Abstract
Macrophages are rapidly conditioned by cognate and soluble signals to acquire phenotypes that deliver specific functions during inflammation, wound healing and angiogenesis. Whether inhibitory CD200R signaling regulates pro-angiogenic macrophage phenotypes with the potential to suppress ocular neovascularization is unknown. CD200R-deficient bone marrow derived macrophages (BMMΦ) were used to demonstrate that macrophages lacking this inhibitory receptor exhibit enhanced levels of Vegfa, Arg-1 and Il-1β when stimulated with PGE2 or RPE-conditioned (PGE2-enriched) media. Endothelial tube formation in HUVECs was increased when co-cultured with PGE2-conditioned CD200R−/− BMMΦ, and laser-induced choroidal neovascularization was enhanced in CD200R-deficient mice. In corroboration, signaling through CD200R results in the down-regulation of BMMΦ angiogenic and pro-inflammatory phenotypes. Translational potential of this pathway was investigated in the laser-induced model of choroidal neovascularization. Local delivery of a CD200R agonist mAb to target myeloid infiltrate alters macrophage phenotype and inhibits pro-angiogenic gene expression, which suppresses pathological angiogenesis and CNV development.
Severe and sudden visual impairment in patients with age-related macular degeneration (AMD) characterized by choroidal neovascularization (CNV), occurs where pathological angiogenesis extends from choriocapillaris into the subretinal space and retina1,2. Recent revised clinical definitions of AMD describe early, intermediate and late disease phases, based on the appearance and size of drusen formation at the RPE/Bruch's membrane interface3. Drusen are immunologically active deposits containing oxidative lipids, complement and other potential immune activating components that develop in response to environmental stress and altered tissue homeostasis4,5,6. Drusen are considered to be precursors of advanced disease, namely RPE dysfunction, death and ultimately geographic atrophy. Whilst the precise mechanisms that initiate the dominant VEGF drive of CNV in AMD remain elusive, the switch towards the neovascular form of disease may occur during any of the clinical stages of AMD.
Although patients with AMD do not display signs of overt ocular inflammation, it is recognized that both innate and adaptive immune responses contribute to the pathogenic pathways in AMD7. The accumulation of myeloid cells, especially macrophages adjacent to and within drusen occurs in all clinical stages of disease, but in particular a larger number are often present at the time of CNV1,4. The function and phenotype of macrophages is conditioned by signals encountered within the tissue microenvironment, and in mouse, the paradigm of M1 and M2 macrophages has been studied with respect to angiogenesis8,9. Classical activation generates M1 macrophages (nitric oxide synthase 2, NOS2+) which have pro-inflammatory functions, whereas alternatively activated M2 macrophages (Arginase-1, Arg-1+) confer responses related to wound healing, and are capable of generating VEGF and promoting angiogenesis. However, pathological angiogenesis is observed most commonly in the presence of M2 macrophages10,11 that generate VEGF either through conditioning by Th2 cytokines, IL-4 or IL-138, or via PGE2 signaling12.
Despite conflicting evidence from studies investigating potential mechanisms whereby macrophage phenotype contributes to CNV progression, the general consensus is that activated macrophages, on the background of a highly complex and dynamic ocular microenvironment are central to the process6,13,14,15,16,17. The role of macrophages driving a VEGF-dependent angiogenic response is supported by recent evidence from studies using the laser-induced CNV model that show early initiation of choroidal angiogenesis is dependent upon macrophage phagocytosis of damaged RPE components which elicits an Arg-1+ VEGF+ M2 phenotype18. These data support the view that targeted interventions that modulate myeloid activation or protect against RPE death may prove beneficial for prevention of these early events that trigger CNV.
The CD200:CD200R interaction provides a normal homeostatic control mechanism by which CD200 binds to the inhibitory CD200R receptor expressed predominantly on cells of myeloid lineage, to limit potentially damaging pro-inflammatory responses19,20. CD200 is expressed abundantly within the eye, on retinal vascular endothelium, neurons and RPE, and is known to regulate myeloid cell activation21,22. Furthermore, previous studies demonstrate that modulating macrophage responses through CD200R with a receptor specific agonist, DX109, can suppress activation and protect against tissue damage in inflammatory ocular environments23.
The purpose of the present study was to interrogate whether CD200R signaling regulates the proangiogenic macrophage phenotype (Arg-1+VEGF+) pivotal to initiation of angiogenesis, and whether targeting the inhibitory CD200R could modulate early infiltrating macrophage phenotype and function, thereby influencing the clinical outcome of disease.
Results
CD200R-deficient macrophages demonstrate a pronounced proangiogenic phenotype following PGE2 stimulation
Vascular endothelial growth factor (VEGF) is critical to the process of vascular remodeling during tissue repair and wound healing responses following inflammation, but under pathological conditions VEGF-mediated angiogenesis may cause tissue damage, as observed in AMD. Alternatively activated macrophages, characterized by canonical arginase-1 (Arg-1) expression, also generate VEGF in response to various environmental signals, including Th2 cytokines (IL-4, IL-13)8,9 as well as immune-modulatory compounds such as PGE212. The inhibitory CD200R, expressed predominantly by cells of myeloid lineage provides a normal homeostatic control mechanism that serves to limit cell activation19,23.
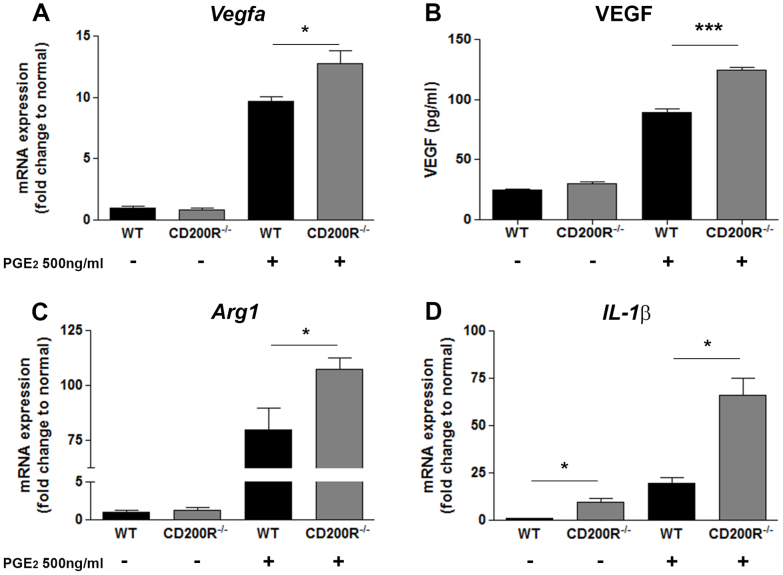
To determine whether and in what context CD200R signaling influences M2 macrophage activation and phenotype we generated bone marrow derived macrophages (BMMΦ) from C57BL/6J (wild-type; WT) and CD200R-deficient (CD200R−/−) animals and cultured them in the presence of PGE2 for 6 hours, before analyzing for gene expression. Previously, it has been shown that PGE2 stimulation of WT macrophages results in increased Vegfa and Arg1 gene expression12. Our initial data not only confirmed this response but also demonstrated that Il-1β mRNA levels are significantly upregulated, following a similar dose-dependent response (Supplementary Figure 1). The greatest induction of gene expression was achieved when macrophages were stimulated with 500 ng/ml PGE2, and this concentration was therefore used for subsequent in vitro assays. Analysis of CD200R−/− macrophages stimulated with PGE2 revealed these cells to have a more pronounced proangiogenic response, with significantly elevated levels of Vegfa, Arg1 and Il-1β mRNA when compared to WT (Figure 1A, C, D). The basal expression of IL-1β from CD200R−/− macrophages was also significantly greater than that from WT cells. Furthermore, PGE2 stimulation resulted in increased VEGF production in CD200R−/− macrophages (Figure 1B). Levels of IL-18 mRNA expression were also analyzed, and although elevated (2-fold change increase) in PGE2-stimulated CD200R−/− macrophages, the overall response was markedly reduced when compared to IL-1β induction (60-fold change) (Supplementary Figure 2).
Figure 1. CD200R-deficient macrophages demonstrate a pronounced proangiogenic phenotype following PGE2 stimulation.
(A), (C), (D) Vegf, Arg1 and Il1β mRNA expression, respectively, determined by quantitative RT-PCR; (B) VEGF protein production from cell supernatants derived from WT and CD200R−/− macrophages stimulated by PGE2 for 6 h, as determined by ELISA. Messenger RNA level was normalized to 18srRNA. Data are presented as mean ± SEM, n = 3. *P < 0.05, and ***P < 0.0005 between two groups.
Lack of CD200R signal results in enhanced macrophage VEGF expression in response to RPE-derived PGE2
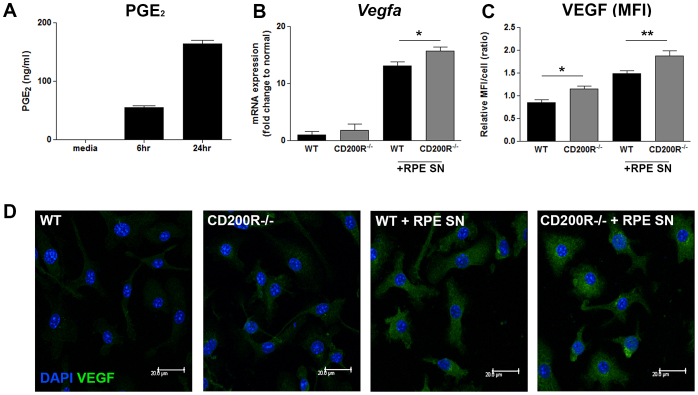
We have shown that CD200R deficiency drives bone marrow-derived M2 macrophage activation in the context of PGE2-mediated Vegfa, Arg and IL-1β expression. In relation to the chorioretinal complex of the eye, retinal pigment epithelium (RPE) cultures secrete PGE2, as demonstrated in studies that use both murine primary cultured RPE cells and the human ARPE-19 cell line24,25. On this basis, we replicated conditions in the subretinal microenvironment to determine how macrophages respond to RPE-derived PGE2, and whether CD200R signaling had any role in modulating this. Utilizing the recently characterized murine RPE cell line (B6-RPE07)26, we examined both PGE2 secretion from cultured RPE cells, and the effect of supernatant derived from these cells on macrophage phenotype. Conditioned media from cultured B6-RPE07 cells, contains high levels of PGE2 after 24 hrs (Figure 2A), and addition of this cell supernatant to WT BMMΦ resulted in upregulated Vegfa mRNA expression as compared to media controls (Figure 2B). Moreover, Vegfa mRNA and VEGF expression, as confirmed by analysis of confocal microscopy images, were elevated in CD200R−/− BMMΦ when compared to WT (Figure 2B–D).
Figure 2. Lack of CD200R signal results in enhanced macrophage VEGF expression in response to RPE-derived PGE2.
(A) PGE2 secretion from murine B6RPE-07 cells cultured for 6–24 hr was determined by ELISA. (B) Vegf mRNA expression from BMMΦ cultured for 6 hr in RPE conditioned media collected after 24 hr. 18srRNA was used as internal control. (C) Relative mean fluorescent intensity (MFI) of VEGF expression in WT or CD200R1−/−macrophages. (D) Representative confocal images of BMMΦ cultured in RPE supernatants or control media for 24 h. Cells were fixed and permeabilized before immuno-staining with anti-VEGF antibody. Scale bar: 20 μm. Data are presented as mean ± SEM, n≧3. *P < 0.05, **P < 0.005 between groups.
The enhanced pro-angiogenic phenotype of CD200R-deficient macrophages promotes angiogenesis
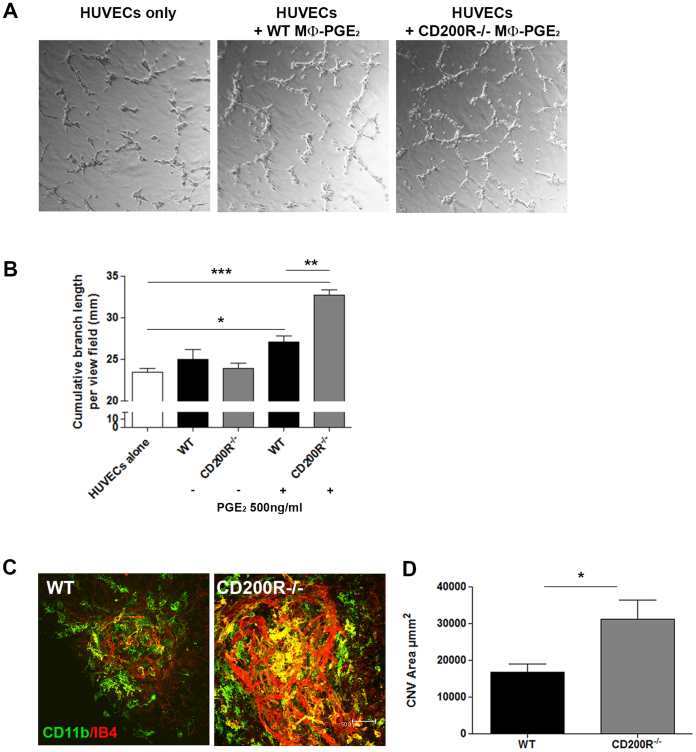
Having established an elevated VEGF response in PGE2-stimulated CD200R-deficient macrophages, we sought to determine whether this phenotype translated to enhanced pro-angiogenic functions. Using the HUVEC angiogenesis assay27, we examined whether co-culture with PGE2 stimulated WT or CD200R−/− macrophages influenced endothelial cell tube formation (Figure 3A, B). The addition of WT macrophages stimulated with PGE2 led to increased HUVEC tube formation measured as the cumulative branch length per field of view, however tube formation following co-culture with PGE2-conditioned CD200R−/− BMMΦ was significantly greater demonstrating the requirement for intact CD200R signaling to suppress macrophage pro-angiogenic function.
Figure 3. Enhanced pro-angiogenic phenotype of CD200R-deficient macrophages promotes angiogenesis.
(A) Representative images of HUVEC cells cultured alone, or in the presence of WT or CD200R−/− macrophages stimulated with PGE2 for 24 h in advance. (B) Cumulative branch length per field of HUVEC cells cultured alone, or in the presence of WT or CD200R−/− macrophages stimulated, or not, with PGE2. Data are presented as mean ± SEM, n = 3, *p < 0.05; **p < 0.005; ***p < 0.0005 between groups. (C) Representative confocal images of RPE/choroid flat-mounts from WT and CD200R−/− mice at day 7 post-laser application, were immunostained with CD11b (green) and Isolectin B4 (IB4) (red). (D) Mean CNV area calculated from confocal images. Data presented as mean ± SEM, n = 26 lesions for each strain; *p < 0.05.
The laser-induced model of choroidal neovascularization (CNV), employs laser photocoagulation to rupture Bruch's membrane (a pentalaminar configuration of elastin and collagen fibres located under the RPE) and stimulate a neovascular response that is associated with acute RPE dysfunction, cell loss and dysregulated immune responses28,29. Given the evidence for a central role of macrophages in driving CNV17,30, we examined whether lack of CD200R signaling and an associated elevated macrophage VEGF response would influence choroidal neovascularization and angiogenic development in this model. Laser-induced CNV was evaluated in WT C57BL/6J and CD200R−/− mice, and CNV lesions assessed at 7 days following laser application, the time when angiogenesis and neovascular membranes are established. Analysis of CNV lesion sizes, based on IB4 immunostaining of RPE/choroid flatmounts, revealed that lesions in CD200R−/− mice were significantly larger compared with WT animals (Figure 3C & D).
Promoting CD200R signaling can suppress pro-angiogenic macrophage gene expression
The current results demonstrate that in the absence of CD200R signaling, both the phenotype and proangiogenic activity of macrophages are altered, suggesting that engagement of CD200R may offer the potential to influence macrophage activity and CNV development. Such an approach using an agonist monoclonal antibody DX109 has been successful in suppressing cell activation and disease severity in several experimental disease models19,23. We therefore examined whether engagement of CD200R, using the DX109 agonist, could modulate the PGE2-induced pro-angiogenic macrophage gene signature. In these experiments, WT BMMΦ were pre-incubated with DX109 or control mAb for 2 hr before PGE2 stimulation. Promoting CD200R signaling did not alter macrophage phenotype, with levels of Vegfa, Arg-1 and IL-1β expression remaining unchanged (Supplementary Figure 3).
Central to CNV development in the laser model are CCR2+ macrophages31. We have recently shown that recruited macrophages are conditioned via uptake of damaged RPE components toward a proangiogenic Arg-1+ VEGF+ phenotype, and the selective depletion of these cells suppresses CNV development18. Therefore we examined whether DX109 could modulate the pro-angiogenic gene expression of macrophages conditioned through the uptake of damaged RPE components. Using an adapted in vitro phagocytosis assay, WT BMMΦ were pre-incubated with DX109 or an isotype control for 2 hrs before the addition of heat-induced necrotic mouse B6-RPE07 cells. After 24 hr, the effect of CD200R engagement on gene expression was assessed. DX109 treatment (10- and 25- μg/ml) was able to inhibit the expression of both Arg-1 and IL-1β when compared with isotype mAb (Figure 4), but this effect did not extend to suppressing Vegfa expression (Supplementary Figure 4).
Figure 4. Promoting CD200R signaling suppresses pro-angiogenic gene expression profile of macrophages.
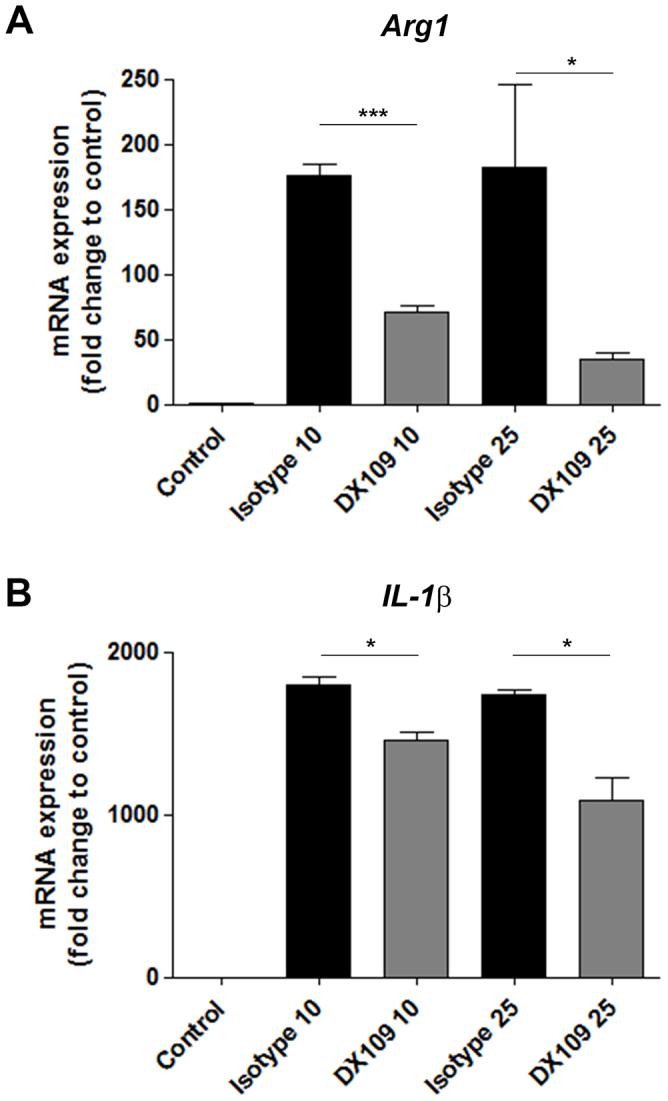
Using an adapted in vitro phagocytosis assay, necrotic RPE cells prepared from B6-RPE07 cells by heating at 95°C for 15 minutes, were added to BMMΦ that were pre-incubated for 2 hours with 10- or 25 μg/ml DX109 or isotype control mAb. BMMΦ were washed and collected after 24 hr incubation with necrotic RPE, and RNA extracted for RT-qPCR analysis to determine Arg-1 and Il-1β gene expression. Gapdh was used as a normalizing control. Data are presented as mean ± SEM, n = 3. *p < 0.05; ***p < 0.0005.
Promoting CD200R signaling can suppress pro-angiogenic gene expression and CNV development
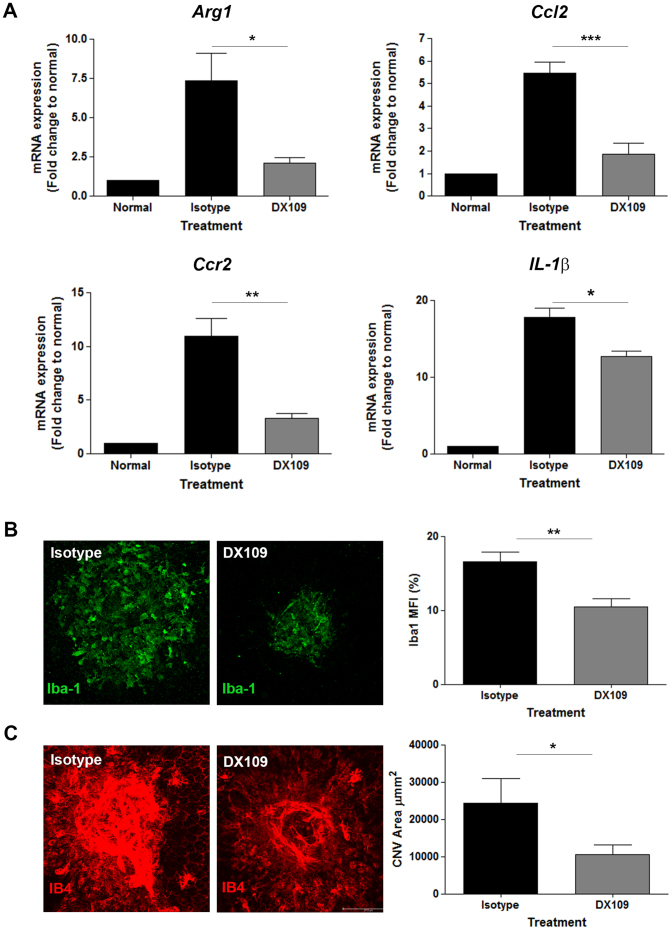
In vitro assessment demonstrates that promoting CD200R signaling with DX109 can directly modulate gene expression of macrophages that are conditioned toward a pro-angiogenic phenotype. We next determined the therapeutic potential of DX109 using the laser-induced CNV model. To this end, DX109 or control isotype mAb were administered to WT mice via intravitreal injection immediately following application of 4 laser lesions to the RPE/choroid. On day 3, the peak time-point for macrophage infiltrate and pro-angiogenic gene expression in this model18, RPE/choroid were dissected for analysis of gene expression. In DX109-treated eyes, expression of Arg-1, Ccl2, Ccr2 and Il-1β in the choroid was significantly reduced compared with choroid from isotype-treated eyes (Figure 5A). The reduction in Ccr2 expression suggests that DX109 treatment impedes, either directly or indirectly, CCR2+ cell recruitment resulting in reduced numbers of pro-angiogenic Arg-1+ macrophages to incipient CNV. To determine the degree of macrophage infiltrate, immunohistochemistry with Iba-1 was performed on choroidal flat-mounts derived from DX109- and isotype-treated eyes (Figure 5B). In DX109-treated eyes, there was a significant reduction in levels of Iba-1 expression, reflecting the observed reduction in Ccr2 gene expression. Furthermore, IB4 flat-mount staining revealed that DX109 treatment also resulted in an overall reduction in the total CNV area at this time-point (Figure 5C).
Figure 5. DX109 treatment alters the proangiogenic gene signature of CNV and reduces infiltration of Iba-1+ macrophages.
Intravitreal administration of DX109 or isotype control mAb was performed immediately following laser induction of CNV in C57BL/6J wild-type mice. (A) RT-pPCR analysis for Arg-1, Ccl2, Ccr2 and Il-1β gene expression in RPE/choroid tissue collected at day 3 post-laser and injection. Gapdh served as a normalizing control. Data are presented as mean ± SEM, n = 6 for each treatment group. *p < 0.05; **p < 0.005, ***p < 0.0005 isotype vs. DX109. (B) Representative RPE/choroid flat-mounts at day 3 were immunostained with Iba-1, and mean fluorescence intensity (MFI) of Iba-1 calculated from confocal images. Data presented as mean ± SEM, n = 12 lesions for each treatment group; **p < 0.005. (C) Representative RPE/choroid flat-mounts were immunostained with IB4, and mean CNV area calculated from confocal images. Data presented as mean ± SEM, n = 8 lesions for each strain; *p < 0.05.
Having demonstrated that promoting CD200R signaling with DX109 treatment leads to reduced proangiogenic gene expression, macrophage infiltration and CNV area, we extended these observations to CD200R-deficient mice. A comparison between WT and CD200R−/− mice was performed to determine whether the gene signature, in particular the Arg-1 signal, was also reduced in the choroid of CD200R-deficient animals following treatment with DX109 (Supplementary Figure 5). The results confirm that the reduction in gene expression following treatment is CD200R specific, and that targeting CD200R can suppress the Arg-1 response associated with the initial stages of laser-induced CNV.
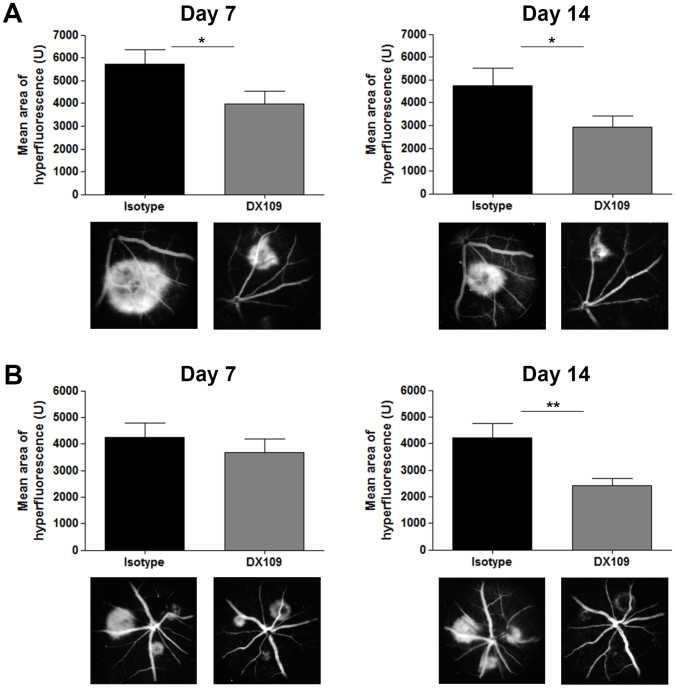
To establish whether these altered levels of expression and activation of macrophages also impacted the development of CNV, mice were treated immediately following laser injury and lesion size assessed by fundus fluorescein angiography at 7 and 14 days (Figure 6A). The results demonstrate that delivery of the CD200R agonist resulted in a significant reduction in lesion size (39% and 30% at 1 and 2 weeks respectively) compared with isotype-treated eyes. Having demonstrated that manipulating macrophage proangiogenic phenotype early in CNV development attenuates the neovascular response, the question remains as to whether intervention at the time of maximal macrophage infiltration (3 days) post laser could modulate late outcome of CNV. We predicted that intervention at day 3 where macrophage drive of angiogenesis was maximal would not influence the early development of CNV but would suppress in the longer term the extent of CNV. We found that administration of DX109 at day 3 had no apparent impact on CNV at day 7, but resulted in a sustained reduction of 43% in CNV area on fluorescein angiography at day 14 (Figure 6B).
Figure 6. DX109 treatment leads to reduced CNV lesion development.
Intravitreal administration of DX109 or isotype control mAb (A) immediately following laser induction of CNV, or (B) at day 3 post-laser in C57BL/6J wild-type mice, and mean lesion size determined by fundus fluorescein angiography performed at 7 and 14 days. Graphs for each time-point show mean lesion sizes following laser and injection of either DX109 or the isotype control mAb. Results are shown from the early phase of fluorescein angiograms (mean area of hyperfluorescence +/− SEM; n = 54 lesions examined), *p < 0.05, **p < 0.005. Representative images from angiograms are shown below each time-point. Mean lesion sizes in mice receiving DX109 compared to isotype were significantly reduced in (A) by 30% and 39% at 7 and 14 days post laser respectively, and (B) by 42% at 14 days.
Discussion
The results of this study demonstrate for the first time that modulation of macrophage phenotype through engagement of CD200R can inhibit proangiogenic function and reduce the extent of pathological angiogenesis in the eye. The laser-induced CNV model used in this study is well established and importantly provides a platform which permits an investigation of the function and behavior of macrophages in the context of an angiogenic drive.
The local administration of a CD200R agonist mAb, known to regulate macrophage function, reveals that targeting early macrophage infiltrate has therapeutic potential to inhibit clinical disease. Indeed our data show that suppression of macrophage activity reduces CNV, but also treating when pro-angiogenic macrophage potential is maximal and VEGF activity is heightened (day 3), results in reduced size of mature lesions. Furthermore, we also report that CD200R deficiency results in augmented macrophage VEGF responses, which generate an increased functional capacity to promote angiogenic HUVEC cell activity, particularly when macrophages are conditioned toward a pro-angiogenic phenotype following stimulation with PGE2 or PGE2-enriched RPE media in vitro. Finally, our data suggest a role for CD200R signaling in maintaining tonic control over VEGF expression, by demonstrating that in absence of CD200R pathological angiogenesis is increased in laser induced CNV. In comparison, in vitro assessment demonstrates that promoting CD200R signaling with DX109 directly modulates macrophage gene expression and subverts conditioning with necrotic RPE components toward a pro-angiogenic Arg-1+ macrophage phenotype.
Despite an increasing focus on the role of macrophages in retinal and choroidal angiogenesis, the question of how macrophages acquire specific VEGF+Arg-1+ phenotypes that promote angiogenesis remains unanswered. One notion is that infiltrating myeloid cells are polarized and conditioned by cytokines in the retina and subretinal space adjacent to the RPE. The RPE serves to support the metabolically active photoreceptor layer and maintain ocular homeostasis, not only through visual cycle participation but also by regulating inflammation in the eye32,33,34,35. Prostaglandin E2 (PGE2) is thought to exert an immunosuppressive effect in the eye and is reported to be secreted in murine primary cultured RPE cells and the human ARPE-19 cell line24,25. This inflammatory mediator is also recognized to enable alternative activation of macrophages and to promote VEGF expression12. In this context, it is conceivable that the RPE is capable of driving a VEGF+ macrophage phenotype, and our evidence that both PGE2 and PGE2-rich RPE cell supernatants induce a VEGF+ pro-angiogenic phenotype in macrophages would support this hypothesis36,37.
The phenotype and functional roles of infiltrating macrophages associated with maintaining vascular integrity and promoting angiogenic responses in the eye are dependent upon how these macrophages are activated14. Understanding the complex nature and mechanisms by which multiple drivers, such as senescence38, accumulation of lipids or other oxidative stress products5,39, or damaged components of damaged RPE18 influence macrophage function is critical when considering their contribution to ocular neovascularization. Whilst IFN-γ and LPS are factors capable of generating NOS2+VEGF+M1 macrophages, PGE2 remains a potent stimulus for the generation Arg1+VEGF+ M2 macrophages12. Conditioning with other cytokines such as IL-4 or IL-13 still generates Arg-1+ M2 macrophages, but these cells express reduced levels of VEGF, and instead secrete large quantities of sFlt-1, an anti-angiogenic factor [Wu W-K, personal communication]. Arg-1 is also an important factor that regulates inflammation, with documented involvement in chronic inflammatory conditions and vascular dysfunction in retinopathy9,40,41. In the context of the current study, the consequence of promoting CD200R signaling is to modulate choroidal gene expression, in particular the Arg-1 signal and subsequent CNV development.
The NLRP3 Inflammasome is implicated as having a central role in the pathogenesis of AMD, specifically components of drusen have been shown to activate NLRP3, causing secretion of IL-1β and IL-1815. It has been postulated that NLRP3 and IL-18 may have a protective and anti-angiogenic effect in terms of regulating the development of CNV. Interestingly, whilst the current data demonstrates that CD200R−/− macrophages predominantly elicit an enhanced IL-1β response, IL-18 levels were also elevated (albeit to a far lower level) when compared to wild-type. Whether CD200R signaling also contributes to such protective mechanisms and plays a potential role in the wider context of regulating inflammasome activation will require further investigation. Previous studies using the laser-induced CNV model demonstrate the importance of IL-1β as a key driver for angiogenesis in AMD, and approaches to inhibit its activity using IL-1 receptor antagonists can prevent neovascular development42. Thus, the observed reduction in IL-1β levels following DX109 treatment further emphasizes the important potential of this approach in modulating macrophage functional responses and their influence on CNV development.
The ability to target inhibitory receptors has emerged as an effective mechanism to modulate and regulate inappropriate immune activation, characteristic of chronic inflammatory and degenerative diseases22,43,44. CD200 and its myeloid receptor CD200R constitute an inhibitory signaling pathway that provides a homeostatic control mechanism that regulates myeloid cell activation20,45,46. Therapeutic targeting of this pathway has proven successful in numerous inflammatory models23,47,48,49,50 and CD200R expression, originally described as restricted to cells of myeloid lineage including macrophages, microglia, granulocytes and dendritic cells, is now recognized to include a wide range of immune cell subsets including T cells, B cells and NK cells19,51,52,53,54. CD200 is expressed abundantly within the normal eye, on retinal vascular endothelium and glial fibrillary acidic protein (GFAP)-negative neurons in retina and optic nerve, and on a subpopulation of CD45-positive perivascular and juxtavascular cells20,21,54,55. Moreover, CD200 expression on RPE56, and vascular endothelial cells57, suggests that its interaction with CD200R-expressing myeloid cells, including resident microglia or infiltrating macrophages may serve to regulate local responses. In our study, we noted that a deficiency of CD200R signaling resulted in augmented VEGF expression and pro-angiogenic functions in macrophages in vitro (under PGE2 enriched conditions) and more pronounced angiogenesis following laser-induced CNV in vivo. Taken together, these data suggest that CD200R signaling regulates myeloid cell phenotypes and consequently ocular neovascularization, specifically via the inhibition of VEGF expression from infiltrating macrophages. Despite in vivo targeting of early macrophage infiltrate with DX109 influencing pro-angiogenic gene expression, macrophage recruitment and subsequent CNV development (Figures 5 & 6), we were unable to directly demonstrate in vitro, CD200R-mediated suppression of RPE-induced Vegfa expression (Supplementary Figure 4). Whether this remains as VEGF independent suppression is still very interesting in itself and may add weight to considering combinatorial approaches in the future for therapy. Therefore manipulation of the CD200-CD200R pathway may prove a beneficial adjunct in the treatment of neovascular AMD.
The monoclonal antibody DX109, originally generated from investigations detailing the cellular distribution of the inhibitory CD200R protein52, was subsequently shown to signal as a functional agonist capable of inhibiting myeloid cell responses19,46. Experimentally, promoting CD200R-mediated signaling with DX109 has been shown to suppress classical macrophage activation and protect against tissue damage during autoimmune responses in the retina23. Here, we provide further evidence of the potential clinical benefits of DX109 application, specifically that early targeting of proangiogenic myeloid infiltrate alters angiogenic development and disease progression, whilst delayed administration, even though it does not circumvent the initial drive, the in vivo half-life of DX109 (5 days) is still capable of attenuating the extent of CNV in the latter stages of development. Thus, despite the presence of RPE-derived VEGF and other pro-angiogenic factors, triggering the CD200R still has clinical benefit in terms of the maintenance of CNV.
In addition, it should also be considered that the observed suppressive effects of DX109 treatment may also arise from altered responses of other CD200R-positive immune cells. Histological studies demonstrate macrophages, lymphocytes, and mast cells, as well as fibroblasts, are associated with both atrophic lesions and neovascularization of the retina58. Mast cells express CD200R, and receptor specific agonists including DX109, can inhibit their inflammatory function, by blocking degranulation and secretion of inflammatory mediators19. Furthermore, recent evidence implicates infiltrating γδT cells, that express IL-17 in response to macrophage- and photoreceptor-derived IL-1β and high-mobility group box 1 (HMGB-1), in CNV development59. Whilst IL-17 is generally considered a pro-inflammatory cytokine linked to autoimmunity, it can also promote angiogenesis60. In this study, both depletion of γδT cells or suppression of IL-1β and HMGB-1 were shown to ameliorate CNV. The inhibitory effect of DX109 in terms of reducing macrophage and choroidal IL-1β gene expression may therefore indirectly impact γδT cell IL-17 production, or alternatively, since γδT cells express CD200R61, the agonist may act directly to suppress activation of this inflammatory cytokine network. Thus, in the current study CD200R receptor-mediated signaling may also regulate mast cell and γδT cell responses, and potentially inhibit their contribution to angiogenesis and cell injury in the laser-CNV model.
In summary, CD200R signaling acts to suppress a PGE2- or RPE fragment-induced pro-angiogenic macrophage phenotype and the loss of such regulation leads to more severe ocular neovascularization in CD200R1−/− mice than WT mice. Harnessing this mechanism, by using the CD200R agonist mAb DX109, attenuates the neovascular response in a model of CNV and indicates a potential novel therapeutic approach to the treatment of neovascular age-related macular degeneration.
Methods
Mice
C57BL/6J (CD200R+/+) mice and CD200R−/− breeding colonies were established in Animal service unit of University of Bristol. All mice were housed under specific pathogen-free conditions with continuously available food and water. The mice were aged between 6 and 8 weeks. Treatment of animals conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments were carried out in compliance with University of Bristol institutional guidelines and under the authority of a project licence from the UK Home Office.
Reagents
The agonist rat IgG1-anti-mouse CD200R mAb (DX109) was generated from rats using immunogenic fusion proteins consisting of the extracellular domains of CD200R gene fused to the Fc domain of human immunoglobulin and was a gift from Joe Phillips & Jonathon Sedgwick (SP Biopharma, California, USA). The control isotype mAb used was a rat anti-human IL-4. Both mAb preparations used contained < 0.1 ng of endotoxin/mg of protein, as determined by the Limulus Amebocyte Lysate Pyrogen Testing kit, QCL-1000 (Cambrex, East Rutherford, NJ).
Complete medium consisted of Dulbecco's modified Eagle's medium(DMEM) supplemented with 10% heat inactivated fetal calf serum, 100 U/ml penicillin–streptomycin, 2 mmol/l L-glutamine, 1 mmol/l sodium pyruvate and 5 × 10−5 mol/l 2-mercaptoethanol (all from PAA, Yeovil, UK).
CNV Induction and therapeutic intervention
CNV was induced by laser photocoagulation in mice aged 6–8 weeks. In brief, the pupils of animals were dilated using topical 1% tropicamide and mice anesthetized by intraperitoneal (i.p.) injection of 200 μl of Vetelar and Rompun mixed with sterile water in the ratio 0.6:1:84. Four laser spots were delivered to the posterior retina using an OculightSlx Krypton Red Laser system (power 200 mW, duration 75 ms, spot size 75 μm).
Local administration of DX109 or isotype control mAb was performed by intravitreal injection, either immediately following laser or at day 3. In brief, the eye was proptosed and held in position with a pair of forceps, while 10 μg of mAb/4 μl of PBS was injected using a 33-gauge hypodermic needle, (Esslab, Essex, UK). The injection site was treated with chloramphenicol and globe reposited.
Cell culture and in vitro assays
Bone marrow derived macrophages (BMMΦ) were generated using a previously described method23. In brief, bone marrow cells were washed in DMEM media and resuspended at 1 × 105 cells/ml in complete medium supplemented with 5% horse serum (PAA, UK) and 50 pg/ml macrophage-colony stimulating factor (M-CSF). Cell suspensions (50 ml) was transferred to Teflon-coated tissue culture bags (supplied by Dr. M. Munder, University of Heidelberg, Germany) and incubated for 8 days at 37°C in 5% CO2. Cells were removed from the Teflon bags and plated at 1 × 106 cells/well in 24-well plates (Corning-Costar, Corning, NY), before stimulation with media alone, PGE2 (Sigma-Aldrich, Poole, UK) or B6-RPE07 cell conditioned media for 24 hrs.
For the HUVEC-BMMΦ co-culture assay, HUVEC cells cultured in EGM-2 media (Lonza, Switzerland) were washed and then co-cultured with or without BMMΦ established from WT mice or CD200R1−/− mice, stimulated or unstimulated by PGE2 (500 ng/ml). Co-culture was conducted on matrigel (Becton Dickinson, UK) in 96-well plate, containing EBM-2 culture media (Lonza, Switzerland). 6000 HUVECs in a well were co-cultured with 5000 BMMΦ for 6 hrs, and cumulative tube length per field was measured by Image Pro Plus software from bright field microscope images. BMMΦ used for co-culture were cultured with PGE2 (500 ng/ml) for 24 hrs in advance in the Teflon coated bag and used directly to co-culture.
For the RPE phagocytosis assay, necrotic RPE was generated from a mouse RPE cell line B6-RPE0726 by heating a known number of cells for 15 minutes at 95°C, and cell death confirmed by trypan blue staining. BMMΦ cells were plated at a concentration of 1 × 106 cells/well in 24-well plates and incubated for 2 hours to allow cell attachment. Cell culture supernatant was replaced with serum-free DMEM medium containing 1 × 106 necrotic RPE cells, for additional 24 hours incubation. Cells were then washed before subsequent downstream gene analysis.
RNA extraction and real time RT-qPCR
Total RNA from RPE/choroid tissue or BMMΦ cell cultures was isolated using TRIzol reagent (Life Technologies). One μg of total RNA was treated with RQ1 RNase-free DNase before reverse-transcription using the ImProm-II™ Reverse Transcription System (Promega). cDNA was amplified using the Power SYBR® Green PCR Master Mix Reagent (Applied Biosystems) on a StepOne™ Real-Time PCR System (Applied Biosystems). Primer sequences for mouse Gapdh, 18 s rRNA, Arg-1, and Vegfa were described previously12,62. Primers for other mouse or human genes were designed using the PrimerQuest online tool (http://eu.idtdna.com/Scitools/Applications/Primerquest/): mouse Il1b: forward 5′-GCCCATCCTCTGTGACTCAT-3′; reverse 5′-AGGCCACAGGTATTTTGTCG-3′; Ccr2: forward 5′-AATGAGAAGAAG AGGCACAGGGCT; reverse 5′-ATGGCCTGGTCTAAGTGCTTGTCA-3′; Ccl2: forward 5′- TCACCTGCTGCTACTCATTCACCA-3′; reverse 5′- TACAGCTTCTTTGGGACACCTGCT-3′.
Cytokine measurements
Cytokine production (VEGF and IL-1β) in culture supernatants from in vitro PGE2 stimulated BMMΦ, and PGE2 concentration of RPE-conditioned media, were assessed by ELISA, following manufacturer's instructions (R&D Systems, UK). Cultured supernatants were immediately frozen after collection and stored at −20°C until use.
RPE/Choroid whole mount and BMMΦ confocal microscopy
Eyes were enucleated and fixed in 2% (wt/vol) paraformaldehyde (PFA). After dissection of retina and RPE/choroid, tissues were blocked and permeabilized in 5% BSA, 5% goat serum with 0.3% Triton X-100 in PBS for 2 hours, followed with incubation of primary antibodies (biotinylated IB4; Sigma-Aldrich: AF-488 rat anti-mouse CD11b; Becton Dickinson: Rabbit polyclonal anti-Iba1; Wako Chemicals) in 1% BSA with 0.15% Triton X-100 at 4°C. After thorough washing, samples were incubated with secondary antibodies (AF-488 goat anti-rabbit IgG; Life Technologies: Streptavidin Rhodamine RedX, Jackson ImmunoResearch Labs) at RT for 3 hours in the dark. Tissues were washed and flat-mounted in Vectashield antifade medium (Vector Laboratories) and examined using a Leica TCS-SP2-AOBS confocal laser scanning microscope. Isotype antibodies or negative controls with primary antibody omitted did not show significant fluorescence signal.
BMMΦ cells cultured on glass coverslips with RPE-conditioned media for 24 hrs were washed and fixed in 2% PFA for 2 hrs, blocked and permeabilized in 5% BSA, 5% goat serum with 0.3% Triton X-100 in PBS for 30 mins, before overnight incubation with chicken polyclonal anti-VEGF antibody (1:100; Abcam, Cambridge, UK) 1% BSA with 0.15% Triton X-100 at 4°C. After washing, cells were further incubated with a secondary antibody, DyLight 650-goat anti-chicken IgY (1:400; Abcam, Cambridge UK) for 2 hours in the dark, washed and mounted in DAPI containing Vectashield antifade medium and examined using a SP5-AOBS confocal laser scanning microscope.
Statistical analyses
Data was analyzed with unpaired Student's t-test (GraphPad Prism software, San Diego, CA). Data are generated as mean ± SEM and representative of at least 2 independent experiments. Values were considered statistically significant at *p < 0.05, **p < 0.005, ***p < 0.0005.
Author Contributions
D.A.C., S.H., S.J.R. and A.D.D. conceived and designed the experiments. D.A.C., S.H., S.J.R., J.L. and W.W. performed the experiments. Data was analyzed by D.A.C., S.H., S.J.R., R.R.A., J.W.B., L.B.N., M.M., A.D.D. and the manuscript written by D.A.C., S.H., S.J.R., A.D.D. All authors reviewed the manuscript.
Supplementary Material
Supplementary figures
Acknowledgments
The authors wish to acknowledge the following contributions: Dr Jon Sedgwick and Dr Joe Phillips (SP Biopharma (formerly DNAX), Palo Alto, California, USA) for providing the DX109 and control isotype mAbs; Dr. Heping Xu (Queens University of Belfast, Belfast, UK) for providing the B6-RPE07 cell line; Dr Ian Humphreys (Cardiff University, Cardiff UK) for providing the CD200R−/− mouse strain; Dr Atsuhiko Oikawa, Mr Jonathan Rowlinson and Prof. Paolo Madeddu, (School of Clinical Sciences, University of Bristol) for providing the HUVEC cell cultures used in this study.
Footnotes
This work was supported by grants from Underwood Trust Endowment Program grant, and the Dunhill Medical Trust [grant number: R128/1109]. A.D.D., R.R.A. are partially supported by the NIHR Biomedical Research Centre at Moorfields Eye Hospital and UCL Institute of Ophthalmology. JWB is supported by NIHR Research Professorship.
References
- Jager R. D., Mieler W. F. & Miller J. W. Age-related macular degeneration. N Engl J Med 358, 2606–2617 (2008). [DOI] [PubMed] [Google Scholar]
- Lim L. S., Mitchell P., Seddon J. M., Holz F. G. & Wong T. Y. Age-related macular degeneration. Lancet 379, 1728–1738 (2012). [DOI] [PubMed] [Google Scholar]
- Ferris 3rd F. L. et al. Clinical classification of age-related macular degeneration. Ophthalmol 120, 844–851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman G. S. et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 20, 705–732 (2001). [DOI] [PubMed] [Google Scholar]
- Ambati J. & Fowler B. J. Mechanisms of age-related macular degeneration. Neuron 75, 26–39 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield J. G. et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med 14, 194–198 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcup S. M. et al. The role of the immune response in age-related macular degeneration. Int J Inflamm 2013, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3, 23–35 (2003). [DOI] [PubMed] [Google Scholar]
- Mosser D. M. & Edwards J. P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958–969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina R. J. et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med 17, 1045–1055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrusiewicz K. et al. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE 6, e23902 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. K., Llewellyn O. P., Bates D. O., Nicholson L. B. & Dick A. D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 215, 796–803 (2010). [DOI] [PubMed] [Google Scholar]
- Tsutsumi C. et al. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol 74, 25–32 (2003). [DOI] [PubMed] [Google Scholar]
- Apte R. S., Richter J., Herndon J. & Ferguson T. A. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med 3, e310 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S. L. et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 18, 791–798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeie J. M. & Mullins R. F. Macrophages in neovascular age-related macular degeneration: friends or foes? Eye (Lond) 23, 747–755 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai E., Anand A., Ambati B. K., van Rooijen N. & Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 44, 3578–3585 (2003). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Myeloid Cells Expressing VEGF and Arginase-1 Following Uptake of Damaged Retinal Pigment Epithelium Suggests Potential Mechanism That Drives the Onset of Choroidal Angiogenesis in Mice. PLoS ONE 8, e72935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenmalm M. C., Cherwinski H., Bowman E. P., Phillips J. H. & Sedgwick J. D. Regulation of myeloid cell function through the CD200 receptor. J Immunol 176, 191–199 (2006). [DOI] [PubMed] [Google Scholar]
- Hoek R. M. et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290, 1768–1771 (2000). [DOI] [PubMed] [Google Scholar]
- Dick A. D., Broderick C., Forrester J. V. & Wright G. J. Distribution of OX2 Antigen and OX2 Receptor within Retina. Invest Ophthalmol Vis Sci 42, 170–176 (2001). [PubMed] [Google Scholar]
- Dick A. D. et al. Control of myeloid activity during retinal inflammation. J Leukoc Biol 74, 161–166 (2003). [DOI] [PubMed] [Google Scholar]
- Copland D. A. et al. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol 171, 580–588 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Futagami Y., Smith S. B., Naggar H. & Mochizuki M. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Exp Eye Res 83, 1459–1471 (2006). [DOI] [PubMed] [Google Scholar]
- Horie S., Sugita S., Futagami Y., Yamada Y. & Mochizuki M. Human retinal pigment epithelium-induced CD4+CD25+ regulatory T cells suppress activation of intraocular effector T cells. Clin Immunol 136, 83–95 (2010). [DOI] [PubMed] [Google Scholar]
- Chen M. et al. Characterization of a spontaneous mouse retinal pigment epithelial cell line B6-RPE07. Invest Ophthalmol Vis Sci 49, 3699–3706 (2008). [DOI] [PubMed] [Google Scholar]
- Spinetti G. et al. Tissue kallikrein is essential for invasive capacity of circulating proangiogenic cells. Circ Res 108, 284–293 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus H. E., Kang S. J. & Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res 29, 500–519 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen K., Petrovski G., Moe M. C., Berta A. & Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol 90, 299–309 (2012). [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann D. G. et al. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 44, 3586–3592 (2003). [DOI] [PubMed] [Google Scholar]
- Xie P. et al. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS ONE 6, e28933 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari J. C. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 32, 125–145 (2012). [DOI] [PubMed] [Google Scholar]
- Streilein J. W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 3, 879–889 (2003). [DOI] [PubMed] [Google Scholar]
- Sugita S. Role of ocular pigment epithelial cells in immune privilege. Arch Immunol Ther Exp (Warsz) 57, 263–268 (2009). [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Sugita S. & Kamoi K. Immunological homeostasis of the eye. Prog Retin Eye Res 33, 10–27 (2013). [DOI] [PubMed] [Google Scholar]
- Yanni S. E., Barnett J. M., Clark M. L. & Penn J. S. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Invest Ophthalmol Vis Sci 50, 5479–5486 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Toma H. S., Barnett J. M. & Penn J. S. Ketorolac inhibits choroidal neovascularization by suppression of retinal VEGF. Exp Eye Res 91, 537–543 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J., Ali Khan A., Yin J., Ferguson T. A. & Apte R. S. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest 117, 3421–3426 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene A. et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab 17, 549–561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. & Martinez F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010). [DOI] [PubMed] [Google Scholar]
- Caldwell R. B., Zhang W., Romero M. J. & Caldwell R. W. Vascular dysfunction in retinopathy-an emerging role for arginase. Brain Res Bull 81, 303–309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalette S. et al. Interleukin-1beta inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol 178, 2416–2423 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R. W., Brauer R., Stuhlmuller B., Palombo-Kinne E. & Burmester G. R. Macrophages in rheumatoid arthritis. Arthritis Res 2, 189–202 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R., Kieseier B. C., Stoll G. & Hartung H. P. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 64, 109–127 (2001). [DOI] [PubMed] [Google Scholar]
- Broderick C. et al. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol 161, 1669–1677 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M. et al. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol 174, 1348–1356 (2005). [DOI] [PubMed] [Google Scholar]
- Banerjee D. & Dick A. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul Immunol Inflamm 12, 115–125 (2004). [DOI] [PubMed] [Google Scholar]
- Chitnis T. et al. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol 170, 1695–1712 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. CD200R1 agonist attenuates mechanisms of chronic disease in a murine model of multiple sclerosis. J Neurosci 30, 2025–2038 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simelyte E. et al. CD200-Fc, a novel antiarthritic biologic agent that targets proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis. Arthritis Rheum 58, 1038–1043 (2008). [DOI] [PubMed] [Google Scholar]
- Preston S., Wright G. J., Starr K., Barclay A. N. & Brown M. H. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur J Immunol 27, 1911–1918 (1997). [DOI] [PubMed] [Google Scholar]
- Wright G. J. et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 171, 3034–3046 (2003). [DOI] [PubMed] [Google Scholar]
- Rijkers E. S. et al. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol Immunol 45, 1126–1135 (2008). [DOI] [PubMed] [Google Scholar]
- Wright G. J. et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13, 233–242 (2000). [DOI] [PubMed] [Google Scholar]
- Barclay A. N. & Ward H. A. Purification and chemical characterisation of membrane glycoproteins from rat thymocytes and brain, recognised by monoclonal antibody MRC OX 2. Eur J Biochem 129, 447–458 (1982). [DOI] [PubMed] [Google Scholar]
- Sugita S. et al. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J Immunol 181, 7525–7536 (2008). [DOI] [PubMed] [Google Scholar]
- Ko Y. C. et al. Endothelial CD200 is heterogeneously distributed, regulated and involved in immune cell-endothelium interactions. J Anat 214, 183–195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold P. L., Madigan M. C., Gillies M. C. & Provis J. M. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res 20, 385–414 (2001). [DOI] [PubMed] [Google Scholar]
- Hasegawa E. et al. IL-23–Independent Induction of IL-17 from γδT Cells and Innate Lymphoid Cells Promotes Experimental Intraocular Neovascularization. J Immunol 190, 1778–1787 (2013). [DOI] [PubMed] [Google Scholar]
- Moran E. M. et al. Interleukin-17A induction of angiogenesis, cell migration, and cytoskeletal rearrangement. Arthritis Rheum 63, 3263–3273 (2011). [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M., Yu K. & Clark D. Receptor engagement on cells expressing a ligand for the tolerance-inducing molecule OX2 induces an immunoregulatory population that inhibits alloreactivity in vitro and in vivo. J Immunol 165, 4854–4860 (2000). [DOI] [PubMed] [Google Scholar]
- Khera T. K., Dick A. D. & Nicholson L. B. Fragile X-related protein FXR1 controls post-transcriptional suppression of lipopolysaccharide-induced tumour necrosis factor-alpha production by transforming growth factor-beta1. FEBS J 277, 2754–2765 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures