Abstract
Purpose:
To compare the localization and density of collagens I, IV, VI, and elastin, the major protein components of connective tissue, in the inferior oblique muscle of patients with overelevation in adduction and in controls and to characterize changes that develop following surgery. Biomechanical studies suggest that the connective tissue matrix plays a critical role in extraocular muscle function, determining tensile strength and force transmission during contraction.
Methods:
Prospective laboratory-based case-control study of inferior oblique muscle specimens from 31 subjects: 16 with primary inferior oblique overaction, 6 with craniofacial dysostosis, and 9 normal controls. Collagen I, IV, VI, and elastin were localized and quantified using immunohistochemical staining. Densities were compared using analysis of variance and post hoc comparisons.
Results:
In primary inferior oblique overaction, all connective tissue components in unoperated specimens were elevated compared to controls (P<.0001). Previously operated muscles showed normal levels of collagens IV and VI (P>.27) but increased collagen I. In unoperated craniofacial dysostosis specimens, only elastin was elevated (P=.03), whereas density of collagens IV and VI was lower in previously operated vs unoperated specimens (P=.015).
Conclusions:
Elevated collagen and elastin levels in the cohort with primary inferior oblique overaction are consistent with the clinical finding of muscle stiffness. Contrarily, normal connective tissue densities in craniofacial dysostosis support the hypothesis that overelevation in this group reflects anomalous muscle vectors rather than tissue changes. Surgical intervention was associated with changes in the connective tissue matrix in both cohorts. These results have ramifications for treating patients with overelevation in adduction.
INTRODUCTION
For many clinical diagnoses of strabismus, the relationship between the pattern of abnormal ocular alignment and possible abnormalities in the extraocular muscles at the molecular level remains poorly understood. In fact, there are a myriad of distinct clinical entities that result in a similar abnormal eye position commonly described as inferior oblique muscle overaction. These include such different conditions as Duane syndrome, superior oblique palsy, dissociated vertical divergence (DVD), globe rotation in craniofacial dysostosis, and mechanical restriction of the inferior rectus muscle.1,2 This thesis tests the hypotheses that the connective tissue phenotype of inferior oblique muscles differs between patients with overelevation in adduction and controls, and that inferior oblique surgery alters the connective tissue phenotype.
As Burton Kushner pointed out: “When an eye moves excessively into the field of action of an extraocular muscle on version testing, that muscle is typically described as being overacting. Because terminology influences understanding, the use of the term overacting can be misleading. . . . In many circumstances, an excessive movement of an eye may in fact be in the field of action of a muscle that is not actually responsible for the abnormal movement.”1
The inferior oblique muscles serve to extort, elevate, and abduct the eyes,3 and the elevating action of the inferior oblique is greatest in the adducted position. Excessive elevation of the eye in adduction has traditionally been termed inferior oblique overaction.3,4 Primary inferior oblique overaction develops commonly in individuals with infantile esotropia (often after surgical treatment of the original horizontal strabismus), as well as in some children with accommodative esotropia, or intermittent exotropia.5 Wilson and Parks6 estimated the incidence at 72% among individuals with infantile esotropia and greater than 30% in those with acquired esotropia or intermittent exotropia. Primary inferior oblique overaction has been reported to represent 16.7%7 of hypertropias. Other, less common types of strabismus that may demonstrate overelevation in adduction include superior oblique palsy8; V-, Y-, or X-pattern exotropia8; Duane syndrome with upshoot9; and ocular deviations associated with craniosynostosis.10–12
The term inferior oblique overaction may in some cases be misleading,4 as it suggests that hypertrophy or hypercontractility causes the muscle to “overact.” However, superior oblique weakness, co-contraction of the horizontal rectus muscles (Duane syndrome), restriction of rectus muscles, aberrant innervation of the extraocular muscles, or anomalies in the insertion of other extraocular muscles may result in overelevation in adduction.1,2 In patients with craniosynostosis, excyclorotation of the globes and orbits alone may cause apparent overaction of the inferior oblique muscles because the medial rectus muscles elevate as well as adduct the globe. Regardless of the cause of overelevation in adduction, traditional surgical management has often included weakening of the inferior oblique. Variations of inferior oblique weakening are myriad, from simple recession to combinations of recession with alterations of the normal muscle pathway, including anterior transposition13–15 or anterior nasal transposition.9,12,16 Additional surgical procedures include myectomy of the temporal portion of the muscle and denervation and extirpation.8,17,18 Recently, it has been shown that nasal myectomy19 as well as fixation of the inferior oblique to the orbital wall20 are effective techniques to weaken the action of the muscle.
Although each of these procedures has been shown to effectively decrease overelevation in adduction, residual or recurrent overelevation often occurs.21–23 In particular, the most common inferior oblique weakening procedures (simple recession, anterior transposition, and myectomy) are known to be followed by recurrent overelevation. Secondary procedures designed to further weaken the muscle include re-recession, nasal transposition, nasal myectomy, and denervation and extirpation. These procedures may be technically challenging, particularly after primary myectomy, which may unpredictably alter muscle anatomy. The high recurrence rate of overelevation after inferior oblique weakening, along with the possibility that the inferior oblique muscle is not the primary cause of the problem, has led to a heightened interest in the anatomy24 and histology of this muscle.
To date, few studies have attempted to correlate the histology of the inferior oblique muscle with specific clinical diagnoses. Antunes-Foschini and colleagues25 reported substantially increased numbers of activated satellite cells in inferior oblique muscle specimens from a series of subjects with inferior oblique overaction. These results suggest that there is active modulation of individual myofibers within the inferior oblique of these subjects that is increased over the level seen in normal inferior oblique muscles. The investigators acknowledge that overelevation in adduction may have multiple causes and describe their cohort as highly heterogeneous.25 Indeed, there were wide variations in the density of muscle precursor cells in the muscle segments removed among their subjects. Nonetheless, the percentages were greater than normal in the muscles from every subject analyzed.
The results of Antunes-Foschini and colleagues25 confirm that extraocular muscles, in contrast to most other skeletal muscles, maintain a process of continuous myofiber remodeling, and that the rate of this remodeling appears to change in patients with inferior oblique overaction. Metabolic labeling studies have demonstrated that there is a continuous and ongoing level of myofiber remodeling in the extraocular muscles.26,27 This phenomenon involves both the addition and removal of single myonuclei in single myofibers, which suggests that segmental regions of individual myofibers undergo local cytoplasmic remodeling as well.28 This process appears to occur in all species examined thus far,26,27,29 even in aging humans.26 The rate of this process is modulated by a number of factors, including thyroid hormone, myogenic growth factors, stretch, and functional denervation.30–33 One prediction from these studies is that alterations in satellite cell number in muscles from subjects with strabismus (compared to normal) reflect the muscle’s attempt to modulate the number or size of myofibers in order to adapt to local cues or to cues derived from the central nervous system.34 Beyond these descriptions of the satellite cell density in patients with inferior oblique overaction, little is known about other potential adaptions of the involved muscles and what role these adaptions might play in the abnormal function of these muscles when ocular motility is disturbed.
It is clear that the inferior oblique muscles from patients with overelevation in adduction are not all the same. Forced duction testing will confirm that inferior oblique muscles from different patients do not have the same passive stiffness or range of motion. Thus, in clinical examination, muscles are described as being either normal, stiff, tight, or slack.35 These differences are actually considered to be a property of the connective tissue matrices within the muscles.36 There is a paucity of information available about the composition of various connective tissue matrix molecules in extraocular muscles and even less in patients with strabismus.
In skeletal muscles and tendons, the extracellular matrix and the connective tissue elements it contains are critical for defining the tensile properties of muscle function. They play a critical role in transmission of force required for movement during muscle contraction. Myofascial force transmission is more important at lower forces,37 emphasizing the critical role the connective tissue matrix plays, particularly in muscles that operate under lower forces, such as the extraocular muscles. The major protein component of connective tissue is collagen. This structural protein can represent 1% to 10% of the dry weight of skeletal muscle.38 Recent studies have demonstrated that the connective tissue matrix within skeletal muscles represents a highly ordered and massively interconnected network including both endomysium39 and perimysium.40 These mechanical properties result in a significant transmission of the force during movements. These studies and others have shown that both the active and passive mechanical properties of muscle are significantly influenced by the composition of the extracellular matrix. The changes in the isoforms of collagen expressed in a muscle would have significant consequences in its function. Almost all muscle pathologies are associated with some degree of fibrosis, and this transformation has been linked to the microenvironment within the muscle tissue.41 Based on these studies, it is clear that clinical examination of the passive range of motion and stiffness in skeletal muscles is actually a reflection of the connective tissue composition.
In addition to the role collagen and extracellular matrix molecular composition plays in determining muscle stiffness and mechanical properties, collagen and related extracellular matrix molecules also play an important role in the sequestration and release of a number of growth factors that control both connective tissue and muscle fiber cell signaling processes. Adaptations of skeletal muscle and its connective tissue components occur rapidly after changes in mechanical load or as a result of disease or injury.42,43 In fact, these changes in connective tissue can occur without affecting myofibrillar protein synthesis.44 Loading or unloading skeletal muscles results in changes in expression of a number of growth factors, including transforming growth factor-β1 45,46 and insulin-like growth factor I.47,48 These factors lead to modulation in collagen isoform expression.49,50 It is becoming increasingly evident that the connective tissue components within skeletal muscles are critically important in both disease and in their response to manipulation.
Few studies have been performed examining connective tissue composition within the extraocular muscles. An examination of the tendon of normal superior oblique muscles showed that they expressed collagens III, V, and VI, with local patches of collagen II.51 Relative to changes after surgical intervention, a study of scar remodeling in stretched scars after strabismus surgery demonstrated that the tendons contain collagen III.52 In another study, a “moderate amount” of connective tissue in the endomysial and perimysial compartments was noted.53
The aim of this study was to investigate whether different diagnostic groups display identifiably different connective tissue phenotypes in their inferior oblique muscles despite their apparent similarity in ocular misalignment patterns. Three collagen isoforms were chosen from the larger collagen superfamily of proteins to represent three of the major collagen types found in skeletal muscle (Kovanen, 200254; Gelse et al, 200355; Ricard-Blum and Ruggiero, 200556). Collagen I is in the group of fibril-forming collagens and is a predominant isoform in skeletal muscle. It is considered to provide tensile strength and rigidity to muscle. Collagen IV is the major basement membrane collagen in the body, and it forms the skeleton of the basement membranes in muscles. Collagen VI is a microfibrillar collagen and forms beaded filaments that are known to extend from the endomysium into myotendinous junctions. Collagen VI plays a role in maintaining muscle contractile strength (Irwin et al, 200357). In addition, elastin was also examined within the inferior oblique muscle specimens. Elastin is the main structural protein responsible for tissue resilience and extensibility, counteracting the stiffness that is provided by collagen. While there are other structural molecules within the extracellular matrix of skeletal muscle, understanding their expression in the inferior oblique muscles from normal and strabismic subjects has the potential to shed light on the different properties seen in these specimens.
Two hypotheses are tested. First, this thesis tests the hypothesis that the connective tissue phenotype of inferior oblique muscles differs between patients with overelevation in adduction and controls. The second hypothesis is that inferior oblique surgery alters connective tissue phenotype. Specific molecular components of the endomysial and perimysial connective tissue matrix were quantified in normal control inferior oblique muscles. These were compared with the connective tissue expression patterns in inferior oblique muscles from patients who had no prior surgery and from patients who had prior inferior oblique surgery. These muscles were also compared to inferior oblique muscles removed from patients with craniofacial dysostosis, including those who did and did not have prior inferior oblique muscle surgery.
MATERIALS AND METHODS
The Institutional Review Board (IRB) of the University of Texas Southwestern Medical Center approved collection of tissue samples under exempt status (approval date April 2009) and approved review of human subjects’ medical records in compliance with the Health Insurance Portability and Accountability Act (HIPAA) guidelines (under file No. 112005-027). This study adhered to the tenets of the Declaration of Helsinki.
Study specimens were collected from patients scheduled to undergo temporal or nasal myectomy of one or both inferior oblique muscles for primary or secondary overaction. Control specimens were obtained from patients at the time of enucleation for other diagnoses.
Inferior oblique muscle tissue was collected at the time of surgery. Four groups of inferior oblique specimens were collected: (1) 9 samples with primary inferior oblique overaction with no prior inferior oblique surgery (NPS) (Table 1); (2) 7 samples with recurrent inferior oblique overaction after previous surgery (Table 1); (3) 6 samples from patients with craniofacial dysostosis (Apert syndrome) and overelevation in adduction (2 patients who had previous surgery and 4 who were naïve to surgical intervention) (Table 2); and (4) 9 control samples removed by an ophthalmologist at the time of enucleation (Table 3).
TABLE 1.
DEMOGRAPHIC AND KEY CLINICAL CHARACTERISTICS OF THE 16 PATIENTS WITH PRIMARY INFERIOR OBLIQUE OVERACTION WHOSE INFERIOR OBLIQUE MUSCLE SPECIMENS WERE STUDIED
| SUBJECT | GENDER |
AGE AT SURGERY (YEARS) |
VISUAL ACUITY (OD, OS) |
IO OVERACTION & UNDERACTION* |
SPECIMEN COLLECTED (OD OR OS) |
PREVIOUS IO SURGERY |
|---|---|---|---|---|---|---|
| 1 | M | 2 | GCM, GCM | +2 LIO | OS | Recession |
| 2 | M | 3 | GCM, GCM | +2 LIO | OS | Recession |
| 3 | F | 5 | GCM, GCM | +2 LIO | OS | Recession |
| 4 | F | 6 | 20/25, 20/20 | +3 LIO | OD | Recession |
| 5 | M | 9 | 20/30, 20/30 | +2 RIO | OD | Recession |
| 6 | M | 9 | 20/20, 20/20 | +3 LIO | OD | Recession |
| 7 | M | 17 | 20/25, 20/25 | +2 RIO, +2 LIO | OD and OS | Myectomy |
| 8 | M | 4 | GCUM, GCM | +3 RIO | OD | NPS |
| 9 | F | 4 | GCM, GCM | +2 RIO, +2 LIO | OD and OS | NPS |
| 10 | M | 4 | 20/30, 20/70 | +4 RIO | OD | NPS |
| 11 | F | 4 | GCUM, GCM | +3 RIO, +2 LIO | OD and OS | NPS |
| 12 | M | 5 | 20/40, 20/50 | +3 RIO, +3 LIO | OD and OS | NPS |
| 13 | M | 6 | GCM, GCM | +3 LIO | OS | NPS |
| 14 | M | 7 | 20/40, 20/50 | +3LIO | OS | NPS† |
| 15 | M | 9 | 20/20, 20/20 | +2 LIO | OS | NPS† |
| 16 | M | 19 | 20/25, 20/25 | +3LIO | OS | NPS |
F, female; GCM, good, central, maintained; GCUM, good, central, unmaintained; IO, inferior oblique muscle; LIO, left inferior oblique muscle; M, male; NPS, no prior surgery; OD, right eye; OS, left eye; RIO, right inferior oblique muscle.
According to the Elliott and Parks grading scale.58
Two patients in the NPS group had previous IO surgery in the contralateral eye.
TABLE 2.
DEMOGRAPHIC AND KEY CLINICAL CHARACTERISTICS OF THE 6 PATIENTS WITH CRANIOFACIAL DYSOSTOSIS (APERT SYNDROME) WHOSE INFERIOR OBLIQUE MUSCLE SPECIMENS WERE STUDIED
| SUBJECT | GENDER |
AGE AT SURGERY (YEARS) |
VISUAL ACUITY (OD, OS) |
IO OVERACTION & UNDERACTION* |
SPECIMEN COLLECTED (OD OR OS) |
PREVIOUS IO SURGERY |
|---|---|---|---|---|---|---|
| 1 | F | 4 | GCM, GCM | N/A | OD and OS | Anterior nasal transposition |
| 2 | M | 8 | HM, 20/50 | +3RIO, +4LIO | OD and OS | Nasal myectomy (bilateral) |
| 3 | F | 2 | GCUM, GCM | +3 RIO, +3 LIO | OD and OS | NPS |
| 4 | F | 3 | GCM, GCM | +3RIO, +3LIO | OD | NPS |
| 5 | F | 5 | 20/40, 20/50 | +3LIO | OD | NPS |
| 6 | F | 7 | 20/25, 20/25 | +2RIO | OS | NPS |
F, female; GCM, good, central, maintained; GCUM, good, central, unmaintained; IO, inferior oblique muscle; LIO, left inferior oblique muscle; M, male; NPS, no prior surgery; OD, right eye; OS, left eye; RIO, right inferior oblique muscle.
According to the Elliott and Parks grading scale.58
TABLE 3.
DEMOGRAPHIC AND KEY CLINICAL CHARACTERISTICS OF THE 9 CONTROL SUBJECTS WHOSE INFERIOR OBLIQUE MUSCLE SPECIMENS WERE STUDIED
| SUBJECT | GENDER |
AGE AT SURGERY (YEARS) |
REASON FOR AVAILABILITY |
|---|---|---|---|
| 1 | M | 0.8 | Intraocular retinoblastoma |
| 2 | M | 2 | Intraocular retinoblastoma |
| 3 | M | 7 | Intraocular retinoblastoma |
| 4 | F | 7 | Cornea donation |
| 5 | F | 11 | Cornea donation |
| 6 | F | 41 | Cornea donation |
| 7 | M | 59 | Cornea donation |
| 8 | M | 74 | Cornea donation |
| 9 | F | 75 | Orbital exenteration |
F, female; M, male.
Mean patient ages were 6.9 years (standard deviation [SD], 4.9) for patients without prior surgery and 7.3 years (SD, 5.1) years for patients with previous surgery. In the craniofacial dysostosis group, the 2 patients who had previous surgery to the inferior oblique muscle had a mean age of 6 years (range, 4–8), and the 4 patients that were naïve to surgical intervention had a mean age of 4.4 years (SD, 2.1) (Table 2). The normal control group had a mean age of 25.2 years (SD, 28) and included 5 children and 4 adults. None of the control subjects had a history of strabismus, and none had undergone prior extraocular muscle surgery.
The severity of overelevation in adduction for each patient was quantified using the grading system outlined by Elliott and Parks58 (Tables 1 and 2).
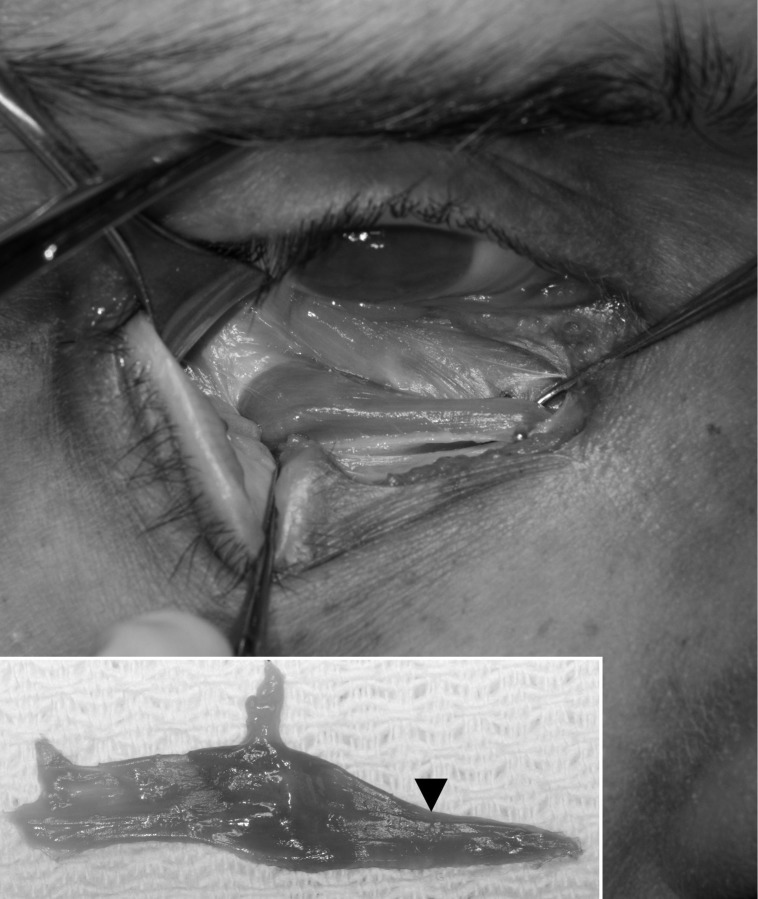
The 9 patients with previous inferior oblique surgery on the temporal portion of the muscle (7 from group 2 and 2 from group 3) underwent nasal myectomy19 of the inferior oblique muscle. This procedure was performed unilaterally in 6 cases and bilaterally in 3 cases (Table 1). For this surgical procedure,19 the patient was placed in the supine position under general anesthesia. An inferonasal cul-de-sac incision was made and carried down to the bare sclera. The inferior rectus muscle was isolated from its nasal border, the eye was rotated superiorly, and the inferior oblique muscle was identified. The insertion of the inferior oblique was exposed, and temporal traction was placed on the muscle while retracting its capsule nasally. The nasal segment of the inferior oblique was visualized nasal to the inferior rectus where the inferior oblique muscle narrows abruptly (Figure 1). The nasal portion was then isolated with 2 muscle hooks and stretched. Hemostats were placed across the muscle near the lateral border of the inferior rectus muscle and approximately 5 mm nasal to that point. The portion of the inferior oblique muscle between the 2 hemostats was excised. The muscle was cauterized near both hemostats; cauterization was particularly heavy on the side of the muscle nearest the neurofibrovascular junction. The distal segment of the muscle was repositioned posteriorly. Any opening in the Tenon capsule was sutured to prevent fat protrusion. The conjunctiva was closed, and a tobramycin and dexamethasone ophthalmic suspension solution (Alcon Laboratories, Inc, Fort Worth, Texas) was instilled.
FIGURE 1.
Gross anatomy of the inferior oblique muscle, which is isolated with a muscle hook. The inset shows characteristic narrowing of the nasal portion of the muscle.
The 13 patients who had no prior surgery to the inferior oblique underwent a standard temporal myectomy (9 from group 1 and 4 from group 3). This procedure was similar to the one described above, except that the temporal portion of the inferior oblique muscle (temporal to the neurofibrovascular junction) was isolated and a 4- or 5-mm section myectomized. This procedure was performed unilaterally in 9 cases and bilaterally in 4 cases (Tables 1 and 2).
Control inferior oblique muscles from 9 individuals were removed by an ophthalmologist at the time of enucleation due to intraocular retinoblastoma (n=3), orbital exenteration for a malignant neoplasm in the posterior aspect of the orbit (n=1), or enucleation for corneal donation (n=5; Table 3).
Four connective tissue molecules were examined: collagen I, collagen IV, collagen VI, and elastin. They were chosen to represent specific types of common connective tissue matrix molecules found within muscle. Collagen I is the major fibrillar collagen in the group of five fibril-forming collagens,56 providing tensile stiffness to tissues.55 Collagen IV is the major collagen of basement membranes.55 In contrast, collagen VI is a microfibrillar collagen that forms an independent microfibrillar network in virtually all connective tissue. It is found in the perimysium and in the endomysium just external to the collagen IV–containing basement membrane. Elastin is an extracellular matrix protein that provides strength and flexibility to connective tissue and was previously shown to be expressed in extraocular muscle.59
All muscles were chilled and embedded in tragacanth gum, snap-frozen in 2-methylbutane chilled to a slurry on liquid nitrogen, and stored at −80°C. The muscles were sectioned at 12 μm and processed for expression of collagen I, IV, and VI (1:1,000; Abcam, Cambridge, Massachusetts) and elastin (1:2,500; Sigma, St Louis, Missouri) immunohistochemically using the Vectastain Elite ABC Kit and colorized using diaminobenzidine with heavy metal intensification (Vector Laboratories, Burlingame, California).
DATA ANALYSIS
The sections were analyzed for percent of extracellular matrix molecule per muscle area using the Bioquant Image Analysis System (Bioquant, Nashville, Tennessee) and its autothresholding mode. Thresholds of staining intensity were used to determine all the elastin or collagen in square micrometers (μm2) compared to the nonstained regions and calculated as a percent. The data were examined statistically by analysis of variance (ANOVA) and Dunn’s multiple comparison tests using Prism software (Graphpad Software Inc, La Jolla, California) for multiple group comparisons. Results were considered statistically significantly different if P<.05.
RESULTS
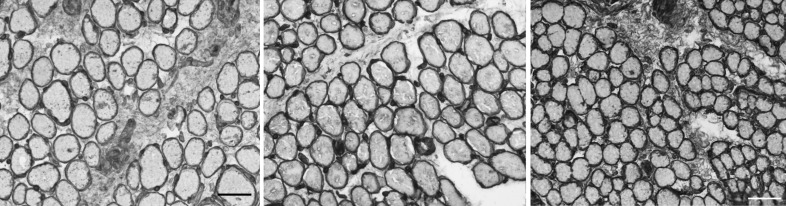
Analysis of the normal inferior oblique muscle pattern of collagen I expression shows that it is primarily located surrounding individual myofibers (left panel in Figure 2); however, a low level of collagen I was found in the perimysial regions of the muscle cross-sections. Collagen IV was located in the basement membrane region surrounding each individual myofiber. It was absent in the perimysial connective tissue in the control muscles (middle panel in Figure 2). Collagen VI appeared to be the densest of the collagen isoforms examined, with intense immunostaining surrounding individual myofibers and significant immunostaining in the perimysial (as shown in right panel in Figure 2) and epimysial (not shown) connective tissues. Each individual myofiber in the inferior oblique muscles is completely surrounded by a basement membrane and endomysium composed of multiple collagen isoforms.
FIGURE 2.
Immunohistochemical visualization of collagen in normal inferior oblique muscles from children. Collagen I in control subject 5 (left); collagen IV in control subject 3 (center); and collagen VI in control subject 3 (right) (stained for collagen I, IV, and VI; bar = 50 μm).
COLLAGEN I
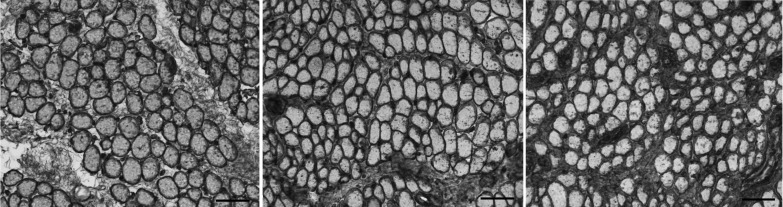
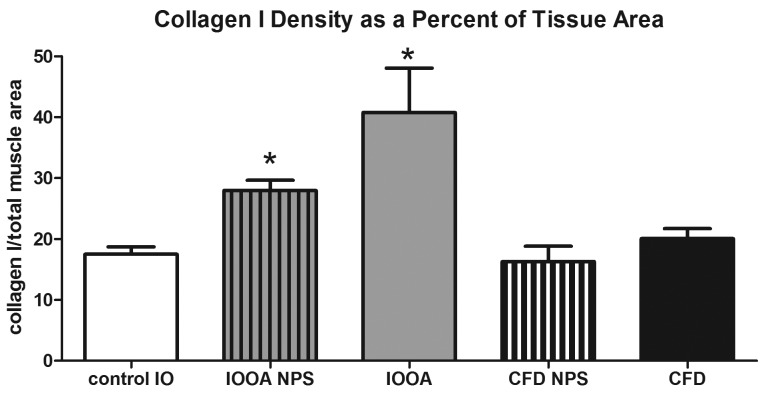
Collagen I immunostaining showed clear increases in the amount of this isoform in the inferior oblique muscles from patients with primary inferior oblique overaction, whether or not they had prior surgery (center and right panels in Figure 3), when compared to the control muscles (left panel in Figure 3). Collagen I appeared to be much more prominent in the perimysial connective tissue in the patients with primary inferior oblique overaction with and without prior surgery, with especially intense expression in the inferior oblique muscles on which prior strabismus surgery had been performed. The collagen I levels in the inferior oblique muscles from patients with no prior surgery were 28.0 (standard error ±1.7) (density units; percent of tissue area), a 37.5% mean increase (P<.0001) compared to the normal control muscles (17.5±1.2 percent of tissue area). Mean collagen I density in previously operated inferior oblique muscles was 40.8±7.3, over twice the density compared to normal control inferior oblique muscles (P=.004) (Figure 4). The muscles from both groups of patients with craniofacial dysostosis showed no difference in the collagen I density compared to the normal inferior oblique muscles (P=.93 and P=.14) (Figure 4).
FIGURE 3.
Expression of collagen I in inferior oblique muscles. Left, normal (control subject 3). Center, a patient who had no prior strabismus surgery (primary inferior oblique overaction subject 10). Right, a patient who had prior strabismus surgery (primary inferior oblique overaction subject 7) (stained for collagen I; bar = 50 μm).
FIGURE 4.
Mean density of collagen I in inferior oblique muscles. Data is expressed as percent expression of collagen I per muscle area and presented as the mean ±1 standard error of the mean (SEM). Asterisk (*) indicates significantly different from control values at P<.05. CFD, craniofacial dysostosis; IO, inferior oblique; IOOA, inferior oblique overaction; NPS, no prior surgery.
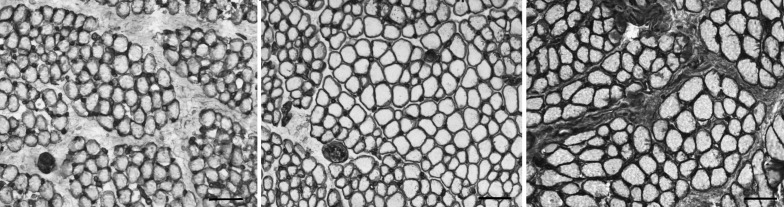
COLLAGEN IV
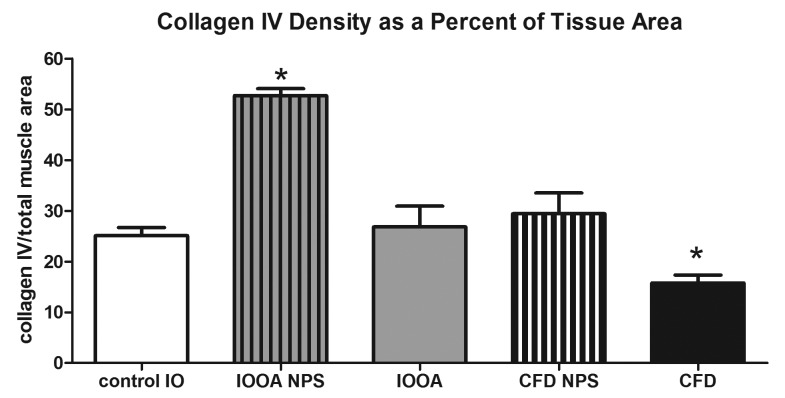
Immunostaining of the inferior oblique muscles naïve to surgery demonstrated more collagen IV around the individual myofibers. Collagen IV also was located in the perimysial connective tissue, as seen in Figure 5. When quantified, the collagen IV content in muscles from patients who had no prior inferior oblique surgery was increased to twice that in the control group (52.7±1.4 in patients with primary inferior oblique overaction, vs 25.1±1.6 in controls; P<.0001; Figure 6). In the inferior oblique muscles where the patients had previous surgery, the collagen IV levels were not different from control values (26.8±4.1; P=.70). The muscles from the patients who had craniofacial dysostosis and no prior surgery had similar levels of collagen IV to control muscles (Figure 6). In the muscles from the craniofacial dysostosis patients who had prior inferior oblique surgery, the collagen IV levels were reduced by approximately 37% compared to the naïve muscles and control muscles. Prior surgical intervention was associated with reduced collagen IV levels in those muscles regardless of the basal levels in the two groups seen in muscles from patients who had not been operated on previously.
FIGURE 5.
Expression of collagen IV in inferior oblique muscles. Left, normal (control subject 1). Center, a patient who had no prior strabismus surgery (primary inferior oblique overaction subject 10). Right, a patient who had prior strabismus surgery (primary inferior oblique overaction subject 8) (stained for collagen IV; bar = 50 μm).
FIGURE 6.
Mean density of collagen IV in inferior oblique muscles. Data is expressed as percent expression of collagen IV per muscle area and presented as the mean ±1 standard error of the mean (SEM). Asterisk (*) indicates significantly different from control values at P<.05. CFD, craniofacial dysostosis; IO, inferior oblique; IOOA, inferior oblique overaction; NPS, no prior surgery.
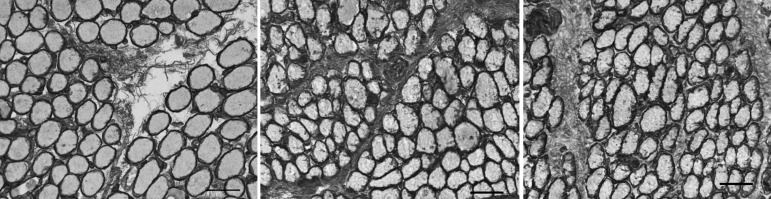
COLLAGEN VI
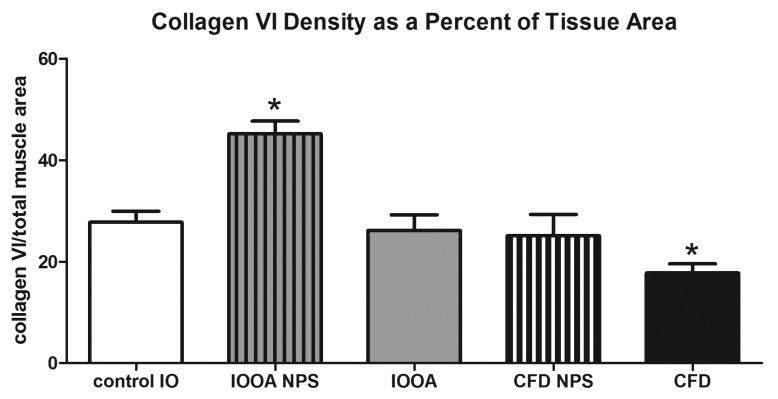
Collagen VI immunostaining showed a similar pattern to that seen in immunostaining for collagen IV (Figure 7). In the muscles from patients without previous inferior oblique surgery, collagen VI levels were 45.2±2.5, approximately 38% more dense than control muscles (27.8±2.1; P<.0001; Figure 8). Those muscles from patients who had previous surgery were not different than controls (P=.44). Examination of the muscles from the craniofacial dysostosis patients also showed a similar trend to that seen with collagen IV. The muscles from patients who had no prior surgery (25.1±4.2) were no different than controls (P=.27). But, in the muscles from patients who had prior surgery, levels of collagen VI were significantly less than control muscles with a density of collagen VI 36% less at 17.8±1.8 (P=.015), as summarized in Figure 8. A number of patients had asymmetric overelevation. Collagen VI levels in the inferior oblique muscle from the side with the larger deviation were significantly greater than those in control muscles and also greater than levels from the side with the smaller deviation (Figure 9). The collagen VI density was also compared in muscles where eye misalignments of the two eyes were similar, and no differences were seen.
FIGURE 7.
Expression of collagen VI in inferior oblique muscles. Left, normal (control subject 5). Center, a patient who had no prior strabismus surgery (primary inferior oblique overaction subject 11). Right, a patient who had prior strabismus surgery (primary inferior oblique overaction subject 5). (stained for collagen VI; bar = 50 μm).
FIGURE 8.
Mean density of collagen VI in inferior oblique muscles. Data is expressed as percent expression of collagen VI per muscle area and presented as the mean ±1 standard error of the mean (SEM). Asterisk (*) indicates significantly different from control values at P<.05. CFD, craniofacial dysostosis; IO, inferior oblique; IOOA, inferior oblique overaction; NPS, no prior surgery.
FIGURE 9.
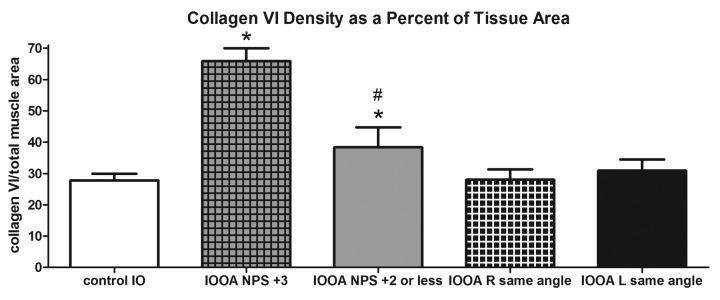
Mean density of collagen VI in inferior oblique muscles. Results are compared from controls, patients whose angle of misalignment varied in their two eyes, and those where similar angles of misalignment were seen. Data is expressed as percent expression of collagen VI per muscle area and presented as the mean ±1 standard error of the mean (SEM). Asterisk (*) indicates significantly different from control values at P<.05; # sign indicates significantly different from contralateral side at P<.05. IO, inferior oblique; IOOA, inferior oblique overaction; NPS, no prior surgery.
ELASTIN
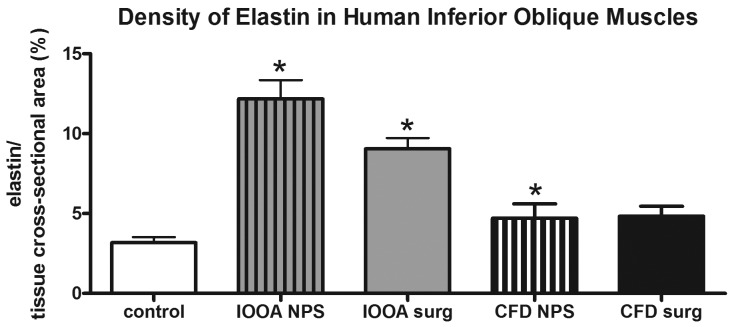
Elastin was found in and around individual myofibers in the control inferior oblique muscles (Figure 10). It ran in both longitudinal and circumferential directions relative to myofiber length. As sections moved progressively toward the muscle that had been in the more nasal position (approaching the origin at the nasal lacrimal crest), increased elastin density was seen (not shown). In both groups of muscles from the patients with primary inferior oblique overaction, elastin levels were significantly elevated compared to control muscles (3.18±0.35), with those with no prior surgery showing a density of 12.2±1.2 (P<.0001) and those with prior surgery showing a density of 9.05±0.68 (P<.0001) (Figure 11). Patients with craniofacial dysostosis also showed increased elastin density compared to the normal control muscles, at 4.7±0.9 for those with no prior surgery (P=.03) and 4.8±0.62 for those with prior surgery.
FIGURE 10.
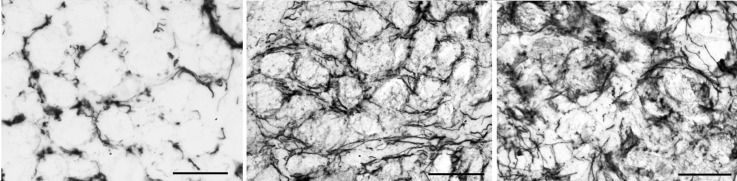
Expression of elastin in inferior oblique muscles. Left, normal (control subject 5). Center, a patient who had no prior strabismus surgery (primary inferior oblique overaction subject 12). Right, a patient who had prior strabismus surgery (primary inferior oblique overaction subject 3) (stained for elastin; bar = 20 μm).
FIGURE 11.
Mean density of elastin in inferior oblique muscles. Data is expressed as percent expression of collagen VI per muscle area and presented as the mean ±1 standard error of the mean (SEM). Asterisk (*) indicates significantly different from control values at P<.05. CFD, craniofacial dysostosis; IO, inferior oblique; IOOA, inferior oblique overaction; NPS, no prior surgery.
DISCUSSION
What does it mean when an extraocular muscle is slack or tight or stiff? While active contraction of the muscle as controlled by the corresponding motor neurons is responsible for the ultimate position of the eye, passive properties and resistance to any given movement are specifically related to the connective tissue components within the muscle. While many studies have focused on the extraocular muscles themselves, little is known about the connective tissues in normal extraocular muscles, and less is known about potential differences in muscles from patients with strabismus.
Several important conclusions can be made from the data presented here. First, significant increases were observed in the levels of the 4 connective tissue molecules—collagens I, IV, and VI, and elastin—within the inferior oblique muscles of the patients in the diagnostic group consisting of primary inferior oblique overaction without prior strabismus surgery when compared to the normal control muscles. These results are consistent with clinical reports of stiff inferior oblique muscles in this condition.1,60 Second, the density of collagens IV and VI was decreased in those muscles that had prior inferior oblique muscle surgery and was similar to control levels of these connective tissue elements despite the fact that the patients still had overelevation in adduction sufficient to require a second surgery. This finding suggests that collagen and elastin levels are not the sole factor responsible for inferior oblique overaction. Third, the density of collagen I was significantly increased in the inferior oblique muscles that had been subjected to prior surgery to treat the patients’ overelevation in adduction. This finding indicates that each of these connective tissue components is individually controlled in the extraocular muscles. Fourth, the patients in the craniofacial dysostosis diagnostic group did not have overexpression of elastin or any of the 3 collagens examined in this study in either the naïve or previously operated muscle. Thus, a similar motility disturbance, overelevation in adduction, does not equal a similar phenotype relative to the type I, IV, and VI collagens and elastin levels examined in this study. In Apert syndrome (craniofacial dysostosis), the overelevation is likely to result from the rotation of the orbits. Further research will be necessary to determine if other strabismus diagnoses featuring overelevation in adduction (e.g., superior oblique palsy) result in phenotypical similarities at the connective tissue level. Finally, while collagen IV and VI levels were similar to controls in the naïve muscles of the craniofacial dysostosis cohort, prior surgery was associated with a reduction of these levels as was observed in the cohort of inferior oblique overaction patients who had surgery.
Examination of control inferior oblique muscles showed that these muscles express collagens I, IV, and VI in overlapping locations. Collagens I and VI were found in both the basement membrane and perimysial locations in the normal inferior oblique muscles, and collagen IV was located in the basement membrane region around individual myofibers. Collagen I is the major fibrillar collagen in the group of five fibril-forming collagens,58 providing tensile stiffness to tissues.56 Collagen IV is the collagen of basement membranes, playing an important structural role integrating laminins, nidogens, and other components of the basement membrane.56 Collagen VI is a microfibrillar collagen that forms an independent microfibrillar network in virtually all connective tissue. It is found in the endomysium just external to the collagen IV–containing basement membrane and in the perimysium.61 It plays an important role anchoring the basement membrane to the sarcolemma of the muscle fibers. Collagen VI is highly expressed in skeletal muscle,62 where it plays a role in tissue remodeling and fibrosis in diseases such as Duchenne muscular dystrophy.63 Its mutation results in myopathy.64
The increased collagen in the cohort of patients with primary inferior oblique overaction without prior inferior oblique muscle surgery would result in muscles that are stiffer than control muscles. It is unclear whether this is a primary effect or is secondary to overexpression of fibrogenic factors such as transforming growth factor-β by the muscle cells, fibroblasts, or nerves. It could also be compensatory due to some other yet-to-be-discovered factor. The sequelae in terms of inferior oblique muscle function, however, are clear. There is increased innervation in the inferior oblique muscles of these patients (McLoon LK, et al. IOVS 2013;54:ARVO E-Abstract 1303). This, in turn, could result in increased release of growth factors into the muscle from the nerves or increased release from the muscle fibers into the connective tissue that would increase fibrogenesis. Fibrogenic factors could include, for example, IGF-147 or TGF-β1.65 Studies are ongoing to assess the levels of these fibrogenic factors in extraocular muscles. In studies of repeated muscle strain, where all other aspects of muscle and nerve function were normal, fibrosis occurs.66 It is also known that mechanical load, as when a muscle contracts with increased force, results in release of these factors with resultant increases in collagen synthesis.44,47
Unloading skeletal muscle can have the opposite effect on collagen density, specifically collagen IV.50 The surgery for treating primary inferior oblique overaction unloads the muscle. Thus, it is interesting that prior surgery in diagnostic groups 2 and 3 resulted in decreased density of collagen types IV and VI and elastin. This finding would be expected based on the published literature. It is also interesting, however, that collagen I levels were significantly increased in the muscles from the patients who had prior surgery to treat their overaction compared to the levels in the muscles from the nonsurgical group. It is known that the factors that control transcription vary for the different collagen isoforms56 and the enzymes that remodel the extracellular matrix, such as metalloproteinases, are collagen isoform specific.67
In the patients with primary inferior oblique overaction, elastin levels were markedly elevated over control levels. Elastin is an extracellular matrix protein that provides strength and flexibility to connective tissue. Previous work examined elastin expression in cow extraocular muscle and showed that elastin is located around all extrafusal fibers, with some gaps in staining, and extended into the perimysium and epimysium.55 Examination of both cross-sections and longitudinally oriented sections showed that the elastin courses along the myofiber length and also circumferentially around individual myofibers. This type of network is not normally described in skeletal muscle. While increased collagen levels would make the patient muscles “stiffer,” increased elastin would make the patient muscle “tighter.” All patient muscles had elevated levels of elastin in their connective tissue matrices compared to control values. This finding suggests that these muscles would be “tighter” in forced duction tests. As with the other connective tissue elements, surgery in the patients with primary inferior oblique overaction resulted in a decrease of elastin density, which suggests that alteration of the muscle length and tension by the surgery modulates the connective tissue matrices of these muscles in a manner that makes them move toward normalcy.
There were significant differences in the connective tissue changes seen in the muscles from the primary inferior oblique overaction diagnostic group compared to those with craniofacial dysostosis, where no collagen up-regulation over control levels was seen. It is surprising that collagen levels are no different than control levels because one common cause of the syndrome is a mutation in the fibroblast growth factor receptor 2 gene, resulting in decreases in glycosaminoglycan and fibronectin content and increases in collagen I content.68 However, the transcriptional control of the various extracellular matrix molecules is clearly tissue specific. Thus, the effects in the extraocular muscles are not necessarily predicted by the effects on bone or cartilage precursor cells. Additionally, despite known orbital dystopia associated with the syndrome, the types of ocular motility disturbances found in these patients suggest that mechanical factors are not the sole cause of their strabismus.69 It is important to stress that a similar phenotype, overelevation in adduction, does not mean similar changes within the connective tissue elements within these muscles. It is also important to stress, however, that the same types of changes that occur to collagen isoforms after surgery in the primary inferior oblique diagnostic group also are seen, albeit to a more moderate extent, in the muscles from the craniofacial dysostosis diagnostic group. Although only 2 inferior oblique muscles were available for analysis in the subgroup of craniofacial dysostosis with prior surgery, both showed differences in collagen I, IV, and VI levels that paralleled the differences seen between the patients with primary inferior oblique overaction with and without prior surgery.
CONCLUSIONS
The normal density of connective tissues found within extraocular muscles in control inferior oblique muscles is significantly altered in the muscles of patients with primary inferior oblique overaction naïve to surgery. This finding supports the role of increased muscle stiffness as a factor in their strabismus. In contrast, in patients with craniofacial dysostosis, overelevation in adduction was not found to be associated with altered levels of connective tissue elements. In these patients, the overelevation is likely a direct result of abnormal muscle vectors due to the rotation of the orbits. Prior surgery on inferior oblique muscles of patients with overelevation in adduction is associated with a decreased density of collagen IV and VI. For collagen I, an increase is observed. Thus, individual isoforms of collagen are individually regulated in the muscles of these patients.
It has long been observed that muscles differ in their stiffness and tightness. Knowing what is happening within the connective tissue elements within these muscles suggests new approaches for treating strabismus. Future treatment modalities could be directed at the transcriptional control of overexpression of these molecules. This thesis represents the first systematic examination of the connective tissue milieu of normal extraocular muscles and muscles obtained from two specific diagnostic groups. These results are also the first to detail how strabismus surgery alters the content of these molecules.
Acknowledgments
Funding/Support: Supported in part by NIH grant EY015313 (L.K.M.), the Minnesota Lions and Lionesses (L.K.M.), and a grant from Research to Prevent Blindness to the Department of Ophthalmology at the University of Minnesota.
Financial Disclosures: Neither the candidate nor any of the coauthors have any financial disclosures other than the funding listed above.
Author Contributions: Design and conduct of the study (D.S., L.M., J.F.); Collection, management, analysis, and interpretation of the data (D.S., L.M., J.F.); Preparation, review, and approval of the manuscript (D.S., L.M., J.F.).
Other Acknowledgments: I would like to express my sincere gratitude to the following individuals: Jeff Stager (photography of cadaver specimens), David Stager Sr, MD (specimen collection), James Merritt, MD (specimen collection), David Weakley, MD, and Edward Wilson, MD.
REFERENCES
- 1.Kushner BJ. Multiple mechanisms of extraocular muscle “overaction”. Arch Ophthalmol. 2006;124(5):680–688. doi: 10.1001/archopht.124.5.680. [DOI] [PubMed] [Google Scholar]
- 2.Kushner BJ. Incomitant strabismus: Does extraocular muscle form denote function? Arch Ophthalmol. 2010;128(12):1604–1609. doi: 10.1001/archophthalmol.2010.301. [DOI] [PubMed] [Google Scholar]
- 3.Von Noorden GK. Atlas of Strabismus. 4th ed. StLouis: CV Mosby Co; 1983. [Google Scholar]
- 4.Hertle RW, National Eye Institute Sponsored Classification of Eye Movement Abnormalities and Strabismus Working Group A next step in naming and classification of eye movement disorders and strabismus. J AAPOS. 2002;6(4):201–202. doi: 10.1067/mpa.2002.126491. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Ophthalmology . Pediatric Ophthalmology and Strabismus. San Francisco: American Academy of Ophthalmology; 2006. [Google Scholar]
- 6.Wilson ME, Parks MM. Primary inferior oblique overaction in congenital esotropia, accommodative esotropia, and intermittent exotropia. Ophthalmology. 1989;96(7):950–955. doi: 10.1016/s0161-6420(89)32774-6. [DOI] [PubMed] [Google Scholar]
- 7.Tollefson MM, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood hypertropia. Ophthalmology. 2006;113(7):1142–1145. doi: 10.1016/j.ophtha.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 8.Isenberg SJ, Apt L. Inferior oblique weakening procedures: technique and indications. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Surgical Techniques. 1st ed. Philadelphia: WB Saunders; 1999. pp. 449–458. [Google Scholar]
- 9.Stager DR, Jr, Beauchamp GR, Wright WW, Felius J, Stager D., Sr Anterior and nasal transposition of the inferior oblique muscles. J AAPOS. 2003;7(3):167–173. doi: 10.1016/s1091-8531(03)00003-x. Erratum in J AAPOS 2003;7(6):450. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers JD. Strabismus in craniofacial dysostosis. Graefes Arch Clin Exp Ophthalmol. 1988;226(3):230–234. doi: 10.1007/BF02181187. [DOI] [PubMed] [Google Scholar]
- 11.Cuttone JM, Brazis PT, Miller MT, Folk ER. Absence of the superior rectus muscle in Apert’s syndrome. J Pediatr Ophthalmol Strabismus. 1979;16(6):349–354. doi: 10.3928/0191-3913-19791101-04. [DOI] [PubMed] [Google Scholar]
- 12.Hussein MA, Stager DR, Sr, Beauchamp GR, Stager DR, Jr, Felius J. Anterior and nasal transposition of the inferior oblique muscles in patients with missing superior oblique tendons. J AAPOS. 2007;11(1):29–33. doi: 10.1016/j.jaapos.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Apt L, Call NB. Inferior oblique muscle recession. Am J Ophthalmol. 1978;85(1):95–100. doi: 10.1016/s0002-9394(14)76672-3. [DOI] [PubMed] [Google Scholar]
- 14.Elliott RL, Nankin SJ. Anterior transposition of the inferior oblique. J Pediatr Ophthalmol Strabismus. 1981;18(3):35–38. doi: 10.3928/0191-3913-19810501-08. [DOI] [PubMed] [Google Scholar]
- 15.Gobin MH. Anteroposition of the inferior oblique muscle in V-esotropia. Ophthalmologica. 1965;149:138–141. doi: 10.1159/000304744. [DOI] [PubMed] [Google Scholar]
- 16.Stager DR, Sr, Beauchamp GR, Stager DR., Jr Anterior and nasal transposition of the inferior oblique muscle: a preliminary case report on a new procedure. Binocul Vis Strabismus Q. 2001;16(1):43–44. [PubMed] [Google Scholar]
- 17.Coats DK, Olitsky SE. Strabismus Surgery and its Complications. 1st ed. Berlin: Springer; 2007. [Google Scholar]
- 18.Helveston EM. Surgical Management of Strabismus. 5th ed. Oostende, Belgium: JP Wayenborgh; 2005. [Google Scholar]
- 19.Stager DR, Jr, Wang X, Stager DR, Sr, Beauchamp GR, Felius J. Nasal myectomy of the inferior oblique muscles for recurrent elevation in adduction. J AAPOS. 2004;8(5):462–465. doi: 10.1016/j.jaapos.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Ela-Dalman N, Velez FG, Felius J, Stager DR, Sr, Rosenbaum AL. Inferior oblique muscle fixation to the orbital wall: a profound weakening procedure. J AAPOS. 2007;11(1):17–22. doi: 10.1016/j.jaapos.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Mims JL, 3rd, Wood RC. Bilateral anterior transposition of the inferior obliques. Arch Ophthalmol. 1989;107(1):41–44. doi: 10.1001/archopht.1989.01070010043024. [DOI] [PubMed] [Google Scholar]
- 22.Parks MM. The weakening surgical procedures for eliminating overaction of the inferior oblique muscle. Am J Ophthalmol. 1972;73(1):107–122. doi: 10.1016/0002-9394(72)90313-3. [DOI] [PubMed] [Google Scholar]
- 23.Ziffer AJ, Isenberg SJ, Elliott RL, Apt L. The effect of anterior transposition of the inferior oblique muscle. Am J Ophthalmol. 1993;116(2):224–227. doi: 10.1016/s0002-9394(14)71290-5. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen SP. Strabismus surgery: What’s next? J AAPOS. 2009;13(3):227–228. doi: 10.1016/j.jaapos.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Antunes-Foschini RM, Ramalho FS, Ramalho LN, Bicas HE. Increased frequency of activated satellite cells in overacting inferior oblique muscles from humans. Invest Ophthalmol Vis Sci. 2006;47(8):3360–3365. doi: 10.1167/iovs.05-0798. [DOI] [PubMed] [Google Scholar]
- 26.McLoon LK, Wirtschafter J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci. 2003;44(5):1927–1932. doi: 10.1167/iovs.02-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve. 2002;25(3):348–358. doi: 10.1002/mus.10056. [DOI] [PubMed] [Google Scholar]
- 28.McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29(5):707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLoon LK, Wirtschafter J. Activated satellite cells are present in uninjured extraocular muscles of mature mice. Trans Am Ophthalmol Soc. 2002;100:119–123. [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47(6):2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christiansen SP, McLoon LK. The effect of resection on satellite cell activity in rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2006;47(2):605–613. doi: 10.1167/iovs.05-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison AR, Lee MS, McLoon LK. Effects of elevated thyroid hormone on adult rabbit extraocular muscles. Invest Ophthalmol Vis Sci. 2010;51(1):183–191. doi: 10.1167/iovs.09-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ugalde I, Christiansen SP, McLoon LK. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest Ophthalmol Vis Sci. 2005;46(11):4114–4120. doi: 10.1167/iovs.05-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antunes-Foschini RS, Miyashita D, Bicas HE, McLoon LK. Activated satellite cells in medial rectus muscles of patients with strabismus. Invest Ophthalmol Vis Sci. 2008;49(1):215–220. doi: 10.1167/iovs.07-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souza-Dias CR. The intimate nature of oculomotor muscles contracture. Arq Bras Oftalmol. 2010;73(2):204–208. doi: 10.1590/s0004-27492010000200022. [DOI] [PubMed] [Google Scholar]
- 36.Schleip R, Naylor IL, Ursu D, et al. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med Hypotheses. 2006;66(1):66–71. doi: 10.1016/j.mehy.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Meijer HJ, Baan GC, Huijing PA. Myofascial force transmission is increasingly important at lower forces: firing frequency-related length-force characteristics of rat extensor digitorum longus. Acta Physiol (Oxford) 2006;186(3):185–195. doi: 10.1111/j.1748-1716.2006.01528.x. [DOI] [PubMed] [Google Scholar]
- 38.Dansfield E. Intramuscular composition and texture of beef muscles. J Sci Food Agr. 1977;28:833–842. [Google Scholar]
- 39.Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations in muscle length. J Muscle Res Cell Motil. 1994;15(3):229–308. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- 40.Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J Struct Biol. 2007;159(1):19–28. doi: 10.1016/j.jsb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Mann CJ, Perdiguero E, Kharraz Y, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1):1–21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams PE, Goldspink G. Connective tissue changes in surgically overloaded muscle. Cell Tissue Res. 1981;221(2):465–470. doi: 10.1007/BF00216749. [DOI] [PubMed] [Google Scholar]
- 43.Williams PE, Goldspink G. Connective tissue changes in immobilized muscle. J Anat. 1984;138(Pt 2):343–350. [PMC free article] [PubMed] [Google Scholar]
- 44.Doessing S, Heinemeier KM, Holm L, et al. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010;588(Pt 2):341–351. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirose T, Nakazato K, Song H, Ishii N. TGF-β1 and TNF-α are involved in the transcription of type I collagen α2 gene in soleus muscle atrophied by mechanical unloading. J Appl Physiol. 2008;104(1):170–177. doi: 10.1152/japplphysiol.00463.2006. [DOI] [PubMed] [Google Scholar]
- 46.Heinemeier KM, Olesen JL, Haddad F, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582(Pt 3):1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olesen JL, Heinemeier KM, Haddad F, et al. Expression of insulin-like growth factors I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol. 2006;101(1):183–188. doi: 10.1152/japplphysiol.00636.2005. [DOI] [PubMed] [Google Scholar]
- 48.Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol. 2009;106(1):178–186. doi: 10.1152/japplphysiol.91092.2008. [DOI] [PubMed] [Google Scholar]
- 49.Miller TA, Lesniewski LA, Muller-Delp JM, Majors AK, Scalise D, Delp MD. Hindlimb unloading induces a collagen isoform shift in the soleus muscle of the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1710–R1717. doi: 10.1152/ajpregu.2001.281.5.R1710. [DOI] [PubMed] [Google Scholar]
- 50.Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48H immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol. 2006;101(4):1136–1148. doi: 10.1152/japplphysiol.00180.2006. [DOI] [PubMed] [Google Scholar]
- 51.Milz S, Regner F, Putz R, Benjamin M. Expression of a wide range of extracellular matrix molecules in the tendon and trochlea of the human superior oblique muscle. Invest Ophthalmol Vis Sci. 2002;43(5):1330–1334. [PubMed] [Google Scholar]
- 52.Ludwig IH. Scar remodeling after strabismus surgery. Trans Am Ophthalmol Soc. 1999;97:583–651. [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez AJ, Biglan AW, Hiles DA. Structural features of extraocular muscles of children with strabismus. Arch Ophthalmol. 1980;98(3):533–539. doi: 10.1001/archopht.1980.01020030529020. [DOI] [PubMed] [Google Scholar]
- 54.Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev. 2002;30(1):20–25. doi: 10.1097/00003677-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Gelse K, Poschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53(7):430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Irwin WA, Bergamin N, Sabatelli P, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nature Genet. 2003;35(4):367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 58.Elliott RL, Parks MM. A comparison of inferior oblique muscle weakening by anterior transposition or denervation-extirpation. Binocular Vis Eye Muscle Surg Q. 1992;7(4):205–210. [Google Scholar]
- 59.Maier A, McDaniels CN, Mayne R. Fibrillin elastin networks in extrafusal tissue and muscle spindles of bovine extraocular muscles. Invest Ophthalmol Vis Sci. 1994;35(7):3103–3110. [PubMed] [Google Scholar]
- 60.Guyton DL. Exaggerated traction test for the oblique muscles. Ophthalmology. 1981;88(10):1035–1040. doi: 10.1016/s0161-6420(81)80033-4. [DOI] [PubMed] [Google Scholar]
- 61.Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272(42):26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- 62.Gara SK, Grumati P, Squarzoni S, et al. Differential and restricted expression of novel collagen VI chains in mouse. Matrix Biol. 2011;30(4):248–257. doi: 10.1016/j.matbio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Sabatelli P, Gualandi F, Gara SK, et al. Expression of collagen VI a5 and a6 chains in human muscle and in Duchenne muscular dystrophy–related muscle fibrosis. Matrix Biol. 2012;31(3):187–196. doi: 10.1016/j.matbio.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42(9):673–685. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao CG, He XJ, Li HP, Kang AJ. Increased expression of collagens, transforming growth factor-β1, and β3 in gluteal muscle contracture. Musculoskel Disord. 2010 Jan 25;11:15–22. doi: 10.1186/1471-2474-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stauber WT, Knack KK, Miller GR, Grimmett JG. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve. 1996;19(4):423–430. doi: 10.1002/mus.880190402. [DOI] [PubMed] [Google Scholar]
- 67.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baroni T, Lilli C, Marinucci L, et al. Crouzon’s syndrome: differential in vitro secretion of bFGF, TGFb isoforms and extracellular matrix macromolecules in patients with FGFR2 gene mutation. Cytokine. 2002;19(2):94–101. doi: 10.1006/cyto.2002.0877. [DOI] [PubMed] [Google Scholar]
- 69.Kreiborg S, Cohen MM. Ocular manifestations of Apert and Crouzon syndromes: qualitative and quantitative findings. J Craniofac Surg. 2010;21(5):1354–1357. doi: 10.1097/SCS.0b013e3181ef2b53. [DOI] [PubMed] [Google Scholar]