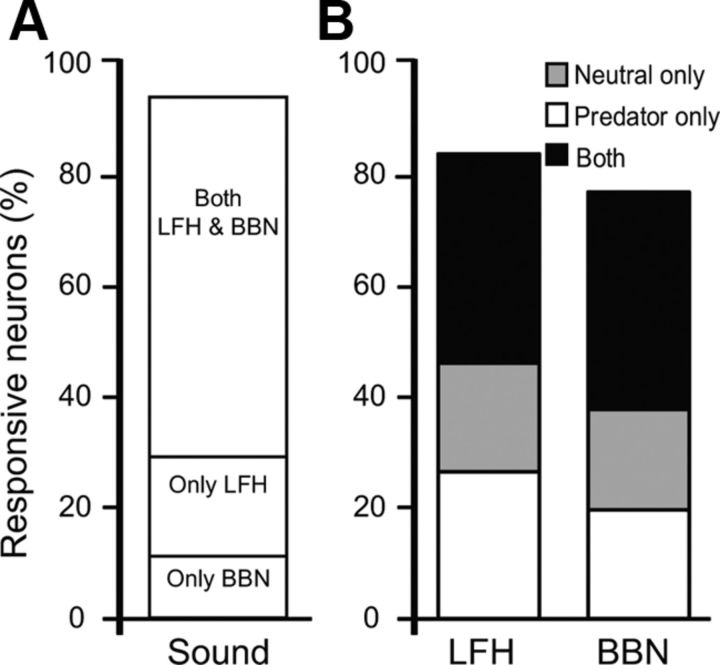
We tested responses to LFH and BBN sounds under two nonauditory contexts: neutral and predator. The mating context was not used because male mice vocalized in response to female urine, confounding the analysis of auditory responses (Grimsley et al., 2011; Roullet et al., 2011). Neurons were considered to be auditory-responsive if firing rates in 10 ms bins changed by >2 SDs from the background activity recorded immediately before the auditory stimulus (see Experimental procedures). Responses could be either excitatory or inhibitory, but note that suppression is difficult to detect with low background firing rates. Based on these criteria, 94% (or 68 of 72) of the neurons responded to either LFH or BBN stimuli under at least one of the test conditions, and nearly two-thirds of the neurons responded to both acoustic stimuli (Fig. 4A). Furthermore, approximately half of the neurons responding to either sound did so under both contexts, while the remainder responded during only one of the contexts (Fig. 4B).
Figure 4.
Auditory responsiveness of amygdalar neurons in different contexts. A, The majority of the recorded neurons responded to both the LFH and BBN sounds. B, Similar proportions of auditory-responsive BLA neurons responded to LFH calls and BBN sounds in each context. Approximately half of auditory-responsive neurons were context-selective, only responding to a stimulus in one context.
The spike discharge patterns of acoustically responsive neurons were diverse (Fig. 5). Both excitatory and inhibitory responses were common for both the LFH call and BBN, and within the same neuron these patterns were often modulated by the presence of the predator cue. Some neurons, like the one illustrated in Figure 5A, showed a combination of excitation and inhibition. This neuron's response was only slightly modulated by context, principally in the excitatory response to the LFH call. For the neuron in Figure 5B, the predator cue had a more complex effect, enhancing an early response and reducing a later response to the LFH call, while strongly suppressing the entire response to BBN. In other neurons (Fig. 5C), the predator cue revealed a suppressive effect of acoustic stimulation. Finally, many neurons displayed a strongly enhanced response to the LFH call in the presence of the predator cue, with no corresponding enhancement to BBN responses (Fig. 5D). Despite this diversity, our subsequent analyses revealed strong population patterns in the effect of the predator cue on acoustic responses.
Figure 5.
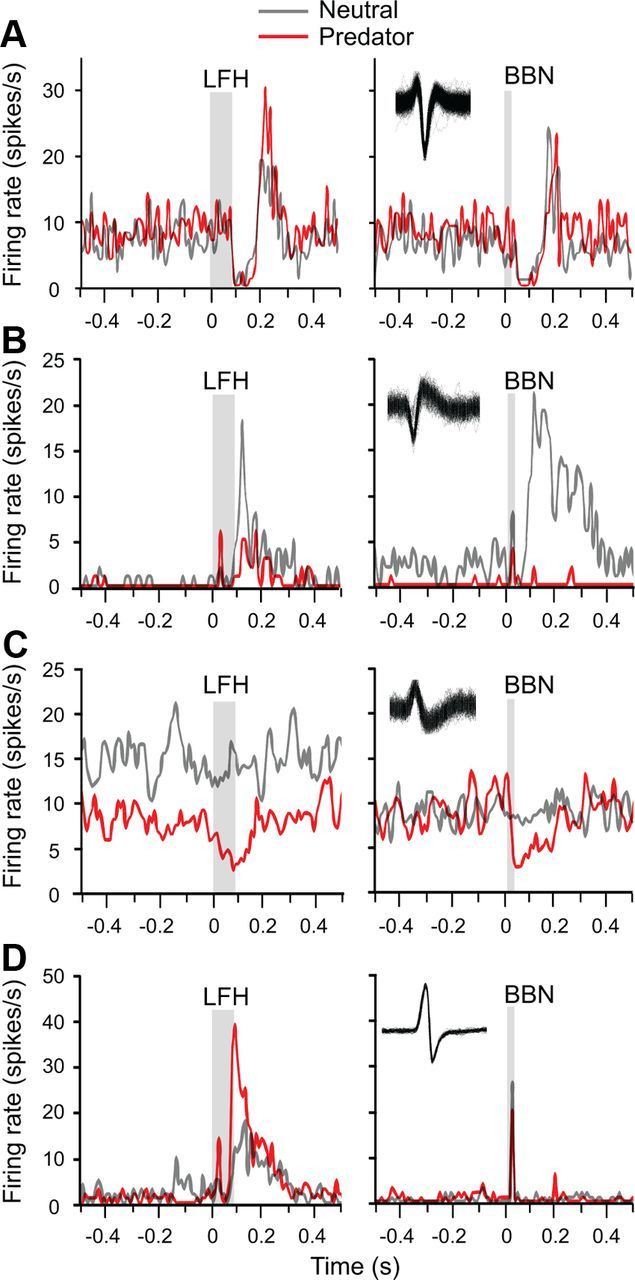
Auditory responses and contextual effects are diverse among amygdalar neurons. Results from four neurons plotted as peristimulus time histograms of responses to the indicated acoustic stimulus in two contexts: neutral (gray) and predator (red). The gray bars represent the duration of the acoustic stimulus. For each neuron, inset shows overlapping spike waveforms. A, This neuron's inhibitory–excitatory auditory response pattern was only weakly modulated by context. B, An early excitatory response was present for the LFH call only in the presence of the predator cue; the later acoustic responses were suppressed by the predator cue. C, Lack of auditory response in the neutral context changed to a suppressive response in the predator context. D, The predator cue substantially increased both the early and the later response to the LFH call. Among all neurons, note the long duration of many responses relative to stimulus duration.
Context dependency of auditory responses in the amygdala
The major finding in neural recordings is that BLA neurons as a population showed differential effects of nonauditory context on their response patterns to different acoustic stimuli. The neuron in Figure 6 exemplifies many of the features of the population response. In particular, the neuron's response to the LFH call shifts from tonic suppression (neutral context) to a strong, early excitation with elevated persistent firing in the predator context (Fig. 6A,B, left). The discharge in the 200 ms before each presentation of the LFH call was significantly higher in the presence of the nonauditory predator cue (mean discharge: LFH neutral, 10.9; SD, 1.9; LFH predator, 12.4, SD 2.9; t(99) = 2.8, p = 0.005). A mutual information analysis for 50 ms time bins (Fig. 6C, left) shows that the neuron carried contextual information throughout its response to the LFH call, but peak information occurred early in the response. In contrast, the neuron's response to the BBN sound was virtually the same in neutral and predator contexts (Fig. 6A,B, right). The discharge in the 200 ms before each presentation of BBN was similar in both contexts (mean discharge: BBN neutral, 10.2, SD 3.7; BBN predator, 9.8, SD 2.6). No information about context was carried in the response to the BBN sound (Fig. 6C, right).
Figure 6.
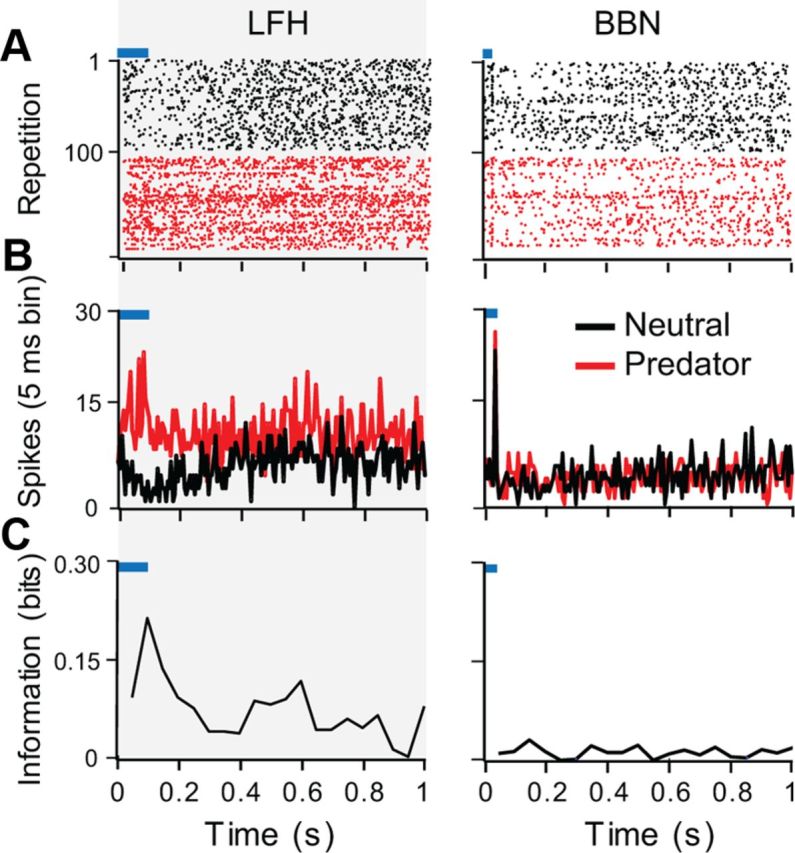
Analysis of contextual modulation of auditory-evoked activity in a single neuron. This neuron epitomizes a common effect of predator context to increase early excitation to the LFH but not to the BBN sound. A, Raster plots showing responses to the indicated acoustic stimulus in the neutral (black) and predator (red) contexts. The blue bars represent the timing of the acoustic stimulus. B, Peristimulus time histograms with 10 ms sliding windows showing the pooled responses across trials in the neutral (black) and predator (red) contexts. C, Mutual information values plotted in 50 ms time windows. This unit had more contextual information in its response to the LFH call than in the response to BBN.
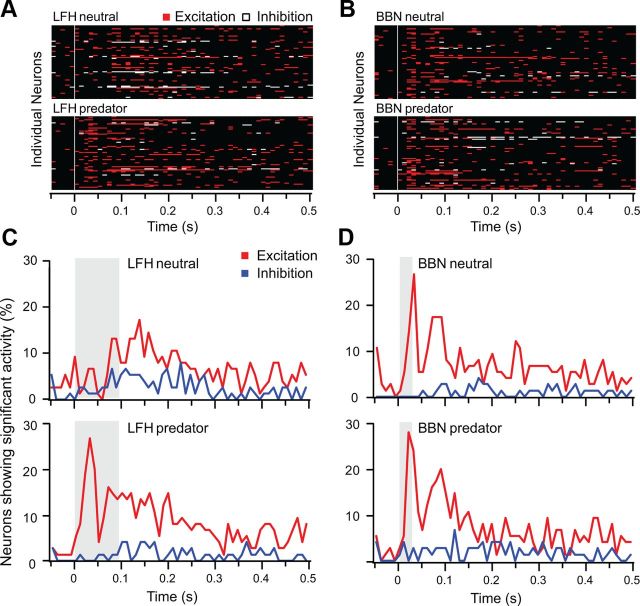
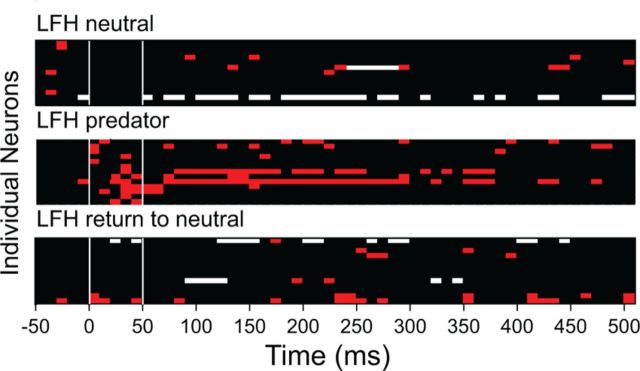
Although the background discharge of neurons was typically modulated by context, 78% of neurons showed significant modulation. There was no consistent pattern of modulation; across the sample of 72 amygdalar neurons, the predator cue could change the response to either LFH or BBN sounds. Further, the overall effects of context on measures of response rate and mutual information did not differ for LFH and BBN responses (Fig. 7A). Nonetheless, there was a systematic, context-dependent shift in the overall population response to the LFH call that was not evident in the BBN responses. Figure 8A,B presents an overview of the responses of each of the 72 sampled neurons; excitatory periods of the response are represented with red bins and inhibitory periods are represented with white bins. For the response to the LFH call (Fig. 8A), there is a striking shift in the response pattern with context. That is, a large number of neurons gained an excitatory response during the initial 50 ms after LFH stimulus onset when combined with a predator cue. By contrast, there is very little contextual change in the overall pattern of response to BBN. Overall, the temporal response patterns of excitation and inhibition were very similar for the LFH predator, BBN neutral, and BBN predator conditions (Fig. 8C,D). Only the LFH neutral condition showed a distinct pattern. This corresponds well with the assessment of approach behavior; BBN was aversive in the neutral context, whereas the LFH call was attractive. Thus, the early excitatory response was only present for those stimuli that were behaviorally aversive. The effect of the predator cue on responses to the LFH call was reversible. Recovery was tested on 42 of 72 neurons by returning the animal to a clean chamber. Of these, 15 of 42 neurons showed a shift to excitation in the first 50 ms for the LFH call when presented in the predator cue context, and 13 of those exhibited recovery (Fig. 9).
Figure 7.
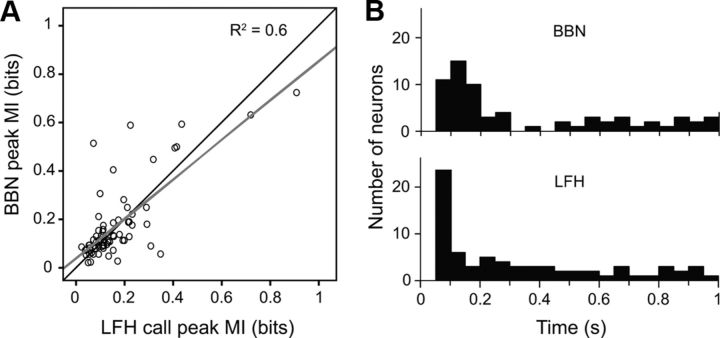
Mutual information values show context-dependent modulation of auditory responses in amygdalar neurons. A, Comparison of contextual mutual information values for LFH and BBN responses revealed no consistent difference in the degree of contextual information in LFH and BBN responses. B, Timing of maximum contextual mutual information for BBN and LFH responses. For BBN responses, most neurons carried maximum contextual information in the first 200 ms after BBN onset. For LFH responses, the majority of neurons held maximum contextual information in the first 50 ms after LFH onset.
Figure 8.
Stimulus-specific contextual modulation of activity among BLA neurons. A, B, These plots represent excitation (red), inhibition (white), or no response (black) in 10 ms periods for each of 72 BLA neurons under four acoustic/context combinations. Each neuron's responsiveness (as defined in Materials and Methods) is plotted along a line; the same order of neurons is maintained in the four plots. White vertical lines indicate stimulus onset. Across the population, early excitation to the LFH call was substantially increased in the presence of the predator cue (A), while no similar effect was observed for BBN responses (B). C, D, Proportions of BLA units showing excitation (red) or inhibition (blue) in 10 ms bins following stimulus onset. Gray bars indicate timing of stimuli. C, Few neurons display early responses to LFH in the neutral context, but many more neurons show early responses in the predator context. D, The population responsiveness to BBN did not change substantially with different contexts; in both contexts there is a substantial early responsiveness component.
Figure 9.
Modulation of LFH responses by a predator cue is reversible. As in Figure 8, these plots represent LFH-evoked excitation (red), inhibition (white), or no response (black) in 10 ms periods for 15 BLA neurons tested for recovery from effects of a predator cue. Of the 42 neurons tested for recovery, these 15 showed a shift to excitation for LFH in the first 50 ms when in the predator context. Thirteen of the 15 neurons exhibited recovery with return to neutral context.
We show that this overall pattern of change is significant by analyzing several features of the response. For instance, a mutual information analysis showed that contextual information is carried earlier in the response to the LFH call than in the response to BBN (Fig. 7B). Although background firing rates in 78% of neurons differed with context (see Materials and Methods), the majority of neurons held their maximum contextual information in the sound-locked portion of their response. This indicated that the majority of neurons show modulation in their responses to sound that goes beyond their response to the nonauditory contextual cue alone. In the sections below, we further show that there is a significant shortening of response latency for the LFH call in the presence of a predator cue that did not generalize to responses to BBN. Finally, we compare a measure of response rate in each context to reveal a significant, systematic shift toward excitation in the early portion of the response to the LFH call in the presence of predator cues.
Contextual changes in response latency
Across the amygdala, an early excitatory period is evident in responses to the LFH call (predator context) and the BBN stimulus (both contexts; Fig. 8). The absence of early excitation in responses to the LFH call under the neutral context is indicated by a longer mean response latency in this condition (Fig. 10A). A one-way ANOVA revealed a significant main effect of stimulus condition on response latency (F(3,169) = 6.4, p < 0.001). The mean latency for LFH responses was 35 ms shorter in the presence of the predator cue [clean (mean ± SD): 78 ± 69 ms; predator: 43 ± 36 ms, p < 0.001]. The mean latency for BBN responses did not change with context, and was similar to the latency of response to the LFH call in the predator context (clean: 49 ± 52 ms; predator: 32 ± 29 ms, p > 0.2 for all post hoc comparisons). Because response duration was highly variable among neurons, a corresponding shift in the duration of LFH responses with context was not observed (Fig. 10B). It is noteworthy that the duration of responses did not differ significantly for the two sound stimuli even though the LFH call was longer in duration than the BBN stimulus (98 vs 30 ms). In all conditions, the mean amygdalar responses outlasted the acoustic stimulus (LFH clean: 127 ± 139 ms; LFH predator: 154 ± 171 ms; BBN clean: 104 ± 123 ms; BBN predator: 169 ± 167 ms).
Figure 10.
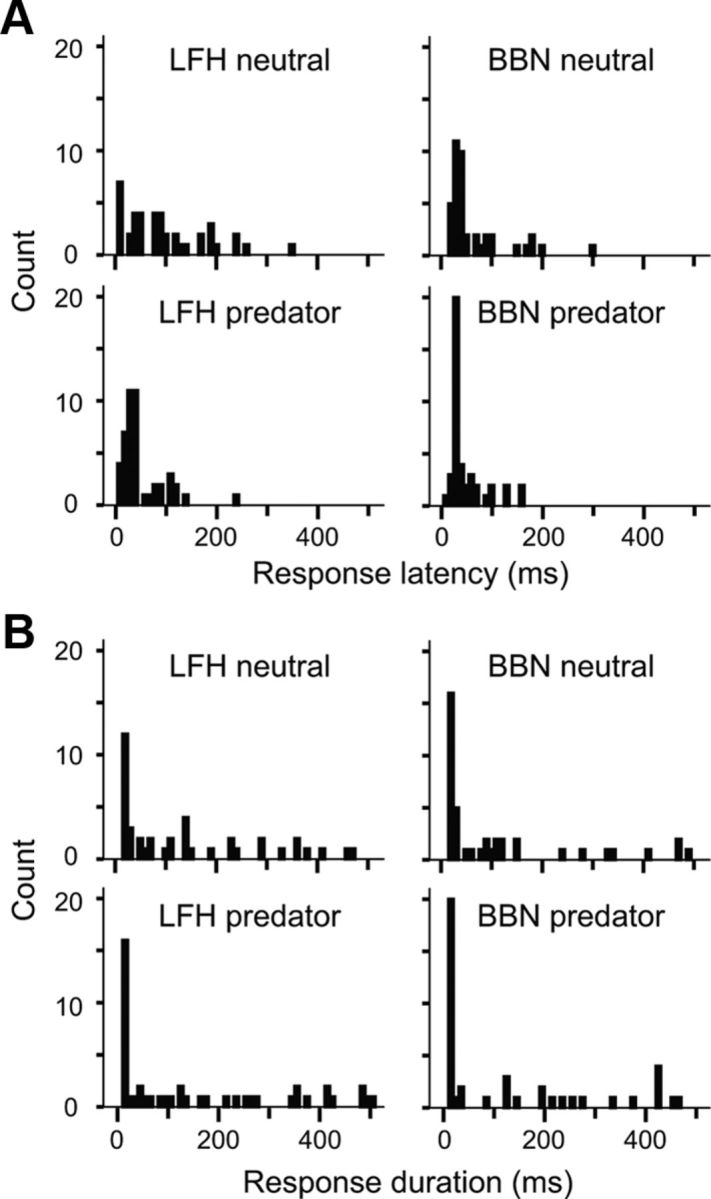
Temporal characteristics of BLA responses. A, Response latencies for LFH and BBN stimuli are shown in 10 ms bins. Latencies were significantly longer for the LFH call in the neutral context than for the LFH call in the predator context or for the BBN in either context. B, Response durations for LFH and BBN stimuli are shown in 10 ms bins. Response durations did not differ significantly between contexts.
Modulation of response rate by stimulus context
The population analyses of amygdalar activity (Fig. 8) and response latency (Fig. 10) suggest that nonauditory contextual cues modulate early responsiveness to the LFH call differently than later responsiveness, and that contextual modulation is less systematic in BBN responses. To examine whether this is reflected in response magnitude measures, we computed an RMI for each neuron's response during an early response period (0–50 ms after stimulus onset) and a later response period (60–200 ms). The RMI expresses firing rate relative to background with values ranging from −1 (strong suppression) to +1 (strong excitation) and serves as a measure of sound-evoked detectability independent of context-evoked changes in background firing.
Examination of context-dependent changes in RMI values support our major finding that, as a population, BLA neurons show differential effects of stimulus context on the responses to different acoustic stimuli. Figure 11 shows how response rates to the LFH call and BBN, expressed as RMI values, changed with context in both early and later response periods. Clearly, many neurons show changes in responsiveness as the result of the predator cue. However, the only significant pattern of change across the sample of BLA neurons occurred for LFH responses during the first 50 ms of the response (Wilcoxon signed rank test: Z(72) = −2.8, p = 0.006). During the early part of the LFH response, 42 of 72 neurons had a greater RMI value in the predator context, 20 neurons had a lower RMI value, and 10 neurons had RMI values that did not differ contextually. Two of the example units in Figure 5B,D exhibit the predominant type of modulation of early responses to the LFH call. Early excitation was typically absent in the neutral context but present in the predator context. These results support analyses in Figures 8 and 10 that indicate that the major effect of a predator cue within BLA was to increase early excitation to the LFH call. Further, this effect was differential for responses to different acoustic stimuli, since no significant modulation of the population was observed in responses to BBN.
Figure 11.
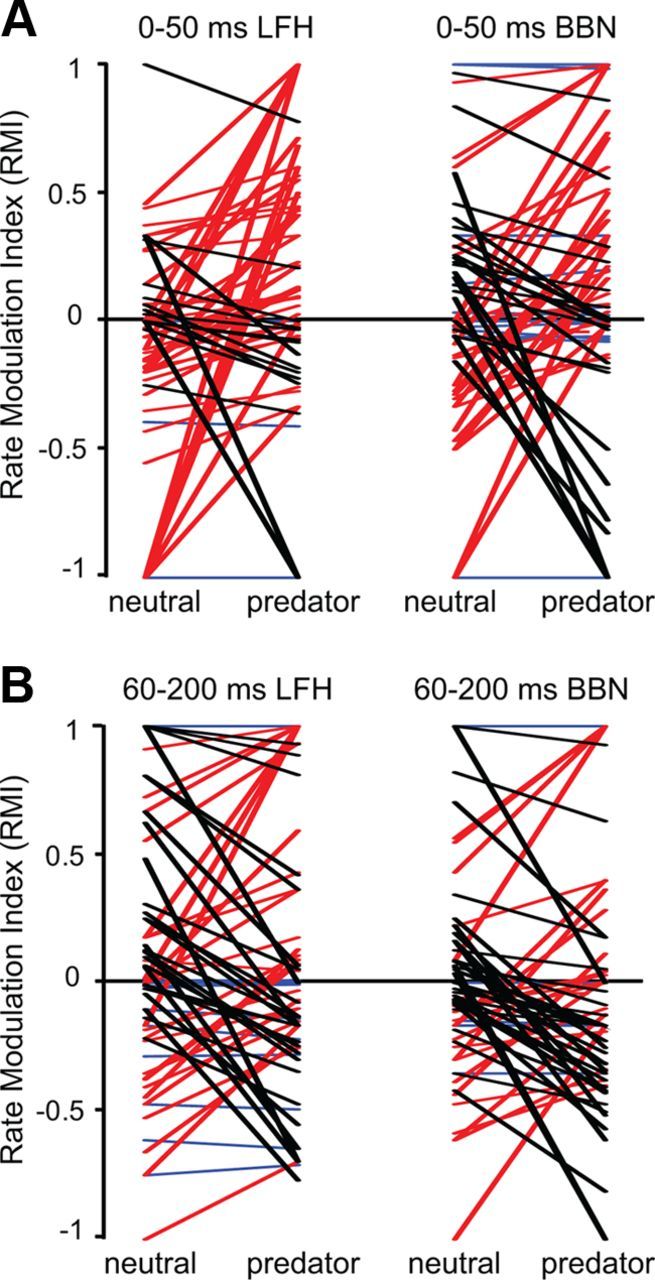
Context-dependent modulation of auditory response rate in amygdalar neurons. The RMI expresses auditory-evoked firing rate; positive values reflect excitation and negative values reflect inhibition. For individual neurons, lines indicate the changes in RMI values with stimulus context; red lines represent neurons displaying a shift toward more positive RMI values in the predator context, black lines represent a negative RMI shift, and blue lines represent no change in RMI with predator context. A, In the early (0–50 ms) window, most neurons showed increased RMI values in response to the LFH call when the predator cue was presented, with many shifting from suppression to excitation. Across the population, the pattern of change in the BBN responses with a predator cue was not significant. The average magnitude of change did not differ between the LFH and the BBN responses. B, In the late (60–200 ms) window, there was no systematic direction of change in RMI for the LFH or the BBN responses when the predator cue was presented.
Discussion
Female mice produce the same LFH call in two distinct behavioral contexts: during mating (Wang et al., 2008) and when threatened or injured (Williams et al., 2008). How does a male mouse interpret this call and respond appropriately? The present study addressed this question using a combination of acoustical, behavioral, and neural analyses. We showed that LFH calls emitted by females during mating interactions with males are not acoustically distinct from those that females emit when agitated by an experimenter. We then demonstrated that males use nonauditory cues to determine the valance of the LFH call; male mice approach LFH calls both in a clean context and in the presence of female mouse urine, but they avoid the LFH call when a nondirectional predator cue is present. Importantly, this effect does not generalize to noncommunication sounds, such as BBN. In neural recordings, we investigated whether neurons in the BLA responded to the LFH call in a manner that reflected its valence. There were two major results. First, the population response of BLA neurons displays early excitation to all forms of behaviorally aversive stimuli tested. Second, context modulates the population response of the BLA to the LFH call but not to a noncommunication sound. These results indicate that the meaning of communication calls and other acoustic signals are reflected in the spike discharge patterns of BLA neurons.
The context of natural and artificial sounds
Prior experience affects both behavioral and neural responses to pup calls in mice (Liu et al., 2006). This long-lasting change caused by motherhood, or pup experience in virgins, affects how the auditory cortex represents these sounds. We demonstrate a similar but rapid and contextually driven change in how mice respond to a communication call behaviorally and neurally. We show that the LFH call is emitted by female mice in two distinct contexts, with no statistical difference in acoustic features. To male mice, however, even the single exemplar that we used is interpreted differently depending on the nonauditory cues that are present. Thus, the LFH call is neutral or slightly attractive to sexually experienced males whether or not the odor of a female mouse is present. In the presence of predator cues, the same LFH call becomes aversive. In male mice, then, the valence of affect evoked by the LFH call is established by nonauditory cues. It is unusual in animal vocalizations for the same communication call to shift so dramatically in meaning for the listener; it thus provides a useful stimulus for studying the neural encoding of meaning. The effect of predator cues on responses to the LFH call does not generalize to the other acoustic stimulus; a predator cue has no consistent effect on the attractiveness of BBN bursts like it has on LFH call attractiveness. This suggests that brain mechanisms influencing the attractiveness of the LFH call have little effect on the attractiveness of BBN.
Amygdalar coding of contextual information in sounds
The amygdala contributes to an analysis of the significance of sensory signals and to the orchestration of emotional responses to those signals (Cardinal et al., 2002; Price, 2003; Sah et al., 2003; Phelps and LeDoux, 2005). Much of the understanding of amygdalar function comes from conditioning experiments, including auditory fear conditioning. A key feature of auditory fear conditioning is that the animal, and amygdalar neurons, respond differently when a previously neutral acoustic signal is paired with an aversive somatosensory signal (Quirk et al., 1995; Herry et al., 2008). As a result of the conditioning, BLA neurons display enhanced short-latency responses to aversively conditioned tones compared with responses to neutral tones (Quirk et al., 1995; Herry et al., 2008). This modulation in the early response can be represented by a reduction in the latency of the response in the lateral amygdala.
Our study shows that this enhanced short-latency response to aversive stimuli is a general feature of BLA neurons that does not depend on conditioning. In our results, inherently aversive stimuli (BBN) evoked early discharge regardless of the nonauditory stimulus present. In contrast, behavioral tests show the LFH call is not inherently aversive in males. In agreement, the LFH call did not generally evoke early excitation among BLA neurons. However, when the LFH call was paired with an innately aversive predator cue, the sound became behaviorally aversive and, correspondingly, BLA neurons as a population displayed an early excitatory response. Our results further show that both the behavioral effect of the pairing of LFH call and predator cues, and the BLA response to the pairing, are rapidly reversible. This provides a strong argument that the early excitation is not due to conditioning, but rather is a general underlying coding principle of BLA responses: the meaning of sounds, both communication calls and synthetic stimuli, are coded in the spike discharge patterns of BLA neurons.
The contextual meaning of LFH calls allowed us to evaluate another element of amygdalar responses to vocal communication signals: that these neurons respond predominantly to vocalizations with negative affect. In rats, most BLA neurons show excitatory responses to 22 kHz vocalizations (Parsana et al., 2012) that are predominantly produced during negative, distressing situations, such as social defeat (Thomas et al., 1983) or predation (Blanchard et al., 1991). In contrast, a separate population of BLA neurons shows predominant suppression when presented with 55 kHz signals (Parsana et al., 2012) that are produced under only positive behavioral contexts, such as play or mating (Knutson et al., 2002; Brudzynski, 2005). In the present study, we show that the same amygdalar neurons often respond to the LFH call in both neutral and negative contexts. In fact, many neurons showed a stronger late response to the LFH call in the neutral context. These results suggest a greater complexity in the amygdalar response to vocal communication signals, in which neurons respond to a broad range of social calls but maintain special elements of their responses to signals with negative affect.
This complexity is supported by our recent study in big brown bats (Gadziola et al., 2012a). Most BLA neurons responded to most of the 10 syllables presented, whether these were associated with aggressive or appeasing social situations (Gadziola et al., 2012b). However, the neurons discriminated well among these syllables, and generally responded with stronger and longer discharge to syllables associated with negative affect. Amygdalar responses to nonlinguistic emotional vocalizations with both positive and negative affect are also observed in the large-scale fMRI activation patterns in humans (Fecteau et al., 2007).
Implications of context-dependent amygdalar activation
Parsana et al. (2012) proposed that early, onset-like portions of BLA responses may be involved in the triggering of motor circuits that control freezing. Further, contextual differences in the activity of the BLA may modulate the internal state of the animal. These contextual spiking patterns may send different messages to the central amygdala, modulating positive affect responses or hormones involved in sexual arousal by differentially activating arousal centers and the cholinergic system.
This contextual difference in spike discharge pattern also has the potential to affect multiple sensory systems. Neurons in the BLA tend not to be modality-specific (Uwano et al., 1995); the amygdala receives inputs from brain areas that separately analyze each modality (auditory, olfactory, gustatory and visceral, somatosensory, visual), but also projects back to many of these areas (for review, see Sah et al., 2003). Observations in a variety of species suggest that principal areas involved in coding each of these domains are subject to influences from other senses (Taylor-Clarke et al., 2002; Bizley and King, 2008; Nishimura and Song, 2012). The amygdala could modulate the ascending auditory system via its direct projections to the inferior colliculus in bats (Marsh et al., 2002), or through its direct and indirect projections to auditory cortices (Amaral and Price, 1984; McDonald and Jackson, 1987; Yukie, 2002).
Conclusion
The mouse LFH call by itself has ambiguous meaning when emitted during mating and distress. We show that nonauditory cues modulate an animal's response to a single example of this call, and this is matched by corresponding modulation of the population response of BLA neurons. Specifically, there is a rapid and reversible shift in the neural code that reflects the changing meaning of the communication call. Behaviorally aversive stimuli, but not appetitive stimuli, evoke early excitation across the BLA, even though the BLA responds to the call in both contexts. It would be interesting to see whether this effect generalizes across different examples of the LFH call. The amygdala is implicated in many disorders that are characterized by deficits in the emotional coding of sounds, and mouse models have been developed for the study of these, including autism (Jamain et al., 2008; Patterson, 2011), schizophrenia (Shen et al., 2008), and tinnitus (Longenecker and Galazyuk, 2011). Understanding how the amygdala brings together information from multiple modalities to determine the emotional valence of communication signals provides a useful tool for further research into the emotional encoding of multisensory events in these disorders.
Footnotes
This work was supported by Research Grant R01 DC00937-22 and Supplement 19S1 (J.J.W.) from the National Institute on Deafness and Other Communication Disorders of the U.S. Public Health Service. We thank C. Magri, K. Whittingstall, V. Singh, N.K. Logothetis, and S. Panzeri for use of their information breakdown toolbox for mutual information analysis (Magri et al., 2009). We also thank S. Shanbhag for his help in integrating these data with the information breakdown toolbox. Thanks also go to M. Gadziola for comments on the manuscript.
The authors declare no competing financial interests.
References
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Andics A, McQueen JM, Petersson KM, Gál V, Rudas G, Vidnyánszky Z. Neural mechanisms for voice recognition. Neuroimage. 2010;52:1528–1540. doi: 10.1016/j.neuroimage.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Berryman JC. Guinea-pig vocalizations—their structure, causation and function. Z Tierpsychol. 1976;41:80–106. doi: 10.1111/j.1439-0310.1976.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Magill PJ, Ferraguti F, Capogna M. Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron. 2012;74:1059–1074. doi: 10.1016/j.neuron.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, King AJ. Visual-auditory spatial processing in auditory cortical neurons. Brain Res. 2008;1242:24–36. doi: 10.1016/j.brainres.2008.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-L. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/S0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Endler JA. Some general-comments on the evolution and design of animal communication-systems. Philos Trans R Soc Lond B Biol Sci. 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Belin P, Joanette Y, Armony JL. Amygdala responses to nonlinguistic emotional vocalizations. Neuroimage. 2007;36:480–487. doi: 10.1016/j.neuroimage.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Gadziola MA, Grimsley JM, Faure PA, Wenstrup JJ. Social vocalizations of big brown bats vary with behavioral context. PLoS One. 2012a;7:e44550. doi: 10.1371/journal.pone.0044550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadziola MA, Grimsley JM, Shanbhag SJ, Wenstrup JJ. A novel coding mechanism for social vocalizations in the lateral amygdala. J Neurophysiol. 2012b;107:1047–1057. doi: 10.1152/jn.00422.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe CM, Kemble ED, Rawleigh JM. Novel odors evoke risk assessment and suppress appetitive behaviors in mice. Aggressive Behav. 1993;19:447–454. doi: 10.1002/1098-2337(1993)19:6<447::AID-AB2480190605>3.0.CO%3B2-Y. [DOI] [Google Scholar]
- García-Cano E, Resende RO, Boiteux LS, Giordano LB, Fernández-Muñoz R, Moriones E. Phenotypic expression, stability, and inheritance of a recessive resistance to monopartite begomoviruses associated with tomato yellow leaf curl disease in tomato. Phytopathology. 2008;98:618–627. doi: 10.1094/PHYTO-98-5-0618. [DOI] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS One. 2011;6:e17460. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Whittingstall K, Singh V, Logothetis NK, Panzeri S. A toolbox for the fast information analysis of multiple-site LFP, EEG and spike train recordings. BMC Neurosci. 2009;10:81. doi: 10.1186/1471-2202-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci. 2002;22:10449–10460. doi: 10.1523/JNEUROSCI.22-23-10449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- Mothes-Lasch M, Mentzel HJ, Miltner WH, Straube T. Visual attention modulates brain activation to angry voices. J Neurosci. 2011;31:9594–9598. doi: 10.1523/JNEUROSCI.6665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Song WJ. Temporal sequence of visuo-auditory interaction in multiple areas of the guinea pig visual cortex. PLoS One. 2012;7:e46339. doi: 10.1371/journal.pone.0046339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Senatore R, Montemurro MA, Petersen RS. Correcting for the sampling bias problem in spike train information measures. J Neurophysiol. 2007;98:1064–1072. doi: 10.1152/jn.00559.2007. [DOI] [PubMed] [Google Scholar]
- Parsana AJ, Li N, Brown TH. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav Brain Res. 2012;226:77–86. doi: 10.1016/j.bbr.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Modeling autistic features in animals. Pediatr Res. 2011;69:34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann NY Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wöhr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2011;216:19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Meaning and emotion in animal vocalizations. Ann NY Acad Sci. 2003;1000:32–55. doi: 10.1196/annals.1280.004. [DOI] [PubMed] [Google Scholar]
- Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, Chatzi C, He S, Mackie I, Brandon NJ, Marquis KL, Day M, Hurko O, McCaig CD, Riedel G, St Clair D. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Taylor-Clarke M, Kennett S, Haggard P. Vision modulates somatosensory cortical processing. Curr Biol. 2002;12:233–236. doi: 10.1016/S0960-9822(01)00681-9. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Takahashi LK, Barfield RJ. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus-Norvegicus) J Comp Psychol. 1983;97:201–206. doi: 10.1037/0735-7036.97.3.201. [DOI] [PubMed] [Google Scholar]
- Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–361. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WO, Riskin DK, Mott KM. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci. 2008;47:8–10. [PMC free article] [PubMed] [Google Scholar]
- Yukie M. Connections between the amygdala and auditory cortical areas in the macaque monkey. Neurosci Res. 2002;42:219–229. doi: 10.1016/S0168-0102(01)00325-X. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/S0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]