ABSTRACT
The identification of “factor H binding protein (fHbp)-null” invasive meningococcal isolates and the realization that widespread use of fHbp-based vaccines could herald selection of such strains prompted us to characterize novel mechanisms of alternative pathway (AP) inhibition on meningococci. Of seven strains engineered to lack four known AP-inhibiting molecules, capsular polysaccharide, lipooligosaccharide sialic acid, fHbp, and neisserial surface protein A (quadruple mutants), four strains inhibited human AP-mediated C3 deposition. All four expressed the porin B2 (PorB2) molecule, and three strains belonged to the hypervirulent ST-11 lineage. Consistent with reduced C3 deposition, the rate of C3a generation by a PorB2 isolate was lower than that by a PorB3 strain. Allelic replacement of PorB3 with PorB2, in both encapsulated and unencapsulated strains, confirmed the role of PorB2 in AP inhibition. Expression of PorB2 increased resistance to complement-dependent killing relative to that seen in an isogenic PorB3-expressing strain. Adult rabbit and mouse APs were unimpeded on all mutants, and human fH inhibited nonhuman C3 deposition on PorB2-expressing strains, which provided functional evidence for human fH-dependent AP regulation by PorB2. Low-affinity binding of full-length human fH to quadruple mutants expressing PorB2 was demonstrated. fH-like protein 1 (FHL-1; contains fH domains 1 through 7) and fH domains 6 and 7 fused to IgG Fc bound to one PorB2-expressing quadruple mutant, which suggested that fH domains 6 and 7 may interact with PorB2. These results associate PorB2 expression with serum resistance and presage the appearance of fHbp-null and hypervirulent ST-11 isolates that may evade killing by fHbp-based vaccines.
IMPORTANCE
The widespread use of antimeningococcal vaccines based on factor H (fH) binding protein (fHbp) is imminent. Meningococci that lack fHbp were recently isolated from persons with invasive disease, and these fHbp-null strains could spawn vaccine failure. Our report provides a molecular basis for an explanation of how fHbp-null strains may evade the host immune system. Meningococci possess several mechanisms to subvert killing by the alternative pathway (AP) of complement, including production of the fHbp and NspA fH binding proteins. Here we show that a meningococcal protein called porin B2 (PorB2) contributes to inhibition of the AP on the bacterial surface. A majority of the “fHbp-null” isolates identified, as well as all members of a “hypervirulent” lineage (called ST-11), express PorB2. Our findings highlight the potential for the emergence of fHbp-negative strains that are able to regulate the AP and may be associated with fHbp vaccine failure.
Introduction
Neisseria meningitidis is an important cause of bacterial meningitis and sepsis worldwide. The complement system is an important component of innate immune defenses against this pathogen. Individuals deficient in terminal complement components or in components of the alternative pathway (AP) are at an increased risk of meningococcal disease (1). A key feature of the AP of complement is a positive-feedback loop that amplifies C3b deposition on microbial surfaces (2). The AP also plays an important role in maximizing the killing activity elicited by select antimeningococcal antibodies (Abs) (3). Under physiological conditions, factor H (fH) plays a major role in limiting unwanted activation of the AP (4). fH acts as a cofactor for the factor I-mediated cleavage of C3b to inactive C3b (iC3b) and also serves to limit C3 activation by irreversibly dissociating the AP C3 convertase (C3b, Bb). Microorganisms can hijack host fH and use this molecule to protect themselves from immune attack by downregulating deposition of C3b and inactivating C3b that is deposited.
The meningococcus possesses several distinct and often redundant mechanisms to limit AP activation. Meningococci directly bind human fH through two surface molecules, fH binding protein (fHbp) (5) and neisserial surface protein A (NspA) (6); both serve to limit C3 deposition and enhance resistance of the organism to complement-dependent killing. In addition, sialylation of meningococcal lacto-N-neotetraose (LNT) lipooligosaccharide (LOS) regulates the AP by enhancing the interactions of the C-terminal domains of fH with surface-bound C3 fragments (7) and the group B and group C capsular polysaccharides limit AP-mediated deposition of C3 (8, 9).
Based on observations that binding of fH to meningococci was human specific (10), we developed a human fH transgenic (Tg) rat which enhanced the ability of H44/76 to cause bacteremia (bacteremia was observed after challenge with 5 × 102 CFU) (11). Rather unexpectedly, deleting both known fH ligands, fHbp, and NspA, did not diminish the ability of H44/76 to cause bacteremia in human fH Tg infant rats (11); this result suggested the existence of an additional human fH-dependent mechanism(s) for complement evasion. LOS sialylation was identified as one such mechanism (7, 11).
Recent work by Lucidarme et al. (12) identified meningococcal isolates that lacked fHbp expression that had been collected from patients with invasive disease, which demonstrated that in select strains, fHbp was dispensable for virulence in humans. These findings imply that certain meningococcal strains possess novel means of inhibiting complement activation in the absence of fHbp. This observation is particularly important given that fHbp-based vaccines are on the verge of clinical use.
The aim of the present study was to identify novel mechanisms meningococci use to regulate the AP that could contribute to the virulence of this pathogen in the absence of fHbp. These mechanisms could contribute to the ability of fHbp-negative meningococci to maintain virulence and escape killing by fHbp-containing vaccines.
RESULTS
Meningococcal PorB2 strains regulate the human AP.
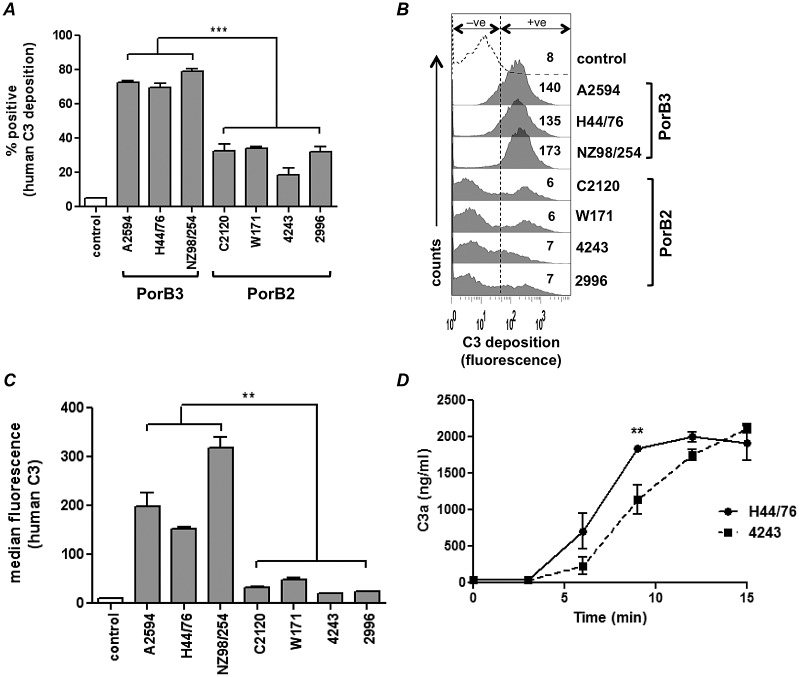
In order to study AP regulation in the absence of factors known to modulate C3 deposition on meningococci, we created mutants that lacked expression of capsular polysaccharide, LOS sialic acid, fHbp, and NspA. Strains harboring these four mutations simultaneously are referred to here as “quadruple mutants.” Quadruple mutants, derived from 7 diverse strains of N. meningitidis (see Table S1 in the supplemental material), were screened for deposition of human C3 following incubation with normal human serum-MgCl2-EGTA (NHS-Mg/EGTA) (20% [vol/vol]); Mg/EGTA blocked classical and lectin pathway activation and allowed assessment of AP activation only (Fig. 1). Significantly lower C3 deposition was seen on quadruple mutants generated from four strains that expressed the PorB2 molecule than on mutants generated from strains that expressed PorB3 (Fig. 1A and B). Similar results were seen with C2-depleted serum (only AP intact; classical and lectin pathways blocked) and with sera from two additional donors (data not shown).
FIG 1 .
Regulation of the human AP by PorB2-expressing N. meningitidis. Seven strains of N. meningitidis that lacked capsular polysaccharide, LOS sialic acid, and fHbp and NspA expression (quadruple mutants) were screened for AP-mediated C3 deposition with NHS-Mg/EGTA (20% [vol/vol]). (A) C3 deposition measured by FACS analysis. The percentage of positive events relative to control organisms incubated with heat-inactivated serum (gated to yield 5% of positive events in the negative-control sample as shown in Fig. 1B [+ve]) is represented on the y axis. Each bar represents the standard error of the mean (SEM) of the results of three independent experiments. ***, P < 0.001 (1-way ANOVA with Tukey’s posttest for pairwise comparisons). (B) Histograms from an experiment representative of those described for panel A; data represent the median fluorescence of C3 binding of the entire bacterial population. (C) AP regulation by PorB2-expressing strains revealed using purified AP components. Quadruple mutants were incubated with purified C3 (500 µg/ml), factor B (100 µg/ml), factor D (2 µg/ml), and factor H (100 µg/ml). C3b deposited on bacteria was measured by FACS analysis. Controls were bacteria incubated with C3 alone. The amount of deposited C3b is represented as median fluorescence (y axis). Each bar represents the mean (range) of the results of two separate experiments. **, P < 0.01. (D) 4243 (PorB2) activates the AP at a lower rate than H44/76 (PorB3). Quadruple mutants of 4243 (PorB2) and H44/76 (PorB3) were incubated with NHS-Mg/EGTA. Supernatants collected at the times indicated (x axis) were assayed for C3a by enzyme-linked immunosorbent assay (ELISA). Each datum point represents the SEM of the results of three independent experiments. **, P < 0.01 (2-way ANOVA).
To ensure that serum factors other than AP components did not contribute significantly to the differential C3 deposition on the meningococcal mutants, we examined C3 deposition on the strains using purified C3, factor B, factor D, and fH (Fig. 1C). Again, each of the four PorB2-expressing quadruple mutants showed significantly (P < 0.01) lower C3 deposition than each of the three PorB3 isolates. These data indicate that despite loss of capsule, LOS sialic acid, fHbp, and NspA, select PorB2 strains effectively regulated the human AP.
Decreased C3 deposition in PorB2 strains is associated with slower C3 activation.
Decreased C3 deposition could result from reduced targets for covalent binding of C3 (i.e., fewer electron-donating –OH groups) or decreased activation of C3 on the bacterial surface (i.e., because of increased fH binding) or a combination of these events. The quantitative differences seen by flow cytometry (Fig. 1) were confirmed by Western blotting (see Fig. S1 in the supplemental material), which revealed both LOS and Opa as major targets for iC3b across all isolates, as has been previously described for Neisseria (13). LOS structure can modulate complement activation on neisseriae (7, 8, 14). All strains expressed an ~4.5-kDa LOS species; NZ98/254 showed an additional 3.6-kDa LOS species (data not shown). Thus, there was no obvious correlation between the LOS migration pattern and the amount of C3 deposited.
Activation of C3 is accompanied by release of the C3a fragment, and measurement of the rate of C3a generation reflects C3 activation kinetics. Based on previously published data that measured the rates of complement activation (9), we focused on C3a production during the first 15 min of incubation. Quadruple mutants of 4243 (PorB2) and H44/76 (PorB3) were chosen as examples of low- and high-level C3 binders, respectively. As shown in Fig. 1D, the reaction mixture containing the 4243 quadruple mutant generated C3 at a rate lower than that seen with H44/76. The total amounts of C3a in the reaction mixture reached similar levels across strains at time points at or beyond 20 min, likely because C3 convertases in the fluid phase contribute to overall complement activation (9). These data suggest that lower C3 deposition on strain 4243 occurs at least in part because of a lower rate of C3 activation.
Human fH regulates the AP of nonhuman complement on PorB2-expressing quadruple mutants.
Binding of fH to the known meningococcal ligands for fH, fHbp, and NspA is human specific (6, 10). To determine if regulation of the AP by PorB2-bearing strains was also specific to humans, we measured deposition of rabbit and mouse C3 using adult sera. PorB2 did not regulate the AP of these adult animals, and similar amounts of C3 were deposited on all quadruple mutants (Fig. 2, black bars). The addition of purified human fH to these nonhuman sera resulted in significantly greater downregulation of the AP on PorB2 strains relative to that seen with PorB3 isolates (Fig. 2, gray bars). Fluorescence-activated cell sorter (FACS) tracings of a representative experiment are shown in Fig. S2 in the supplemental material. These data provide strong evidence for human fH-dependent regulation of the AP by PorB2-bearing strains compared to PorB3-bearing strains.
FIG 2 .
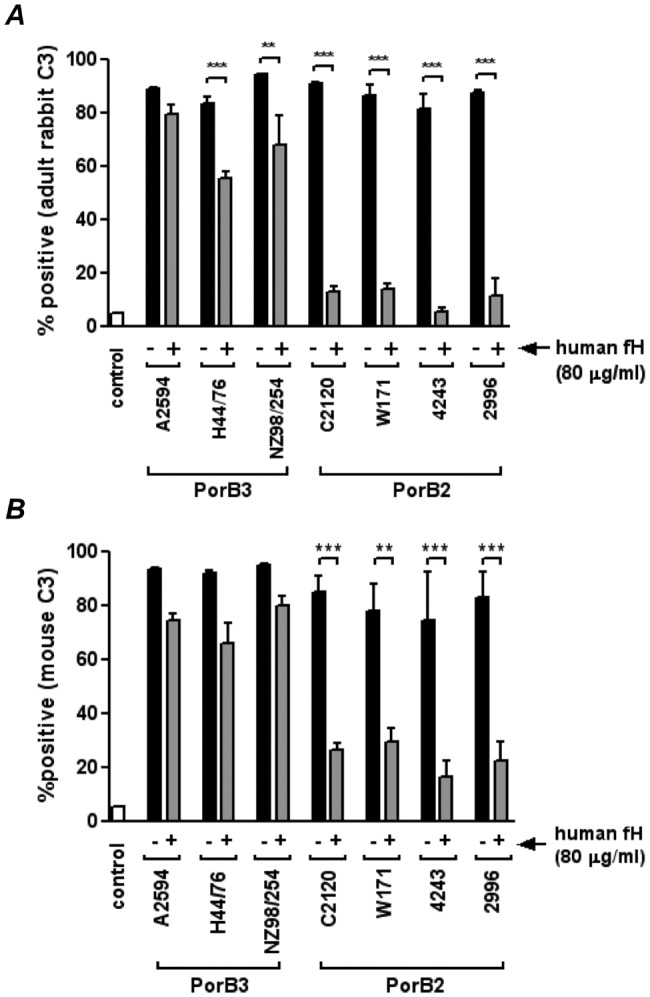
Human fH inhibits C3 deposition by adult nonhuman complement on PorB2-expressing quadruple mutants. Quadruple mutants of 7 strains of N. meningitidis were incubated with adult rabbit complement (A) or adult mouse serum (B) in either the absence (“−”; solid black bars) or the presence (“+”; gray bars) of human fH (80 µg/ml), and levels of C3 fragments deposited on bacteria were measured by FACS analysis. The control (open bar) represents C3 deposition when bacteria were incubated with heat-inactivated complement. The percentage of positive events relative to controls (bacteria plus heat-inactivated complement) is shown on the y axis. Each bar represents the SEM of the results of 2 to 3 independent observations. **, P < 0.01; ***, P < 0.001 (1-way ANOVA).
Confirmation of the role of PorB2 in regulating the human AP.
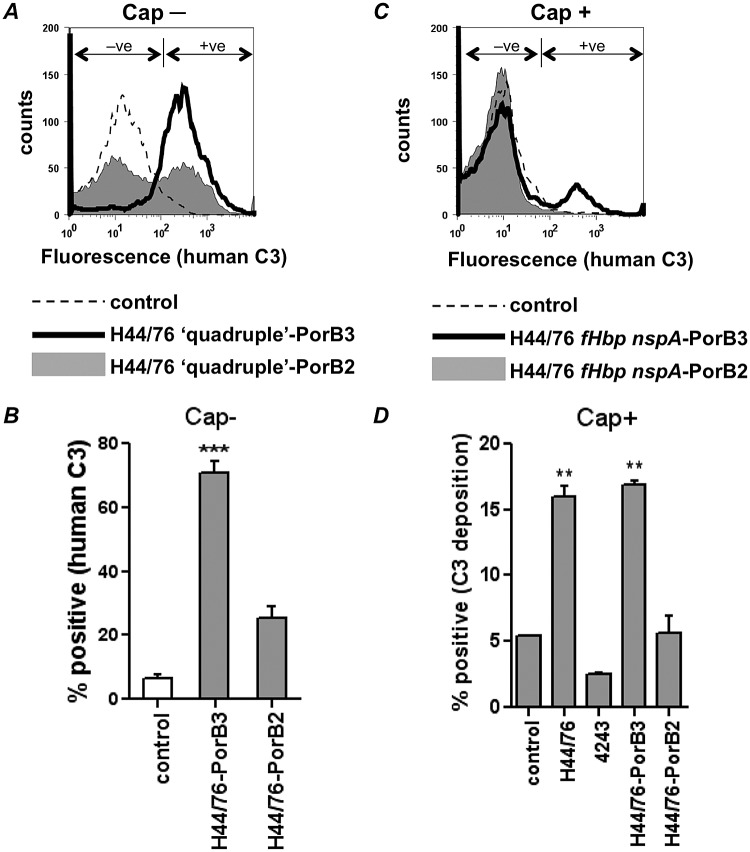
PorB is a major outer membrane protein that contributes to the fitness of a strain (15), and its deletion could have pleiotropic effects with respect to bacterial growth in addition to any interactions with the complement system. To avoid these concerns, we created allelic exchanges of the entire porB2 and porB3 sequences in a homologous background and compared C3 depositions in these isogenic strains that differed only in PorB types. Two sets of mutants were created; the first set lacked fHbp, NspA, capsule, and LOS sialic acid (H44/76 “quadruple”-PorB3 and H44/76 “quadruple”-PorB2), while the second set lacked fHbp and NspA but elaborated capsule and sialylated LOS (H44/76 fHbp nspA-PorB3 and H44/76 fHbp nspA-PorB2).
H44/76 that expressed PorB2 showed greater downregulation of C3 deposition than H44/76 that expressed its own PorB3 molecule (Fig. 3). The effect was most pronounced in the absence of capsule (Fig. 3A; “Cap−”) but was also observed in the encapsulated (Fig. 3C; “Cap+”) background (quantitative comparisons are shown in Fig. 3B and D, respectively). These data provide strong evidence for a role for PorB2 in regulating the human AP on meningococci.
FIG 3 .
PorB2 mediates regulation of the human AP. Isogenic strains that differed only in PorB expression (either PorB3H44/76 or PorB24243) were created in H44/76. All mutants lacked fHbp and NspA and were either unencapsulated with no LOS sialic acid (“quadruple”; “Cap−”) or possessed a capsule and LOS acid (H44/76 fHbp nspA-PorB3 and H44/76 fHbp nspA-PorB2; “Cap+”). Bacteria were incubated with NHS-Mg/EGTA (20% [vol/vol]), and C3 deposited on the bacterial surface was measured by FACS analysis. (A and C) Representative histogram tracings of C3 deposition on the unencapsulated (Cap-) and encapsulated (Cap+) strains. Bacteria incubated with heat-inactivated serum served as negative controls (dashed lines). (B and D) Quantitative representation (percentage of positive events on the y axis; mean [SEM] of the results of three independent experiments) of C3 deposition on the Cap- and Cap+ isogenic PorB mutants, respectively. The percentage of positive events relative to control organisms incubated with heat-inactivated serum (gated to yield 5% of positive events in the negative-control sample as shown in Fig. 1B [+ve]) is represented on the y axis. Wild-type parent strains H44/76 and 4243 are shown as comparators. Note the different y-axis scales in panels B and D. ***, P < 0.001; **, P < 0.01.
C3 deposition on PorB2-expressing strains displays a bimodal distribution (Fig. 1B and 3A; see also Fig. S3 in the supplemental material). These data suggest that there are two populations that differentially regulate the AP. PorB2 expression was normally distributed (unimodal) and did not correlate with variation in C3 deposition. Regions of low, intermediate, and high PorB2 expression from within the normally distributed PorB2-positive population all revealed bimodal C3 deposition (Fig. S3). Thus, molecules other than PorB2 could contribute to the observed heterogeneity in C3 deposition.
Binding of fH, fH/Fc fusion proteins, and fH-like protein 1 (FHL-1) to meningococci lacking fHbp and NspA.
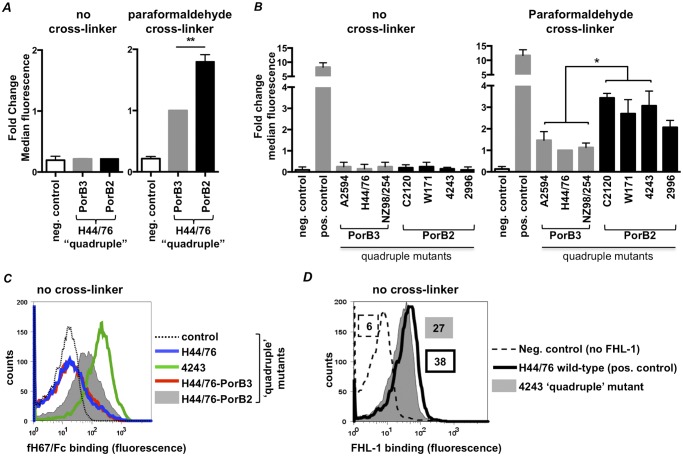
Having provided evidence for human fH-dependent downregulation of the AP by PorB2-bearing strains, we compared the abilities of H44/76 “quadruple”-PorB3 and H44/76 “quadruple”-PorB2 to bind to human fH. Using standard FACS analysis (Fig. 4A [see also Fig. S4A in the supplemental material]; labeled “no cross-linker”) and Western blot (data not shown) methods, we did not detect binding of full-length human fH (100 µg/ml) to either strain. Similar negative results were obtained with the 7 quadruple mutants that express either PorB2 (C2120, W171, 4243, and 2996) or PorB3 (A2594, H44/76, and NZ98/254) (Fig. 4B [see also Fig. S4B]; labeled “no cross-linker”). To enhance detection of low-affinity interactions between fH and PorB, we added paraformaldehyde to cross-link bound fH to the bacterial surface prior to washing and then detected bound fH by FACS analysis (the terms “low” and “high” affinity are henceforth used to refer to low-level fH binding that can be detected only with a cross-linker and high-level fH binding that can be detected without a cross-linker). In the presence of the cross-linker, the quadruple mutant of H44/76 expressing PorB2 bound greater amounts of fH than the isogenic H44/76 bearing PorB3 (Fig. 4A [see also Fig. S4A]; labeled “paraformaldehyde cross-linker”). In other experiments, quadruple mutants of 3 of 4 strains that expressed PorB2 (C2120, W171, and 4243) bound statistically significantly more fH than each of the 3 strains that expressed PorB3 (A2594, H44/76, and NZ98/254); the fourth PorB2 strain, the quadruple mutant of 2996, also bound more fH than strain H44/76 at a statistically significant level (PorB3; P < 0.05) (Fig. 4B [see also Fig. S4B]; labeled “paraformaldehyde cross-linker”). However, the higher fH binding by the quadruple mutant of strain 2996 (PorB2) and each of the two other PorB3-expressing strains was not statistically different (P > 0.05).
FIG 4 .
PorB2 binds full-length fH, fH domains 6 and 7, and FHL-1. (A and B) Binding of human fH to quadruple mutants of H44/76 that express either PorB344/76 (H44/76-PorB3) or PorB24243 (H44/76-PorB2) (A) and quadruple mutants of N. meningitidis strains that express either PorB3 (A2594, H44/76, and NZ98/254) or PorB2 (C2120, W171, 4243, and 2996) (B). Quadruple mutants were incubated with purified fH (100 µg/ml), and bound fH was measured by FACS analysis (graphs labeled “no cross-linker”). In some experiments, bound fH was cross-linked to the surface with paraformaldehyde (final concentration, 1% [vol/vol]) prior to detection by FACS analysis (graphs labeled “paraformaldehyde cross-linker”). The negative control (control) was a reaction mixture that lacked fH (neg. control). Binding of fH to wild-type H44/76 that expresses fHbp, NspA, capsule, and LOS sialic acid is shown as a positive control in panel B (pos. control). The amount of bound fH is represented on the y axis as fold change in median fluorescence relative to the H44/76 quadruple mutant that expresses PorB3 (in panel A) or the H44/76 quadruple mutant (in panel B) with paraformaldehyde cross-linker. Each bar represents the SEM of the results of 2 to 3 independent experiments (note that some error bars are small and are not easily seen on the figure). **, P < 0.01; *, P < 0.05 (1-way ANOVA with Tukey’s posttest for pairwise comparisons). (C) PorB24243 binds to fH67/Fc. Binding of human fH67/Fc to quadruple mutants of H44/76 (blue line), 4243 (green line), and H44/76 that express either PorB344/76 (H44/76-PorB3; red line) or PorB24243 (H44/76-PorB2; gray shading) was measured by FACS analysis. The negative control (broken line) was a reaction mixture that lacked fH67/Fc. x axis, fluorescence on a log10 scale; y axis, counts. Data from an experiment representative of at least two reproducible repeats are shown. (D) A quadruple mutant of strain 4243 binds FHL-1. Binding of FHL-1 (10 µg/ml) to the 4243 quadruple mutant (gray-shaded histogram) was measured by FACS analysis using polyclonal goat anti-human fH. FHL-1 was omitted in the negative control (broken line). The positive control shows binding of FHL-1 to wild-type H44/76 (solid black line). Numbers alongside histograms represent the median fluorescence, and the fill/outline corresponds to that of the histograms.
Prior observations have shown that most pathogens interact with fH domains 6 and 7 or domains 18 through 20 (16). Neisseria that express fHbp (17) or NspA (6) bind fH67/Fc but not fH18-20/Fc. Binding of two recombinant molecules that comprised either fH domains 6 and 7 or fH domains 18 through 20 fused to murine IgG2a Fc (fH67/Fc and fH18-20/Fc) to H44/76 “quadruple”-PorB3 and H44/76 “quadruple”-PorB2 demonstrated that fH67/Fc specifically bound to H44/76 expressing PorB24243 (Fig. 4C; gray-shaded histogram) but not to H44/76 expressing the homologous PorB3 molecule (Fig. 4C; red histogram). The quadruple mutant of 4243, but not other strains (data not shown), also bound fH67/Fc (Fig. 4C; green histogram). Binding of fH67/Fc to H44/76 “quadruple”-PorB2 (Fig. 4C; gray histogram) was decreased relative to binding of the quadruple mutant of 4243 (Fig. 4C; green histogram), suggesting that the strain background may be important for this interaction. fH18-20/Fc did not bind to any quadruple mutant tested (data not shown).
N. meningitidis can also bind to fH-like protein 1 (FHL-1), a molecule that contains the first seven N-terminal domains of fH (18) and regulates the AP. Consistent with binding of fH67/Fc, the quadruple mutant of 4243, but not other strains (data not shown), bound purified FHL-1 (Fig. 4D). Binding of FHL-1 and fH67/Fc to wild-type H44/76 expressing both fHbp and NspA is shown as a positive control. All isolates expressed similar amounts of PorB as assessed using Coomassie-stained gels, which suggested that differences in PorB expression did not account for differences in AP regulation or binding of FHL-1 or fH67/Fc (data not shown).
To better understand the differences in binding of FHL-1 and fH67/Fc among the PorB2 isolates, the translated amino acid sequences were aligned (see Fig. S5 in the supplemental material). The only difference between PorB2 of 4243, which bound FHL-1 and fH67/Fc with high affinity, and the PorB2 molecules of W171 and C2120, which did not, was the absence of a 3-amino-acid stretch in predicted surface-loop 7 in the latter 2 strains (VKD deleted in W171 and NGV deleted in C2120). The 4243 loop 7 sequence alone likely was not sufficient to mediate high-affinity binding of fH67/Fc to PorB2, because the amino acid sequence of loop 7 of PorB2 2996 was identical to that of 4243; yet 2996 did not bind detectable amounts of fH67/Fc in the absence of cross-linker. Outside loop 7, the 2996 PorB2 was the most divergent from 4243, with 12 amino acid differences (see Fig. S5). The amino acid sequence of PorB2 4243 was identical to that of a group C strain called FAM18; a quadruple mutant of FAM18 also bound fH67/Fc and regulated the human AP (see Fig. S6 in the supplemental material). This observation further supports the idea of a role of PorB2 in regulating the AP.
All PorB2 strains tested regulated the AP (Fig. 1), and in all cases this regulation was dependent on the presence of human fH (Fig. 2). All PorB2-expressing strains bound full-length fH when a cross-linker was used to detect low-affinity binding, and 4243 bound FHL-1 with high affinity in the absence of a cross-linker. Collectively, these data suggest that AP regulation by human fH involves low-affinity PorB2-fH interactions.
Expression of PorB2 enhances serum resistance.
To determine the relative roles of PorB2 and PorB3 in serum resistance, we compared the abilities of H44/76 fHbp nspA-PorB3 and H44/76 fHbp nspA-PorB2 to resist killing by human serum. Strains lacking fHbp and NspA were used to avoid the confounding effects of fH binding to these ligands. Serum was absorbed against a mixture of H44/76 fHbp nspA-PorB3 and H44/76 fHbp nspA-PorB2 to remove strain-specific antibodies. Absorbed serum (20%) alone did not kill either strain (Fig. 5). Expression of PorB2 by H44/76 fHbp nspA resulted in greater resistance to complement-dependent killing mediated by anti-group B capsule IgG antibody SEAM 12 (19) than expression of PorB3 (Fig. 5). Absorbed serum treated with Mg/EGTA to selectively activate the AP did not initiate killing of either strain in the presence or absence of SEAM 12 (>100% survival in both instances). As previously reported for other antimeningococcal Abs (3), the AP was required for maximal killing; blocking the AP with an anti-factor Bb monoclonal antibody (MAb) decreased killing of H44/76 fHbp nspA-PorB2 (SEAM 12 [1 µg/ml] plus absorbed serum yielded 35% survival versus 71% survival when anti-Bb blocked the AP; P < 0.01). These data correlate expression of PorB2 with the evasion of immune killing seen when all pathways of complement were intact.
FIG 5 .
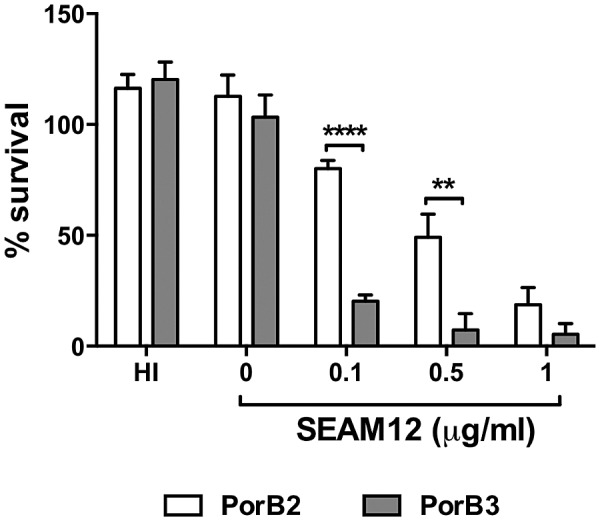
PorB2 expression enhances serum resistance. Data represent percent survival of isogenic H44/76 fHbp nspA expressing either PorB24243 (PorB2; open bar) or PorB3H44/76 (PorB3; gray bar) in 20% absorbed serum that contained anti-group B capsule antibody, SEAM 12, at the concentrations indicated. Heat-inactivated (HI) sera and NHS without added SEAM 12 (0 µg/ml) served as controls. Percent survival relative to time 0 is plotted on the y axis. Each bar represents the SEM of the results of three independent observations. **, P < 0.01; ****, P < 0.0001 (2-way ANOVA).
DISCUSSION
PorB, one of the most abundant proteins in the neisserial outer membrane, is an integral membrane protein organized as a β barrel with 16 transmembrane domains and 8 predicted surface loops (20). PorB functions as a porin, allowing the passage of small molecules across the outer membrane, and has also been implicated in virulence. Gonococcal PorB has been shown to play a key role in regulation of both the AP and classical pathways of complement by virtue of its ability to bind to fH and C4BP, respectively (21, 22). Meningococcal PorB is divided into two classes, PorB2 and PorB3, which are mutually exclusive and are expressed from alternate alleles (porB2 and porB3) at the porB locus. PorB3 of N. meningitidis serves as a ligand for Toll-like receptor 2 (TLR2) and activates cells through a TLR2/TLR1-dependent pathway (23).
The goal of our study was to examine AP regulation in strains that did not rely on fHbp or NspA expression. We have identified a novel role for meningococcal PorB2 in regulation of the AP of complement. Concurrent with our studies, Lucidarme and colleagues described 29 invasive meningococcal isolates that lacked fHbp expression (12). Of these strains, nine belonged to the “hypervirulent” ST-11 lineage. It is noteworthy that strains that belong to the hypervirulent ST-11 clonal complex express PorB2. In an ongoing analysis of select strains reported by Lucidarme et al., three of four non-ST-11 isolates also expressed PorB2 (24). Although anti-capsule MAb SEAM 12 could overcome AP regulation and initiate in vitro killing of meningococci, maximal killing required the AP. Further, our studies demonstrated that expression of PorB2, relative to PorB3, enhanced the ability of H44/76 fHbp nspA to evade complement-mediated killing. Similarly, expression of PorB2 may render strains more resistant to complement-dependent killing in humans. The ability to regulate complement and cause invasive disease in the absence of fHbp expression may offer these strains a distinct advantage when faced with immune pressure by anti-fHbp antibodies, which is a likely occurrence with clinical use of fHbp-containing vaccines (25, 26). Natural disruption of PorA (27, 28) and NadA (IS1301) (29), which are both components of the proposed vaccine, has also been reported in the ET-15 subset of the ST-11 complex.
We have provided considerable functional evidence for human fH-dependent inhibition of the AP by PorB2. A novel finding of this study was the low affinity of the interaction between full-length fH and PorB2 on intact bacteria. fH is present in serum at concentrations of 200 to 500 µg/ml (~1.3 to 3.3 µM), and, given these high ligand concentrations, it is plausible that low-affinity interactions between PorB2 and fH suffice for complement inhibition. Higher-affinity fH-ligand interactions may be important in niches where the concentration of fH is lower, such as at mucosal surfaces. Several studies have highlighted a lack of correlation between the amount of fH binding to meningococci as revealed by flow cytometry and complement inhibition on the bacterial surface. Seib et al. (30) and Dunphy et al. (31) have shown that neither differences in fHbp-fH affinity (over 2 orders of magnitude) nor differences in the amount of fH bound to bacteria by FACS predicted resistance to complement-dependent killing. Another example is the NspA-fH interaction; despite the fact that capsule and full-length LOS expression decreased fH binding to NspA to levels barely detectable by FACS analysis, complement-inhibiting function was demonstrated under these circumstances (6). As an additional point, we previously demonstrated that a H44/76 mutant lacking fHbp and NspA, which did not bind human fH at detectable levels in vitro, caused bacteremia in human fH transgenic, but not wild-type, infant rats. These data highlighted the in vivo significance of fH function even in the absence of high-affinity fH binding in vitro (11). This “double” fHbp NspA mutant was also sensitive to infant rat complement in vitro, but dose-responsive resistance was observed upon addition of human fH (11). The paradigm for studying regulation of the AP may shift from relying on demonstration of high-affinity binding of fH to examining more functional readouts of complement activation (such as, downregulation of C3 deposition).
We were able to demonstrate high-affinity binding of FHL-1 and fH67/Fc to strains expressing PorB4243. These findings were confirmed in strain FAM18, which expresses a PorB2 with an amino acid sequence identical to that of 4243. High-affinity binding of these “fragments” of fH, but not the full-length fH, to strains expressing PorB4243 may result from structural constraints in intact fH (32, 33) that reduce its affinity for intact bacteria. A more detailed understanding of the regions of PorB that are required for functional interactions with fH may aid in the prediction of specific PorB molecules that may be involved in complement evasion.
This report sheds light on an important immune evasion function mediated by meningococcal PorB. In light of the functional role of PorB in complement evasion, it is conceivable that implementation of fHbp-containing vaccines (25, 26) may drive (or select for) loss of fHbp expression in combination with positive selection for specific PorB molecules that are optimized for AP regulation and functional interaction with fH.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical School. All subjects who donated blood for this study provided written informed consent.
Bacterial strains and culture conditions.
Characteristics of the wild-type strains used to derive the mutants used in this study are listed in Table S1 in the supplemental material. N. meningitidis bacteria were routinely grown on chocolate agar plates supplemented with IsoVitaleX equivalent at 37°C in an atmosphere with 5% CO2. GC plates supplemented with IsoVitaleX equivalent were used for antibiotic selection. Escherichia coli bacteria (Invitrogen, Carlsbad, CA) were cultured in Luria-Bertani broth or agar with antibiotics as needed. Antibiotics were used at the concentrations indicated in Table S2 in the supplemental material.
Strains were rendered unencapsulated by interruption of mynB (group A) or siaD (group B, C, or W135) (6, 34). Insertional inactivation of lst (lst::kan) abrogated LOS sialylation of group B, C, and W135 isolates as previously described (6, 34). Group A strains do not sialylate their LOS unless cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA) is added to growth media. fHbp and NspA expression was abrogated (fHbp::erm [5] and nspA::spc [6], respectively) as previously described.
PorB deletion mutants of H44/76 fHbp nspA were constructed using a plasmid that contained (5′ to 3′) an 800-bp region 5′ to PorB3H44/76 (amplified with porMC58up_F_ApaI and porMC58up_R_SacII; see Table S3 in the supplemental material), the AphA3 kanamycin resistance determinant, and a 1,100-bp region 3′ to PorB3H44/76 (amplified with PorMC58Down_F_SpeI and PorMC58Down_R_SacI; see Table S3). Meningococcal strains were transformed as previously described.
PorB allelic replacements in H44/76 were made using the H44/76 PorB3 deletion strain and chromosomal DNA isolated from either 4243 or H44/76 containing the TetM tetracycline resistance cassette downstream of porB2 or porB3, respectively. This ensured complete replacement of porB and eliminated the potential formation of hybrid PorB2/PorB3 molecules. TetM was inserted downstream of porB in each strain using plasmids that contained either porB3H44/76 (amplified with MC58_porB3R_SacII and MC58_porB3F_ApaI; see Table S3 in the supplemental material) or porB24243 (amplified with FAM18_porB2F_ApaI and Nl_porR_SacII; see Table S3) followed by TetM and the 1,100-bp region 3′ to porB3H44/76 described above. Transformants were selected for tetracycline resistance and checked for sensitivity to kanamycin. All clones were verified by DNA sequencing.
Sera.
Normal human serum (NHS) was obtained from healthy adult volunteers, divided into aliquots, and stored (as individual sera) at −80°C till used. To inactivate selectively the classical and lectin complement pathways and isolate the AP as the only active pathway, MgCl2 and EGTA (10 mM each) were added to NHS (NHS-Mg/EGTA).
Absorbed sera, in which antibodies specific to H44/76 fHbp nspA PorB3 and H44/76 fHbp nspA PorB2 were removed, were prepared as follows. EDTA (10 mM) was added to fresh NHS, and the sera were dialyzed against phosphate-buffered saline (PBS)–1 mM EDTA for 4 h at 4°C using a 10-kDa molecular mass cutoff. Meningococcal strains were subjected to passage on overnight plates and added onto fresh chocolate agar plates and grown for 5 h. Bacteria were harvested and washed once with PBS–1 mM EDTA. Dialyzed serum was absorbed twice with bacteria (1-ml serum with a mixture of 100 µl of packed cells from each of the 2 mutant strains) processed end over end for 30 min at 4°C. The serum was filtered (0.22-µm-pore-size filter), divided into aliquots, and stored at −80°C. Ca2+ and Mg2+ (2 mM each) were added prior to use. Similar amounts of fH were detected (MAb 90X) in NHS and absorbed sera spotted on polyvinylidene difluoride (PVDF), which suggested that the fH was not depleted by the absorption. The hemolytic activity of serum was confirmed using a Total hemolytic complement kit.
Mouse complement was obtained by cardiac puncture of adult BALB/c mice. Blood was allowed to clot at 22°C for 20 min, followed by incubation at 4°C for 20 min, and then spun, and the supernatant was divided into aliquots and stored at −80°C. Adult rabbit complement was purchased from Cedarlane Laboratories.
Bacterial killing by NHS.
Susceptibility of neisserial strains to complement-mediated killing was determined using a serum bactericidal assay as described previously (6). Anti-group B capsule IgG antibody SEAM 12 (19) was added to absorbed sera at the concentrations indicated. Survival was calculated as the number of viable colonies at time 30 min relative to time 0.
Purified complement components.
Factors B and D were from Complement Technology, Inc. (Tyler, TX). C3 was isolated from human plasma by polyethylene glycol (PEG) precipitation and chromatography over DEAE Sephacel using a modification of previously established methods (35). NHS immunodepleted of C3 (Complement Technology, Inc.) regained full hemolytic activity upon addition of the purified C3 to a concentration of 500 µg/ml, using a Total hemolytic complement kit (The Binding Site, Birmingham, United Kingdom).
C3 deposition on bacteria using purified complement proteins.
To deposit C3 on bacteria using purified AP components, ~108 bacteria in Hanks balanced salt solution (HBSS)–1 mM MgCl2–0.1% bovine serum albumin (BSA) were incubated with C3 (500 µg/ml), factor B (100 µg/ml), and fH (100 µg/ml) followed by factor D (2 µg/ml) in a final reaction volume of 50 µl for 30 min at 37°C. Deposited C3 was measured by FACS analysis.
Antibodies.
Human, rabbit, and mouse C3 deposition on bacteria was measured using fluorescein isothiocyanate (FITC)-conjugated anti-human C3 (BioDesign/Meridian Life Sciences), anti-guinea pig C3, and anti-mouse C3 (the latter 2 from Cappel/MP Biomedical) at a dilution of 1:200 in HBSS–0.1% BSA, respectively. Preliminary FACS and Western blotting experiments confirmed the specificity and cross-reactivity of anti-guinea pig C3 FITC with rabbit C3.
Recombinant human fH/Fc fusion proteins.
fH domains 18 through 20 fused in frame to the N terminus of the Fc fragment of murine IgG2a (called fH/Fc) have been described previously (36). Similarly, we generated fH domains 6 and 7 fused to murine IgG2a Fc. fH domains 6 and 7 were amplified (primers, six AscI and seven NotI) from human fH cDNA (gift of M. K. Pangburn, University of Texas Health Science Center, Tyler, TX) and cloned into AscI and NotI sites of pcDNA3 (Invitrogen Life Technologies) encoding mouse IgG2a Fc (37). Transfection of CHO cells and protein purification using protein A Sepharose were performed as described previously (36).
Flow cytometry.
Human, rabbit, and mouse C3 deposition on the bacterial surface and FHL-1, fH, and fH/Fc construct binding were measured using flow cytometry as described previously (10, 17, 36). To detect low-affinity binding of fH, organisms incubated with human fH (100 µg/ml) for 30 min were fixed by the addition of paraformaldehyde (final concentration, 1%). The organisms were pelleted after incubation for 10 min at room temperature, and bound fH was detected by flow cytometry as described above. Data were analyzed using FlowJo (version 7.2.5; Tree Star, Inc.). Binding is represented either as a percentage of positive events (relative to a negative control that was gated to yield 5% of positive events) when histogram tracings showed a non-Gaussian distribution or as median fluorescence of the entire bacterial population when all samples yielded normally distributed histograms.
C3 generation assay.
Bacteria (~2 × 108) were incubated with 20% NHS-Mg/EGTA in an initial reaction volume of 200 µl. Samples (10 µl) were collected at specified intervals between 0 min and 15 min and diluted immediately in 90 µl PBS–50 mM EDTA. C3a in the reaction mixture was measured using a MicroVueC3a Plus EIA kit (Quidel) according to the manufacturer’s instructions.
Statistical analyses.
Statistical comparisons across multiple groups were carried out using 1-way analysis of variance (ANOVA) with Tukey’s posttest for pairwise comparisons. Differences in C3a generation and bacterial survival between strains were measured using 2-way ANOVA with Bonferroni’s posttest. All probability values, with the exception of the 1-way ANOVA, are two tailed.
SUPPLEMENTAL MATERIAL
iC3b deposition on quadruple mutants of seven isolates of N. meningitidis assessed by Western blotting. Incubation of bacteria with serum results in complement activation and deposition of C3b on the bacteria. C3b deposited on bacteria is converted to iC3b by fH (cofactor) and factor I (enzyme). Following 30 min of incubation with serum, almost all the C3b deposited on meningococci is converted to iC3b, even on meningococcal strains that lack fHbp, NspA, and lipo-oligosaccharide (LOS) sialic acid (S. Ram, L. A. Lewis, and S. Agarwal, J. Biol. Chem. 286:8297-8307, 2011). Alternative pathway-mediated iC3b deposited on bacteria was assessed by Western blotting as described previously (S. Ram, L. A. Lewis, and S. Agarwal, J. Biol. Chem. 286:8297-8307, 2011; L. A. Lewis, S. Ram, A. Prasad, S. Gulati, S. Getzlaff, A. M. Blom, U. Vogel, and P. A. Rice, Infect. Immun. 76:339-350, 2008). Briefly, 108 bacteria suspended in Hanks balanced salt solution (HBSS) containing 1 mM MgCl2 and 1 mM EGTA were incubated with normal human serum-MgCl2-EGTA (NHS-Mg/EGTA) (final concentration, 25% [vol/vol]) in a final reaction volume of 80 µl for 10 or 30 min at 37°C. Bacteria were washed twice in HBSS and lysed in 4× LDS sample buffer (Invitrogen) containing 10% 2-mercaptoethanol (2-ME). Proteins were separated on NuPAGE Novex 4–12% bis-Tris gradient gels using NuPAGE 3-morpholino propane sulfonic acid (MOPS) running buffer (Invitrogen). Proteins were transferred to a 0.45-µm-pore-size polyvinylidene difluoride (PVDF) membrane (Millipore) by Western blotting. iC3b was detected using G-3E monoclonal antibody (MAb) (tissue culture supernatants containing approximately 20 µg/ml of antibody diluted 1:4 in Tris-buffered saline [TBS] followed by anti-mouse IgG conjugated to alkaline phosphatase; this MAb specifically recognizes a neoepitope in the 68-kDa α1′ chain of iC3b [K. Iida, K. Mitomo, T. Fujita, and N. Tamura, Immunology 62:413-417, 1987]). Under these electrophoresis conditions, the α1′ chain of iC3b (68 kDa, indicated as “α1”) migrates as part of a covalent complex with its bacterial target or independently as a 68-kDa molecule in the lane that contains pure iC3b. A portion of iC3b detected in lanes containing bacteria treated with serum migrates as a free (or released) α1″ fragment. Spontaneous release of C3b previously covalently linked by ester bonds to surfaces such as sheep red blood cells (RBCs), glycerol, IgG aggregates, or tyrosine has been reported (A. Sahu and M. K. Pangburn, Mol. Immunol. 32:711-716, 1995; Y. P. Venkatesh and R. P. Levine, Mol. Immunol. 25:821-828, 1988; Y. P. Venkatesh, T. M. Minich, S. K. Law, and R. P. Levine, Immunology 132:1435-1439, 1984). Similarly, spontaneous release of part of the covalently linked α1″ fragment from meningococcal targets has been reported by us previously (S. Ram, L. A. Lewis, and S. Agarwal, J. Biol. Chem. 286:8297-8307, 2011). Complexes of the α1′ chain of iC3b with LOS or opacity protein (Opa) have previously been characterized (L. A. Lewis, S. Ram, A. Prasad, S. Gulati, S. Getzlaff, A. M. Blom, U. Vogel, and P. A. Rice, Infect. Immun. 76:339-350, 2008) and are indicated. The lower section of the blot (proteins migrating faster than the rate corresponding to~40 kDa) was stained with Coomassie blue (labeled as “load”) and served to illustrate similar levels of loading of bacteria across lanes. Download
Addition of human fH to adult nonhuman complement selectively regulates C3 deposition on PorB2 quadruple mutants. Histograms from a representative experiment performed as described for Fig. 2 are shown. Quadruple mutants of 7 strains of N. meningitidis were incubated with adult rabbit complement (A) or adult mouse serum (B) in either the absence (solid black line) or the presence (gray-shaded histograms) of human fH (80 µg/ml). Controls (bacteria incubated with the respective heat-inactivated sera) are shown as histograms with the broken lines. Download
Heterogeneity in C3 fragment deposition on PorB2-expressing strains occurs independently of the PorB2 expression level. (A) PorB2 is expressed as a single population with a unimodal (Gaussian) distribution on strains that display bimodal C3 fragment distribution. Quadruple mutants of 4243, 2996, and H44/76-PorB2 (all lack capsule, Lst, fHbp, and NspA) were incubated with NHS-Mg/EGTA (at concentrations optimized for each strain), and bacteria were stained simultaneously for C3 and PorB2. C3 was detected using anti-C3c conjugated to fluorescein isothiocyanate (FITC), while PorB2 was detected using either MAb P2.2a (4243 and H44/76-porB2) or MAb P2.2b (2996) followed by goat anti-mouse IgG conjugated to Alexa Fluor 647 (A 647). A total of 100,000 events were acquired and analyzed. The upper panel shows dot plot graphs where C3 deposition is shown on the x axis and PorB2 expression on the y axis. The control represents a reaction mixture that lacked serum and was used to generate quadrant gates. Corresponding C3 deposition and PorB2 expression histograms (gray-shaded graphs) are shown in the middle and lower panels, respectively. The numbers alongside the PorB2 histograms represent the median fluorescence values for the entire bacterial population. The median fluorescence value for the controls (dashed lines) in all cases was ~5. (B) Dual “high”- and “low”-expression C3 deposition patterns are seen in diverse strata of PorB2 expression. Based on the intensity of staining with anti-PorB2, bacterial populations representing low, medium, and high PorB2 expression levels were analyzed separately for C3 deposition. All gated populations showed distinct biphasic C3 deposition patterns, suggesting that PorB2 expression levels did not account for heterogenous C3 deposition patterns. Download
Enhanced binding of full-length fH to quadruple mutants of meningococcal strains expressing PorB2 compared to those expressing PorB3. Histograms from representative experiments represented in Fig. 4A and B are shown. (A and B) Binding of human fH to quadruple mutants of H44/76 that express either PorB344/76 (H44/76-PorB3) or PorB24243 (H44/76-PorB2) (A) and quadruple mutants of N. meningitidis strains that express either PorB3 (A2594, H44/76, and NZ98/254) or PorB2 (C2120, W171, 4243, and 2996) (B). Quadruple mutants were incubated with purified fH (100 µg/ml), and bound fH was measured by FACS analysis (labeled “no cross-linker”). In some experiments, bound fH was cross-linked to the surface with paraformaldehyde (final concentration, 1% [vol/vol]) prior to detection by FACS analysis (labeled “paraformaldehyde cross-linker”). The negative control (control) was a reaction mixture that lacked fH (neg. control). Binding of fH to wild-type H44/76 that expresses fHbp, NspA, capsule, and LOS sialic acid is shown as a positive control in panel B (pos. control). Histograms from a representative experiment are shown in panels A and B; data represent the median fluorescence of fH binding of the entire bacterial population. Download
Sequences of PorB2 isolates used in this study. (A) ClustalW alignment of PorB2 molecules used in this study. Predicted surface-exposed loops are shaded gray. Bolded amino acid residues represent differences in loop amino acids compared to PorB24243 results. Below the alignment, asterisks (*) indicate regions of identity, colons (:) indicate conservative substitutions, periods (.) indicate semiconservative substitutions, and blanks indicate regions of nonidentity. (B) A rooted phylogram tree showing the relatedness among the PorB2 molecules used in this study. The number of sequence character changes is related to the horizontal branch length. PorB2W171 and PorB2C2120 differ from PorB24243 by the loss of 3 amino acids from loop 7. Loop 7 of PorB22996 is identical to loop7 of PorB24243; however, PorB22996 is divergent from PorB24243 in loops 4, 5, and 6. Download
Binding of fH67/Fc to and AP regulation by the quadruple mutant of FAM18. (Left panel) Binding of fH67/Fc (20 µg/ml) to the quadruple mutant of FAM18 (PorB2) shown by a flow cytometry (gray-shaded) histogram. Also shown are fH67/Fc binding to the quadruple mutants of 4243 (red line) and H44/76 (solid black line) as positive and negative controls, respectively. The dotted line represents a reaction mixture with FAM18 (quadruple mutant) and anti-mouse IgG-FITC (no added fH67/Fc). (Right panel) The quadruple mutant of FAM18 regulates the human AP. The quadruple mutant of FAM18 was incubated with NHS-Mg/EGTA (20%), and deposited C3 was measured by FACS analysis (gray-shaded histogram). The quadruple mutants of H44/76 (PorB3; does not regulate the human AP; solid black line) and 4243 (PorB2; regulates the human AP; red line) were used as controls. Baseline fluorescence (FAM18 “quadruple” plus heat-inactivated NHS) is depicted by the dotted line. Download
Strains used in this study and their relevant characteristics.
Antibiotic and concentrations used.
Primers used in this study.
ACKNOWLEDGMENTS
This work was supported by grants AI054544, AI084048, AI32725, AI 046464, and AI 082263 from the National Institute of Allergy and Infectious Diseases, NIH. The work at the Children’s Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant number C06 RR 016226 from the National Center for Research Resources, NIH.
We thank Ulrich Vogel (Universität Würzburg, Germany) for A2594, H44/76, C2120, and W171 and their mutants lacking capsule and LOS sialic acid.
Footnotes
Citation Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. 2013. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio 4(5):e00339-13. doi:10.1128/mBio.00339-13.
REFERENCES
- 1. Ram S, Lewis LA, Rice PA. 2010. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin. Microbiol. Rev. 23:740–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Austen KF, Fearon DT. 1979. A molecular basis of activation of the alternative pathway of human complement. Adv. Exp. Med. Biol. 120B:3–17 [PubMed] [Google Scholar]
- 3. Giuntini S, Reason DC, Granoff DM. 2012. The combined roles of human IgG subclass, alternative complement pathway activation, and epitope density on bactericidal activity of antibodies to meningococcal factor H binding protein. Infect. Immun. 80:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreira VP, Pangburn MK, Cortés C. 2010. Complement control protein factor H: the good, the bad, and the inadequate. Mol. Immunol. 47:2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 6:e1001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis LA, Carter M, Ram S. 2012. The relative roles of factor H binding protein, neisserial surface protein A, and lipooligosaccharide sialylation in regulation of the alternative pathway of complement on meningococci. J. Immunol. 188:5063–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarvis GA, Vedros NA. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ram S, Lewis LA, Agarwal S. 2011. Meningococcal group W-135 and Y capsular polysaccharides paradoxically enhance activation of the alternative pathway of complement. J. Biol. Chem. 286:8297–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vu DM, Shaughnessy J, Lewis LA, Ram S, Rice PA, Granoff DM. 2012. Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis. Infect. Immun. 80:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. 2011. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin. Vaccine Immunol. 18:1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis LA, Ram S, Prasad A, Gulati S, Getzlaff S, Blom AM, Vogel U, Rice PA. 2008. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect. Immun. 76:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ram S, Cox AD, Wright JC, Vogel U, Getzlaff S, Boden R, Li J, Plested JS, Meri S, Gulati S, Stein DC, Richards JC, Moxon ER, Rice PA. 2003. Neisserial lipooligosaccharide is a target for complement component C4b: inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278:50853–50862 [DOI] [PubMed] [Google Scholar]
- 15. Nawar H, Smith K, Amer L, Vann W, Bash M. 2012. Analysis of the effect of antigenic diversity of the Neisseria meningitidis outer membrane porin B protein (PorB) on strain fitness, p 202 In Frosch M, Rudel T, Vogel U. (ed), XVIIIth International Pathogenic Neisseria Conference. Wuerzburg, Germany; Conventus Congressmanagement and Marketing GmbH, Jena, Germany [Google Scholar]
- 16. Blom AM, Hallström T, Riesbeck K. 2009. Complement evasion strategies of pathogens—acquisition of inhibitors and beyond. Mol. Immunol. 46:2808–2817 [DOI] [PubMed] [Google Scholar]
- 17. Shaughnessy J, Lewis LA, Jarva H, Ram S. 2009. Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect. Immun. 77:2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zipfel PF, Skerka C. 1999. FHL-1/reconnection: a human complement and immune regulator with cell-adhesive function. Immunol. Today 20:135–140 [DOI] [PubMed] [Google Scholar]
- 19. Granoff DM, Bartoloni A, Ricci S, Gallo E, Rosa D, Ravenscroft N, Guarnieri V, Seid RC, Shan A, Usinger WR, Tan S, McHugh YE, Moe GR. 1998. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J. Immunol. 160:5028–5036 [PubMed] [Google Scholar]
- 20. Tanabe M, Nimigean CM, Iverson TM. 2010. Structural basis for solute transport, nucleotide regulation, and immunological recognition of Neisseria meningitidis PorB. Proc. Natl. Acad. Sci. U. S. A. 107:6811–6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O’Connell C, Boden R, Elkins C, Pangburn MK, Dahlbäck B, Rice PA. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193:281–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176:2373–2380 [DOI] [PubMed] [Google Scholar]
- 24. Giuntini S, Vu DM, Granoff DM. 2013. fH-dependent complement evasion by disease-causing meningococcal strains with absent fHbp genes or frameshift mutations. Vaccine 31:4192–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nissen MD, Marshall HS, Richmond PC, Jiang Q, Harris SL, Jones TR, Jansen KU, Perez JL. 2013. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr. Infect. Dis. J. 32:364–371 [DOI] [PubMed] [Google Scholar]
- 26. Santolaya ME, O’Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R, Dull PM, V72P10 Meningococcal B Adolescent Vaccine Study Group; 2012. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 379:617–624 [DOI] [PubMed] [Google Scholar]
- 27. Jelfs J, Munro R, Wedege E, Caugant DA. 2000. Sequence variation in the porA gene of a clone of Neisseria meningitidis during epidemic spread. Clin. Diagn. Lab. Immunol. 7:390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van der Ende A, Hopman CT, Dankert J. 1999. Deletion of porA by recombination between clusters of repetitive extragenic palindromic sequences in Neisseria meningitidis. Infect. Immun. 67:2928–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elias J, Vogel U. 2007. IS1301 fingerprint analysis of Neisseria meningitidis strains belonging to the ET-15 clone. J. Clin. Microbiol. 45:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seib KL, Brunelli B, Brogioni B, Palumbo E, Bambini S, Muzzi A, DiMarcello F, Marchi S, van der Ende A, Aricó B, Savino S, Scarselli M, Comanducci M, Rappuoli R, Giuliani MM, Pizza M. 2011. Characterization of diverse subvariants of the meningococcal factor H (fH) binding protein for their ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. Infect. Immun. 79:970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunphy KY, Beernink PT, Brogioni B, Granoff DM. 2011. Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood. Infect. Immun. 79:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makou E, Mertens HD, Maciejewski M, Soares DC, Matis I, Schmidt CQ, Herbert AP, Svergun DI, Barlow PN. 2012. Solution structure of CCP modules 10–12 illuminates functional architecture of the complement regulator, factor H. J. Mol. Biol. 424:295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okemefuna AI, Nan R, Gor J, Perkins SJ. 2009. Electrostatic interactions contribute to the folded-back conformation of wild type human factor H. J. Mol. Biol. 391:98–118 [DOI] [PubMed] [Google Scholar]
- 34. Madico G, Ngampasutadol J, Gulati S, Vogel U, Rice PA, Ram S. 2007. Factor H binding and function in sialylated pathogenic neisseriae is influenced by gonococcal, but not meningococcal, porin. J. Immunol. 178:4489–4497 [DOI] [PubMed] [Google Scholar]
- 35. Hammer CH, Wirtz GH, Renfer L, Gresham HD, Tack BF. 1981. Large scale isolation of functionally active components of the human complement system. J. Biol. Chem. 256:3995–4006 [PubMed] [Google Scholar]
- 36. Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, Visintin A, Monks B, Madico G, Rice PA. 2008. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J. Immunol. 180:3426–3435 [DOI] [PubMed] [Google Scholar]
- 37. Visintin A, Halmen KA, Latz E, Monks BG, Golenbock DT. 2005. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J. Immunol. 175:6465–6472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
iC3b deposition on quadruple mutants of seven isolates of N. meningitidis assessed by Western blotting. Incubation of bacteria with serum results in complement activation and deposition of C3b on the bacteria. C3b deposited on bacteria is converted to iC3b by fH (cofactor) and factor I (enzyme). Following 30 min of incubation with serum, almost all the C3b deposited on meningococci is converted to iC3b, even on meningococcal strains that lack fHbp, NspA, and lipo-oligosaccharide (LOS) sialic acid (S. Ram, L. A. Lewis, and S. Agarwal, J. Biol. Chem. 286:8297-8307, 2011). Alternative pathway-mediated iC3b deposited on bacteria was assessed by Western blotting as described previously (S. Ram, L. A. Lewis, and S. Agarwal, J. Biol. Chem. 286:8297-8307, 2011; L. A. Lewis, S. Ram, A. Prasad, S. Gulati, S. Getzlaff, A. M. Blom, U. Vogel, and P. A. Rice, Infect. Immun. 76:339-350, 2008). Briefly, 108 bacteria suspended in Hanks balanced salt solution (HBSS) containing 1 mM MgCl2 and 1 mM EGTA were incubated with normal human serum-MgCl2-EGTA (NHS-Mg/EGTA) (final concentration, 25% [vol/vol]) in a final reaction volume of 80 µl for 10 or 30 min at 37°C. Bacteria were washed twice in HBSS and lysed in 4× LDS sample buffer (Invitrogen) containing 10% 2-mercaptoethanol (2-ME). Proteins were separated on NuPAGE Novex 4–12% bis-Tris gradient gels using NuPAGE 3-morpholino propane sulfonic acid (MOPS) running buffer (Invitrogen). Proteins were transferred to a 0.45-µm-pore-size polyvinylidene difluoride (PVDF) membrane (Millipore) by Western blotting. iC3b was detected using G-3E monoclonal antibody (MAb) (tissue culture supernatants containing approximately 20 µg/ml of antibody diluted 1:4 in Tris-buffered saline [TBS] followed by anti-mouse IgG conjugated to alkaline phosphatase; this MAb specifically recognizes a neoepitope in the 68-kDa α1′ chain of iC3b [K. Iida, K. Mitomo, T. Fujita, and N. Tamura, Immunology 62:413-417, 1987]). Under these electrophoresis conditions, the α1′ chain of iC3b (68 kDa, indicated as “α1”) migrates as part of a covalent complex with its bacterial target or independently as a 68-kDa molecule in the lane that contains pure iC3b. A portion of iC3b detected in lanes containing bacteria treated with serum migrates as a free (or released) α1″ fragment. Spontaneous release of C3b previously covalently linked by ester bonds to surfaces such as sheep red blood cells (RBCs), glycerol, IgG aggregates, or tyrosine has been reported (A. Sahu and M. K. Pangburn, Mol. Immunol. 32:711-716, 1995; Y. P. Venkatesh and R. P. Levine, Mol. Immunol. 25:821-828, 1988; Y. P. Venkatesh, T. M. Minich, S. K. Law, and R. P. Levine, Immunology 132:1435-1439, 1984). Similarly, spontaneous release of part of the covalently linked α1″ fragment from meningococcal targets has been reported by us previously (S. Ram, L. A. Lewis, and S. Agarwal, J. Biol. Chem. 286:8297-8307, 2011). Complexes of the α1′ chain of iC3b with LOS or opacity protein (Opa) have previously been characterized (L. A. Lewis, S. Ram, A. Prasad, S. Gulati, S. Getzlaff, A. M. Blom, U. Vogel, and P. A. Rice, Infect. Immun. 76:339-350, 2008) and are indicated. The lower section of the blot (proteins migrating faster than the rate corresponding to~40 kDa) was stained with Coomassie blue (labeled as “load”) and served to illustrate similar levels of loading of bacteria across lanes. Download
Addition of human fH to adult nonhuman complement selectively regulates C3 deposition on PorB2 quadruple mutants. Histograms from a representative experiment performed as described for Fig. 2 are shown. Quadruple mutants of 7 strains of N. meningitidis were incubated with adult rabbit complement (A) or adult mouse serum (B) in either the absence (solid black line) or the presence (gray-shaded histograms) of human fH (80 µg/ml). Controls (bacteria incubated with the respective heat-inactivated sera) are shown as histograms with the broken lines. Download
Heterogeneity in C3 fragment deposition on PorB2-expressing strains occurs independently of the PorB2 expression level. (A) PorB2 is expressed as a single population with a unimodal (Gaussian) distribution on strains that display bimodal C3 fragment distribution. Quadruple mutants of 4243, 2996, and H44/76-PorB2 (all lack capsule, Lst, fHbp, and NspA) were incubated with NHS-Mg/EGTA (at concentrations optimized for each strain), and bacteria were stained simultaneously for C3 and PorB2. C3 was detected using anti-C3c conjugated to fluorescein isothiocyanate (FITC), while PorB2 was detected using either MAb P2.2a (4243 and H44/76-porB2) or MAb P2.2b (2996) followed by goat anti-mouse IgG conjugated to Alexa Fluor 647 (A 647). A total of 100,000 events were acquired and analyzed. The upper panel shows dot plot graphs where C3 deposition is shown on the x axis and PorB2 expression on the y axis. The control represents a reaction mixture that lacked serum and was used to generate quadrant gates. Corresponding C3 deposition and PorB2 expression histograms (gray-shaded graphs) are shown in the middle and lower panels, respectively. The numbers alongside the PorB2 histograms represent the median fluorescence values for the entire bacterial population. The median fluorescence value for the controls (dashed lines) in all cases was ~5. (B) Dual “high”- and “low”-expression C3 deposition patterns are seen in diverse strata of PorB2 expression. Based on the intensity of staining with anti-PorB2, bacterial populations representing low, medium, and high PorB2 expression levels were analyzed separately for C3 deposition. All gated populations showed distinct biphasic C3 deposition patterns, suggesting that PorB2 expression levels did not account for heterogenous C3 deposition patterns. Download
Enhanced binding of full-length fH to quadruple mutants of meningococcal strains expressing PorB2 compared to those expressing PorB3. Histograms from representative experiments represented in Fig. 4A and B are shown. (A and B) Binding of human fH to quadruple mutants of H44/76 that express either PorB344/76 (H44/76-PorB3) or PorB24243 (H44/76-PorB2) (A) and quadruple mutants of N. meningitidis strains that express either PorB3 (A2594, H44/76, and NZ98/254) or PorB2 (C2120, W171, 4243, and 2996) (B). Quadruple mutants were incubated with purified fH (100 µg/ml), and bound fH was measured by FACS analysis (labeled “no cross-linker”). In some experiments, bound fH was cross-linked to the surface with paraformaldehyde (final concentration, 1% [vol/vol]) prior to detection by FACS analysis (labeled “paraformaldehyde cross-linker”). The negative control (control) was a reaction mixture that lacked fH (neg. control). Binding of fH to wild-type H44/76 that expresses fHbp, NspA, capsule, and LOS sialic acid is shown as a positive control in panel B (pos. control). Histograms from a representative experiment are shown in panels A and B; data represent the median fluorescence of fH binding of the entire bacterial population. Download
Sequences of PorB2 isolates used in this study. (A) ClustalW alignment of PorB2 molecules used in this study. Predicted surface-exposed loops are shaded gray. Bolded amino acid residues represent differences in loop amino acids compared to PorB24243 results. Below the alignment, asterisks (*) indicate regions of identity, colons (:) indicate conservative substitutions, periods (.) indicate semiconservative substitutions, and blanks indicate regions of nonidentity. (B) A rooted phylogram tree showing the relatedness among the PorB2 molecules used in this study. The number of sequence character changes is related to the horizontal branch length. PorB2W171 and PorB2C2120 differ from PorB24243 by the loss of 3 amino acids from loop 7. Loop 7 of PorB22996 is identical to loop7 of PorB24243; however, PorB22996 is divergent from PorB24243 in loops 4, 5, and 6. Download
Binding of fH67/Fc to and AP regulation by the quadruple mutant of FAM18. (Left panel) Binding of fH67/Fc (20 µg/ml) to the quadruple mutant of FAM18 (PorB2) shown by a flow cytometry (gray-shaded) histogram. Also shown are fH67/Fc binding to the quadruple mutants of 4243 (red line) and H44/76 (solid black line) as positive and negative controls, respectively. The dotted line represents a reaction mixture with FAM18 (quadruple mutant) and anti-mouse IgG-FITC (no added fH67/Fc). (Right panel) The quadruple mutant of FAM18 regulates the human AP. The quadruple mutant of FAM18 was incubated with NHS-Mg/EGTA (20%), and deposited C3 was measured by FACS analysis (gray-shaded histogram). The quadruple mutants of H44/76 (PorB3; does not regulate the human AP; solid black line) and 4243 (PorB2; regulates the human AP; red line) were used as controls. Baseline fluorescence (FAM18 “quadruple” plus heat-inactivated NHS) is depicted by the dotted line. Download
Strains used in this study and their relevant characteristics.
Antibiotic and concentrations used.
Primers used in this study.