Abstract
Postoperative pain slows surgical recovery, impacting the return of normal function for weeks, months, or longer. Here we report the antihyperalgesic actions of a new compound, resolvin D1 (RvD1), known to reduce inflammation and to suppress pain after peripheral nerve injury, on the acute pain occurring after paw incision and the prolonged pain after skin-muscle retraction. Injection of RvD1 (20–40 ng) into the L5–L6 intrathecal space 30 minutes before surgery reduces the postincisional primary mechanical hypersensitivity, lowering the peak change by approximately 70% (with 40 ng) and reducing the area under the curve (AUC) for the entire 10-day postincisional course by approximately 60%. Intrathecal injection of RvD1 on postoperative day (POD) 1 reduces the hyperalgesia to the same level as that from preoperative injection within a few hours, an effect that persists for the remaining PODs. Tactile allodynia and hyperalgesia following the skin/muscle incision retraction procedure, measured at the maximum values 12 to 14 days, is totally prevented by intrathecal RvD1 (40 ng) given at POD 2. However, delaying the injection until POD 9 or POD 17 results in RvD1 causing only transient and incomplete reversal of hyperalgesia, lasting for <1 day. These findings demonstrate the potent, effective reduction of postoperative pain by intrathecal RvD1 given before or shortly after surgery. The much more limited effect of this compound on retraction-induced pain, when given 1 to 2 weeks later, suggests that the receptors or pathways for resolvins are more important in the early than the later stages of postoperative pain.
Keywords: Postoperative pain, Inflammation, Glia, Intrathecal, MAP kinases
1. Introduction
Postoperative pain is an acute obstacle to rapid functional recovery and earlier hospital discharge after surgery. Furthermore, the incidence of ensuing chronic postoperative pain is substantial after certain procedures [32]. Surgery can also result in pain that only resolves over months, or persists longer, causing incapacitance and psychological stress for an extended time [24,29]. For both medical and economic reasons, therefore, it is desirable to minimize postoperative pain.
Both peripheral and central factors contribute to postoperative pain [11,33]. The initial discharge of local nerves caused by the incision [46] and the later, delayed activation of impulses from peripheral nerve that may be conducted by injured or uninjured fibers [4,5,17,30] probably contribute to the establishment of longer-lasting hyperalgesia [28], and there is little doubt that changes in the central nervous system (CNS), at least at the spinal cord (and probably also in the brain), are essential for the maintained chronic pain [10,23,24]. The differential ability of different drugs, applied at the incision site or delivered systemically, to prevent or reverse postoperative pain supports the concept of at least 2 stages of postoperative pain, an induction stage and a maintenance stage, which involve different mechanisms and occur at different locations [2,12,27]. Experiments with glutamate receptor antagonists delivered intrathecally around the time of skin incision show the importance of AMPA/kainate and mGluR5 receptors for the induction of postincisional pain [26,31,50].
Resolvins are novel lipid mediators produced by cells of inflammatory exudates that stimulate resolution of inflammation [34–37]. Resolvin D1 was first identified in resolving inflammatory exudates, murine brain and microglial cells [18,22,37]. Its complete stereochemistry was confirmed and assigned, as well as its potent anti-inflammatory and proresolving actions [34,35]. Recent publications report an antihyperalgesic effect of resolvins in several different pain models. Resolvins D1 and E1 were shown to reduce pain after nerve injury or peripheral inflammation, through both peripheral and central (spinal) actions [45]. Here it was noted that subcutaneous resolvin E1 (RvE1) was able to reduce the local hypersensitivity from paw incision. Resolvin D1 (RvD1) was shown to have selective effects in vitro on the cellular activities of transient receptor potential channels (TRPs) TRPA1, TRPV3, and TRPV4, but not on TRPV1 or TRPM8, and to reduce the pain behavior caused by subcutaneous injection of substances known to activate these TRP channels [3].
In the present study, we combined the spinal (intrathecal) application of RvD1 with 2 different surgical models in the rat to determine RvD1’s ability to alter postoperative pain when applied to the spinal cord. This compound was delivered before or shortly after skin incision, or weeks after an incision-retraction procedure known to induce prolonged mechano-sensitivity [13], to determine its respective effectiveness on the induction or the maintenance stage of postoperative pain.
2. Materials and methods
2.1. Animals
The protocol for animal experimentation was reviewed and approved by the Harvard Medical Area Standing Committee on Animals, Boston, MA. USA. Male Sprague–Dawley rats were purchased from Charles River Laboratory (Wilmington, MA, USA) and kept in the animal housing facilities at Brigham and Women’s Hospital, with controlled humidity (20% to 30% relative humidity), room temperature (24°C), a 12-hour (6:00 am to 6:00 pm) light-dark cycle, and unlimited access to food and water. Before the experiments, the animals were handled to familiarize them with the behavioral investigator, experimental environment, and specific experimental procedures for reduction of stress during experiments. At the time of injection, rats weighed approximately 250 to 300 g.
2.2. Surgery
2.2.1. Lateral paw incision (LPI) surgery
Rats were anesthetized with nose-inhaled, rapidly reversible sevoflurane inhalation anesthetic (Sevofrane, Abbott Laboratory, North Chicago, IL, USA). A 1-cm longitudinal incision was made with a No. 11 blade through the skin and fascia along the hairs bordering the lateral plantar surface of the hind paw, beginning 0.5 cm distal from the end of the heel [39]. The underlying flexor muscle was longitudinally sectioned, as far along its length as possible. Skin was closed with 5–0 nylon sutures, and topical antibiotics were administered. Animals were allowed to recover in their cages, and sutures were removed on the third postoperative day.
2.2.2. Skin/muscle incision and retraction (SMIR) surgery
The SMIR surgery was performed as previously described [13]. Rats (250 to 300 g) were anesthetized with intraperitoneal Nembutal (sodium pentobarbital, Sigma–Aldrich, St. Louis, MO, USA, 50 mg/mL), at doses of 65 to 75 mg/kg, laid on their back, and the medial side of left lower limb shaved. The shaved skin was sterilized with beta-iodine then alcohol to enable visualization of the saphenous vein. A 1.2- to 1.5-cm skin incision was made approximately 4 mm medial to the saphenous vein to reveal the muscle of the leg. An incision (7 – 10 mm long) was then made in the superficial muscle layer, approximately 4 mm medial to the saphenous nerve. The superficial muscle was then parted further by spreading blunt scissors within the muscle incision site to allow the insertion of a microdissecting retractor. The retractor had 4 prongs spaced over an 8-mm distance, and each prong was 4 mm deep (catalog no. 13-1090, Biomedical Research Instruments Inc., Silver Spring MD, USA). The retractor was inserted into the incision site to position all prongs underneath the superficial layer of the thigh muscle. The skin and superficial muscle of the thigh were then retracted by 2 cm, revealing the fascia of the underlying adductor muscles. With wet sterile phosphate buffered saline (PBS) dressing over the open wound, this retraction was maintained for 1 hour. The animals were closely monitored during the retraction period, and if required, additional anesthesia was provided using sevoflurane. Animals were also completely covered (apart from the top of the head) with a large absorbent bench pad (VMR International, catalog no. 56616-031) to minimize heat loss during anesthesia and to prevent dehydration of the surgical site. After the SMIR procedure, drying of the muscle and skin of the surgical site was not observed, and these tissues were closed with 4–0 vicryl and 3–0 silk sutures, respectively. Sham-operated rats underwent the same procedure with the exception of the skin/muscle retraction. After recovery from anesthesia, all animals could ambulate normally and rise up on their hind limbs to reach food and water.
2.3. Chemicals
Resolvin D1 was purchased as a solution in 100% ethanol from Cayman Chemical Company (Ann Arbor, MI, USA) and stored at −80°C. Less than 1 hour before injection, the aqueous solution of RvD1 was prepared by evaporating the stock RvD1 to dryness under a gentle steam of nitrogen and immediately adding the vehicle solution, PBS with 0.1% ethanol, while minimizing exposure to light.
2.4. Schedules for resolvin dosing, by percutaneous intrathecal injection
Each experimental group included 8 to 10 rats.
2.4.1. LPI experimental groups
20 ng RvD1 (in 30 μL, trathecal), followed 30 minutes later by LPI.
Vehicle (PBS plus 0.1% ethanol, 30 μL, intratracheal), followed 30 minutes later by LPI.
LPI followed 1 day later by 10, 20, or 40 ng RvD1, in 30 μL, trathecal.
LPI followed 1 day later by 30 μL PBS (0.1% ethanol), trathecal.
2.4.2. SMIR experimental groups
SMIR followed on POD 2 with vehicle (PBS plus 0.1% ethanol), trathecal.
SMIR followed on POD 2 by RvD1 (40 ng/30 μL), trathecal.
SMIR followed on PODs 9 and 17 by vehicle, trathecal.
SMIR followed on POD 9 by RvD1 (40 ng/30 μL) and on POD 17 by RvD1 (40 ng/30 μL), trathecal.
2.4.3. Percutaneous intrathecal injection
Rats were anesthetized with brief isoflurane inhalation and shaved at the lower back, then placed in the prone position with lower back elevated and flexed ventrally. A lumbar puncture was performed between the L4–L5 or L5–L6 intervertebral space (located in relation to the superior iliac line), perpendicular to the skin, using a 30-gauge needle attached to a 50-μL Hamilton syringe. A slight movement of the tail or hind limb indicated the proper approach and entry into the subarachnoid space. Solutions were injected over <1 minute.
2.5. Behavioral testing of mechanical sensitivity
Before behavioral testing, all animals were habituated 3 times to the testing environment on 3 different days. Throughout the behavioral testing time courses, the experimenter was blind to the preceding surgical procedure (SMIR or sham) and to the injection agents (RvD1 or vehicle solution).
Animals were placed on an elevated wire mesh floor and confined underneath individual overturned plastic boxes. Mechanical allodynia/hyperalgesia was assessed using 4 von Frey filaments with bending forces of 4, 6, 10, and 15 g (Touch-Test Sensory Evaluators, Stoelting Co., USA). In ascending order of force, each filament was applied 10 times, to the mid-plantar/central area of the hind paw encircled by tori/footpads. Each press with the filament was spaced from the others of that force by approximately 30 seconds, and the time between application of the different von Frey hairs (VFHs) was several minutes. There was no pattern of increasing response with the number of applications in each set, from 1 to 10, evidence for an absence of any behavioral “wind up”-like process. Withdrawal responses to each of the von Frey filaments from both hind paws were counted and recorded. The total number of responses, per each 10 stimuli in a set, are presented graphically and compared for statistical analysis between treatment groups. Thus, 0.2 of 10 is the sensitivity score for the 4-g VFH in naïve, preoperative rats, and a maximum hyperalgesic score of 8 of 10 is observed in rats at the peak of SMIR-induced pain. Prior to the surgical procedures, 3 baseline measurements of mechanical sensitivity were taken on separate days and then averaged to provide the presurgery baseline response, denoted on postoperative day 0.
2.6. Statistical analysis
Data are presented as mean ± SEM. One-way repeated measures ANOVA followed by Bonferroni post hoc analysis was used to compare presurgery baseline responses to postsurgery and postinjection responses in the behavioral time course of responses to mechanical stimulation. One-way ANOVA was used to compare the means of the area under the curve (AUC), above the presurgery baseline, of the responses to mechanical stimulation among experimental groups for LPI and for SMIR procedures (see Section 2.4, above). Given a significant F test (P < .05), a Bonferroni adjustment was performed to obtain post hoc pairwise tests for contrasts of interest. Significance was assigned at P < .05.
3. Results
3.1. RvD1 impact on pain after lateral paw incision
A modification of the paw incision model by Brennan et al. [6] was used in these studies to localize the area of tactile postoperative sensitivity to the lateral margin of the hind paw. This area is exclusively innervated by the sciatic nerve [38]. Recent findings in our laboratory indicated that the Brennan model’s incision, along the midline of the paw, involved responses mediated through both sciatic and saphenous nerve innervation and raised the likelihood of an interaction of inputs through central sensitization [39]. In contrast, incision at the lateral edge of the paw in this new LPI model instigates changes that appear to depend only on sciatic innervation. In the present experiments, the lateral periincisional region was the site primarily tested, by tactile probing with VFHs, with an occasional test of the central area that involves some saphenous input and indicates a contribution from central integration [39].
3.1.1. Preoperative delivery of RvD1 reduces postincisional paw pain
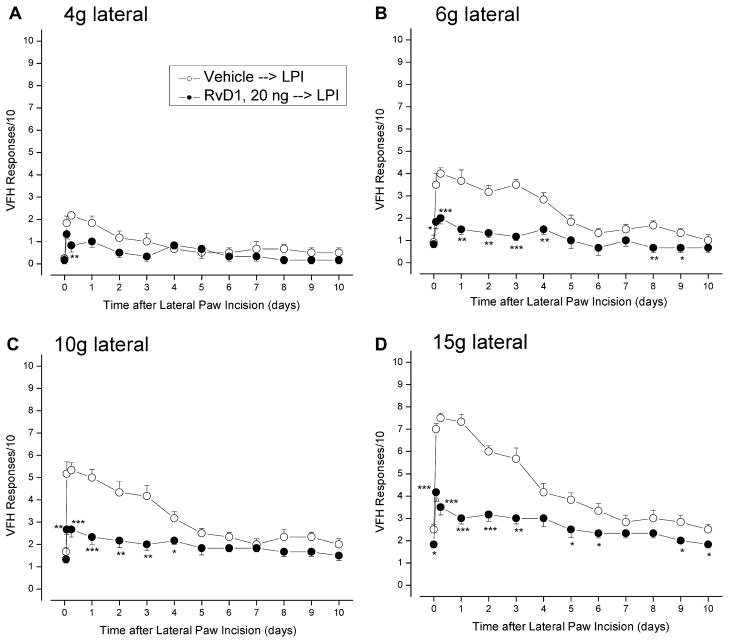
Intrathecal delivery of 20 ng of RvD1 30 minutes before the incision of the lateral paw (all done under sevoflurane general anesthesia) significantly reduced tactile hypersensitivity throughout the following 10 days (Fig. 1). The earliest postincisional responses to strong force stimulation by 10 and 15 g VFH, measured at 2 hours showed that this dose of RvD1 reduced initial hyperalgesia by at least 50%. In contrast, RvD1 caused no reduction of the initial allodynia, measured at 2 hours by the response to a 4-g VFH.
Fig. 1.
Lateral paw incision increases the response rates to simulation by von Frey hairs of the lateral paw area that are reduced by preincisional injection of RvD1. Thirty minutes before the incision, 20 ng of RvD1 was injected intrathecally; testing occurred at 1 and 6 hours postincisional, and then at each day including POD 10. Appearance of responses to weak von Frey hairs (A, B: 4 and 6 g) indicates allodynia, and the increase in response to strong von Frey hairs (C, D: 10 and 15 g) indicates hyperalgesia. Comparison of vehicle-injected and RvD1-injected rats, at respective times after incision: *P < .05; **P < .01; ***P < .001 (1-way ANOVA).
Reductions of hypersensitivity were significant for the later stages of postincisional pain. Allodynia was suppressed at 6 hours and later, in both lateral and central test regions of the paw. Hyperalgesia continued to be suppressed over the full postoperative period, with an approximately 75% reduction in the response to a 15-g VFH at POD 1, the peak time of response (Fig. 1D). The integrated antihyperalgesic effect of RvD1 can be quantitated by the reduction of AUC for the elevation of response above the baseline preoperative response level. For the antiallodynia measured in the lateral area by the 6-g VFH, the AUC is reduced from 14.6 ± 6.3 (area is in units of days) to 4.3 ± 5.2 days (71% reduction, P = .012, 2-tailed t-test) for 20-ng RvD1, and for the lateral area’s antihyperalgesia, measured by the 15-g VFH, the control area of 19.8 ± 8.1 days is reduced to 8.2 ± 4.9 days (59% reduction, P = .013). Results from tests in the central test area were similar in extent and statistical strength, but the modest allodynia and hyperalgesia tested by these VFHs applied at the medial area were unaffected by preoperative injection of RvD1 (data not shown).
3.1.2. Postoperative delivery of RvD1 dose-dependently reduces postincisional paw pain
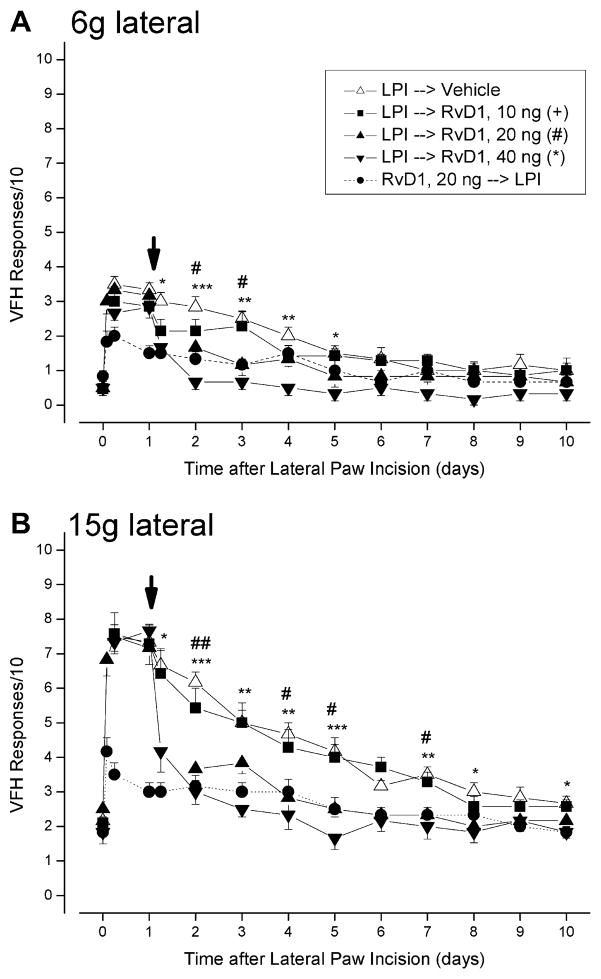
The enduring effect over 10 postincisional days implies that an RvD1-sensitive state exists during the first few hours after surgery. To test whether such a condition of RvD1 sensitivity lasts longer than the first few hours after surgery, we delivered the first intrathecal injection of the agent 1 day after the incision (POD 1). The effects were rapid and persistent, with both allodynia (Fig. 2A) and hyperalgesia (Fig. 2B) falling within 1 hour to the low levels that occurred when the same dose (20 ng) of RvD1 had been given preoperatively, and remaining at that level for the remaining postoperative test period.
Fig. 2.
Intrathecal injection of RvD1 1 day after lateral paw incision (at down-pointing arrow) causes a sharply dose-dependent decrease in allodynia (A) and hyperalgesia (B). The dotted line (filled circles) reproduces the data from Fig. 1, showing the effect of preincisional 20 ng RvD1. Comparison of vehicle-injected and different doses of RvD1-injected rats, at respective times after injections: *P < .05; **P < .01; ***P < .001; #1-way ANOVA. RvD1, resolvin D1.
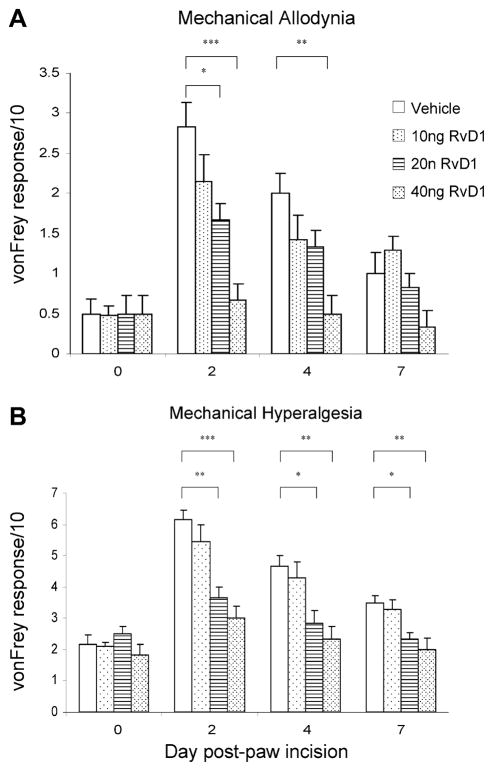
A sharp dose dependence of RvD1’s actions was apparent when different doses were injected intrathecally on POD 1. When the overall postoperative time course was viewed, the dose of 10 ng was ineffective, whereas the 20-ng dose appeared almost as effective as the 40-ng dose. Comparison of the responses at different postoperative days with the baseline response (Fig. 3) shows a significant reduction in allodynia from 20 and 40 ng RvD1 at POD 2, and from 40 ng persisting to POD 4 (Fig. 3A). A similar pattern, with an additional significant reduction from 20 ng RvD1 at POD 4, and for 20 and 40 ng at POD 7, occurred for the hyperalgesic responses (Fig. 3B). Indeed, delivery of 40 ng of RvD1 at POD 2 prevented the subsequently measured tactile sensitivity from rising above the preoperative baseline level.
Fig. 3.
RvD1 effects at different doses given at POD 1 on (A) the allodynia responses to a 6-g VFH and (B) the hyperalgesia responses to a 15-g VFH, assessed at PODs 2, 4, and 7. Preoperative values are designated at day 0. *P < .05; **P < .01 (multigroup 1-way ANOVA followed by post hoc t-test of comparisons between vehicle- and RvD1-injected rats). For the 40-ng dose, the responses at all 3 PODs were not different than the preoperative baseline response. RvD1, resolvin D1; POD, postoperative day; VFH, von Frey hairs.
3.2. RvD1 reduces tactile hypersensitivity from SMIR
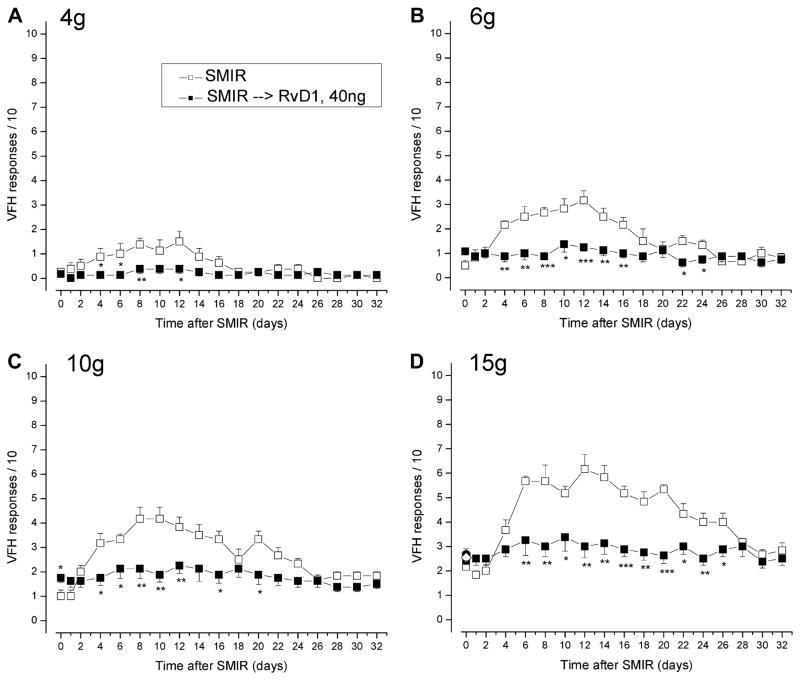
To examine the effects of RvD1 on a more persistent postoperative pain, such as that from inguinal herniorrhaphy or other abdominal procedures, we used the SMIR model developed by Flatters in 2008 [13]. SMIR surgery evokes weeks of exclusively mechanical hypersensitivity, when compared with presurgery baseline responses, to changes after sham surgery that include incision but no retraction, or to changes in the contralateral paw [13]. This hypersensitivity is exclusively mechanical and does not result from nerve injury, as judged by neuroanatomical investigation at and adjacent to the site of the retractors and by the negative immunocytochemical evidence for upregulated transcription factors signaling neural injury, ie, ATF-3, in the L3 and L4 dorsal root ganglia [12]. Becasue the testing VFHs are applied to areas of the plantar paw that are normally innervated by the sciatic nerve [10,38] and the surgical manipulation of incision-retraction involves only the saphenous nerve, the hypersensitivity tested here is almost certainly an indication of central sensitization. This sensitization is behaviorally characterized by the earliest increase in responsiveness at POD 3, a maximum change at PODs 10 to 14 and a return to preoperative sensitivity by POD 28 to 35 (Fig. 4).
Fig. 4.
The SMIR procedure induces allodynia (A, B) and hyperalgesia (C, D) that last for 3 to 4 weeks. Both aspects of mechano-hypersensitivity are effectively prevented by injection of RvD1 (40 ng) 2 days after the procedure. RvD1, resolvin D1; SMIR, skin/muscle incision retraction.
No signs of autotomy were evident in any of the SMIR or sham-operated animals. All SMIR-operated rats gained weight normally, comparable to the sham-operated group, and there were no gait abnormalities or postural adjustments obvious from visual examination, suggesting that tonic, spontaneous pain at the foot surface was minimal.
3.2.1. Early delivery of intrathecal RvD1 prevents SMIR-induced pain
Intrathecal injection of 40 ng RvD1 at POD 2, before the appearance of tactile sensitivity, effectively prevented the development of SMIR-induced pain, with no significant elevation of responses to weak or strong VFHs during the next 30 days (Fig. 4). Comparison of the highest responses in vehicle- and RvD1-injected rats, averaged over PODs 10, 12, and 14, shows that this dose of RvD1 effectively abolishes postoperative hypersensitivity, maintaining the responses at their preoperative levels (Fig. 5).
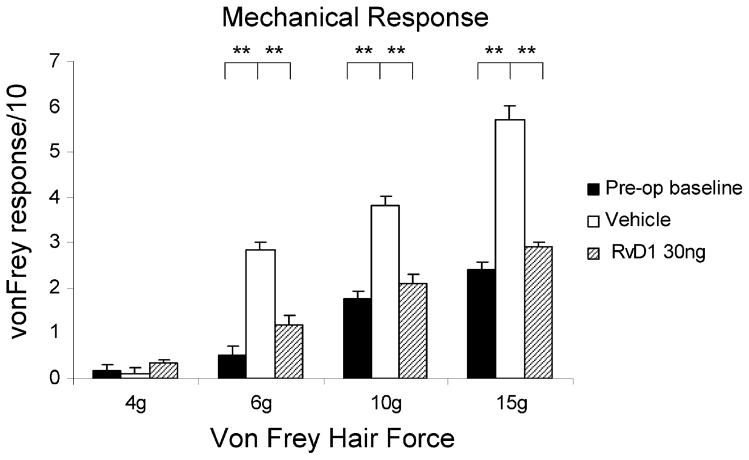
Fig. 5.
Responses to tactile stimulation by von Frey hairs in intact (preoperative) rats are graded with increasing force (solid bars), elevated by the SMIR procedure to maximum levels averaged over postoperative days 10 to 12 (open columns), but kept at the preoperative levels by the intratracheal injection of 30 ng RvD1 2 days after the procedure (patterned columns). RvD1, resolvin D1; SMIR, skin/muscle incision retraction.
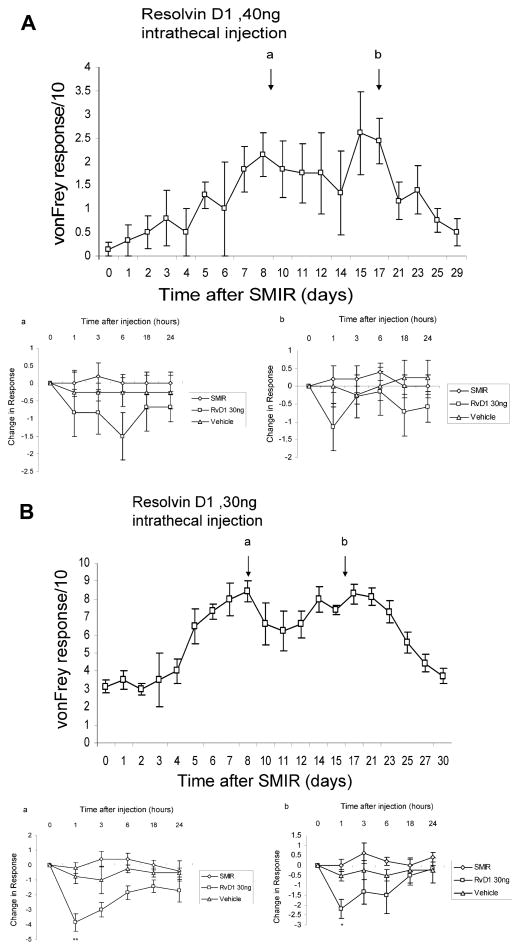
3.2.2. Later delivery of intrathecal RvD1 results in a transient reversal of pain
In contrast to the enduring antihyperalgesia from early delivery of RvD1, intrathecal injections of the same dose 1 or 2 weeks later had only a transient effect. Injections at POD 9, near the time of maximum hypersensitivity, and at POD 17, when hypersensitivity was beginning to wane, resulted in acute but temporary reductions in hyperalgesia (Fig. 6B). The decline of hypersensitivity after POD 17 is a spontaneous phenomenon that occurs in SMIR-treated rats receiving no drugs (see Fig. 4 and Flatters [13]). A similar-sized decrease in allodynia did not reach significance (Fig. 6A). The earlier time of delivery, POD 9, gives a larger antihypersensitive effect than the later time, POD 17 (−3.8 response units vs −2.1 units). The overall AUC for the entire postoperative period is reduced by 20% to 25% as a result of the effects of the 2 injections, at POD 9 and 17. This is still far less than the virtual prevention of hypersensitivity that results from the early administration of RvD1 at POD 3.
Fig. 6.
(A) SMIR-induced allodynia, assessed by responses to paw stimulation by a 6-g von Frey hair, are transiently but insignificantly reduced by intratracheal injection of 40 ng RvD1 on postoperative day 9 (lower panel a) and on postoperative day 17 (lower panel b). Injection of vehicle at these times also has no effect on the response. (B) SMIR-induced hyperalgesia, assessed by responses to a 15-g von Frey hair, are transiently reduced at 1 hour after RvD1 injection on (a) POD 9 and (b) POD 17, compared with untreated SMIR animals (ANOVA, *P < .05; **P < .01). Injection of vehicle on these days causes no change in response. RvD1, resolvin D1; SMIR, skin/muscle incision retraction; POD, postoperative day.
4. Discussion
Both peripheral and central nervous system changes contribute to postoperative pain. For example, nerve growth factor [41,42,49], tumor necrosis factor [49], glutamate [49], H+ [25,40], and endo-thelin-1 (ET-1) [27], and the activation of TRPV1 receptors [5,43] have all been implicated as peripheral factors in postincisional pain. Preoperative injection of ET-1 receptor antagonists at the skin incision site strongly suppressed postincisional pain, but identical delivery of the same antagonist after tactile hypersensitivity had reached its maximum value (4 to 6 hours postincision, on the hairy skin of the rat’s back) was far less effective, indicating a role for peripheral ET-1 in the induction but not the maintenance stage of this pain [27].
Central nervous system responses to incision involve glutamate and its receptors, and glutamatergic transmission in is affected by resolvins. Plantar paw incision causes a rapid, transient release of glutamate and aspartate in the spinal cord for the first hour after incision [48], and intrathecal delivery of antagonists of AMPA/kainate receptors [26,31] and of mGluR5 receptors [50], but antagonists of NMDA receptors [47], around the time of incision can reduce the pain elevated by paw incision. These findings suggest that the activation of AMPA/kainate and certain metabotropic glutamate receptors in the spinal cord is important for the induction of pain after skin incision [23,44].
Recent observations in the SMIR model show that the NMDA-R antagonist MK-801, given systemically (0.1 mg/kg, intraperitoneally) on PODs 9 to 13, is ineffective in reversing tactile hypersensitivity, although morphine (6 mg/kg, intraperitoneally) or gabapentin (100 mg/kg, intraperitoneally) each effect a significant reversal of this pain [14]. These findings show that maintained pain after the SMIR procedure does not require NMDA-Rs, but the results are uninformative about the role of other glutamate receptors for SMIR-evoked pain during the induction or the maintenance stages.
Resolvins, including RvD1, inhibit pain from inflammation by both peripheral and central actions [3,45]. Peripheral actions are known to suppress inflammation [34,35], to reduce postincisional pain [45], and to inhibit selectively certain thermo-TRP receptors [3], although not TRPV1, which has been shown to contribute to spontaneous firing of sensory neurons and to pain after paw incision [5,43]. When given centrally, into the intrathecal space, as RvD1 was during the current experiments, an almost identical molecule, RvE1, has both presynaptic and postsynaptic actions. Intrathecal RvE1 acts to reduce the elevated presynaptic release of glutamate, shown by a reduction to control values in the frequency of miniature excitatory postsynaptic currents recorded in lamina II cells of the dorsal horn in spinal cord slices, after that miniature excitatory postsynaptic current frequency is elevated by tumor necrosis factor (TNF)-α or capsaicin. The same concentrations of RvE1 also reduce the amplitude of NMDA-R currents recorded in these postsynaptic neurons after they have been exposed to TNF-α [45]. These respective presynaptic and postsynaptic effects of the resolvins are attributable to these compounds’ ability to interfere with the upregulation of these activities by activated extracellular-activated receptor kinase (pERK), a MAP kinase enzyme known to be critical for the sensitization of central pain pathways by emphatic nociceptor activation [19–21].
Resolvins and related local mediators are biosynthesized from dihydro-hexanoic acid in resolving inflammatory exudates [37], and are also produced in murine brain homogenates and cultured microglial cells [18,35]. Activation of microglial cells in vitro by a foreign product, bacterial zymosan A, and by the Ca+2 ionophone A23187 stimulated the release of compounds generated from dihydro-hexanoic acid, including ones structurally related to the resolvins, indicating an activation of the essential biosynthetic pathways enzymes [18]. A reciprocal action of these released substances on these microglia is relevant to the current discussion. Nanomolar concentrations of the 17S resolvins were able to inhibit 80% of the production of the transcript for the cytokine interleukin 1β stimulated by exposure of the astrocytes to TNF-α [18]. The activation of spinal microglia and astrocytes by peripheral nerve injury, and likely also by incision, and their role in cytokine production is well known [9,15,16,21]. That such cells can synthesize and release resolvins that in turn prevent this cytokine production indicates a role for endogenous resolvins and implies another target for the intrathecally delivered agents in postoperative pain suppression.
Mice undergoing Brennan-type incision of the hind paw show increased activation of spinal ERK, which is further elevated when the mice receive perioperative opiates [8]. It has been suggested that both elevated pain and MAP kinase activities from this procedure are related to a downregulation of spinal opiate receptors [7] resulting from enhanced levels of activated spinal p38 and ERK and resulting in a slower resolution of postincisional pain [1,19]. Although all of these studies involve a skin incision model, with hypersensitivity that normally resolves within 1 week, the downregulation of the opiate receptors slows this resolution by at least 2-fold [7], resulting in durations of postoperative pain that approach that from the SMIR procedure. Thus it is possible that the prolonged recovery seen in the latter surgery is also due to opiate receptor downregulation, a consequence of the activation of ERK and/or p38 pathways. Therefore, by decreasing pERK-driven (and possibly p-p38-driven) pathways, resolvins might both maintain opioid receptor numbers and inhibit the elevation in release of glutamate in exerting their antihyperalgesic actions after surgery.
There may be useful information from the difference in RvD1 effectiveness when given at different times after surgery. For the paw incision, RvD1 produced identical effects, measured at PODs 2 to 10, whether it was administered just before the incision or 1 day later. For the SMIR procedure, RvD1 given as late as POD 2 still effectively abolished surgery-evoked hypersensitivity, a slow process that normally only begins at POD 3 and reaches its maximum value 1 to 2 weeks later [13]. But when RvD1 was given near the height of this hypersensitivity (POD 9), its effects were partial and temporary, and when it was given yet a week later (POD 17), it had no significant effect. There are 2 explanations for this time-dependent effect. First, if RvD1’s actions on target pathways are invariable, regardless of the time of administration, then the selective, complete, and persistent effects from early administration imply that those targeted pathways are important in pain induction, and the diminishing effects at 1 to 2 weeks later imply that these pathways have decreasing importance as the pain is maintained. This may be a reflection of the slowly developing irreversibility of pathways that contribute to chronic postoperative pain, as well as to chronic pain from nerve injury [2]. Second, the difference in effectiveness could be the result of a time-dependent pharmacology, eg, reflecting local metabolic inactivation of RvD1. RvD1 is eventually metabolized to less active or inactive products by enzymatic dehydration through pathways known to be present in activated macrophages [22,34,36]. Because activated microglial cells have functions somewhat analogous to circulating macrophages that are known to biosynthesize and release resolvin precursors, and to metabolically inactivate them [18,34,36], it is likely that microglial cells (and possibly astrocytes) also increase their capacity to metabolically inactivate resolvins in the spinal cord. If this were the case, the decreasing antihyperalgesic effectiveness of exogenous RvD1 that occurs over weeks after the surgical procedure could be attributable to an increase in inactivating pathways accompanying the well-documented elevation of activated microglia in the spinal cord [8,14].
Acknowledgments
This work was supported by funds from the BWH Anesthesia Foundation for Education and Research. C.N.S. is supported by National Institutes of Health grants GM38765 and NS67686. We also acknowledge help in the preparation and handling of resolvin D1 by Dr. Rong Yang from the Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women’s Hospital. The advice of Dr. Ru-Rong Ji, Pain Research Center, Brigham and Women’s Hospital, for some of these experiments and on reading the manuscript is appreciated. C.N.S. is inventor on patents assigned to Brigham and Women’s Hospital and Partners Healthcare on the composition of matter, uses and clinical development of anti-inflammatory and pro-resolving mediators, including resolvins. These are licensed for clinical development. C.N.S. retains founder’s stock in Resolvyx Pharmaceuticals.
Footnotes
Conflict of interest statement
None of the other authors have any financial interest in the molecules or animal models described here.
References
- 1.Alkaitis MS, Solorzano C, Landry RP, Piomelli D, DeLeo JA, Romero-Sandoval EA. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS ONE. 2010;5:1–15. e10891. doi: 10.1371/journal.pone.0010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo MC, Sinnott CJ, Strichartz GR. Multiple phases of relief from experimental mechanical allodynia by systemic lidocaine: responses to early and late infusions. Pain. 2003;103:21–9. doi: 10.1016/s0304-3959(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 3.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010;161:707–20. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008;138:380–91. doi: 10.1016/j.pain.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain. 2009;141:41–51. doi: 10.1016/j.pain.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 7.Cabañero D, Célérier E, Garcia-Nogales P, Mata M, Roques BP, Maldonado R, Puig MM. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: prevention of the nociceptive response by on-site delivery of enkephalins. Pain. 2009;141:88–96. doi: 10.1016/j.pain.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Campillo A, González-Cuello A, Cabañero D, Garcia-Nogales P, Romero A, Milanés MV, Laorden ML, Puis MM. Increased spinal dynorphin levels and phosphor-extracellular signal-related kinases 1 and 2 and c-Fos immunoreactivity after surgery under remifentanil anesthesia in mice. Mol Pharmacol. 2010;77:185–94. doi: 10.1124/mol.109.059790. [DOI] [PubMed] [Google Scholar]
- 9.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 10.Devor M, Schonfield D, Seltzer Z, Wall PD. Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol. 1979;185:211–20. doi: 10.1002/cne.901850113. [DOI] [PubMed] [Google Scholar]
- 11.Dirks J, Moiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002;97:1591–6. doi: 10.1097/00000542-200212000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103:113–25. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Flatters SJL. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Pain. 2008;135:119–30. doi: 10.1016/j.pain.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flatters SJL. Effect of analgesic standards on persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Neurosci Lett. 2010;477:43–7. doi: 10.1016/j.neulet.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y-J, Ji R-R. Chemokines, neuronal–glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:279–90. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamalainen MM, Gebhart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hind paw. J Neurophysiol. 2002;87:712–20. doi: 10.1152/jn.00207.2001. [DOI] [PubMed] [Google Scholar]
- 18.Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autocoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 19.Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.05.030. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–9. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 21.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis N, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–87. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamata M, Koshizaki M, Shimada SG, Narimatsu E, Kozuka Y, Takahashi T, Namiki A, Collins JG. Changes in response properties and receptive fields of spinal dorsal horn neurons in rats after surgical incision in hairy skin. Anesthesiology. 2005;102:141–51. doi: 10.1097/00000542-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 25.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, Pogatzki-Zahn EM, Brennan TJ. The effect of the AMPA/kainate receptor antagonist LY293558 in a rat model of postoperative pain. J Pain. 2006;7:768–77. doi: 10.1016/j.jpain.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Mujenda FH, Duarte AM, Reilly EK, Strichartz GR. Cutaneous endothelin-A receptors elevate postincisional pain. Pain. 2007;133:161–73. doi: 10.1016/j.pain.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long-term postthoracotomy pain. Can J Anaesth. 1999;46:1127–32. doi: 10.1007/BF03015520. [DOI] [PubMed] [Google Scholar]
- 29.Perkins F, Kehlet H. Chronic pain as an outcome of surgery: a review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 30.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Aδ- and C-fibers innervating the plantar rat hind paw one day after an incision. J Neurophysiol. 2001;87:721–31. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 31.Pogatzki EM, Niemeier JS, Sorkin LS, Brennan TJ. Spinal glutamate receptor antagonists differentiate primary and secondary mechanical hyperalgesia caused by incision. Pain. 2003;105:97–107. doi: 10.1016/s0304-3959(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 32.Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH, Chambers WA. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain. 2003;19:48–54. doi: 10.1097/00002508-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Scholz J, Yaksh TL. Preclinical research on persistent postsurgical pain: what we don’t know, but should start studying. Anesthesiology. 2010;112:511–3. doi: 10.1097/ALN.0b013e3181cf4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, Fiore S, Brezinski DA, Lynch S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry. 1993;32:6313–9. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- 37.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;8:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- 39.Wang C-F, Pancaro C, Gerner P, Strichartz G. Prolonged suppression of postincisional pain by a slow-release formulation of lidocaine. Anesthesiology. 2011 doi: 10.1097/ALN.0b013e3182001996. in press. [DOI] [PubMed] [Google Scholar]
- 40.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–35. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 42.Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology. 2009;110:140–9. doi: 10.1097/ALN.0b013e318190bc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology. 2008;108:1100–8. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Richebe P, Brennan TJ. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. Eur J Pain. 2009;8:820–8. doi: 10.1016/j.ejpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Xu Z-Z, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji R-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto T, Shimoyama N, Mizuguchi T. Role of the injury discharge in the development of thermal hyperesthesia after sciatic nerve constriction injury in the rat. Anesthesiology. 1993;79:993–1002. doi: 10.1097/00000542-199311000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology. 1998;88:143–56. doi: 10.1097/00000542-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 48.Zahn PK, Sluka KA, Brennan TJ. Excitatory amino acid release in the spinal cord caused by plantar incision in the rat. Pain. 2002;100:65–76. doi: 10.1016/s0304-3959(02)00241-5. [DOI] [PubMed] [Google Scholar]
- 49.Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of nerve growth factor and tumor necrosis factor on pain behaviors after plantar incision. J Pain. 2004;5:157–63. doi: 10.1016/j.jpain.2004.02.538. [DOI] [PubMed] [Google Scholar]
- 50.Zhu CZ, Hsieh G, EI-Kouhen O, Wilson SG, Mikusa JP, Hollingsworth PR, Chang R, Moreland RB, Brioni J, Decker MW, Honore P. Role of central and peripheral mGluR5 receptors in the postoperative pain in rats. Pain. 2005;114:195–202. doi: 10.1016/j.pain.2004.12.016. [DOI] [PubMed] [Google Scholar]