In 1994 Petit reported the isolation and anti-cancer activity of the marine sponge-derived macrolide dictyostatin.[1] Wright subsequently isolated a sample that allowed initial biological characterization of dictyostatin as a potent inducer of tubulin polymerization,[2] and that was used by Wright and Paterson to make a full structural assignment in 2004.[3] This assignment was confirmed soon thereafter by total syntheses by Paterson[4] and Curran,[5] and the material thus obtained facilitated more detailed characterization of dictyostatin’s mechanism of action.[6,7] Total syntheses by Phillips[8] and Ramachandran,[9] formal syntheses by Micalizio[10] and Cossy,[11] a synthesis of C(9)-epi-dictyostatin by Gennari,[12] second generation syntheses by Paterson[13] and Curran,[14] and several fragment syntheses[15] followed these initial reports. In addition, the Paterson/Wright[16] and Curran/Day[17] teams have reported extensive SAR studies, while the Paterson/Díaz/Jiménez-Barbero[18] and Curran/Snyder[19] teams have advanced models for the interaction of dictyostatin with the taxane binding site on β-tubulin. Because dictyostatin and some of the prepared analogs are among the most potent microtubule-stabilizing agents characterized to date, there has been and continues to be intense interest in the possibility of advancing dictyostatin or an analog thereof into the clinic, a goal which might be facilitated by the development of a significantly more efficient and step-economical synthesis. As part of a larger program devoted to the development of new strategies and methods for the synthesis of complex and precious marine macrolides with high levels of step-economy, efficiency, and scalability,[20] we have developed and report herein a synthesis of dictyostatin that comprises just 14 steps in the longest linear sequence.
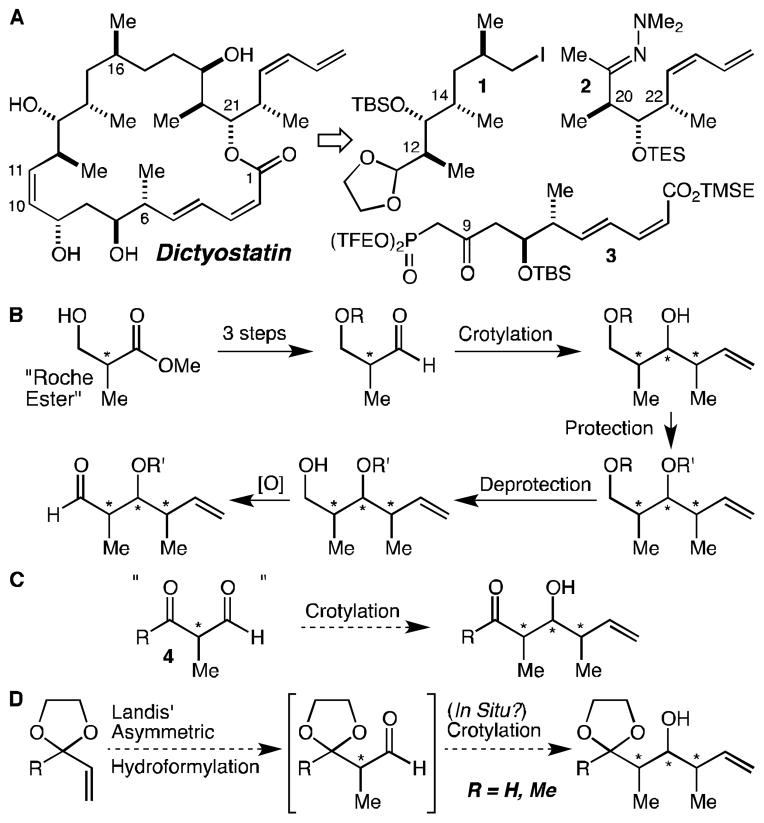
Similarly to the previous syntheses of dictyostatin, our retrosynthesis disconnected the target into three roughly equally complex fragments, 1, 2, and 3 (Fig. 1A). It was in the synthesis of the fragments, and especially the C(12)-C(14) and C(20)-C(22) stereotriad-containing fragments 1 and 2 that we saw an opportunity for a streamlining of the synthesis. Ever since its introduction by Roush more than 20 years ago, what might be called the “Roche ester strategy” has reigned supreme for the synthesis of such stereotriads,[21] and indeed was employed in the Paterson, Curran, and Ramachandran syntheses, in most of the approaches reported by others, and in most of the syntheses of the related natural product discodermolide.[22] In this approach, the requisite enantiomer of the Roche ester is protected, reduced, and oxidized to the corresponding aldehyde, which is then subjected to diastereoselective crotylation, followed by several additional functional group manipulations (Fig. 1B). Thus, these stereotriad syntheses typically comprise at least 6–7 steps of which all but one are protecting group or redox reactions. The crotylation of a configurationally stable aldehyde such as 4 would represent an ideal alternative, but of course, no such aldehyde exists (Fig. 1C). Protected versions of 4 do exist, however, and can be prepared using Landis’ ligand for asymmetric hydroformylation reactions.[23] Given that hydroformylation reactions tend to be clean, we were optimistic that the crotylation reactions could be carried out in operationally simple one-pot procedures (Fig. 1D). After we began our investigations, Burke reported a similar sequence employing an ortho ester-protected acrylate, in which the product aldehyde was reacted in situ with trans-crotylpinacolboronate.[24] This elegant demonstration of the power of the concept notwithstanding, significant work remained to establish whether externally controlled crotylations would work well with the products of the hydroformylation reactions and, more importantly, to establish whether and how these reactions could be easily and inexpensively scaled.
Figure 1.
(A) Disconnection of dictyostatin into 3 fragments of similar complexity; (B) The “Roche ester strategy” for the synthesis of functionalized stereotriads; (C) A hypothetical one-step stereotriad synthesis from aldehyde 4; (D) A proposal for the one-pot synthesis of the C(12)-C(14) and C(20)-C(22) stereotriads of dictyostatin.
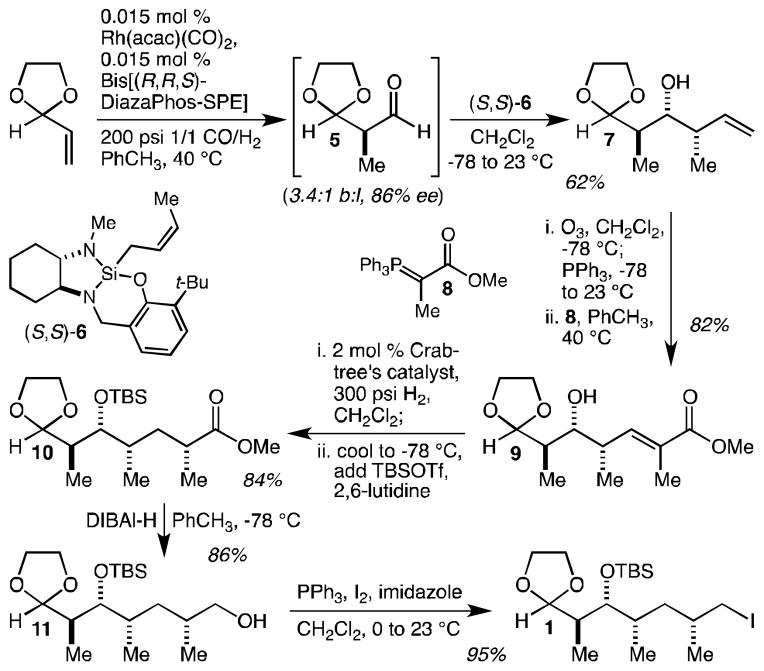
The synthesis of iodide 1 commenced with the rhodium-catalyzed hydroformylation of 2-vinyl-1,3-dioxolane with Landis’ bis[(R,R,S)-DiazaPhos-SPE] ligand[23] to produce aldehyde 5 (Scheme 1). At first, we employed Rh(acac)(CO)2 and ligand loadings of 0.50 and 0.55 mol % respectively, according to Landis’ original procedure, and these conditions produced 5 with 4.2:1 regioselectivity and in 92% ee. Because the ligand is far and away the most expensive component of the reaction, however, and because Sigma-Aldrich discontinued the sale of the ligand during the course of this project, we were highly motivated to investigate how low we could get the catalyst/ligand loadings and eventually settled on a loading of 0.015 mol %.[25] This did result in a small drop in both regioselectivity (3.4:1) and ee (86%), but in terms of the amount of 5 produced per unit of ligand, this represented a truly significant increase in efficiency. Asymmetric crotylation of 5 using our recently reported and more easily scaled crotylsilane (S,S)-6[26] was effectively carried out in situ, and this one-pot procedure resulted in the isolation of 7 in 62% yield. It is worth emphasizing again that because the starting dioxolane is relatively inexpensive, this yield figure is not particularly meaningful as a measure of the true efficiency of this reaction. It should be noted instead that this optimized procedure allowed the production of more than a gram of 7 using just 2 mg of the Landis ligand. Ozonolysis of 7 and subsequent Wittig reaction with stabilized ylide 8 could be rendered as a simple one-pot procedure and resulted in the isolation of 9 in 83% yield (≥10:1 E:Z). Alcohol-directed hydrogenation[27] with Crabtree’s catalyst[28] proceeded highly diastereoselectively (≥20:1 dr) and led, after in situ alcohol silylation with tert-butyldimethylsilyl triflate (TBSOTf), to the isolation of 10 in 80% yield. Finally, ester reduction with DIBAl-H gave alcohol 11 (86% yield), which was converted to iodide 1 in 95% yield. This 5-step synthesis of 1 proved readily scalable and was used to prepare multi-gram amounts of both 11 and 1 in just a few weeks.
Scheme 1.
An efficient synthesis of iodide 1 in just 5 steps. TBSOTf = tert-butyldimethylsilyl triflate, DIBAl-H = di-iso-butylaluminum hydride.
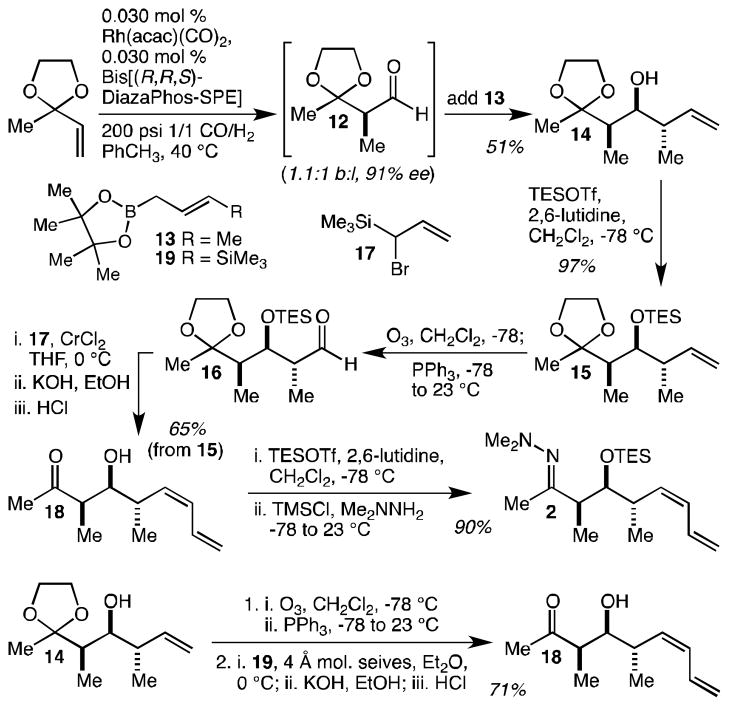
The synthesis of hydrazone 2 began in a similar fashion with the asymmetric hydroformylation of 2-methyl-2-vinyl-1,3-dioxolane (Scheme 2). Because this substrate is more hindered, the catalyst/ligand loading could not be reduced quite as much, but could nevertheless be effectively dropped to 0.030 mol %. These conditions resulted in the production of 12 with 1.1:1 regioselectivity and in 91% ee. Felkin-selective crotylation of aldehyde 12 with trans-crotylpinacolboronate 13 was highly diastereoselective (≥20:1) and was easily performed in one-pot in analogy to the Burke procedure.[24] Once optimized, this sequence produced 14 in 51% yield (as above, we note that a more meaningful measure of the efficiency of this reaction is the fact that more than a gram of 14 may be produced using only 4 mg of the Landis ligand). On larger scales, however, we found it more convenient to chromatographically separate the aldehyde regioisomers prior to the crotylation reaction, both to avoid wasting large amounts of 13 and to avoid a more tedious separation of the crotylation products. Alcohol silylation with triethylsilyl triflate (TESOTf) proceeded smoothly to give 15 in 97% yield and was followed by ozonolysis to give aldehyde 16. Without further purification, 16 was subjected to the cis-selective diene synthesis using allylsilane 17 that was developed by Paterson as part of a discodermolide synthesis.[29] Following in situ base-promoted Peterson elimination,[30] HCl was added to hydrolyze the ethylene ketal and this resulted in TES ether hydrolysis as well, giving 18 in 65% overall yield from 15. Resilylation with TESOTf was followed in situ by hydrazone formation to give 2 in 90% yield. This synthesis of 2 requires just 5 or 6 steps (depending on whether or not 12 is isolated) and allowed the preparation of more than 21 g of 2 in a campaign carried out by a single chemist in less than 3 weeks. Because this route required two separate silylations of the same alcohol, we have developed an alternative route: 14 may be directly subjected to ozonolysis, and the resulting aldehyde may be treated with Matteson’s reagent (19)[31] followed by in situ base-promoted Peterson elimination, to deliver 18 in 71% yield. This modification results in a 4 or 5 step synthesis of 2 and obviates the use of large amounts of CrCl2 during scale-up.
Scheme 2.
An efficient synthesis of hydrazone 2 in just 5 steps. TESOTf = triethylsilyl triflate, TMSCl = trimethylsilyl chloride.
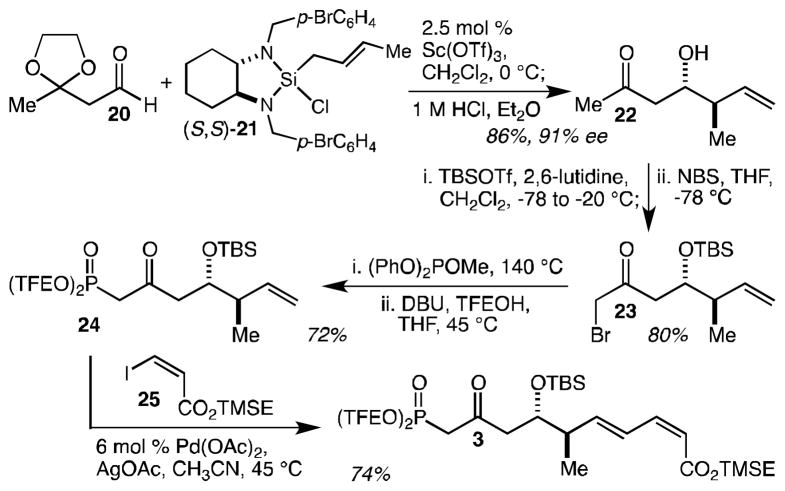
The two most significant challenges in the synthesis of phosphonate 3 are the cis-dienoate and the Still-Gennari type phosphonate. The former task has typically required multi-step solutions, while the latter – due to the failure of tris(trifluoroethyl)phosphite to engage productively in Arbuzov reactions – has been accomplished by the (in our hands, technically difficult, capricious, and poorly scalable) addition of the metallated methyl phosphonate to an acid chloride at −100 °C. As described here, we have devised novel single step solutions for both of these challenges. Sc(OTf)3-catalyzed crotylation[32] of known (and commercially available) aldehyde 20[33] with (S,S)-21[34] (with an HCl work-up optimized for ketal hydrolysis) gave ketone 22 in 86% yield and 91% ee (Scheme 3). Treatment of 22 with 2.1 equiv of TBSOTf both silylated the alcohol and converted the ketone into the corresponding TBS enol ether, and was followed by in situ silyl enol ether bromination with N-bromosuccinimide (NBS) to give bromoketone 23 in 80% yield. To get around the failure of tris(trifluoroethyl)phosphite to engage productively in Arbuzov reactions, we wondered whether it might be possible to first perform an Arbuzov reaction with a different phosphite, and then simply transesterify the product with trifluoroethanol. Indeed, we have reduced this idea to practice: 23 was subjected to an Arbuzov reaction with (PhO)2POMe, and the resulting diphenylphosphonate was treated in situ with trifluoroethanol and DBU to give the desired Still-Gennari-type phosphonate 24 in 72% yield. Finally, a Pd(OAc)2-catalyzed Heck reaction with cis-iodoacrylate 25 gave cis-dienoate 3 in 74% yield.[35] This last reaction results in a significant improvement in step-economy and overall efficiency relative to, for example, the corresponding cross-coupling approach to the dienoate synthesis that requires pre-activation of the alkene coupling partner. Principally due to the development of the one-pot Arbuzov/transesterification reaction and the Heck approach to the cis-dienoate, this synthesis of 3 requires just 4 steps, and made possible the preparation of multi-gram quantities of 24 (we did not want to store large amounts of cis-dienoate 3 and made only what was necessary for the completion of the synthesis) in less than 2 weeks.
Scheme 3.
An efficient synthesis of phosphonate 3 in just 4 steps. DIBAl-H = di-iso-butylaluminum hydride, TBSOTf = tert-butyldimethylsilyl triflate, NBS = N-bromosuccinimide, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, TFEOH = 2,2,2-trifluoroethanol, TMSE = 2-(trimethylsilyl)ethyl.
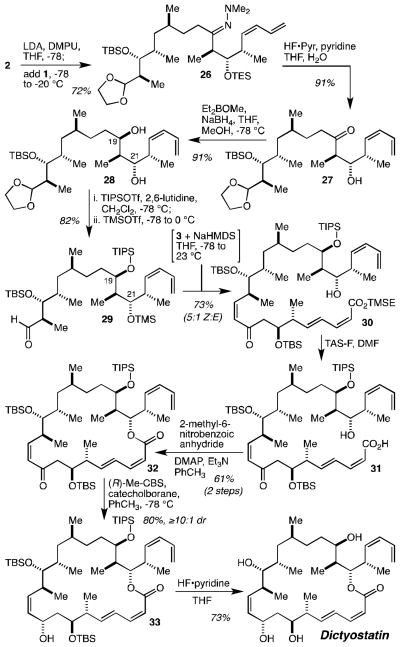
Fragment coupling and completion of the synthesis proceeded as described in Scheme 4. Deprotonation of hydrazone 2 with LDA under Evans’ carefully designed hexane-free conditions[36] and alkylation of the resultant metalloenhydrazide with iodide 1 gave 26 in 72% yield. Treatment of 26 with HF•pyridine in wet THF led to hydrolysis of the TES ether and the hydrazone to give 27 in 91% yield. Narasaka’s syn-selective β-hydroxyketone reduction[37] using Prasad’s protocol[38] proceeded highly diastereoselectively (≥20:1 dr) to give diol 28 in 91% yield. Selective silylation of the C(19) hydroxyl group with tri-iso-propylsilyl triflate (TIPSOTf) was followed in situ by silylation of the C(21) hydroxyl group with trimethylsilyl triflate (TMSOTf), which also resulted in acetal hydrolysis[39] to produce aldehyde 29 in 82% yield. Still-Gennari olefination[40] of 29 with phosphonate 3 proceeded with ~5:1 Z:E selectivity, and the desired Z-enone 30 (the C(21) TMS ether was hydrolyzed in the workup) was isolated in 73% yield. Deprotection of the 2-(trimethylsilyl)ethyl (TMSE) ester was accomplished with tris(dimethylamino)-sulfonium difluorotrimethylsilicate (TAS-F)[41] to deliver hydroxy acid 31, which was directly subjected to the macrolactonization reaction without purification. Following Curran’s demonstration of its superiority in this context,[42] the macrolactonization was carried out using Shiina’s protocol with 2-methyl-6-nitrobenzoic anhydride,[43] and led to the isolation of macrolactone 32 in 61% yield over two steps from 30 and without significant isomerization of the C(2)-C(3) Z-alkene. Diastereoselective reduction of the C(9) ketone was accomplished using the CBS protocol with catecholborane[44] and gave 33 in 80% yield. Finally, global silyl ether deprotection with unbuffered HF•pyridine delivered dictyostatin in 73% yield.
Scheme 4.
Fragment coupling and completion of the synthesis in 9 steps from 1 and 2, and in 5 steps from 3. LDA = lithium di-iso-propylamide, DMPU = 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone, TIPSOTf = tri-iso-propylsilyl triflate, TMSOTf = trimethylsilyl triflate, NaHMDS = sodium hexamethyldisilazide, TAS-F = tris(dimethylamino)-sulfonium difluorotrimethylsilicate, DMAP = 4-dimethylamino pyridine.
Our synthesis of dictyostatin proceeds with a longest linear sequence of 14 steps. Each of the 3 fragments (1–3) was prepared in just 4 or 5 steps, and this unrivalled step-economy was achieved through a combination of methodological and strategic innovation and the successful telescoping of steps into technically simple and effective one-pot procedures at several points in the route. The important innovations realized in the course of this project include the one-pot syntheses of stereotriads 7 and 14, the one-pot Arbuzov/transesterification route to Still-Gennari-type β-ketophosphonate 24, and the Heck approach to the synthesis of the C(1)-C(5) cis-dienoate. The ready scalability of the 3 fragment syntheses that accrued from this step-economy was amply demonstrated by the preparation of multi-gram quantities of each of them in just a few weeks or less. Should a dictyostatin analog emerge that merits clinical evaluation, we believe our synthesis could serve as the starting point for the development of a process that could deliver large amounts of each of the fragments with significantly reduced costs in terms of time, effort, and money. The step-economy of our synthesis, and more specifically the multi-gram quantities of the 3 fragments that we have been able to stockpile as a direct result, may also have the far more immediate and tangible benefit of greatly facilitating our own efforts to design and then rapidly synthesize analogs in pursuit of a clinical candidate.
Supplementary Material
Acknowledgments
This work was supported by a grant form the National Institute of General Medical Sciences (GM058133).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Pettit GR, Cichacz ZA, Gao F, Boyd MR, Schmidt JM. J Chem Soc, Chem Commun. 1994:1111–1112. [Google Scholar]
- 2.Isbrucker RA, Cummins J, Pomponi SA, Longley RE, Wright AE. Biochem Pharmacol. 2003;66:75–82. doi: 10.1016/s0006-2952(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 3.Paterson I, Britton R, Delgado O, Wright AE. Chem Commun. 2004:632–633. doi: 10.1039/b316390c. [DOI] [PubMed] [Google Scholar]
- 4.Paterson I, Britton R, Delgado O, Meyer A, Poullennec KG. Angew Chem. 2004;116:4729–4733. doi: 10.1002/anie.200460589. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:4629–4633. doi: 10.1002/anie.200460589. [DOI] [PubMed] [Google Scholar]
- 5.Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP. Angew Chem. 2004;116:4734–4737. doi: 10.1002/anie.200460593. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:4634–4637. doi: 10.1002/anie.200460593. [DOI] [PubMed] [Google Scholar]
- 6.Buey R, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, Mooberry S, Andreu JM, Díaz JF. Chem Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Madiraju C, Edler MC, Hamel E, Raccor BS, Balachandran R, Zhu G, Giuliano KA, Vogt A, Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP, Day BW. Biochemistry. 2005;44:15053–15063. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]
- 8.O’Neil GW, Phillips AJ. J Am Chem Soc. 2006;128:5340–5341. doi: 10.1021/ja0609708. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran PV, Srivastava A, Hazra D. Org Lett. 2007;9:157–160. doi: 10.1021/ol062737k. [DOI] [PubMed] [Google Scholar]
- 10.Shimp HL, Micalizio GC. Tetrahedron. 2009;65:5908–5915. [Google Scholar]
- 11.Gallon J, Esteban J, Bouzbouz S, Campbell M, Reymond S, Cossy J. Chem Eur J. 2012;18:11788–11797. doi: 10.1002/chem.201201001. [DOI] [PubMed] [Google Scholar]
- 12.Zanato C, Pignataro L, Ambrosi A, Hao Z, Trigili C, Díaz JF, Barasoain I, Gennari C. Eur J Org Chem. 2011:2643–2661. [Google Scholar]
- 13.Paterson I, Britton R, Delgado O, Gardner NM, Meyer A, Naylor GJ, Poullennec KG. Tetrahedron. 2010;66:6534–6545. [Google Scholar]
- 14.Zhu W, Jiménez M, Jung WH, Camarco DP, Balachandran R, Vogt A, Day BW, Curran DP. J Am Chem Soc. 2010;132:9175–9187. doi: 10.1021/ja103537u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Prusov E, Röhm H, Maier ME. Org Lett. 2006;8:1025–1028. doi: 10.1021/ol052917e. [DOI] [PubMed] [Google Scholar]; b) Jägel J, Maier ME. Synlett. 2006:693–696. [Google Scholar]; c) Shaw SJ, Zhang D, Sunderman KF, Myles DC. Synth Commun. 2006;36:1735–1743. [Google Scholar]; d) Baba VS, Das P, Mukkanti K, Iqbal J. Tetrahedron Lett. 2006;47:7927–7930. [Google Scholar]; e) Sharon O, Monti C, Gennari C. Tetrahedron. 2007;63:5873–5878. [Google Scholar]; f) Saibaba V, Sampath A, Mukkanti K, Iqbal J, Das P. Synthesis. 2007:2797–2802. [Google Scholar]; g) Dilger AK, Gopalsamuthiram V, Burke SD. J Am Chem Soc. 2007;129:16273–16277. doi: 10.1021/ja077336u. [DOI] [PubMed] [Google Scholar]; h) Dias LC, Lima DJP, Gonçalves CCS, Andricopulo AD. Eur J Org Chem. 2009:1491–1494. [Google Scholar]; i) Yadav JS, Rajender V. Eur J Org Chem. 2010:2148–2156. [Google Scholar]; j) Ferreiro-Mederos L, Vila-Gisbert S, Urbano A, Carreño MC, Colobert F. Org Biomol Chem. 2011;9:758–764. doi: 10.1039/c0ob00491j. [DOI] [PubMed] [Google Scholar]; k) Dias LC, Sant’Ana DP, Viera YW, Gonçalves CCS, Lima DJP. J Braz Chem Soc. 2012;23:344–348. [Google Scholar]
- 16.a) Paterson I, Gardner NM, Poullennec KG, Wright AE. Bioorg Med Chem Lett. 2007;17:2443–2447. doi: 10.1016/j.bmcl.2007.02.031. [DOI] [PubMed] [Google Scholar]; b) Paterson I, Gardner NM, Guzmán E, Wright AE. Bioorg Med Chem Lett. 2008;18:6268–6272. doi: 10.1016/j.bmcl.2008.09.109. [DOI] [PubMed] [Google Scholar]; c) Paterson I, Gardner NM, Poullennec KG, Wright AE. J Nat Prod. 2008;71:364–369. doi: 10.1021/np070547s. [DOI] [PubMed] [Google Scholar]; d) Paterson I, Naylor GJ, Wright AE. Chem Commun. 2008:4628–4630. doi: 10.1039/b811575c. [DOI] [PubMed] [Google Scholar]; e) Paterson I, Gardner NM, Guzmán E, Wright AE. Bioorg Med Chem. 2009;17:2282–2289. doi: 10.1016/j.bmc.2008.10.084. [DOI] [PubMed] [Google Scholar]; f) Paterson I, Naylor GJ, Fujita T, Guzmán, Wright AE. Chem Commun. 2010;46:261–263. doi: 10.1039/b921237j. [DOI] [PubMed] [Google Scholar]; g) Paterson I, Naylor GJ, Gardner NM, Guzmán E, Wright AE. Chem-Asian J. 2011;6:459–473. doi: 10.1002/asia.201000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Shin Y, Choy N, Turner TR, Balachandran R, Madiraju C, Day BW, Curran DP. Org Lett. 2002;4:4443–4446. doi: 10.1021/ol026942l. [DOI] [PubMed] [Google Scholar]; b) Shin Y, Fournier JH, Balachandran R, Madiraju C, Raccor BS, Zhu G, Edler MC, Hamel E, Day BW, Curran DP. Org Lett. 2005;7:2873–2876. doi: 10.1021/ol050808u. [DOI] [PubMed] [Google Scholar]; c) Fukui Y, Brückner AM, Shin Y, Balachandran, Day BW, Curran DP. Org Lett. 2006;8:301–304. doi: 10.1021/ol0526827. [DOI] [PubMed] [Google Scholar]; d) Jung WH, Harrison C, Shin Y, Fournier JH, Balachandran R, Raccor BS, Sikorski RP, Vogt A, Curran DP, Day BW. J Med Chem. 2007;50:2951–2966. doi: 10.1021/jm061385k. [DOI] [PubMed] [Google Scholar]; e) Shin Y, Fournier JH, Brückner AM, Madiraju C, Balachandran R, Raccor BS, Edler MC, Hamel E, Sikorski RP, Vogt A, Day BW, Curran DP. Tetrahedron. 2007;63:8537–8562. doi: 10.1016/j.tet.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Jiménez M, Zhu W, Vogt A, Day BW, Curran DP. Beilstein J Org Chem. 2011;7:1372–1378. doi: 10.3762/bjoc.7.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canales A, Matesanz R, Gardner NM, Andreu JM, Paterson I, Díaz JF, Jiménez-Barbero J. Chem Eur J. 2008;14:7557–7569. doi: 10.1002/chem.200800039. [DOI] [PubMed] [Google Scholar]
- 19.Jogalekar AS, Damodaran K, Kriel FH, Jung WH, Alcaraz AA, Zhong S, Curran DP, Snyder JP. J Am Chem Soc. 2011;133:2427–2436. doi: 10.1021/ja1023817. [DOI] [PubMed] [Google Scholar]
- 20.a) Spletstoser JT, Zacuto MJ, Leighton JL. Org Lett. 2008;10:5593–5596. doi: 10.1021/ol802489w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Harrison TJ, Ho S, Leighton JL. J Am Chem Soc. 2011;133:7308–7311. doi: 10.1021/ja201467z. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chalifoux WA, Reznik SK, Leighton JL. Nature. 2012;487:86–89. doi: 10.1038/nature11189. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Harrison TJ, Rabbat PMA, Leighton JL. Org Lett. 2012;14:4890–4893. doi: 10.1021/ol302221s. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Reznik SK, Marcus BS, Leighton JL. Chem Sci. 2012;3:3326–3330. doi: 10.1039/C2SC21325G. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Reznik SK, Leighton JL. Chem Sci. 2013;4:1497–1501. doi: 10.1039/C3SC22186E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roush WR, Palkowitz AD, Ando K. J Am Chem Soc. 1990;112:6348–6359. [Google Scholar]
- 22.Smith AB, III, Freeze BS. Tetrahedron. 2008;64:261–298. doi: 10.1016/j.tet.2007.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald RI, Wong GW, Neupane RP, Stahl SS, Landis CR. J Am Chem Soc. 2010;132:14027–14029. doi: 10.1021/ja106674n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risi RM, Burke SD. Org Lett. 2012;14:2572–2575. doi: 10.1021/ol3008765. [DOI] [PubMed] [Google Scholar]
- 25.Wong GW, Adint TT, Landis CR. Org Synth. 2012;89:243–254. [Google Scholar]
- 26.Suen LM, Steigerwald ML, Leighton JL. Chem Sci. doi: 10.1039/C3SC50714A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307–1370. [Google Scholar]
- 28.a) Crabtree RH, Morris GE. J Organomet Chem. 1977;135:395–403. [Google Scholar]; b) Crabtree RH, Davis MW. Organometallics. 1983;2:681–682. [Google Scholar]; c) Crabtree RH, Morehouse SM. Inorg Synth. 1986;24:173–176. [Google Scholar]
- 29.a) Paterson I, Schlapbach A. Synlett. 1995:498–500. [Google Scholar]; b) Paterson I, Florence GJ, Gerlach K, Scott JP, Sereinig N. J Am Chem Soc. 2001;123:9535–9544. doi: 10.1021/ja011211m. [DOI] [PubMed] [Google Scholar]
- 30.Mickel SJ, Sedelmeier GH, Niederer D, Schuerch F, Seger M, Schreiner K, Daeffler R, Osmani A, Bixel D, Loiseleur O, Cercus J, Stettler H, Schaer K, Gamboni R, Bach A, Chen GP, Chen WC, Geng P, Lee GT, Loeser E, McKenna J, Kinder FR, Konigsberger K, Prasad K, Ramsey TM, Reel N, Repic O, Rogers L, Shieh WC, Wang RM, Waykole L, Xue S, Florence G, Paterson I. Org Process Res Dev. 2004;8:113–121. [Google Scholar]
- 31.Tsai DJS, Matteson DS. Tetrahedron Lett. 1981;22:2751–2752. [Google Scholar]
- 32.Kim H, Ho S, Leighton JL. J Am Chem Soc. 2011;133:6517–6520. doi: 10.1021/ja200712f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly TR, Ananthasubramanian L, Bovah K, Gillard JW, Goerner RN, King PF, Lyding JM, Tsang WG, Vaya J. Tetrahedron. 1984;40:4569–4577. [Google Scholar]
- 34.Hackman BM, Lombardi PJ, Leighton JL. Org Lett. 2004;6:4375–4377. doi: 10.1021/ol0480731. [DOI] [PubMed] [Google Scholar]
- 35.a) Lu X, Huang X, Ma S. Tetrahedron Lett. 1992;33:2535–2538. [Google Scholar]; b) Knowles JP, O’Connor VE, Whiting A. Org Biomol Chem. 2011;9:1876–1886. doi: 10.1039/c0ob00977f. [DOI] [PubMed] [Google Scholar]
- 36.Evans DA, Rieger DL, Jones TK, Kaldor SW. J Org Chem. 1990;55:6260–6268. [Google Scholar]
- 37.Narasaka K, Pai FC. Tetrahedron. 1984;40:2233–2238. [Google Scholar]
- 38.Chen KM, Hardtmann GE, Prasad K, Repič O, Shapiro MJ. Tetrahedron Lett. 1987;28:155–158. [Google Scholar]
- 39.Fujioka H, Okitsu T, Sawama Y, Murata N, Li R, Kita Y. J Am Chem Soc. 2006;128:5930–5938. doi: 10.1021/ja060328d. [DOI] [PubMed] [Google Scholar]
- 40.Still WC, Gennari C. Tetrahedron Lett. 1983;24:4405–4408. [Google Scholar]
- 41.Scheidt KA, Chen H, Follows BC, Chemler SR, Coffey DS, Roush WR. J Org Chem. 1998;63:6436–6437. [Google Scholar]
- 42.Eiseman JL, Bai L, Jung WH, Moura-Letts G, Day BW, Curran DP. J Med Chem. 2008;51:6650–6653. doi: 10.1021/jm800979v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.a) Shiina I, Ibuka R, Kubota M. Chem Lett. 2002:286–287. [Google Scholar]; b) Shiina I, Kubota M, Ibuka R. Tetrahedron Lett. 2002;43:7535–7539. [Google Scholar]; c) Shiina I, Kubota M, Oshiumi H, Hashizume M. J Org Chem. 2004;69:1822–1830. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
- 44.a) Corey EJ, Bakshi RK. Tetrahedron Lett. 1990;31:611–614. [Google Scholar]; b) Corey EJ, Helal CJ. Angew Chem. 1998;110:2092–2118. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.