Abstract
Introduction
We previously reported that simvastatin and enamel matrix derivative (EMD) have a dentinogenic effect. However, there is little information about the combined effects of these 2 agents on odontoblastic differentiation. The aim of this study was to investigate the effects of combined treatment with simvastatin and EMD on odontoblastic differentiation of human dental pulp cells (hDPCs). This study further explored the role of extracellular signal-regulated kinase (ERK) as a target and mediator of the differentiation induced by simvastatin in hDPCs.
Methods
The odontoblastic differentiation was analyzed by alkaline phosphatase activity, real-time polymerase chain reaction (PCR) for odontoblastic/osteoblastic markers (ie, dentin sialophosphoprotein, dentin matrix protein 1, and osteonectin), and alizarin red S staining. We also explored the role of ERK signaling as a mediator of simvastatin by Western blotting and real-time PCR. The expression of osteoblast-specific transcription factors was detected by reverse-transcription PCR.
Results
The alkaline phosphatase activity and the expression of odontoblastic markers (ie, dentin sialophosphoprotein and dentin matrix protein 1) increased in simvastatin/EMD-treated cells. Mineralized nodule formation increased in EMD- and simvastatin/EMD-treated cells. Notably, the combined use of both simvastatin and EMD resulted in more potent differentiation than that observed after a single therapy. Simvastatin activated ERK phosphorylation and treatment with ERK inhibitor blocked the messenger RNA expression of odontoblastic markers. However, in simvastatin/EMD-treated cells, the expression of these genes did not decrease significantly. Compared with other groups, the EMD- and simvastatin/EMD-treated group showed a greater expression of osterix.
Conclusions
Simvastatin promotes odontoblastic differentiation of hDPCs via the ERK signaling pathway. In addition, simvastatin-induced differentiation is facilitated by co-treatment with EMD. Collectively, these results suggest a new strategy to induce odontoblastic differentiation of hDPCs.
Keywords: Combination, enamel matrix derivative, extracellular signal–regulated kinase, simvastatin, odontoblastic
Direct pulp capping or partial pulpotomy is a method of treatment in which the exposed dental pulp is covered with a material protecting the pulp from additional injury and stimulating healing and repair. Ultimately, the goal of treating the exposed pulp with an appropriate pulp-capping material is to promote the dentinogenic potential of the pulpal cells (1). In this respect, new and effective strategies/agents should be developed to accelerate and improve the repair process.
Simvastatin, a 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor, is a well-established cholesterol-lowering drug able to inhibit cholesterol synthesis. Since Mundy et al (2) first reported that statins may have anabolic effects on bone, strong evidence has been reported that simvastatin promotes osteoblastic differentiation in various cell types 3, 4 and 5. Recently, Okamoto et al (6) reported that simvastatin-treated dental pulp stem cells exhibit enhanced odontogenic differentiation and accelerated mineralized tissue formation. Min et al (7) showed that simvastatin promotes odontogenesis in human dental pulp cells (hDPCs) and suggested simvastatin as a potential supplemental pulp-capping agent.
Although simvastatin can induce osteoblastic/odontoblastic differentiation, a single growth factor cannot always induce maximal differentiation; therefore, combinations of growth factors may be required for evaluation in clinical trials (8). The growth factors that will be used in clinical trials in the future would be more practical if they were already approved for human use in other medical/dental applications. Thus, additional clinically permitted agents can potentially be used with simvastatin for more desirable results.
Enamel matrix proteins have the potential to induce the regeneration of cementum tissue (9). These proteins, as commercial preparations of porcine fetal enamel matrix derivative (EMD), have been used in patients with periodontitis to promote cementogenesis and periodontal ligament regeneration 10 and 11. EMD also induces a process mimicking normal odontogenesis and can thereby serve as a biologically active pulp-dressing agent that specifically induces pulpal wound healing and hard-tissue formation without affecting healthy pulp 12 and 13. Recently, Min et al (14) reported that EMD promotes more rapid differentiation in combination with mineral trioxide aggregate (MTA) in hDPCs. Lee et al (15) suggested that simvastatin and EMD improved cell growth and differentiation in hDPCs cultured in the presence of Portland cement and may be useful ingredients in Portland cement as a pulp-capping material. In this respect, EMD can be considered a strong supplementary agent for pulp-capping procedures if it is used with simvastatin, a well-known odontogenic/osteogenic agent. However, the combined effect of simvastatin and EMD in odontoblastic differentiation is not well understood and remains under investigation.
Dental pulp cells can differentiate into odontoblasts and produce a mineralizing matrix, particularly during reparative dentinogenesis associated with injury or disease. During the differentiation process, the cells secrete type I collagen and other noncollagenous proteins. Among these, dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1) play important roles in hard-tissue development, and they are positive regulators of hard-tissue mineralization with DSPP acting on dentin and DMP1 acting on both bone and dentin (16). Osteonectin (ON) is a major noncollagenous protein of bone and dentin and is responsible for the mineralization properties of these tissues (17).
Extracellular signal–regulated kinase (ERK) is an essential mediator of growth factor–induced cell proliferation and differentiation (18). Several studies have suggested that ERK is involved in cell differentiation in various cell types 19, 20 and 21. However, there is little information about the role of the ERK signaling pathway involved in simvastatin-induced odontoblastic/osteoblastic differentiation in hDPCs.
Therefore, the aim of this study was to investigate whether the combined use of simvastatin and EMD has more positive effects on odontoblastic differentiation in hDPCs compared with their use alone. This study further explored the role of ERK as a target and mediator of the differentiation induced by simvastatin in hDPCs. Two null hypotheses were tested:
There is no difference between the combined use of these 2 agents and their separate use in odontoblastic differentiation of hDPCs.
ERK inhibition does not affect the odontoblastic differentiation induced by simvastatin.
Materials and Methods
Primary Culture of hDPCs
Human dental pulp tissues obtained from sectioned teeth were removed aseptically, rinsed with phosphate-buffered saline solution (PBS; Invitrogen, Carlsbad, CA), and placed in a 60-mm dish (Nunc, Roskilde, Denmark). The dental pulp tissues were then minced with a blade into small fragments and cultured in high-glucose Dulbecco modified Eagle medium (Invitrogen) containing 10% fetal bovine serum (Invitrogen) along with 100 U/mL penicillin and 100 U/mL streptomycin (Invitrogen). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cell cultures between the third to fifth passages were used in this study. All experimental procedures were approved by the Institutional Review Board of the Wonkwang University Dental Hospital, Iksan, Republic of Korea.
Cell Viability Test
hDPCs were seeded in 24-well culture plates at a density of 2 × 104 cells per well and preincubated in a growth medium for 24 hours. Then, the cells were exposed to 0.01 μmol/L simvastatin (Sigma-Aldrich, St Louis, MO) and/or 100 μg/mL EMD (Emdogain; Biora AB, Malmö, Sweden) for up to 72 hours. Cell viability was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, 200 μL MTT solution (0.5 mg/mL in PBS) was added to each well, and the wells were incubated for 2 hours. Subsequently, 200 μL dimethyl sulfoxide (DMSO; Amresco, Solon, OH) was added to each well. The plates were then shaken until the crystals had dissolved, and the solution in each well was transferred to a 96-well tissue culture plate. Reduced MTT was then measured spectrophotometrically at 540 nm in a dual-beam microtiter plate reader.
Alkaline Phosphatase Activity
hDPCs (1 × 105) were inoculated in 6-well culture plates and preincubated in a medium for 24 hours. After the hDPCs were incubated for 1, 3, 7, and 14 days in the presence of simvastatin (0.01 μmol/L) and/or EMD (100 μg/mL), the cells were scraped into cold PBS and then sonicated with a cell disruptor (Heat System-Ultrasonics, Plainview, NJ) in an ice-cold bath. Alkaline phosphatase activity (ALP) in the supernatant was determined using the method reported by Lowry et al (22), with p-nitrophenyl phosphate as a substrate. Absorbance was measured at 410 nm with an enzyme-linked immunosorbent assay reader (Beckman DU-650; Beckman Coulter, Fullerton, CA).
Reverse-transcription Polymerase Chain Reaction Gene Expression Analysis
The cells were placed in a 6-well culture plate at a density of 1 × 105 cells per well and incubated for 24 hours. After 1 and 3 days of culture under the presence of simvastatin (0.01 μmol/L) and/or EMD (100 μg/mL), total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. In brief, the cells were lysed directly in the plates using 1.0 mL Trizol reagent. After chloroform extraction, the total RNA was recovered from the aqueous phase and precipitated with isopropanol and RNAase-free distilled water. Then, reverse transcription of RNA was performed using the Superscript First-Strand Synthesis Kit (Invitrogen). Thereafter, the reverse-transcription–generated first-strand DNA was amplified. The primer sequences are detailed in Table 1. Amplification conditions were as follows: denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds for 30 cycles. The polymerase chain reaction (PCR) products were subjected to electrophoresis on 1.5% agarose gels and stained with ethidium bromide.
Table 1.
PCR primers
| Genes | Sequence | Size |
|---|---|---|
| Runx2 | Forward: 5′-AGACCAACAGAGTCAGTGAG-3′ | 317 |
| Reverse: 5′-TGGTGTCACTGTGCTGAAGA-3′ | ||
| Osx | Forward: 5′- GAGAGACTCGGGACAGCCAGCC-3′ | 105 |
| Reverse: 5′- CCTCAAGCAGGGAGGACGCC-3′ | ||
| GAPDH | Forward: 5′-GACCCCTTCATTGACCTCAACT-3′ | 693 |
| Reverse: 5′-CACCACCTTCTTGATGTCATC-3′ |
Alizarin Red S Staining for Mineralized Nodule Formation
The cells were placed in a 24-well plate at a density of 1 × 105 cells per well and cultured for 24 hours. After an initial attachment period of 24 hours, the medium was switched to osteogenic medium containing Dulbecco modified Eagle medium, 10% fetal bovine serum, 50 μg/mL L-ascorbic acid (Sigma-Aldrich), 10 mmol/L β-glycerophosphate (Sigma-Aldrich), and 100 nmol/L dexamethasone (Sigma-Aldrich) for the duration of the experiment. After culturing hDPCs under the presence of simvastatin and/or EMD for 14 days, mineralization was assessed by staining with alizarin red S (Sigma-Aldrich). In brief, 40 mmol/L of alizarin red S was prepared in distilled water, adjusted to a pH of 4.2 with ammonium hydroxide, and then applied to the cells for 10 minutes at room temperature with gentle agitation. The cells were then washed with distilled water and allowed to dry. Images of tissue stained with alizarin red S were obtained using a scanner, and stain intensity was analyzed using an image-analysis program (Image J; National Institutes of Health, Bethesda, MD).
Western Blotting
Cell extracts from hDPCs were solubilized with protein lysis buffer (Proprep; iNtRon Biotechnology, Seongnam, Korea) for 10 minutes on ice. The cell lysates were centrifuged at 13,000 rpm for 10 minutes, and protein concentrations were determined with Bradford reagent (Bio-Rad Laboratories, Hercules, CA). The protein samples were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis and blotted onto a membrane. The membrane was incubated at 4°C overnight with diluted antibodies against anti-phospho-ERK (or pERK) (Cell Signaling Technology, Danvers, MA) and anti-ERK (Cell Signaling Technology). Blots were incubated with peroxidase-coupled secondary antibodies (Promega, Madison, WI) for 1 hour. Bands were detected using a chemiluminescence system (SuperSignal; Thermo Scientific, Rockford, IL) according to the manufacturer's instructions and exposed to x-ray film.
Real-time PCR Analysis
A SYBR Green-based real-time PCR was optimized and performed using the TOPreal qPCR Premix Kit (Enzynomics, Cheongju, Korea). The final PCR mixture contained 2 μL each forward and reverse primers (final concentration of each = 0.4 μmol/L), 2 μL SYBR Green (2×), 1.6 μL MgCl2 (final concentration = 3 mmol/L), 5 μL template, and the quantity was made up to 20 μL with nuclease-free water. The sequences of the primers are detailed in Table 2. All real-time PCR reactions (ie, reactions, unknown samples, and controls) were performed in duplicate and conducted on the StepOne Real-Time PCR System (Applied Biosystem, Singapore) instrument. The following protocol was used: 10 minutes at 95°C followed by 30 cycles at 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. After the PCR cycles, a DNA melting curve was generated (0 seconds at 95°C, 15 seconds at 65°C, with a ramping time of 20°C/s and 0 seconds at 95°C with a ramping time of 0.1°C/s) in order to discriminate between specific and nonspecific amplification products.
Table 2.
Real time PCR primers
| Genes | Sequence | Size |
|---|---|---|
| DSPP | Forward: 5′-GGGATGTTGGCGATGCA-3′ | 70 |
| Reverse: 5′-CCAGCTACTTGAGGTCCATCTTC-3′ | ||
| DMP1 | Forward: 5′-AGCATCCTGCTCATGTTCCTTT-3′ | 106 |
| Reverse: 5′- GAGCCAAATGACCCTTCCATT-3′ | ||
| ON | Forward: 5′-ACCAGCACCCCATTGACG-3′ | 109 |
| Reverse: 5′-AGGTCACAGGTCTCGAAAAAGC-3′ | ||
| GAPDH | Forward: 5′-AAGGTGAAGGTCGGAGTCAAC-3′ | 102 |
| Reverse: 5′-GGGGTCATTGATGGCAACAATA-3′ |
Effect of ERK Inhibition on Expression of Odontoblastic/Osteoblastic Markers
The cells were cultured in 60-mm plates for 3 days with or without 10 μmol/L U0126, an inhibitor of phosphor-ERK (Promega). Cells were preincubated with U0126 for 1 hour before treatment with simvastatin. The inhibitor was dissolved in DMSO, and control cells were preincubated with equivalent amounts of DMSO alone.
Statistical Analysis
Statistical analysis of the data was performed by 1-way analysis of variance followed by a multiple-comparison Duncan test with the use of the SPSS program (SPSS 12.0; SPSS GmbH, Munich, Germany). Statistical significance was determined at P < .05.
Results
Effects of Simvastatin and/or EMD on Cell Viability
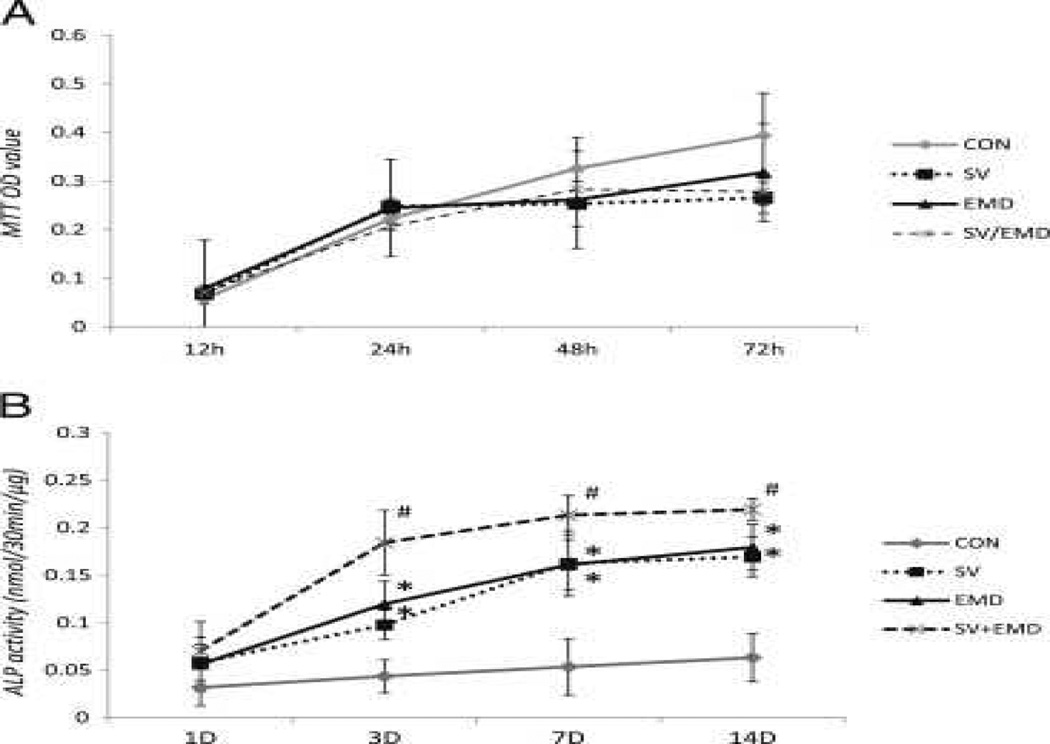
Cell viability tests for simvastatin and EMD indicated that these concentrations of simvastatin, EMD, or the combination of both agents did not inhibit cell viability until 72 hours. As shown in Figure 1A, there was no significant difference between the control and experimental groups (P > .05).
Figure 1.
Effects of Simvastatin and/or EMD on ALP Activity
Compared with untreated control cells, cells treated with simvastatin and/or EMD increased in ALP activity. The increase in ALP activity was particularly pronounced in simvastatin/EMD-treated cells; it was greater than that in simvastatin- and EMD-treated cells on days 3, 7, and 14 (Fig. 1B).
Odontoblast/Osteoblast-related Gene Expression Analysis
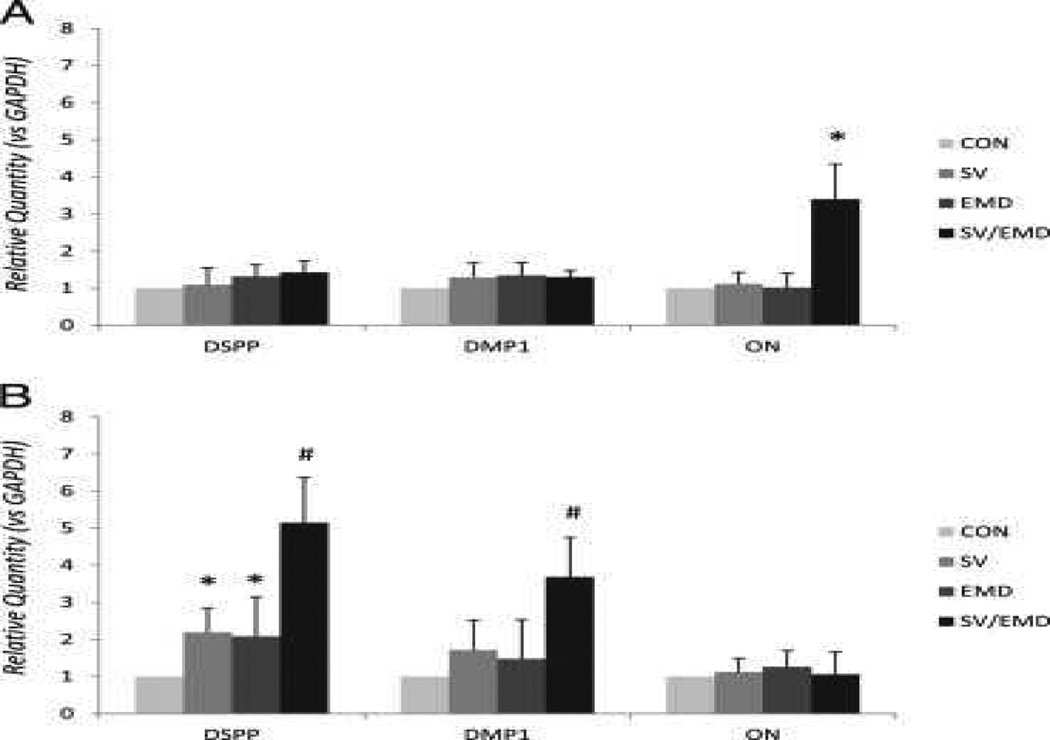
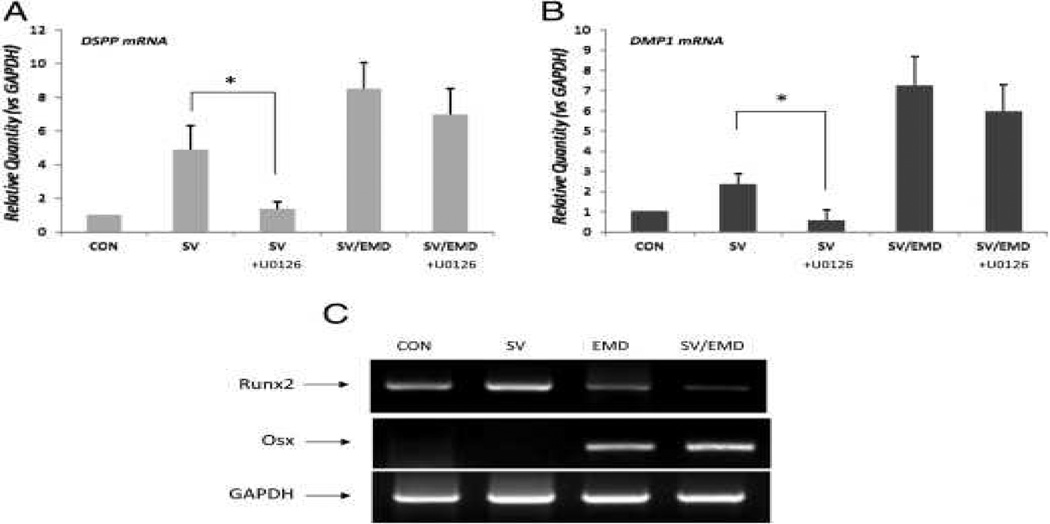
DMP1 and ON served as osteogenic markers, and DSPP served as a specific marker for the odontoblast phenotype. As shown in Figure 2A and B, on day 3 there was a significant level of DSPP and DMP1 expression in simvastatin/EMD-treated cells compared with simvastatin- and EMD-treated cells. A significant level of ON expression was observed only in simvastatin/EMD-treated cells on day 1.
Figure 2.
Effects of Simvastatin and EMD on Mineralization
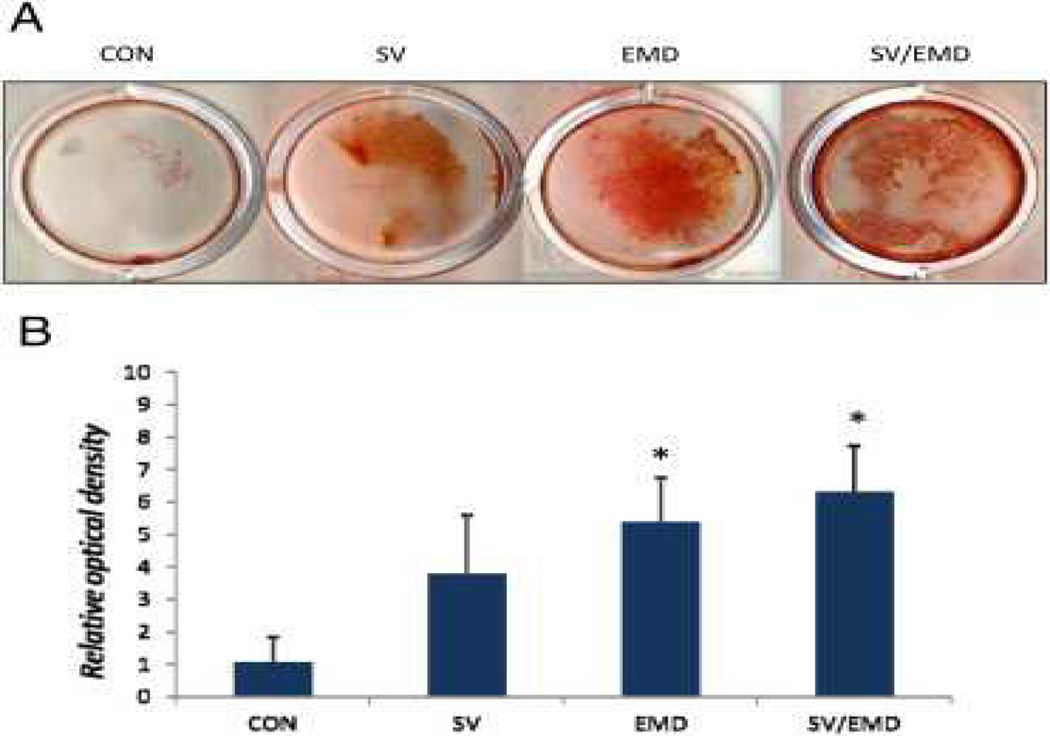
As shown in Figure 3A and B, alizarin red S staining for calcium showed a significant increase in mineralization with EMD and simvastatin/EMD treatments compared with the control. However, the simvastatin-treated group did not show a significant increase compared with the control group (P < .05).
Figure 3.
Effect of the ERK Inhibitor on Simvastatin-induced Odontoblastic/Osteoblastic Marker Expressions
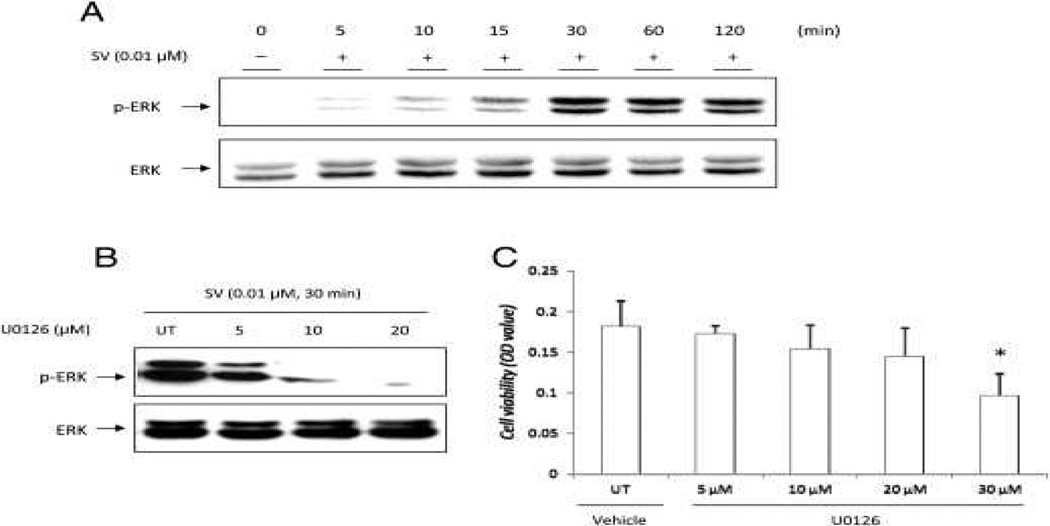
Simvastatin at a concentration of 0.01 μmol/L caused the accumulation of phospho-ERK in hDPCs within 30 minutes of treatment (Fig. 4A). In hDPCs treated with 10 μmol/L U0126, the effects of simvastatin-induced ERK phosphorylation were blocked completely (Fig. 4B). As shown in Figure 4C, cell viability did not decrease significantly in the presence of 10 μmol/L U0126 compared with the control for the cultivation time (P > .05). To address whether the ERK pathway affects odontoblastic/osteoblastic differentiation, real-time PCR was performed on hDPCs treated with 0.01 μmol/L simvastatin in a medium with or without 10 μmol/L U0126 for 3 days. The results showed that the expression of DSPP and DMP1 decreased significantly with U0126 treatment in simvastatin-treated hDPCs (Fig. 5A and B). However, in simvastatin/EMD-treated cells, the expression of these genes did not decrease significantly. The expression of ON did not decrease significantly either in simvastatin- or simvastatin/EMD-treated hDPCs (data are not shown).
Figure 4.
Figure 5.
Effects of Simvastatin and EMD on Osteoblast-specific Transcriptional Factors
To gain additional insight on the odontogenic potential of simvastatin and EMD, we examined the messenger RNA expression of osteoblast-specific transcriptional factors such as Runt-related gene 2 (Runx2) and osterix (Osx). After 3 days of cultivation, the EMD groups showed a decreased expression of Runx2 compared with that in the simvastatin and control groups. The EMD- and simvastatin/EMD-treated groups showed a greater expression of Osx compared with that in the other groups (Fig. 5C).
Discussion
The aim of the current study was to investigate the effect of simvastatin in inducing the differentiation of hDPCs into odontoblast/osteoblast-like cells and whether its effect was facilitated by EMD. In the present study, simvastatin and EMD were used as possible supplemental pulp-capping agents because they showed odontogenic/osteogenic potential in previous studies and have already been approved for clinical use. Therefore, they can be easily used by clinicians if they are prepared commercially in suitable forms for clinical application. This is the advantage of these agents over other agents in terms of the clinical use under investigation. Moreover, unlike liquid agents used in previous studies 23 and 24, EMD is a type of gel that does not rapidly diffuse into the pulp tissue (14). This property of EMD may retain its odontogenic/osteogenic effect and increase the effect of simvastatin when the 2 agents are used together in the form of a mixture.
In this study, we showed that the combined use of simvastatin and EMD has superior potential in odontoblastic/osteoblastic differentiation compared with their separate use, as evidenced by the induction of ALP activity, the formation of mineralization nodules, and the expression of DSPP and DMP1. DSPP, primarily expressed in odontoblasts, is a specific marker for functional odontoblasts (25). DMP1 was initially identified in odontoblasts during embryonic and postnatal development, and it is required in both the early and late stages of odontogenesis (26). DMP1 is also considered an odontoblastic marker even though it is less specific compared with DSPP. Our results suggest that the combined use of these 2 agents has more potent effects in terms of odontoblastic differentiation in hDPCs compared with the separate use of each agent. In addition, the amount of mineralization was greater in EMD- and simvastatin/EMD-treated cells than in the control cells (Fig. 3). This result shows that EMD may contribute to mineralization or odontoblastic/osteoblastic differentiation of hDPCs. The idea of using multiple agents for promoting differentiation is also consistent with a previous report on hDPCs treated by MTA and EMD (14).
The mechanism of how simvastatin or EMD influences the function of hDPCs is not fully understood. One of the potential signal transduction pathways that might regulate the proliferation and differentiation of various cell types is the mitogen-activated protein kinase family, which includes ERK, c-Jun N-terminal kinase, and p38 (27). Among these, ERK is an essential mediator of growth factor–induced cell differentiation in various cell types. Chen et al (28) reported that ERK is one of the signaling pathways that may be involved in simvastatin-induced differentiation in mouse osteoblasts. Kim et al (29) also documented that simvastatin may stimulate the osteoblastic differentiation of periodontal ligament cells through the regulation of the ERK pathway. In this respect, the present study examined whether the ERK pathway is involved in simvastatin-induced odontoblastic/osteoblastic differentiation in hDPCs. However, Emdogain is not an agent with a single formula and consists of many types of protein; therefore, it is difficult to resolve which ingredient mainly affects the differentiation. Thus, in this study, we focused on the signaling pathway involved only in simvastatin-induced differentiation. As shown in Figure 4A, simvastatin enhanced the phosphorylation of ERK within 30 minutes in hDPCs (Fig. 4A). This means that simvastatin stimulates ERK phosphorylation and might affect the differentiation of hDPCs. Notably, blocking of the ERK signaling pathway by U0126, a specific antagonist of ERK, inhibited the expression of DSPP and DMP1 (Fig. 5A and B) and the phosphorylation of ERK (Fig. 4B). These results suggest that the ERK signaling pathway is a potential positive regulator of the simvastatin-induced endothelial differentiation in hDPCs.
In our study, the inhibition of the ERK pathway did not decrease the expression of odontoblastic markers in simvastatin/EMD-treated cells. These results may explain that EMD compensated the blocking effect of U0126 on simvastatin-induced differentiation. To gain additional insight on whether EMD induces osteoblast-specific transcriptional factors that may contribute to odontoblastic differentiation even in the presence of an ERK inhibitor, we investigated the expression of Runx2 and Osx messenger RNA using reverse-transcription PCR. The transcription factor Runx2 is necessary and regulates the expression of many bone- and tooth-related genes (30). Runx2-deficient mice show impaired tooth formation, progressing only to the cap/early bell stages (31). Osx, another transcription factor, is also essential for osteoblast differentiation and bone formation because Osx-deficient mice also lack bone formation because of the arrested maturation of osteoblasts (32). In the present study, the expression of Runx2 decreased during treatment with EMD (Fig. 5C). Recently, Jiang et al (33) reported that Runx2 was inhibited by EMD in human alveolar osteoblasts and stated that Runx2 has dual regulatory activity in repressing and activating osteogenic gene expression, and these effects depend on the maturational stage of cells and cofactors involved at that stage. Weishaupt et al (34) also reported that Runx2 expression was not influenced by stimulation with 100 μg/mL EMD. In contrast, a recent study showed increased messenger RNA levels for osteogenesis-related transcription factors such as Runx2 (35). Because the mechanism of EMD regulation on Runx 2 is still unclear, it needs to be explored further. In this study, the expression of Osx increased in EMD and simvastatin/EMD-treated cells (Fig. 5C). Narukawa et al (35) showed that EMD up-regulated the expression of Osx messenger RNA within 3 days in mouse fibroblastic cells. Recently, Lee et al (15) reported that the expression of Osx was up-regulated after EMD treatment in hDPCs. In our mineralization assay using alizarin red S staining, EMD-treated groups showed more mineralization nodule formation (Fig. 3). These results also support the premise that EMD can contribute to the expression of odontoblastic differentiation markers even in the presence of an ERK inhibitor. In accordance with the results of previous studies, our results show that EMD also plays an important role in odontoblastic differentiation by contributing to the transcription of Osx and mineralization.
Collectively, the present study indicates that simvastatin increases the potential for odontoblastic differentiation in hDPCs via the ERK signaling pathway. In addition, simvastatin-induced odontoblastic differentiation of hDPCs is potentiated by combining simvastatin with EMD. Based on the current results, both null hypotheses were rejected. These results suggest a new strategy for the induction of odontoblastic differentiation, and this strategy might be useful in the formation of reparative dentin for successful vital pulp therapy.
Acknowledgments
The authors thank Professors Joo-Cheol Park and Seoung-Hoon Lee for providing valuable suggestions. Supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (no. 2011-0013825).
Footnotes
The authors deny any conflicts of interest related to this study.
References
- 1.Schroder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64:541–548. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 2.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 3.Baek KH, Lee WY, Oh KW, et al. The effect of simvastatin on the proliferation and differentiation of human bone marrow stromal cells. J Korean Med Sci. 2005;20:438–444. doi: 10.3346/jkms.2005.20.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C, Guo Z, Ma Q, et al. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun. 2003;308:458–462. doi: 10.1016/s0006-291x(03)01408-6. [DOI] [PubMed] [Google Scholar]
- 5.Lai EH, Hong CY, Kok SH, et al. Simvastatin alleviates the progression of periapical lesions by modulating autophagy and apoptosis in osteoblasts. J Endod. 2012;38:757–763. doi: 10.1016/j.joen.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto Y, Sonoyama W, Ono M, et al. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod. 2009;35:367–372. doi: 10.1016/j.joen.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Min KS, Lee YM, Hong SO, Kim EC. Simvastatin promotes odntoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod. 2010;36:447–452. doi: 10.1016/j.joen.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Hargreaves KM, Cohen S. Pathways of the Pulp. 10th ed. St Louis, MO: Mosby Year Book; 2011. p. 604. [Google Scholar]
- 9.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 10.Heijl L, Heden G, Svärdström G, Östgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705–714. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 11.Gestrelius S, Lyngstadaas SP, Hammarström L. Emdogain-periodontal regeneration based on biomimicry. Clin Oral Investig. 2000;4:120–125. doi: 10.1007/s007840050127. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Hammarström L, Matsumoto K, Lyngstadaas SP. The induction of reparative dentine by enamel proteins. Int Endod J. 2002;35:407–417. doi: 10.1046/j.1365-2591.2002.00556.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Slaby I, Matsumoto K, et al. Immunohistochemical characterization of rapid dentin formation induced by enamel matrix derivative. Calcif Tissue Int. 2004;75:243–252. doi: 10.1007/s00223-003-0153-y. [DOI] [PubMed] [Google Scholar]
- 14.Min KS, Yang SH, Kim EC. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endod. 2009;35:847–851. doi: 10.1016/j.joen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Min KS, Choi GW, et al. Effects of simvastain and enamel matrix derivative on Portland cement with bismuth oxide-induced growth and odontoblastic differentiation in human dental pulp cells. J Endod. 2012;38:405–410. doi: 10.1016/j.joen.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Haruyama N, Nishimura F, Kulkarni AB. Dentin sialophosphoprotein and dentin matrix protein-1: Two highly phosphorylated proteins in mineralized tissues. Arch Oral Biol. 2012;57:1165–1175. doi: 10.1016/j.archoralbio.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papagerakis P, Berdal A, Mesbah M, et al. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002;30:377–385. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Choi YR, Park MS, et al. ERK 1/2 activation in enhanced osteogenesis of human mesenchymal stem cells in poly(lactic-glycolic acid) by cyclic hydrostatic pressure. J Biomed Mater Res A. 2007;80:826–836. doi: 10.1002/jbm.a.30945. [DOI] [PubMed] [Google Scholar]
- 19.Lai CF, Chaudhary L, Fausto A, et al. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- 20.Palaez D, Arita N, Cheung HS. Extracellular signal-regulated kinase (ERK) dictates osteogenic and/or chondrogenic lineage commitment of mesenchymal stem cells under dynamic compression. Biochem Biophys Res Commun. 2012;417:1286–1291. doi: 10.1016/j.bbrc.2011.12.131. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal RK, Jaiswal N, Bruder SP, et al. Adult human mesenchymal stem cell differentiation to osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Roberts NR, Wu ML, et al. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954;207:19–37. [PubMed] [Google Scholar]
- 23.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 24.Jepsen S, Albers HK, Fleiner B, et al. Recombinant human osteogenic protein-1 induces dentin formation: an experimental study in miniature swine. J Endod. 1997;23:378–382. doi: 10.1016/S0099-2399(97)80187-2. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza RN, Bachman T, Baumgardner KR, et al. Characterization of cellular responses involved in reparative dentinogenesis in rat molars. J Dent Res. 1995;74:702–709. doi: 10.1177/00220345950740021301. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Ye L, Yu S, et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007;303:191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min KS, Kim HI, Chang HS, et al. Involvement of mitogen-activated protein kinases and nuclear factor-kappa B activation in nitric oxide-induced interleukin-8 expression in human pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:654–660. doi: 10.1016/j.tripleo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Chen PY, Sun JS, Tsuang YH, et al. Simvastatin promotes osteoblast viability anddifferentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res. 2010;30:191–199. doi: 10.1016/j.nutres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Kim IS, Jeong BC, Kim OS, et al. Lactone form 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) stimulate the osteoblastic differentiation of mouse periodontal ligament cells via the ERK pathway. J Periodontal Res. 2011;46:204–213. doi: 10.1111/j.1600-0765.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Gluhak-Heinrich J, Wang YH, et al. Runx2, Osx, and Dspp in tooth development. J Dent Res. 2009;88:904–909. doi: 10.1177/0022034509342873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Souza RN, Aberg T, Gaikwad J, et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 33.Jiang SY, Shu R, Song ZC, Xie YF. Effects of enamel matrix proteins on proliferation,differentiation and attachment of human alveolar osteoblasts. Cell Prolif. 2011;44:372–379. doi: 10.1111/j.1365-2184.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weishaupt P, Bernimoulin JP, Trackman P, Hagewald S. Stimulation of osteoblastswith Emdogain increases the expression of specific mineralization markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:304–308. doi: 10.1016/j.tripleo.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Narukawa M, Suzuki N, Takayama T, et al. Enamel matrix derivative stimulates osteogenesis- and chondrogenesis-related transcription factors in C3H10T1/2 cells. Acta Biochim Biophys Sin. 2007;39:1–7. doi: 10.1111/j.1745-7270.2007.00250.x. [DOI] [PubMed] [Google Scholar]