Abstract
OBJECTIVE:
To test whether weight and the weight gain rate during different age periods are associated with being overweight/obese at 10 years of age.
METHODS:
A nested case-control study was performed in a clinical historic cohort that was selected based on medical records from the Albert Einstein Hospital Social Program in São Paulo, Brazil. A sample of 378 eutrophic and overweight/obese children was analyzed.
RESULTS:
After adjusting for birth weight and gestational age, the likelihood of being overweight/obese at 10 years of age was 4.04-fold greater when progressing from one quartile of weight gain to the immediately superior quartile in the first semester of life and 3.24-fold greater when this occurred from 2-5 years of age. A one-quartile change in weight gain in the first semester was associated with a 0.5 z-score increase in BMI at age 10. A robust independent effect of weight at age 5 confirmed that earlier weight gain was an important predictor.
CONCLUSIONS:
The amount of weight gain during the first 6 months of life and between 2 and 5 years of age and weight at age 5 were important predictors of overweight/obesity at 10 years of age.
Keywords: Obesity, Weight Gain, Weight, Pediatrics, Low Income
INTRODUCTION
In the last three decades, a possible link between low birth weight and noncommunicable chronic diseases, such as obesity, in adulthood has often been discussed in the literature and remains very controversial. In their studies, Barker et al. (1,2) have suggested that a tendency toward obesity in adulthood may be “programmed” in utero or during childhood. It is not clear whether it is the prenatal or postnatal growth rate that is more predictive of future obesity (3).
In the Hertfordshire cohort, weight at 1 year of age was strongly and inversely associated with obesity and cardiovascular disease (CVD) in adults (4). In the Helsinki cohort, men who developed obesity and CVD had low birth weights, remained at low weights during childhood, and presented an increased weight gain rate after 5 years of age (5).
In cohort studies with adolescents, low birth weight, which was associated with high weight gain rate in the first year of life, was positively correlated with an elevated body mass index (BMI) (6-7). This association was stronger when concurrent with improved social economic conditions or migration from rural to urban areas (8).
Few studies on this topic have been published about the development of obesity in regions in which malnutrition is historically prevalent (8-9). These transitional nutrition regions are very relevant to this type of research because these areas contain a large number of children with low birth weights who are further exposed to diets containing foods of low cost, high caloric value, and low nutrient quality, which may increase the incidence of obesity and diseases associated with metabolic syndrome (9).
The present study aimed to assess whether weight and the weight gain rates during different age periods (0-6 months, 6-12 months, 12-24 months, 2-5 years, and 5-10 years) are associated with overweight/obesity at 10 years of age.
METHODS
Population and sampling
A nested case-control study designed within a historic clinical cohort was based on medical records from the Primary Health Center of the Einstein Hospital Social Program in the Paraisopolis slum. The studied children were born between October 1998 and August 1999. Data were collected in August 2009.
This social program covers children between 0 and 10 years of age who live in the Paraisopolis slum. Children attend the clinic for medical appointments, during which periodic anthropometric measurements are taken.
The inclusion criterion was to have been followed up from birth to 10 years of age. The exclusion criteria were the presence of major comorbidities (neurological or endocrine disease) and the presence of malnourishment at 10 years of age. This last criterion was considered a possible marker of a current but undiagnosed disease and was defined as being below the -2.00 z-score of the WHO reference curve (10).
The outcome was excess weight (being overweight/obese) at age 10. The proportions of cases and controls were established by their natural distribution in the studied population. All subjects came from the same poor neighborhood and shared similar social and cultural conditions.
The eligible population comprised 378 subjects. Complete data for every studied period, however, were present in only 147 of the records. Supposing a probability of having an alpha error of 5%, a prevalence of overweight/obesity at 10 years of 25%, and a prevalence of exposure of 25% (being in the highest quartile of weight gain), the power of this final sample would be 80%. The complete data group was compared with the missing data group to test for selection bias. The chi-square test and the Wilcoxon rank test were used for this purpose, given the nonparametric distribution.
The present study was submitted to and approved by the Research Ethics Committee of the Hospital Israelita Albert Einstein.
Study protocol
The study protocol consisted of collecting data from the children's medical records, including sociodemographic information, gestational history, family history, and physical exam results at 6 months and 1, 2, 5, and 10 years of age.
Anthropometric measurements
To measure their body weights, the adolescent participants stood on a platform-type scale without clothes or with only light clothing. A Filizola® brand scale (São Paulo, SP, Brazil) with a minimum/maximum capacity of 2.5/150 kg and an accuracy of 50 g was used. Heights were measured with a stadiometer that was attached to the wall, and these measurements were taken to the closest 0.1 cm (11).
Anthropometric index
BMI was calculated by dividing a participant's weight in kilograms by their height in meters squared. At age 10, obesity was diagnosed when a participant's BMI was greater than or equal to the 2.00 z-score of the WHO reference. Overweight was diagnosed when the BMI was between the 1.00 and 2.00 z-scores of the WHO reference (10). For participants younger than 5 years of age, the cut-off points for the same reference were higher: 2.00 and 3.00 for overweight and obesity, respectively (10).
Statistical analysis
The z-scores for the birth weights and BMIs in each age group were calculated using WHO-Anthro v3.0.1 and WHO AnthroPlus v1.0.2 software (Geneva, Switzerland). Descriptive analyses were performed using EPI-INFO v3.4.3 software (Atlanta, GA, USA).
The main exposure variable was weight gain between different age periods, which was analyzed according to quartiles. The other explanatory variables were birth weight and prematurity. A forward stepwise logistic regression model was built, in which being overweight or obese was the dependent variable. A linear regression model was also built, in which the BMI at age 10, treated as a continuous variable, was the dependent variable.
Because a given weight depends on weight gain during the period immediately before, the independent association of weight at each age with the outcome was tested through linear regression. To overcome the difficulty of modeling highly correlated variables, such as weights in sequential age groups, we used conditional weight variables, which represent weight at a given age in a way that is uncorrelated with earlier weight measures. This technique works with the residuals and provides the necessary independence to build a regression model. This methodology has been proposed for cohort studies (12). STATA 10.0® (College Station, TX, USA) was used for all modeling analyses.
RESULTS
A description of the sample is presented in Table 1. There was no significant difference between the entire eligible cohort and the studied cohort.
Table 1.
Description of the characteristics of the eligible population and the studied subsample.
| Variable | Category or unit | Eligible subjects (n = 378) | Studied subsample (n = 147) | p-value |
| Gender | ♂ | 50.8 | 44.9 | 0.225 |
| Gestational age | preterm | 4.9 | 6.1 | 0.576 |
| Delivery | vaginal | 66.5 | 68.9 | 0.832 |
| cesarean | 28.8 | 27.4 | ||
| forceps | 4.7 | 3.7 | ||
| Excess weight prevalence | overweight | 16.7 | 19.1 | 0.518 |
| obesity | 5.2 | 6.1 | 0.895 | |
| Exclusive breastfeeding | months | 2 (0.54-4) | 2 (0-4) | 0.247 |
| Birth weight | kg | 3.11 (2.82-3.5) | 3.09 (2.8-3.4) | 0.069 |
| BMI 6 months | kg/m2 | 17.89 (16.73-18.77) | 17.93 (16.82-18.81) | 0.952 |
| BMI 1 year | kg/m2 | 17.63 (16.64-18.66) | 17.68 (16.87-18.6) | 0.367 |
| BMI 2 years | kg/m2 | 16.44 (15.62-17.26) | 16.54 (15.73-17.41) | 0.112 |
| BMI 5 years | kg/m2 | 15.4 (14.62-16.29) | 15.37 (14.43-16.3) | 0.903 |
| BMI 10 years | kg/m2 | 16.53 (15.60-17.99) | 16.36 (15.3-18.51) | 0.121 |
BMI = body mass index; categorical variables reported as the% and tested with chi-square; continuous variables reported as the median and interquartile interval and tested with the Wilcoxon test.
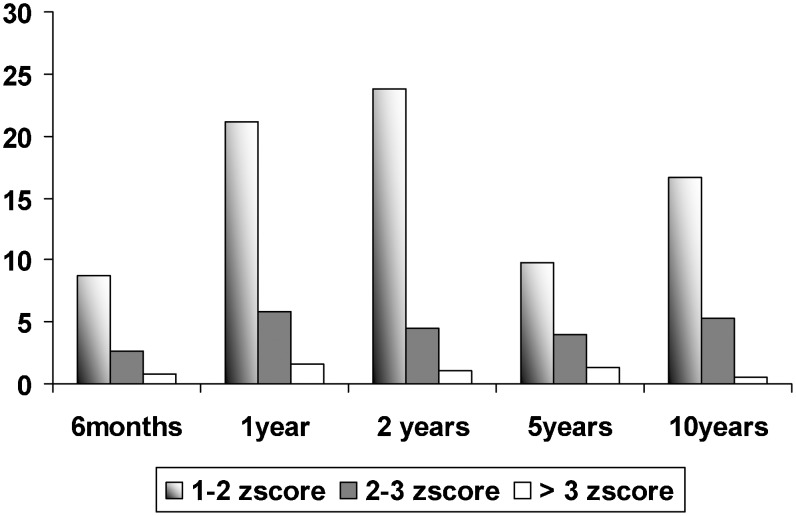
Figure 1 shows the prevalence rates of overweight/obesity at different ages in the same subjects. At 10 years of age, the rates were 16.7% and 5.8%, respectively. Together, these two rates resulted in an excess weight rate of 22.5%. At ages 1, 2, and 5, the estimated frequency of excess weight was 7.4%, 5.6%, and 11.5%, respectively.
Figure 1.
Distribution of overweight/obesity in different ages among the studied subjects.
The social condition data for this population indicate that half of the children's mothers remained at home and did not provide any source of family income, that 70% of the fathers had temporary work, and that 20% of the families lived in precarious homes that were made of wood.
In Table 2, it can be observed that after adjusting for birth weight and gestational age, the likelihood of being overweight/obese at 10 years of age was 4.04-fold greater when progressing from one quartile of weight gain to the immediately superior quartile in the first semester of life, 1.96-fold greater in the second semester, 3.24-fold greater from 2-5 years of age, and 2.98-fold greater from 5-10 years. In the linear regression, a one-quartile change in weight gain in the first semester was associated with a 0.5 z-score increase in BMI at age 10.
Table 2.
Univariate and multivariate analyses of the association between weight gain in different age periods and overweight/obesity at 10 years of age (n = 147).
| Weight Gain Period†) Logistic regression | Univariate Analyses | Multivariate Analyses*) | ||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Birth to 6 months | 1.30 | 0.94-1.81 | 0.112 | 4.04 | 2.01-8.15 | <0.000 |
| 6-12 months | 1.22 | 0.88-1.69 | 0.235 | 1.96 | 1.15-3.32 | 0.013 |
| 1-2 years | 1.32 | 1.01-1.73 | 0.042 | 1.26 | 0.80-1.98 | 0.323 |
| 2-5 years | 1.69 | 1.33-2.14 | <0.001 | 3.24 | 1.93-5.42 | <0.001 |
| 5-10 years | 1.75 | 1.38-2.22 | <0.001 | 2.98 | 1.79-4.95 | <0.001 |
| Linear regression | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Birth to 6 months | 0.12 | -0.01-0.24 | 0.064 | 0.50 | 0.36-0.63 | <0.000 |
| 6-12 months | 0.10 | -0.03-0.22 | 0.128 | 0.27 | 0.15-0.38 | <0.000 |
| 1-2 years | 0.23 | 0.12-0.34 | <0.000 | 0.21 | 0.11-0.31 | <0.000 |
| 2-5 years | 0.25 | 0.17-0.33 | <0.001 | 0.39 | 0.29-0.49 | <0.000 |
| 5-10 years | 0.26 | 0.17-0.34 | <0.001 | 0.30 | 0.20-0.41 | <0.000 |
Weight gain in quartiles; in the logistic regression, OR indicates the risk of progressing from one quartile to the immediately superior quartile; in the linear regression, the coefficient indicates by how many z-score units the BMI at age 10 would increase by progressing from one quartile to the immediately superior quartile.
Adjusted reciprocally and for birth weight and prematurity.
The conditional weight analysis is shown in Table 3. For each age, weight was independently associated with the outcome. The regression coefficients were 0.35, 0.54, and 0.89 at 1, 2, and 5 years, respectively. A robust independent effect of weight at age 5 confirmed that early weight gain was an important predictor of future excess weight.
Table 3.
Linear multiple regression to test the independent association between weight at each age (transformed into conditional weight variable) and later overweight/obesity.
| Coefficient | Overweight/obesity, age 10, 95% CI | p-value | |
| Birth weight | 0.011 | -0.07-0.09 | 0.782 |
| Weight at 1 year | 0.35 | 0.26-0.45 | <0.001 |
| Weight at 2 years | 0.54 | 0.37-0.70 | <0.001 |
| Weight at 5 years | 0.89 | 0.76-1.03 | <0.001 |
Weight analyzed as conditional weight, i.e., standardized residual representing greater than expected weight in the prior interval.
DISCUSSION
In the present study, which was performed in a low socioeconomic region of São Paulo, a high rate of weight gain in an early-weaning infant's first 6 months of life was strongly associated with obesity at 10 years of age, as was weight gain between 2 and 5 years of age and weight at age 5.
In Figure 1, the range between 1.00 and 2.00 z-scores in our curve shows a progressive trend in the first 2 years of life. These children are considered normal according to the reference WHO standard. However, according to this same standard, our curve is shifted to the right. This finding indicates a higher prevalence of excess weight in later years.
The most recent national study (Family Income Survey, 2008-2009) (13) reported prevalences of overweight/obesity according to BMI of 33.5%/14.3% among children who were 5-9 years old and 20.5%/4.9% among adolescents who were 10-19 years old. Our determined prevalence of overweight/obesity was threefold lower than this reported value in children who were 5 years of age (9.8%/5.3%). A possible explanation for this discrepancy is that our estimate reflects the nutritional status at 5 years of age, whereas the national study refers to the 5- to 9-year age range and does not report estimates for younger children. In addition, in the present study, the prevalence of overweight/obesity at 10 years of age (22.5%) corresponded to the prevalence observed in the general Brazilian population (14). This rate indicates the presence of an obesity epidemic that is comparable with that of developed countries, which have a prevalence of overweight/obesity of 22% (15).
In the present study, the variable that assessed dietary habits in early life was breastfeeding. Our subjects received exclusive breastfeeding for an average of 2.0 months. National data have shown that the Brazilian population averages 2 months of exclusive breastfeeding (16). In other countries, the duration of exclusive breastfeeding and the timing of introducing new foods have been shown to vary. The pattern depends on cultural factors, economic factors, and maternal motivations. For example, it is known that in Italy, 35% of mothers introduce foods in addition to breast milk before 4 months of age (17); in Germany, 16% of mothers introduce foods in addition to breast milk before 3 months (18); and in the United Kingdom, 51% of mothers who were surveyed in 2005 followed the same pattern before their children were 4 months old (19).
The studies of Koletzko (20) and Taveras (21) have emphasized that feeding in the first years of life may contribute more than birth weight to future obesity, as determined by the programming hypothesis, which has recently been renamed as the developmental origins of health and disease (DOHaD). In our study, rapid and excessive weight gain in the first 6 months of life was associated with a 4.04-fold higher risk of obesity at 10 years of age than for children who remained in the first quartile. Regarding the critical period for weight gain in childhood and adolescence, our results are similar to the findings reported by Wells in the Pelotas cohort (9) and by Taveras in the Viva Project cohort (21). Other authors have determined that rapid weight gain in the first 3 months of life is associated with several factors that are linked to CVD and diabetes in early adulthood (22). Moreover, rapid changes in BMI between 6 and 24 months of age predict a higher CVD risk at age 4 (23).
Interestingly, our results suggest that a different age group than that identified in previous studies is at risk of future excess weight. In the present study, rapid weight gain between 2 and 5 years of age was associated with a 3.24-fold higher risk of overweight at 10 years of age. Normally, the first 2 years of life are considered to be critical for a child's healthy development. However, children between 2 and 5 years old are not routinely assessed via primary care visits and do not receive sufficient care or nutritional information from health staff or their families. The period between 2 and 5 years is rarely assessed in the literature, although certain studies have detected an association between rapid weight gain in this period and later weight excess ()(24-25). More recently, a pooled analysis of data from five low- and middle-income countries showed that weight in mid-childhood was an important predictor of adult obesity (26). Therefore, obesity prevention programs should consider that children in their first 5 years of life deserve special, differentiated attention compared with other age groups, and these children should be the focus of new public policies.
Another finding that supports the previous observation is that the linear regression that used weights at different ages after transformation into conditional weight variables showed a significant effect on later excess weight. In any regression analysis, an important assumption is that the variables are independent. We know that weight at age 10 depends on weight at age 5, which in turn depends on weight at age 2, and so forth, indicating that the variables are not independent. Recently, conditional analysis was proposed to solve this problem (12). In our analysis, we demonstrated that a higher weight at each point in time was independent and had a more robust effect at age 5. This last finding confirms the importance of weight gain in the period immediately before, i.e., between 2 and 5 years of age.
This paper offers two different and complementary pieces of information. The first is that rapid weight gain during specific periods in childhood is associated with a greater risk of obesity in adolescence. Second, greater weight at age 5 is an important predictor of obesity at age 10.
The main limitation of our study was the risk of selection bias, given the missing information for part of the randomly selected sample. To attempt to overcome this bias, we demonstrated that the sample and subsample did not differ statistically when the main variables were compared. We do not know whether this is also true of other unmeasured characteristics. In addition, breastfeeding information was only provided for exclusive breastfeeding; we did not have data on the introduction of other foods. Because the study data were extracted from medical records, we were unable to include information that was not routinely collected during patient appointments.
Although our sample cannot be considered representative of the entire population of the city of São Paulo, the results of our study identified two critical age periods during which rapid weight gain put children at a higher risk of obesity in early adolescence, 0-6 months and 2-5 years, together with weight at age 5 years. In low-income populations, which are likely exposed to inadequate nutrition during the gestational period, factors that may be associated with feeding during the first year of life, including early weaning and the introduction of new foods, may favor the development of metabolic programming in adolescence.
The rate of weight gain during the first 6 months of life and between 2 and 5 years of age, together with weight at age 5, were important predictors of overweight/obesity at 10 years of age in children from a low-income community in São Paulo, Brazil.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25(5):735–40. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes MTB, Sesso R, Martins PA, Sawaya AL. Increased blood pressure in adolescents of low socioeconomic status with short stature. Pediatr Nephrol. 2003;18(5):435–9. doi: 10.1007/s00467-003-1117-1. [DOI] [PubMed] [Google Scholar]
- 3.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. BMJ. 1999;319(7204):245–9. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJP, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who later have coronary event. N Engl J Med. 2005;353(170):1802–9. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 6.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of african americans. Am J Clin Nutr. 2003;77(6):1374–8. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro POA, Victora CG, Barros FC, Monteiro LMA. Birth size, early childhood growth, and adolescent obesity in a brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27(10):1274–82. doi: 10.1038/sj.ijo.0802409. [DOI] [PubMed] [Google Scholar]
- 8.Forsdahl A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease. Br J Prev Soc Med. 1977;31(2):91–5. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells JCK, Hallal PC, Wright A, Singhal A, Victora CG. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes. 2005;29(10):1192–8. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 10.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisancho RA. The University of Michigan Press; 1990. In: Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor. [Google Scholar]
- 12.Adair LS, Martorell R, Stein AD, Hallal PC, Sachdev HS, Prabhakaran D, et al. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: when does weight gain matter. Am J Clin Nutr. 2009;89(5):1383–92. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antropometria e Estado Nutricional de Crianças, Adolescentes e Adultos no Brasil. 2010 Pesquisa de Orçamentos Familiares 2008-2009. Rio de Janeiro: Ministério da Saúde, IBGE. [Google Scholar]
- 14.Antropometria e Análise do Estado Nutricional de Crianças e Adolescentes no Brasil. 2006 Pesquisa de Orçamentos Familiares 2002-2003. Rio de Janeiro: Ministério da Saúde, IBGE. [Google Scholar]
- 15.Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr. 2002;75(6):971–7. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]
- 16.Segall-Corrêa AM, Marín-Léon L. Amamentação e Alimentação. 2008:242–51. In: PNDS 2006- Pesquisa Nacional de Demografia e Saúde da Criança e da Mulher. Brasília/DF: Ministério da Saúde. p. [Google Scholar]
- 17.Giovannini M, Riva E, Banderali G, Scaglioni S, Veehof SH, Sala M, et al. Feeding practices of infants through the first year of life in Italy. Acta Paediatr. 2004;93(4):492–7. doi: 10.1080/08035250410025591. [DOI] [PubMed] [Google Scholar]
- 18.Koletzko B, Dokoupil K, Reitmayr S, Weimert-Harendza B, Keller E. Dietary fat intakes in infants and primary school children in Germany. Am J Clin Nutr. 2000;72(Suppl 5) :1392S–8S. doi: 10.1093/ajcn/72.5.1392s. [DOI] [PubMed] [Google Scholar]
- 19.Bolling K, Grant C, Hamlyn B, Thornton A. Infant feeding survey 2005. 2007 PL London: PN The Information Centre, National Health Service. [Google Scholar]
- 20.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, et al. Can infant feeding choices modulate later obesity risk. Am J Clin Nutr. 2009;89(5):1502S–8S. doi: 10.3945/ajcn.2009.27113D. [DOI] [PubMed] [Google Scholar]
- 21.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leunissen RWJ, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301(21):2234–42. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 23.Corvalán C, Uauy R, Stein AD, Kain J, Martorell R. Effect of growth on cardiometabolic status at 4 y of age. Am J Clin Nutr. 2009;90(3):547–55. doi: 10.3945/ajcn.2008.27318. [DOI] [PubMed] [Google Scholar]
- 24.Nader PR, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118(3):e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 25.Botton J, Heude B, Maccario J, Ducimetière P, Charles MA the FLVS Study group. Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. Am J Clin Nutr. 2008;87(6):1760–8. doi: 10.1093/ajcn/87.6.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzawa CW, Hallal PC, Adair L, Bhargava SK, Fall CHD, Lee N, et al. Birth weight, postnatal weight gain, and adult body composition in five low and middle income countries. Am J Hum Biol. 2012;24(1):5–13. doi: 10.1002/ajhb.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]