Abstract
Current cancer vaccines induce tumor-specific T cell responses without sustained tumor regression because immunosuppressive elements within the tumor induce exhaustion of effector T cells and infiltration of immune-suppressive regulatory T cells (Tregs). Therefore, much effort has been made to generate agonistic Abs targeting members of the TNFR superfamily, such as OX40, 4- 1BB, and GITR, expressed on effector T cells and Tregs, to reinvigorate T cell effector function and block Treg-suppressive function. In this article, we describe the development of a panel of anti-human OX40 agonistic mouse mAbs that could promote effector CD4+ and CD8+ T cell proliferation, inhibit the induction of CD4+ IL-10 -producing type 1 regulatory T cells, inhibit the expansion of ICOS+IL-10+ Tregs, inhibit TGF-b–induced FOXP3 expression on naive CD4+ T cells, and block natural Treg–suppressive function. We humanized two anti–human OX40 mAb clones, and they retained the potency of their parental clones. These Abs should provide broad opportunities for potential combination therapy to treat a wide realm of cancers and preventative vaccines against infectious diseases.
Introduction
Numerous therapeutic cancer vaccines have been developed that induce tumor-specific T-cell responses in patients (1–4); however, patient clinical response rates following vaccination have been low. This low response rate has been attributed largely to the presence of immunosuppressive elements at the tumor sites that induce exhaustion of tumor-infiltrating lymphocytes (TILs), influx of immune-suppressive CD4+ regulatory T cells (Tregs), secretion of the anti-inflammatory cytokines TGF-β and IL-10 that induce the generation of regulatory DCs (DCregs) and maintain CD4+ natural occurring FOXP3+ regulatory T cells (nTregs) or convert CD4+ T cells into inducible IL-10+/TGF-β+Tregs (iTregs) (5–11). Indeed, recent reports showed that tumor-specific CD8+ T cells from melanoma patients were functionally impaired and expressed high levels of the inhibitory receptors PD-1, TIM-3, CTLA4, and LAG3 (5, 12). Besides impaired CD8+ T cells, a large number of CD25+CD4+Tregs were found in the tumors and draining lymph nodes of many cancer patients (13). The accumulation of Tregs at tumor sites has been attributed to the secretion of the chemokine CCL22 by cancer cells and macrophages that actively recruit Tregs expressing the C-C chemokine receptor 4 (CCR4) (14, 15). Furthermore, TGF-β and IL-10 secreted by cancer cells can induce TGF-β-producing Tregs or Tregs, which actively suppress effector T cell function and expansion (10, 16), either directly or indirectly through the induction of DCregs (10). These two cytokines not only can induce iTregs, they were also shown to maintain the expression of the transcription factor FOXP3 and suppressive function of Tregs (17, 18). As a consequence of these negative factors in the tumor environment, the majority of tumor infiltrating lymphocytes (TILs) are either functionally impaired CD4+/CD8+ T cells (5, 12) or have been converted into (19, 20) or TGF-β– producing (21) Tregs that prevent anti-tumor immune responses.
Evidence from the literature suggests that these negative elements within the tumor microenvironment can be modulated by triggering members of the TNF-R superfamily, such as OX40, 4-1BB and GITR, that are highly expressed on effector T cells and Tregs to reinvigorate T-cell effector function and block Treg suppressive function (13, 22–27). Therefore, intense research over the last decade has focused on generating reagents that trigger these molecules. We recently showed that triggering of human OX40 with OX40 ligand shuts down the generation and function of type one regulatory T cells (Tr1), while agonists of 4-1BB and GITR were ineffective (28). Recent studies by others further showed that triggering of OX40 turns off FOXP3+ Tregs and inhibits TGF-β- and antigen-driven conversion of naive CD4+ T cells into CD25+FOXP3+ T cells (29, 30). Studies from mice have demonstrated that targeting OX40 by using agonistic monoclonal antibodies (31, 32) could promote effector T cell function and memory by promoting T cell survival and clonal expansion (33, 34), inhibit the function or survival of normal and tumor-derived FOXP3+ natural Tregs (23, 31), and induce changes in the tumor stroma, including a decrease in the number of macrophages and myeloid-derived suppressor cells (35). Antibodies against human OX40 generated by using phage antibodies (U.S. Pat. No. 7,550,140) or mice immunized with either human OX40 DNA (36) or OX40-transfected L929 cells (37) are capable of promoting effector T cell function. In particular, the antibodies generated by Weinberg and colleagues have in vivo efficacy in prolonging T cell survival in nonhuman primates (36).
In this report, we describe the successful generation of a new panel of agonistic anti-human OX40 mAbs that can potently enhance CD4+ and CD8+ effector T cell function, inhibit induction of Tr1 cells and FOXP3+ Tregs, and completely block the suppressive function of nTreg cells. We also show that these antibodies can block the function of Tregs derived from follicular lymphoma tumors. Further characterization involving mechanistic studies suggest that our anti-hOX40 mAbs block Treg suppression by directly inhibiting the function of Tregs and indirectly by making effector T cells resistent to suppression by Tregs. In summary, our antibodies can simultaneously promote human effector T cell function and block human Treg suppressive function, both key targets for developing effective immunotherapy strategies to cure cancers.
Materials and Methods
Reagents and cell lines
The following reagents were purchased from the indicated manufacturers: TLR ligands Pam3CSK4 and flagellin (FLA-ST ultrapure, Invivogen); IL-2 and IL-10 ELISA kits (R&D Systems); fetal calf serum (FCS) and human serum (Gemini); carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen); Phytohemagglutinin (PHA-P, Sigma-Aldrich) Mouse fibroblast L cell lines expressing human OX40 (hOX40-L), CD32 alone (CD32-L) or with ICOS ligand, or OX40 ligand- (ICOSL-CD32-L, OX40L-CD32-L) were generated in our laboratory and maintained with RPMI cultured medium containing 10% FCS, 1% glutamine, and 1% penicillin-streptomycin.
Generation and screening of anti-hOX40 mAbs
Anti-hOX40 mAbs antibodies were generated using BALB/c female mice immunized with hOX40-L cells at the MD Anderson mAb Core facility following established protocols. Hybridomas secreting monoclonal antibodies recognizing hOX40 were identified by ELISA and flow cytometry and agonistic function was identified by screening clones for the ability to shut down IL-10+Tr1 cell induction and block the suppressive function of nTregs. For functional assays, antibodies were purified using FPLC-protein A FF HiTrap (GE healthcare) and eluted with Gentle Ag/Ab elution buffer (Pierce). The generation of humanized anti-hOX40 mAbs was performed by JN Biosciences.
Antibodies, FACS analysis, and cell sorting
The following antibodies were used for flow cytometry analysis: IL-2-PE, CD4-APC-Cy7, CD127-PE, CD25-PE-Cy7, CD14-FITC, CD16-FITC, CD20-FITC, CD56-FITC, CD11C-FITC, TCRγδ-FITC (BD Pharmingen). FOXP3-APC was from Biolegend (Clone 259D). Functional grade antibodies were anti-CD3 (OKT3) (Centocor Ortho), rhesus monkey cross-reactive anti-CD3 (Clone SP34, BD Biosciences), anti-ICOS and anti-CD28 (eBioscience). F(ab’)2 goat anti-human IgG, Fcγ fragment specific secondary antibody and ChromPure goat IgG, F(ab’)2 fragment control antibody were from Jackson ImmunoResearch Laboratories, Inc. Human IgG1 isotype control was purchased from Sigma. Anti-human CD134 (clone ACT35) was purchased from BD Biosciences. Flow cytometry was performed on a flow cytometer (FACS Calibur, BD). FACS sorting was conducted on a cell sorter (FACS Aria IIU. BD). All flow cytometry analyses were gated on live T cells.
Isolation of CD4+ T-cell subsets from healthy donors and FL patient
Buffy coat samples prepared from the peripheral blood of healthy, adult donors were obtained from the Gulf Coast Regional Blood Center in Texas (IRB LAB03-0390 “Isolation of human dendritic cells, T cells and hematopoietic progenitor cells from human blood and tissue samples”). CD4+ naïve T cells, Teff and Tregs cells (purity 99% for all) were isolated from PBMCs by using a CD4+ T-cell enrichment cocktail (STEMCELL Technologies) followed by gating out lineage negative markers (CD14, CD16, CD20, CD56, CD11C, TCRγδ) and cell sorting CD4+CD127LowCD25high (top 4–6%) fraction as nTregs), CD4+CD127+CD25lowCD45RA−CD45RO+ fraction as Teff and CD4+CD127+CD25low CD45RO−CD45RA+ fraction as naïve T cells. To isolate T cells from FL patients, single cell suspensions were prepared from FL patient tumor tissue samples obtained from the NCI (IRB LAB04-0717). Single cells were sorted into two fractions of CD4+CD127LowCD25high (Tregs) and CD4+CD127+CD25low(Teff).
Generation Tr1 cells from CD4+ T cells and IL-10 Assays
A total of 2 × 105 freshly isolated CD4+ T cells were cultured with irradiated (60 Gy) ICOSL-expressing CD32-L cells (8 × 104), which were pre-coated with anti-CD3 (0.2 μg/ml) in the presence of dexamethasone (5 × 10−8 M; Life Technologies) and 1α,25-dihydroxyvitamin D3 (1 × 10−7 M; Life Technologies) in T cell culture medium containing 10% FCS, RPMI GlutaMAX (Gibco), 1% penicillin-streptomycin plus IL-2 (50 IU/ml) plus soluble anti-CD28 (0.2 μg/ml) for 7 days in a 48-well tissue culture plate. Expanded T cells were re-stimulated with 50 ng/mL phorbol myristate acetate (PMA, Sigma) and 2 μg/mL ionomycin (ION, Sigma) for 6 h. During the last 4 h, 10 μg/mL brefeldin A (BFA, Sigma) was added. The cells were stained with AlexaFluor647-IL-10 antibody (clone JES3-9D7, eBioscience) using Caltag FIX and PERM kit. For the evaluation of the effects of OX40 signaling on IL-10+ nTregs, freshly sorted CD4+CD127lowCD25high ICOS+Tregs were stimulated with anti-CD3 (0.2 μg/ml) and anti-CD28 (1μg/ml) in the presence of IL-2 (300 IU IL-2/ml) and OX40L-CD32-L cells, or CD32-L cells plus anti-hOX40 mAbs or control Ab for 5 days. Cells were then either re-stimulated with plate-bound anti-CD3 (2 μg/ml) plus soluble anti-CD28 (1 μg/ml) for 24 h and supernatants were assayed for IL-10 by ELISA, or stimulated with PMA/ION and stained with IL-10 antibody as described above.
Proliferation assays
Two systems were used to evaluate T cell proliferation in the presence or absence of Tregs.
Plate-bound proliferation system in the absence of accessory cells: anti-CD3 (3μg/ml) and anti-hOX40 mAbs (2μg/ml) in PBS were coated together on a non-tissue culture treated 96-well flat-bottom plate for 1 h at 37°C in a CO2 incubator. To determine the proliferation of naïve T cells, 105 naïve T cells were added per well containing T cell complete medium (RPMI GlutaMAX), 10% human AB serum, 1% penicillin-streptomycin). 1.0 μCi/well of methyl-3H-thymidine was added on the third day and after 15h, proliferation was assessed by thymidine incorporation. To determine the proliferation of Teff in the presence of Tregs, CFSE (4μM)-labeled Teff and nTregs, each at 8 × 104, were added per well.
CD14+ monocyte-based proliferation system: CFSE-labeled Teff (8 × 104) and Tregs cells at a 1:1 or 2:1 ratio were cultured in T cell medium in the presence of irradiated (60Gy) monocytes at a 1:1 monocyte to Teff ratio for 3.5 days in the presence of 0.3μg/ml of anti-CD3 antibody. (1,2) Teff proliferation in the presence of Tregs was determined after 3.5 days of stimulation by CFSE dilution assessed by flow cytometry.
Direct stimulation of T effector and nTreg with anti-hOX40 mAbs
To directly treat nTregs, nTregs were pre-stimulated with plate-bound anti-CD3 (2μg/ml) in T cell culture medium plus IL-2 (300 IU/ml) for 12h to up-regulate OX40 on all Treg cells in a 24-well plate. Activated nTregs were then pulsed with the anti-hOX40 antibodies (20μg/0.5 × 106 cells) in T cell culture medium for 4h at 37°C in CO2 incubator. Cells were then washed and cultured with CFSE-Teff cells in the presence of monocytes and soluble anti-CD3 (0.3μg/ml). To directly treat Teff, Teff cells were stimulated with plate-bound anti-CD3 (0.8μg/ml) in T cell culture medium containing 30 IU/ml of IL-2 for 12h to up-regulate OX40, then pulsed with the anti-hOX40 antibodies (20μg/0.5 × 106 cells) in T cell culture medium for 4h as above, washed 3 times and labeled with CFSE as described. Next, 8 × 104 of CFSE-Teff were cultured with nTregs cells in the presence of monocytes and soluble anti-CD3.
Induction of FOXP3 expression from naïve T cells
CFSE-labeled freshly sorted naïve CD4+ T cells (105) were stimulated with plate-bound anti-CD3 (3 μg/ml) and an anti-hOX40 mAb clone (2 μg/ml) 119-122, 106-222 or 120-270 in the presence of soluble anti-CD28 (1 μg/ml) and increasing concentrations of TGF-β in serum free AIM-V lymphocyte culture medium (Life Technologies). Three days after stimulation, expanded cells were stained with FoxP3 antibody using FOXP3 stain buffer system (eBioscience).
Generation of activated human PBMCs and Rhesus T cells for OX40 mAb binding assays
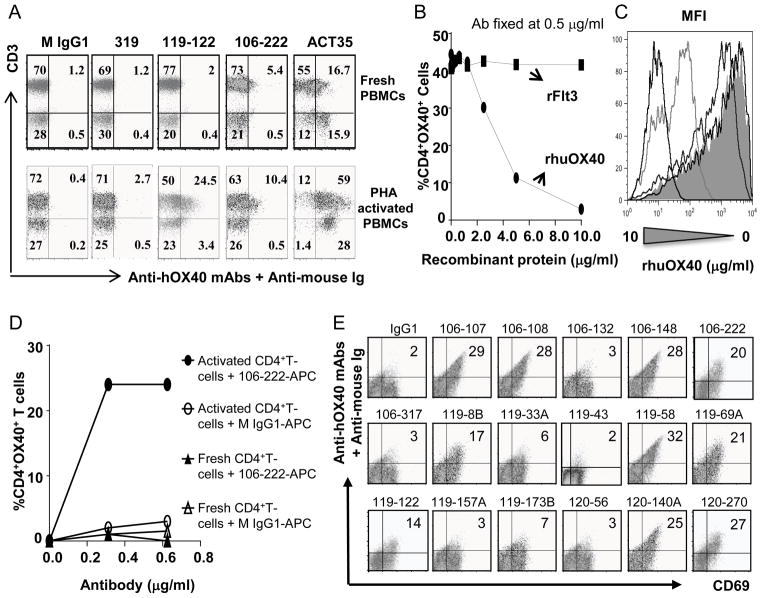
Human PBMCs were stimulated with soluble PHA (10 μg/ml) for 2 days in 10% FCS/1640 RPMI. Staining of CD3+OX40+ T cells was performed using primary anti-hOX40 mAbs followed by a secondary antibody against mouse Ig-PE conjugated. CD3+ T cells were detected using anti-CD3-Pacific Blue conjugated antibody. Antigen binding competition assays were performed by pre-incubating for 20 minutes 0.5 μg/ml of APC conjugated 106-222 antibody (Life Technologies- Antibody labeling kit- A20186) with increasing concentrations of recombinant human OX40 protein (Origene, TP311253) in FACS buffer (1% FCS/2mM EDTA). The antibody-antigen complex formed was later added to 5 × 105 of unstimulated or activated human T cells and further incubated for another 20 minutes. The binding was then analyzed on the CD4+ cell subpopulation. The indicated mean fluorescence was plotted for concentrations 0, 0.078, 0.625, 2.5 and 10 ug/ml of the recombinant protein. Rhesus CD4+ T cells were isolated from rhesus PBMC using anti-CD4 microbeads (Clone M-T466, Miltenyi Biotec). Enriched CD4+ T cells were stimulated with 8 μg/ml of plate-bound anti-CD3 (Clone SP34) for 72 hrs in the presence of IL-2 (50 IU/ml). Staining of OX40 expressing T cells was performed with 106-222-APC conjugated and compared with isotype control mouse IgG1-APC (BD Biosciences).
Statistical analysis
Statistical difference between experimental groups was determined by either paired or unpaired t tests or two-way ANOVA test using Prism software (Graphpad Software, Inc).
Results
Generation and identification of potent agonistic anti-hOX40 mAbs
Although OX40 signaling can break immune tolerance, the commercially available anti-human OX40 mAb ACT35 is ineffective in blocking the function of natural Tregs. We therefore decided to immunize mice with mouse fibroblast L cells stably expressing recombinant human OX40 on the cell surface. We performed three fusions, and screened the generated hybridoma clones by ELISA for binding to plate-bound L cells expressing human OX40 (hOX40-L). We found that over 500 hybridoma clones bound to hOX40-L cells but not the control L cells (data not shown). To demonstrate that these clones bound specifically to human OX40 expressed on the cell surface, we performed flow cytometry analysis. Supernatants from hybridomas were screened by staining a mixture of hOX40-L cells and parental L cells at a 1:1 ratio. We found that only 20 out of 500 of the anti-hOX40 mAbs stained OX40-expressing L cells but not the parental L cells (Fig. 1A), indicating that they bind specifically to surface hOX40.
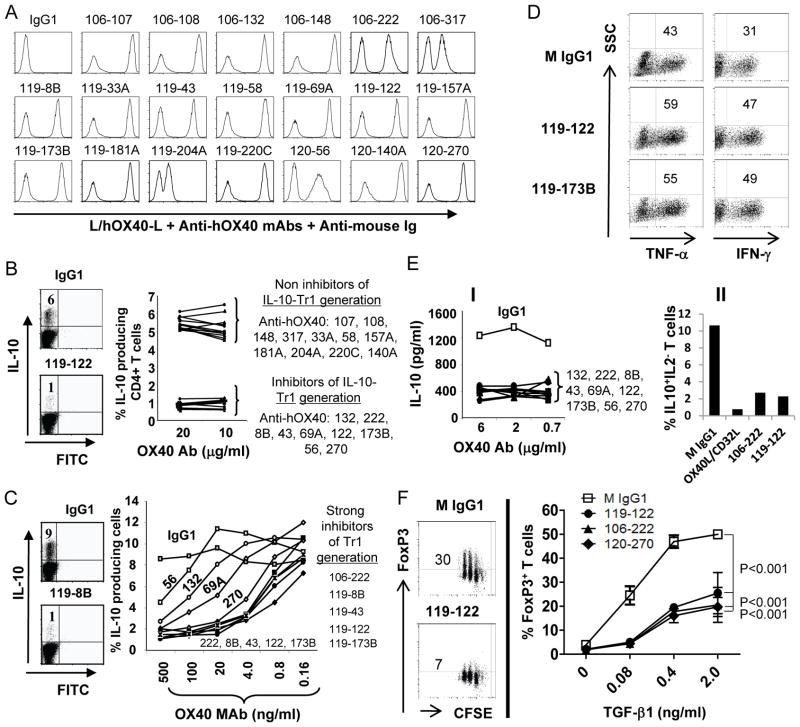
FIGURE 1.
Identification of anti-hOX40 mAbs that inhibit induction of T cells. (A) Flow cytometry analysis of L cells and L cells expressing human OX40 (hOX40-L) mixed (1:1) in FACS buffer and incubated with 0.5 μg of FPLC-purified, anti-hOX40 mAb. Anti-hOX40 mAbs were derived from three hybridoma fusions - 106, 119 and 120. Numbers following fusion number denote a specific antibody. Two peaks indicate positive and negative staining by the mAb. A single peak indicates no binding or non-specific binding of antibodies. Shown is a representative of two independent experiments. (B) Screening procedure to identify anti-hOX40 mAbs that inhibit the generation of cells (Tr1) from CD4+ T cells stimulated by anti-CD3/CD28 in the presence of vitamin D3/dexamethasone. An OX40 mAb was added on day 0 of cell culture. After 7 days of stimulation, IL-10 intracellular staining followed by flow cytometry analysis was performed on the CD4+ T cells. Left panel: representative FACS data Right panel: percentage of Tr1 cells after treatment with indicated anti-hOX40 mAb. (C) Titration of anti-hOX40 mAbs for their ability to inhibit the induction of Tr1 cells from CD4+ T cells. Experiment same as in B but with decreasing amounts of mAb. Left panel: representative FACS data. Right panel: percentage of Tr1 cells after treatment with decreasing concentrations of indicated anti-hOX40 mAb. (D) OX40 triggering induces TNF-α and IFN-γ–producing T cells from the IL-10 induction assay. Experiments were performed as in B and cells were stained with TNF-α and IFN-γ antibodies – followed by flow cytometry. Two representative FACS analyses from 6 antibodies with similar results are shown. (E) The OX40 mAbs that inhibit induction of Tr1 cells also inhibit expansion of IL-10+nTregs. Freshly sorted CD4+CD127lowCD25high ICOS+Tregs were stimulated with anti-CD3 and anti-CD28 in the presence of indicated anti-hOX40 mAb or control isotype for 5 days. I) Cells were then restimulated with anti-CD3/CD28 for 24 hours and supernatants were assayed for IL-10 by ELISA. II) Cells were stimulated with PMA/ION and stained with IL-10 and IL-2-antibodies, –followed by flow cytometry. (D–E) Data are representative of two independent experiments. (F) OX40 antibodies shut down TGF-β1-mediated induction of FOXP3+ expression. Freshly sorted CFSE-labeled naïve CD4+ T cells were stimulated with plate-bound anti-CD3 in the presence of indicated OX40 mAb and increasing concentrations of TGF-β1 in serum-free lymphocyte culture medium for 3 days. Expanded cells were stained with FoxP3 antibody. Results are from two independent experiments, with standard deviation of the mean shown as error bars. P values were calculated by two-way ANOVA test.
OX40 signaling has been shown to block the induction of Tr1 cells, TGF-β mediated conversion of naïve CD4+ T cells to FOXP3+ Tregs, and suppressive function of nTregs, but augment proliferation of CD4+ and CD8+ T cells (28, 29, 34, 38). To evaluate the agonistic activity of the 20 anti-hOX40 antibodies, we purified the antibodies by protein A/G-mediated affinity chromatography, and then first tested them for their ability to inhibit the induction of Tr1 cells. We found that nine of the twenty hOX40-specific antibodies could block vitamin D3/dexamethasone-mediated generation of IL-10–producing Tr1 cells from total CD4+ T cells (Fig. 1B). We next titrated the antibodies to determine their potency. As shown in Fig. 1C, five out of the nine antibodies potently suppressed the generation of IL-10–producing Tr1 cells at a concentration as low as 4 ng/ml. Figure 1D further shows that while the induction of IL-10+Tr1 cells was inhibited by anti-hOX40 mAbs, the percentages of TNF-α+ and IFN-γ+ cells were in fact increased. To evaluate the ability of our anti-hOX40 mAbs to also inhibit expansion of IL-10+FOXP3+ Tregs, we stimulated freshly sorted CD4+CD127lowCD25high ICOS+Tregs with anti-CD3 Ab-coated human CD32-L cells in the presence of anti-CD28 and an anti-hOX40 mAb or control IgG1 isotype for 5 days. Cells were then either re-stimulated with anti-CD3/CD28 Abs for IL-10 cytokine secretion or PMA/ION for staining of IL-10+IL-2−T cells. Figure 1E(I) shows that all nine anti-hOX40 mAbs tested blocked secretion of IL-10 from expanded IL-10+FOXP3+ Tregs. This reduction of IL-10 release was reflected by the 80 percent decrease in the number of IL10+IL−2Tregs (Fig. 1E(II)). To further evaluate the ability of our anti-hOX40 mAbs to inhibit TGF-β-mediated conversion of naïve CD4+ T cells to FOXP3+Tregs, we stimulated naïve CD4+ T cells with plate-bound anti-CD3 and an anti-hOX40 mAb with increasing concentrations of TGF-β in serum-free medium. Figure 1F shows that an anti-hOX40 mAb (three represented in figure) potently inhibited the induction of FOXP3+ T cells. These results, together, suggest that our anti-hOX40 mAbs inhibit the induction and expansion of IL-10+Tregsand FOXP3+ Tregs.
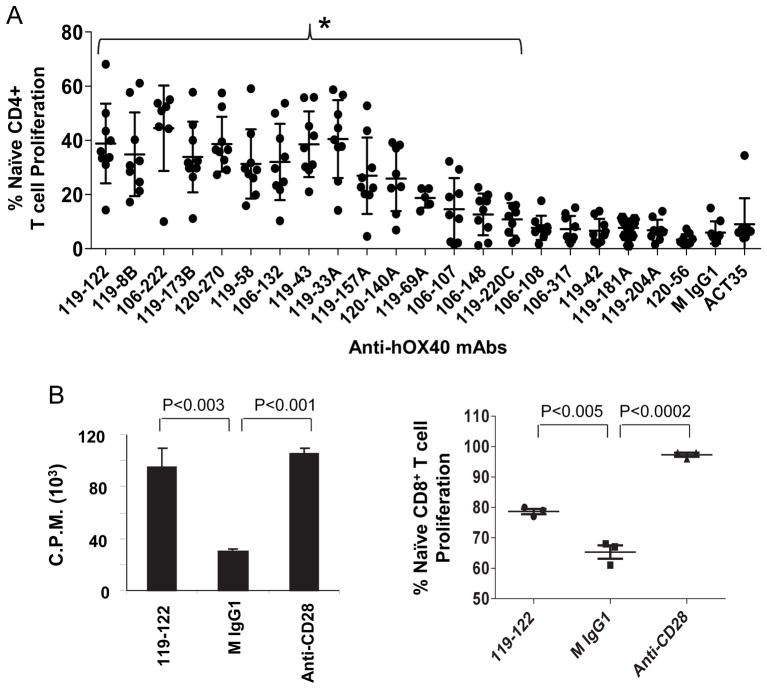
We further determined which of the twenty originally selected antibodies could augment TCR-triggered naïve CD4+ or CD8+ T cell proliferation. Plate bound anti-hOX40 and anti-CD3 mAbs were used to stimulate CFSE-labeled T cells. We found that eleven of the anti-hOX40 mAbs potently enhanced (5–9 fold) proliferation of naïve CD4+ T cells, while the only commercially available anti-hOX40 mAb, ACT35, had no such effect (Fig. 2A). Beside this stimulation of CD4+ T cells, one of the clones tested, 119-122, also stimulated CD8+ T cell proliferation (Fig. 2B). We next investigated whether these anti-hOX40 mAbs could inhibit nTreg suppressive function. We performed similar proliferation assays at a 1:0 or 1:2 ratio of CFSE-labeled CD4+CD25−CD45RA−CD45RO+ T effector cells (Teff) to CD4+CD127lowCD25high Tregs (nTregs). We found that the eleven antibodies that were strong activators of naïve CD4+ T cell proliferation were also strong activators of memory Teff proliferation (Fig. 3A) and could also completely block the suppression of nTregs (Fig. 3B). We note that the commercial anti-hOX40 mAb ACT35 is a relatively weak activator of Teff proliferation and weak blocker of nTreg suppressive function. Together these results indicate that several of our anti-hOX40 mAbs are potent agonists that can potently inhibit the generation and suppressive function of induced and natural Tregs.
FIGURE 2.
Anti-hOX40 antibodies enhance CD4+ and CD8+ T cell proliferation. (A) CFSE- labeled freshly sorted 1 × 105 CD4+CD25lowCD45RO−CD45RA+ naïve T cells were stimulated with plate-bound anti-CD3 (3 μg/ml) and indicated anti-hOX40 mAb (2 μg/ml). T cell proliferation was evaluated 4 days after stimulation. Mouse IgG1 and 119-42 served as negative controls. Results from the commercially available anti-hOX40 mAb ACT35 are shown to the far right. Fifteen anti-hOX40 mAbs (under the bracket {;119-122 to 119-220C) significantly enhanced T cell proliferation compared to control M IgG1 (P<0.05). Error bars represent means + SD. P values were calculated by paired t tests. (B, left panel) CD3+CD45RA+CD27+ CD8+ T cells were stimulated as in A and 3H-thymidine was added on the third day of culture and cells were harvested after another 15 hours of incubation. Proliferation of T cells was evaluated by thymidine incorporation. (B, right panel) CFSE-labeled CD8+ T cells were stimulated with plate-bound anti-CD3 (1.5 μg/ml) and an anti-hOX40 mAb (0.5 μg/ml). T cell proliferation was evaluated 3.5 days after stimulation. Mouse IgG1 and anti-CD28 served as negative and positive controls, respectively. Data are representative of two donors. (B) Error bars represent means + SD. P values were calculated by unpaired (B) t tests.
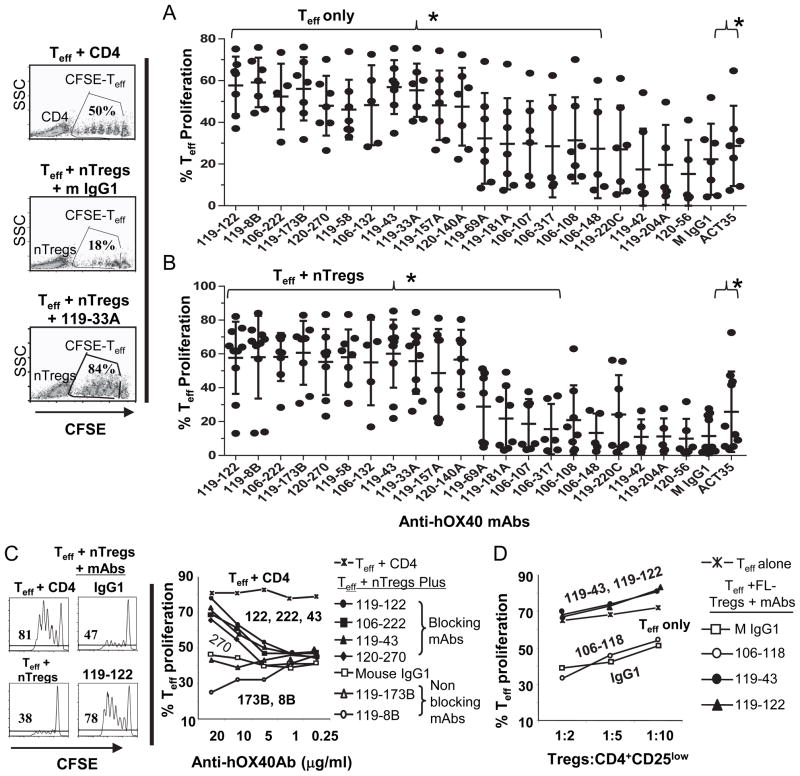
FIGURE 3.
Anti-hOX40 mAbs block the activity of CD4+CD25high nTregs. (A–B) Blocking of nTreg function in the absence of accessory cells. Syngeneic CFSE-labeled CD4+CD25lowCD127+CD45RA-CD45RO+ Teff and CD4+CD25high CD127− nTregs were cultured at a 1:2 ratio with soluble anti-CD28 (0.4 μg/ml), plate-bound anti-CD3 (3 μg/ml) and indicated anti-hOX40 mAb or isotype control (2 μg/ml). (Left panel) Representative FACS data showing the proliferation of Teff in the presence of naïve CD4+ T cells, nTregs or nTregs plus the anti-hOX40 mAb 119-33A. Right panels, percentage of T effector cell proliferation from 6–9 donors in the absence (A) or presence (B) of nTregs after treatment with indicated anti-hOX40 mAb. (A–B) Error bars represent means + SD. Anti-hOX40 mAbs under a bracket ({) on the top significantly enhanced T cell proliferation and block nTreg suppressive function (P<0.05 compared to control M IgG1). P values were calculated by paired t test. (C) Anti-hOX40 mAbs inhibit normal donor-derived nTreg function in the presence of accessory cells. Freshly sorted healthy donor-derived nTregs (3.5 × 104) were cultured 3–4 days with CFSE-Teff (7 × 104), irradiated CD14+ monocytes (7 × 104; 60Gy), anti-CD3 (0.3μg/ml) and increasing concentrations of anti-hOX40 mAb. (D) Inhibition of FL-derived Treg function. CD4+CD127lowCD25high Tregs were sorted from FL-infiltrating lymphocytes. CFSE-labeled CD4+CD127+CD25− T effector cells and monocytes were derived from a healthy donor. In A–C, representative FACS analyses are shown in left panels, unlabeled CD4+ T cells replace nTregs as control and cell proliferation was assessed by flow cytometry for CFSE dilution. Percentages of effector T cell proliferation after treatment with indicated antibody are shown in the right panels. All experiments were performed in duplicates. Data are representative from 2–3 donors.
Anti-hOX40 mAbs block normal donor and cancer patient-derived Treg function in the presence of accessory cells
Because antibodies are likely to interact with accessory cells in vivo to block Treg function, we evaluated the ability of our anti-hOX40 mAbs to block Treg suppression in the presence of CD14+ monocytes, CFSE-labeled Teff, nTregs and soluble anti-CD3 Abs. We found that 20 μg/ml of clone 106-222, 119-122, 119-43 or 120-270 blocked the suppressive function of nTregs (Fig. 3C). Surprisingly, clones 119-173B and 119-8B, which blocked nTreg function in the plate-bound proliferation system, could not block nTreg function in this system. We next wondered whether the anti-hOX40 mAbs could also block the function of allogeneic CD4+CD127lowCD25high Tregs isolated from a follicular lymphoma tumor sample in the presence of monocytes. We found that 119-43 and 119-122 antibodies completely restored proliferation of effector T cells in the three Treg: Teff ratios tested, while the control antibodies IgG1 and 106-118 (does not bind to hOX40) did not (Fig. 3D). These results demonstrate that some of the anti-hOX40 mAbs are capable of blocking Tregs isolated from healthy donors as well as cancer patients.
Anti-hOX40 mAbs act directly on T cells to block Treg suppression
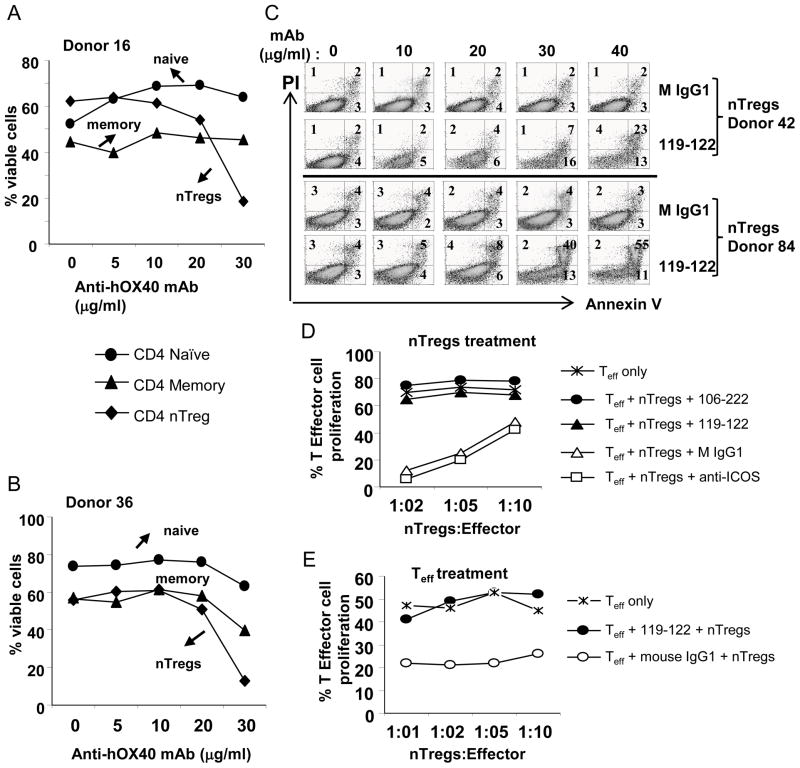
Evidence from the literature suggests that a strong OX40 triggering signal can block Treg suppression either directly or indirectly (23, 39). To test these possibilities, we first determined the sensitivity of a T cell subset to strong OX40 triggering. Each T cell subset, including naïve, memory, and nTreg cells, was stimulated with soluble anti-CD3 Abs in the presence of CD14+ monocytes and increasing concentrations of the anti-hOX40 mAb 106-222 that blocks nTreg suppression function. We found that the anti-hOX40 mAb stimulated proliferation of naïve T cells and nTregs in a dose-dependent manner (data not shown). However, when the antibody concentration reached 20 μg/ml, the percentage of viable nTregs evaluated by gating out live cells on a FSC and SSC plot decreased significantly (Fig. 4A–B). With an antibody concentration of 30 μg/ml, the viability of nTregs was reduced from 60% to less than 20%, while the viability of memory T cells was only reduced 10–15% and was unaffected in naïve T cells. To further monitor the precise apoptotic effect of OX40 triggering, we stained nTregs with annexin V and PI. Consistently, we found that at high doses of anti-hOX40 mAb a significant number nTreg cells were stained positive for annexin V or annexin V and PI (Fig. 4C). These results suggest that a stronger OX40 triggering can preferentialy induce apoptosis on nTregs leading to the loss of suppressive function. We next asked whether a brief pulse of pre-activated nTregs with anti-hOX40 mAb was sufficient to block suppression activity. We pulsed pre-activated nTregs with anti-hOX40 mAbs for 4 h and then co-cultured them with CFSE-labeled Teff in the presence of monocytes. We found that the brief pulse was sufficient to completely block nTreg suppression while control antibodies had no such effect (Fig. 4D). Because effector T cells are resistant to killing by OX40 triggering, we next determined whether OX40 triggering could instead render effector cells resistant to suppression by nTregs. We found that indeed pre-activated Teff became resistant to suppression by nTregs when they were subjected to a brief pulse with the anti-hOX40 mAb clone 119-122, while a control antibody had no such effect (Fig. 4E). These results, together, suggest that several of our anti-hOX40 mAbs act on both effector and nTregs to block nTreg function.
FIGURE 4.
High concentrations of anti-hOX40 mAb kill nTregs. (A–B) T cell subsets naïve, CD4+CD25lowCD127+CD45RO−CD45RA+; T effector memory (Teff), CD4+CD25lowCD127+CD45RA−CD45RO+; or nTregs, CD4+CD25highCD127low were cultured with CD14+ monocytes (1:1) plus soluble anti-CD3 (0.3 μg/ml) and increasing concentrations of the 106-222 anti-hOX40 mAb. Cell viability was determined after 3 days of culture by flow cytometry by gating out live cells on a FSC and SSC plot (two donors). (C) nTregs were stimulated as in A except that, after 3 days of culture, the nTregs were stained with annexin V and PI. (D) Anti-hOX40-treated Tregs are unable to suppress Teff cell proliferation. nTregs were pre-stimulated with plate-bound anti-CD3 (2 μg/ml) for 12 hours, then pulsed with anti-hOX40 mAb, 119-122 or 106-222, or a control antibody, anti-ICOS or IgG1, washed and cultured with CFSE-labeled Teff plus monocytes and soluble anti-CD3 (0.3μg/ml). Proliferation was evaluated by CFSE dilution. (E) Anti-hOX40 mAb confers Teff cell resistance to suppression by nTregs. Teff were stimulated with plate-bound anti-CD3 (0.8 μg/ml) for 12 hours, then pulsed with the anti-hOX40 mAb 119-122 (20 μg/0.5M cells) for 4 hours, and 8 x104 CFSE-Teff were cultured with decreasing ratios of nTreg cells in the presence of monocytes and soluble anti-CD3. Proliferation of Teff cells was evaluated by CFSE dilution (two experiments/two donors).
Anti-hOX40 mAbs bind specifically to human and rhesus OX40
For the purpose of future preclinical testing of the efficacy and toxicity of our anti-hOX40 mAbs, we asked whether anti-hOX40 mAbs could bind specifically to 1) activated CD3+ T cells in human PBMCs; and 2) rhesus OX40 expressed on rhesus activated CD4+ cells. We obtained fresh human PBMCs from healthy donors and performed antibody binding assays. We found that two of our best antibodies (119-122 and 106-222) specifically bound to 10 to 25% of activated CD3+ T cells but not CD3 negative cells, while the same antibodies bound to only 2 to 5% of unstimulated CD3+ T cells (Fig. 5A). In contrast, no such binding was observed with control antibodies, M IgG1 and 319. Surprisingly, we found that the commercially available anti-hOX40 mAb ACT35 bound to both CD3− and CD3+ T cells in unstimulated PBMCs (Fig 5A), suggesting that the antibody is less specific for OX40 binding. To further assess the specificity of OX40 mAbs, we performed a competition assay in which a fixed amount of fluorochrome conjugated 106-222 OX40 antibody was pre-incubated with increasing concentrations of recombinant human OX40 protein. The antibody-antigen complex formed was later added to unstimulated or activated human T cells and the binding was analyzed on the CD4+ cell subpopulation. We found that the binding of 106-222 to activated CD4+ T cells could be blocked by rhOX40 protein in a dose dependent manner, while control protein has no such effect (Fig. 5B and C). Mean fluorescence was plotted for only four of the concentrations used of the recombinant protein (0, 0.078, 0.625, 2.5 and 10 ug/ml) showing that, even if the percentage of CD4+proliferating cells at lower concentrations is similar, (>40%) the mean fluorescence was reduced (Fig. 5C). Taken together, these results demonstrate that our anti-hOX40 mAb binds specifically to human OX40 expressed on activated CD3+ T cells. To determine whether our anti-hOX40 mAbs could bind specifically to rhesus OX40, we performed similar binding assays using fresh and anti-CD3 polyclonally stimulated CD4+ T cells. We found that the APC-conjugated anti-hOX40 mAb 106-222 bound only to activated CD4+ T cells (Fig. 5D), while no such binding was observed using M IgG1 isotype control, suggesting that our OX40 mAb also bind specifically to rhesus OX40. We next extended our study to all the anti-hOX40 mAbs. We purified CD4+ T cells from rhesus PBMCs and stimulated them with PHA for 2 days. Then, anti-hOX40 mAbs were tested for binding to the activated CD4+ T cells. We found that ten antibodies (107, 108, 148, 8B, 58, 69A, 122, 140A, 222 and 270) could bind to rhesus CD69+CD4+ T cells (Fig. 5E), while control antibody IgG1 did not bind, suggesting that these antibodies recognize rhesus OX40 on activated T cells.
FIGURE 5.
Anti-hOX40 mAbs bind specifically to human and rhesus OX40: functional data. (A) Anti-hOX40 mAbs bind specifically to activated human CD3+ cells. Fresh and PHA- activated PBMCs were tested with two agonistic anti-hOX40 mAbs (1μg/106 cells) and negative controls (mouse IgG1 and mAb 106-319, which do not bind to hOX40). Bound cells were determined by flow cytometry. Data are representative from three donors. (B–C) Antigen binding competition assay. APC conjugated 106-222 antibody (0.5 μg/ml) was pre-incubated with increasing concentrations of rhOX40 protein. The antibody-antigen complex formed was later added to unstimulated or activated human T cells and the binding was analyzed on the CD4+ cell subpopulation. Bound cells were expressed as %CD4+OX40+ cells (B) or Mean fluorescence intensity (C). (D) 106-222 mAb binds specifically to activated rhesus CD4+ T cells. CD4+ T cells were activated using plate-bound anti-CD3 and binding was detected using increasing concentrations of APC-106-222 or APC-IgG1. Bound cells were determined by flow cytometry and shown as %CD4+OX40+ cells. (E) Ten anti-hOX40 mAbs bind to activated rhesus CD4+ cells. CD4+ T cells were stimulated with 10 μg/ml PHA. After two days, cells were stained with indicated anti-hOX40 mAb followed by goat anti-mouse IgG-APC and CD69-PE. Mouse IgG1 and 106-317 served as negative controls. Bound cells were determined by flow cytometry. Data are representative from two donors.
Humanized mouse anti-hOX40 mAbs retained the functional activities of their parental mAbs
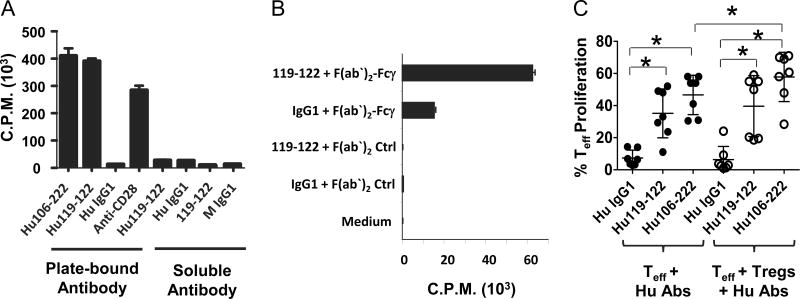
In view of a future clinical trial, we selected the anti-hOX40 mAbs 119-122 and 106-222 for humanization based on their ability to inhibit induction of IL-10+Tr1 cells and FOXP3+ Tregs, enhance proliferation of naïve CD4+ T cells, block suppressive function of nTregs in the absence or presence of accessory cells, and bind to rhesus OX40. The humanized anti-hOX40 mAbs Hu106-222 and Hu119-122 bound to OX40 with similar binding affinity (Supplemental Fig. 1). We next tested the ability of the humanized antibodies to enhance naïve CD4+ T cell proliferation and block nTreg function. As shown in Fig. 6A, plate-bound Hu106-222 and Hu119-122 mAbs have similar potency in enhancing CD4+ T cell proliferation. They both stimulated naïve CD4+ T cell proliferation to a level similar to that induced in the presence of anti-CD28 antibody (Fig. 6A). However, when the humanized or mouse anti-hOX40 mAbs were tested as soluble antibodies, they failed to stimulate T cell proliferation (Fig. 6A), suggesting that cross-linking is required for antibody activity. To demonstrate that cross-linking was required for antibody activity, naïve CD4+ T cells were stimulated with plate-bound-anti-CD3 Abs plus soluble mouse anti-hOX40 mAb 119-122 in the presence or absence of a secondary antibody against Fcγ. We found that the addition of a secondary antibody against Fcγ to the cell culture restored the antibody’s ability to enhance T cell proliferation, while control antibodies were ineffective (Fig. 6B), thus demonstrating that cross-linking is required for anti-hOX40 mAb function. We further determined whether Hu119-122 and Hu106-222 could block nTreg function. Figure 6C shows that both plate-bound antibodies potently stimulated Teff proliferation and blocked nTreg suppressive function. Interestingly, in the presence of Hu106-222 mAb and nTregs, the percentage of Teff proliferation exceeded that in the absence of nTregs, suggesting that in these conditions, OX40 signaling blocks nTregs function by the combined action of reversing nTreg function and inducing Teff resistance to suppression. These results suggest that the Hu106-222 and Hu119-122 mAbs retained the functional activities of their parental mAbs.
FIGURE 6.
Anti-human OX40 mAbs require cross-linking to enhance T cell proliferation and block Treg suppressive function. (A) Only plate-bound anti-hOX40 mAbs enhance CD4+ T cell proliferation. Freshly sorted naïve CD4+ T cells were stimulated with plate-bound anti-CD3 (3 μg/ml) plus indicated humanized or mouse anti-hOX40 mAb (2 μg/ml), plate-bound or soluble, in the absence of accessory cells. After three days, 3H-thymidine was added to the culture and cell proliferation was assessed after 15 hours by thymidine incorporation. Human and mouse IgG1 and anti-CD28 served as negative and positive controls, respectively. (B) Cross-linked soluble anti-hOX40 mAbs augment CD4+ T cell proliferation. Naïve CD4+ T cells were stimulated with plate-bound anti-CD3 (3 μg/ml). Soluble anti-hOX40 mAb clone 119-122 (2 μg/ml) was added alone or in combination with an equal amount of a secondary antibody against Fc. Mouse IgG1 and ChromPure goat IgG, F(ab’)2 fragment served as negative controls. Cell proliferation was evaluated as described in panel A. (A–B) Each treatment was performed in triplicate and data are representative from two donors. (C) Plate-bound humanized anti-hOX40 mAbs Hu106-222 and Hu119-122 block nTreg suppressive function. Teff and nTregs cells were prepared and cultured as described in 3A–B. Figure shows percentage of Teff proliferation taken from 7 donors in the absence (●) or presence (○) of nTregs after treatment with Hu106-222 or Hu119-122. Human IgG1 served as a negative control. Error bars represent means + SD. Statistical significance between treatment groups (*P<0.05 compared to control Hu IgG1) is indicated by a top bracket ([). P values were calculated by paired t test.
Discussion
A key question in the development of effective immunotherapy against cancer is how to reinvigorate effector T cell function and eliminate or block the suppressive function of Tregs. Recent studies have focused on targeting OX40 because it is highly expressed on the surface of Tregs and activated CD4+ and CD8+ T cells, and OX40 activation on effector CD4+ and CD8+ T cells was shown to promote their survival and expansion (33). Importantly, triggering of mouse OX40 on Tregs by using agonistic anti-mouse OX40 antibodies has been shown to block Treg suppressive function and induce Treg cell-specific apoptosis and tumor rejection in mice previously subjected to chemotherapy (23, 24, 27, 30, 33). Therefore, OX40 represents an attractive target molecule to develop immunotherapy for a wide realm of cancers. In this study, we performed an exhaustive immunization strategy using murine fibroblast L cells expressing hOX40 and innovative multitask screening procedures to identify a set of robust agonistic anti-hOX40 mAbs that: 1) enhance naïve CD4+ and CD8+ T cell proliferation; 2) inhibit expansion of IL-10+FOXP3+nTregs; 3) shut down induction of Tr1 cells and FOXP3+ Tregs from CD4+ T cells; and 4) act directly on Tregs to inhibit their function. We found that eleven anti-hOX40 mAbs (132, 222, 8B, 33A, 43, 58, 122, 173B, 157A, 140A, 270) enhanced levels of naïve CD4+ T cell proliferation, each over 5–10 fold compared to the control antibody. Although less robust, the anti-hOX40 mAb 119-122 also enhanced CD3+CD8+ T cell proliferation. This effect might explain why some of our anti-hOX40 mAbs could completely inhibit nTreg suppression of Teff proliferation. Importantly, we found that activated nTregs were highly susceptible to anti-hOX40 mAb or OX40L triggered cell death, while other T cell subtypes such as naïve and memory CD4+ T cells were less susceptible. These results are in agreement with two previous reports showing that stimulation with OX40L or OX40L plus TNF induced apoptosis of an OX40-expressing CD4+ T cell line and that pretreatment of mice with the alkylating agent cyclophosphamide (CTX), which upregulates the expression of OX40 on CD4+ FOXP3+ Tregs, combined with OX40 triggering could induce apoptosis in vivo (23, 39). These observations might also explain why memory T cells treated with a high concentration of an anti-hOX40 mAb maintained the ability to proliferate and became resistant to nTreg suppression, while treatment of nTregs with the same concentration of the anti-hOX40 mAb resulted in decreased cell number and loss of suppressive function. These results suggest that shutting down of IL-10 expression and triggering of apoptosis of the Tregs, and induced resistance of Teff to suppression by Tregs by the OX40 mAbs contributes to their overall inhibition of nTreg suppressive function, depending on the concentration of the antibodies used. Our finding that strong OX40 signaling preferentially kills Tregs is novel and significant. It opens up the possibility to preferentially expand effector T cells at tumor sites to induce potent immune response against cancer cells. These results suggest a new biological role of OX40 signaling in controlling the fate of Tregs in the tumor microenvironment. These results demonstrate that we have identified a set of anti-hOX40 mAbs with potent activity to stimulate T cell proliferation and block the induction or suppressive function of Tregs.
With the purpose of generating reagents suitable for preclinical testing, we further examined the ability of our antibodies in terms of their: a) cell-binding specificity; b) ability to block normal donor and cancer patient-derived Treg suppressive function in the presence of accessory cells; and c) binding to rhesus CD4+ T cells. We found that the majority of our antibodies could bind specifically to CD3+ T cells from human PBMCs, demonstrating that there is no off target binding molecules for our antibody in the human system. We further showed that ten of the anti-hOX40 mAbs could bind to rhesus activated CD4+ T cells, suggesting that they will most likely bind to rhesus OX40 and activate OX40 signaling, allowing us to test their toxicity in monkeys. Although our antibodies require cross-linking for activity, we found that they are able to restore T cell proliferation in the presence of Tregs and monocytes, suggesting that other accessory cells in the peripheral blood might be responsible for the cross-linking and trigger of OX40 signaling. Based on these results, two mouse anti-hOX40 mAbs, 119-122 and 106-222, were chosen for humanization. We found that in comparison with their parental mouse antibodies, the humanized anti-hOX40 mAbs Hu119-122 and Hu106-222 retained the same potency in enhancing naïve CD4+ T cell proliferation and blocking nTreg suppressive function. Collectively, these results indicate that we have generated potent anti-hOX40 mAbs that are suitable for promoting clonal expansion and survival of effector T cells as well as blocking normal donor and cancer patient-derived Treg suppressive function. Thus, these novel reagents are a suitable option for developing robust immunotherapy strategies to cure human cancers.
Supplementary Material
Acknowledgments
We thank Drs Jagannadha K. Sastry and Sattva S Neelapu for the gifts of rhesus PBMCs and follicular lymphoma TILs. We thank Karen Ramirez and Zhiwei He for cell sorting and support and Janis Johnson from antibody core facility for antibody purification. We thank Melissa Wentz for careful reading of the manuscript.
This work was supported by National Institutes of Health Grant U19 AI071130 (YJ Liu); R01 AI061645 (YJ Liu); UT MD Anderson Cancer Foundation (YJ Liu); and UTMDACC Lymphoma SPORE (to K.S.V). Larry Kwak was supported by Leukemia & Lymphoma Society (Specialized Center of Research grant 7262-08).
Abbreviations
- iTreg
IL-10+/TGF-b+ regulatory T cells
- nTreg
naturally occurring FOXP3+ Tregs
- Treg
CD4+ regulatory T cell
- mAb
monoclonal antibody
- Tr1
IL-10-producing type one regulatory T cell
- FOXP3
forkhead box P3
- hOX40-L
mouse fibroblast L cell expressing human OX40
- OX40L-L
L cell expressing OX40 ligand
- PBMC
peripheral blood mononuclear cell
- Teff
effector T cell
- ICOS
Inducible T-cell costimulator
Footnotes
Disclosures
K.V., L.B., and Y.J.L developed the mAb described in this article and are named inventors on a patent application from which they received royalty payments, exclusively licensed to GlaxoSmithKline. The other authors have no financial conflicts of interest.
References
- 1.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, McGaughey DS, Jaffe ES, Chong EA, Reynolds CW, Berry DA, Santos CF, Popa MA, McCord AM, Kwak LW. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)-and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 10.Yaguchi T, Sumimoto H, Kudo-Saito C, Tsukamoto N, Ueda R, Iwata-Kajihara T, Nishio H, Kawamura N, Kawakami Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol. 2011;93:294–300. doi: 10.1007/s12185-011-0799-6. [DOI] [PubMed] [Google Scholar]
- 11.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 12.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 15.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Research. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 -precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J Leukoc Biol. 2003;74:471–478. doi: 10.1189/jlb.0503228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 21.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 22.Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 23.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 26.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 27.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 30.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol. 2008;181:8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 33.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol. 2009;29:187–201. doi: 10.1615/critrevimmunol.v29.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Research. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, Axthelm MK, Picker LJ, Urba WJ. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 37.Xie F, Wang Q, Chen Y, Gu Y, Shi Q, Ge Y, Yu G, Wu H, Mao Y, Wang X, Zhou Y, Zhang X. Characterization and application of two novel monoclonal antibodies against human OX40: costimulation of T cells and expression on tumor as well as normal gland tissues. Tissue Antigens. 2006;67:307–317. doi: 10.1111/j.1399-0039.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer A, Xiao X, Vu MD, Gao W, Minamimura K, Chen M, Maki T, Li XC. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol. 2007;179:5584–5591. doi: 10.4049/jimmunol.179.8.5584. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi Y, Tanaka R, Yamamoto N, Tanaka Y. Enhancement of OX40-induced apoptosis by TNF coactivation in OX40-expressing T cell lines in vitro leading to decreased targets for HIV type 1 production. AIDS Res Hum Retroviruses. 2008;24:423–435. doi: 10.1089/aid.2007.0092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.