Summary
Differences in the lateralization of language processes between healthy subjects and patients with neurological complaints other than epilepsy have been less documented than those between healthy subjects and epilepsy patients. Moreover, the contribution of factors such as the location and type of lesion in determining interhemispheric shift of language function is poorly understood.
Sixty-seven patients who underwent presurgical evaluations at the Medical Imaging Center of the Imam Khomeini University Hospital, Tehran, and the same number of healthy controls, were recruited. The laterality index (LI) of language activation, calculated from two separate functional magnetic resonance imaging tasks, was compared between the patients and the age-/gender-/handedness-matched controls.
Chi square testing showed that the percentages of subjects with “typical” and “atypical” language dominance in the patient group were significantly different from the percentages recorded in the matched healthy controls for both tasks (p<0.005). Lesion type, lesion location, lesion hemisphere, presenting symptom and patient gender had no statistically significant effect on the hemispheric LI (p>0.05). In a logistic regression model including all potential determinants of atypical LI, age emerged as the only independent predictor (p<0.05, odds ratio=0.9).
Abnormal language lateralization is found in patients with a variety of cerebral lesions and with a diversity of clinical manifestations. In our selected population, symptom duration, lesion hemisphere and anatomical site of the lesion were not found to impact significantly on the development of an abnormal LI while patient age can independently predict the presence of an atypical LI.
Keywords: brain lesion , functional magnetic resonance imaging , language lateralization
Introduction
The localization and lateralization of language processing in the healthy and diseased brain has become an interesting and important area of clinical neuroscience research. The “classical model” of language organization, which was proposed based on lesion studies of the brain, although it has been extensively revised, is still popular ( Yuan et al., 2006).
It has been disclosed that injury to the left hemisphere in early childhood often results in a shift of language functions to the opposite hemisphere ( Saltzman et al., 2002; Rathore et al., 2009; Rasmussen and Milner, 1977; Müller et al.,1999 ; Duchowny et al., 1996). Whereas studies in children have focused on a variety of brain lesions, most of the reports in the adult population have focused on epilepsy patients. Epilepsy, being one of the common neurological disorders that can have a far-reaching and prolonged impact on language function, has been the focus of interest in many investigations ( Rathore et al., 2009; Rasmussen and Milner, 1977; Duchowny et al., 1996; Rausch and Walsh, 1984; Helmstaedter et al., 1997; Springer et al., 1999; Brázdil et al., 2003; Woods et al., 1988). These studies provide evidence that lesions of speech centers may induce atypical speech lateralization. Different studies have shown that injury affecting the left hemisphere results in an interhemispheric shift of language function to the right hemisphere ( Rasmussen and Milner, 1977; Hécaen, 1976). However, these reports do not allow adequate investigation and separation of the different factors potentially responsible for abnormal language organization (i.e. age, localization and lateralization of the lesion, and seizure as a trigger).
In fact, in contrast to the wealth of studies on epilepsy in temporal and hippocampal pathologies, the differences in the laterality of language processes between healthy subjects and patients with complaints other than epilepsy have been less extensively investigated. The current functional neuroimaging literature on the impact of different brain lesions (including tumor, previous brain insults, vascular malformations, brain atrophy, etc.) on language lateralization has either been clinical research that focused on evaluating the accuracy of presurgical and postsurgical functional magnetic resonance imaging (fMRI) assessment, or studies comparing language organization patterns among epilepsy patients ( Hertz-Pannier et al., 2002; Liegeois et al., 2002 , 2004 ; Hertz-Pannier et al., 1997).To date, no fMRI study has provided quantitative comparisons, in terms of language dominance patterns, between patients with various pathologies/symptoms and healthy subjects.
Hence, the contribution of factors such as the location and type of lesion in determining interhemispheric shift of language function is poorly understood.
These poorly investigated factors could have implications for language function from both theoretical and clinical perspectives. It can be hypothesized that a collective study of a wide range of brain pathologies and presenting signs may help us to understand whether language is more impaired after lesions to the left rather than the right hemisphere and the degree to which language is left-lateralized in patients versus the normal population. From the clinical standpoint, establishing a possible role of presenting symptoms other than seizures in changing normal language lateralization would have important implications for predicting how a neurosurgical intervention will affect a patient’s post-operative language abilities.
The present study addressed whether a patient’s age, sex, lesion type, lesion site, or presenting symptoms are related to the development of typical speech organization. The novelty of our study is that, by evaluating different brain pathologies, we were able to investigate the effect of any kind of brain insult on speech organization. We also hypothesized that this effect is independent of the anatomical site of brain injury and the presenting symptom of the patient.
To test our hypotheses, we measured and compared the laterality indices of a large and heterogeneous population of patients with those of gender-/age-/handedness-matched healthy controls. Moreover, unlike previous studies, by utilizing two different language tasks simultaneously and by mapping multiple language functions, we tried to provide a more comprehensive view of language laterality.
These comparisons may offer new insights into the symptom-independent nature of language plasticity and show the extent to which the brain re-structures to compensate for damage associated with any kind of brain insult. Moreover, as many patients with new-onset seizures will ultimately be diagnosed with brain tumor, it is important to identify whether or not rapidly growing lesions (such as tumors) are capable of changing the language network in a short time interval.
Materials and methods
Subjects
This study included national fMRI presurgical planning data. Indeed, our institution is the main imaging center in Iran that evaluates patients for neurosurgical planning. Neurosurgeons and neurologists throughout the country refer potential candidates for surgical interventions to our research center as part of their presurgical battery of evaluations.
Between June 2008 and February 2012, 67 consecutive patients who underwent presurgical evaluations at the Imaging Center of the Imam Khomeini University Hospital, Tehran, were recruited irrespective of their presenting symptom, gender, age, lesion type and location, and handedness. Fifty-three left hemisphere-injured patients, 9 right hemisphere-injured patients, five patients with abnormal brain MRI, and 67 age-/gender-/handedness-matched healthy controls participated in the study. All of the participants were right-handed, monolingual and native Farsi speakers. The normal subjects had no prior history of neurological or psychiatric conditions. The patients suffered at least one of the following symptoms: seizures, raised intracranial pressure symptoms (headache, nausea, vomiting or diplopia), paresis or paresthesia. In patients with more than one symptom, the chief complaint was taken as the presenting symptom. Brain lesions were verified by CT or MR imaging. Patients with bilateral brain lesions and those with dementing illness were excluded from the study to avoid confounding variables.
The patients ranged in age from 11 to 65 years (mean 31.9); the time since onset of their first symptom ranged from 1 to 37 years (mean 4.76), and their education from 1 to 21 years (mean 11.3). Forty-six patients were female while the rest were male. Types of brain lesion in the patients were defined according to their T1- and T2-weighted brain MRI. Tumoral lesions, vascular malformations, gliosis, cerebral malacia due to previous infarction, sclerosis, atrophy and cystic lesions were visible brain insults in more than 85% of the patients. In seven cases with signal abnormalities on brain MRI, the radiologist was unable to identify the lesion type. Not considering these unidentified brain lesions, five patients had a totally normal MRI. Lesion data and locations are detailed in table I .
Table I .
Patients’ demographics.
| Descriptive statistics | ||
|---|---|---|
| Mean (SD) | ||
| Age (years) | 31.91 (12.3) | |
| Symptom age (years) | 4.76 (6.2) | |
|
| ||
| Sex | ||
| Frequency | ||
| Male | 46 (68.7%) | |
| Female | 21 (31.3%) | |
|
| ||
| Lesion type | ||
| Normal | 5 (7.5%) | |
| Gliosis | 4 (6%) | |
| Sclerosis | 7 (10.4%) | |
| Tumor | 38 (56.7%) | |
| Cystic lesion | 2 (3%) | |
| Atrophy | 3 (4.5%) | |
| Infarction | 3 (4.5%) | |
| Vascular lesion | 1 (1.5%) | |
| Unidentified | 7 (10.4%) | |
|
| ||
| Location | ||
| Frontal | 16 (23.9%) | |
| Parietal | 6 (9%) | |
| Temporal | 12 (17.9%) | |
| Hippocampus | 7 (10.4%) | |
| Frontoparietal | 4 (6%) | |
| Frontotemporal | 3 (4.5%) | |
| Parietotemporal | 7 (10.4%) | |
| Parietooccipital | 2 (3%) | |
| Occipitotemporal | 5 (7.5%) | |
| N.A. * | 5 (7.5%) | |
|
| ||
| Hemisphere | ||
| Right | 9 (13.4%) | |
| Left | 53 (79.1%) | |
| N.A. | 5 (7.5%) | |
|
| ||
| Presenting symptom | ||
| Seizure | 54 (80.6%) | |
| Paresis | 1 (1.5%) | |
| Paresthesia | 2 (3%) | |
| Raised ICP symptoms | 21 (31.3%) | |
Not applicable (when normal imaging study was present); ICP=intracranial pressure.
The patients and controls gave their written consent to the study after it had been fully explained to them. The study was approved by the Institutional Review Board of the Tehran University of Medical Sciences.
Functional MRI paradigms
In an attempt to develop an optimized fMRI protocol for clinical use, five different Persian language tasks have previously been compared at our research center ( Mahdavi et al., 2011).The results showed that word production (WP) and reverse word reading (RWR) tasks are the best type for localization of language areas in Persian speakers. Each simple block-design task consisted of eight blocks (four rest blocks and four activation blocks in an alternating sequence) and lasted 3 minutes and 20 seconds (25 seconds per block). In each subject, the tasks, the trials within each block, and the letters within each trial were presented randomly. The colors used in all five tasks were black and white, and we always used the same font and size of letters in order to minimize differences due to factors other than linguistic processes.
Word production (WP)
Each activation block consisted of five word trials (5 seconds each). In each of these trials the subject was exposed to a four-letter Persian word presented letter by letter in the correct order. Each letter replaced the previous one after an interval of 1 second. The subjects were then asked to read the four-letter word silently (without any movement of the vocal organs to minimize motion artifacts) during a 1-second delay following the presentation of the fourth letter. Unlike English, vowels are usually omitted in written Persian, and when a word is written, letters are not separate but joined with each other. For this reason, we thought reading Persian might involve an additional process of adding the single letters together and guessing vowels (on the basis of previous learning) to produce a meaningful word. We decided to present the words as separate letters in order to take this difference into account ( Mahdavi et al., 2011).
Reverse word reading (RWR)
In the RWR task, each activation block consisted of 10 word trials. In each trial (2.5 seconds) the subject was presented with a five-letter Persian word. In this case the letters were presented in the reverse order. They were asked to read each word silently once. Rest blocks were included as in the WP task. As with the WP task, the orthographic to phonological transformation process played an important role during performance of the RWR ( Mahdavi et al., 2011). Since the WP and RWR are complementary tasks, we used both in order to obtain a global view of the active brain areas in Persian. WP is a more convenient and sensitive task but exhibits strongest activation in frontal regions of the language network while RWR, although more complex, activates both anterior and posterior language areas ( Mahdavi et al., 2011).
Functional MRI and MRI data acquisition
According to our institutional pre-defined protocol for fMRI experiments ( Mahdavi et al., 2011), all participants were screened for the following exclusion criteria for fMRI experimentation: i) ocular problems affecting visual acuity at the time of scan; ii) cochlear implants or any metal objects in the body; iii) cardiac or neural pacemakers; and iv) a history of musculoskeletal disorder in any limb. The MRI apparatus was a 1.5-Tesla GE® Signa scanner (General Electric; Milwaukee, WI, USA). A T1-weighted spin-echo sequence was used to generate high-resolution structural maps of participants’ brains having the same dimension and orientation as the fMRI scans (TR=1800 ms; TE=90 ms; flip angle=90°). The fMRI data were obtained with a gradient-echo echoplanar imaging (EPI) protocol (TE=60.3 ms; TR=3125 ms; flip angle=90°; field of view=22 cm 2 ; number of slices=15; slice thickness=6 mm; spacing=0 mm; bandwidth=5.62 kHz). A standard quadrate head coil was used. Fifteen contiguous axial slices, relatively parallel to the “anterior commissure-posterior commissure” line according to the Talairach and Touroux atlas ( Lacadie et al., 2008), were taken beginning from the vertex.
The patients’ fMRI images were analyzed using the fMRI Expert Analysis Tool (FEAT), part of FMRIB’s Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). The following pre-processing steps were performed on EPI data before final analyses: motion correction, using Motion Correction from FMRIB’s Linear Image Registration Tool (MCFLIRT; FSL), removal of non-brain tissue signals from anatomical images, using the Brain Extraction Tool (BET, Version 2.1; FSL), and spatial smoothing, using a Gaussian kernel of full-width half-maximum of 8 mm and non-linear high-pass temporal filtering. The parametric statistical analysis was based on a general linear model and performed using FEAT, version 5.90; FSL ( Friston et al., 1995).
Time-series statistical analysis was carried out using FILM (FMRIB Improved Linear Model) pre-whitening to make the statistical approaches valid and maximally efficient. The corresponding BOLDsignal was characterized by a “z-score”. Finally, cluster thresholding was carried out to reveal clusters that are activated significantly. Only clusters with a z-stat greater than 2.3 and a cluster threshold p value of less than 0.05 were considered to be significantly activated.
Higher-level intrasubject analysis was carried out using a fixed effect model, by forcing the random effect variances to zero. All activation maps were overlaid and registered onto a standard Montreal Neurological Institute (MNI) brain template embedded in FSL software. The registration process helped to unify the functional maps and validated the feasibility of the comparison method between patients with different brain lesions.
Laterality index calculation
A hemispheric laterality index (LI) was calculated for each subject by first counting the activated pixels within the pre-defined region of interest (ROI). The ROIs used for the calculation included all pixels over the whole hemisphere after excluding the cerebellum and mesial part of the brain (i.e., around the inter-hemispheric fissure). A threshold was determined by calculating the mean value of the z-scores for all pixels within the ROIs. The number of pixels surpassing this z-score was counted for both the left-and right-side ROI. Final z-score thresholds were not statistically different between patients and controls and the same threshold was used for both hemispheres. For calculation of the LI, we used the following equation: LI= (DL-DR) / (DL+DR)*100 where DL is the number of activated voxels (pixels that exceeded the threshold) in the left hemisphere and DR is the same for the right hemisphere.
This method of calculating the LI avoided the biases imposed by uninformed thresholding and clustering patterns. This approach yields LIs that range between −1 and 1 (maximum right or left dominant respectively). Values close to “0” (i.e., −0.1 ≤ LI ≤0.1) indicate bilateral language distribution ( Holland et al., 2001). A subject with LI >0.1 is categorized as left-side dominant, while a subject with LI ≤0.1 is categorized as right-side dominant ( Yuan et al., 2006).
Statistical analysis
The quantitative data were expressed as mean values ± standard deviation and qualitative data as percentages. For each patient, two LIs were measured (one for the WP task and the other for the RWR task). Using the t-test, patients’ LIs were statistically compared to those of the age-/gender-/handedness-matched healthy controls. After this between-group comparison, the impact of gender, age, symptom duration, site of lesion, and type of lesion on LI was assessed in the patient group. Repeated measures analysis of variance (ANOVA) and linear regression were used to show the possible impact of nominal and interval variables respectively. Since it is likely that any relationship between abnormal LI and potential determinants of abnormal language lateralization such as lesion properties or patient age might be “masked” by the variability in the patients, we also performed multivariable analysis. Hence, in the final stage of our analysis, a logistic regression model was used to monitor variable interactions and find independent predictors of abnormal LI. A p-value less than 0.05 was considered statistically significant.
Results
Cortical activation in the classic language regions, including the Broca and Wernicke areas, was observed during both language tasks in all the subjects, both the patients and the age-/gender-/handedness-matched healthy controls.
Language lateralization in normal subjects and patients
Comparison of LI between healthy controls and patients with cerebral lesions revealed notable differences. On the WP task, 26 patients (38.8%) had bilateral language distribution (−0.1 < LI <0.1); 26 (38.8%) patients had right-sided language dominance (LI <−0.1); and only 15 (22.4%) showed left-sided language dominance (LI >0.1). Conversely, in the age- and sex-matched healthy volunteers, only 8 subjects (11.9%) showed bilateral language lateralization, whereas the majority (n=59, 88.1%) were classed as left-side dominant (LI >0.1). No right-lateralized language pattern was found in the healthy subjects on the WP task. Similar results were obtained for the RWR task, on which 22 patients (32.8%) showed bilateral language distribution; 28 (41.8%) showed right-sided language dominance; and 17 (25.4%) had normal (left) language dominance. Both “bilateral language dominance” and “right-side dominance” were considered “atypical” language lateralization. Chi square test showed that the percentages of subjects with “typical” and “atypical” language dominance in the patient group were significantly different from the percentages in the group of age-/gender-/handedness-matched healthy controls for both tasks (p<0.005).
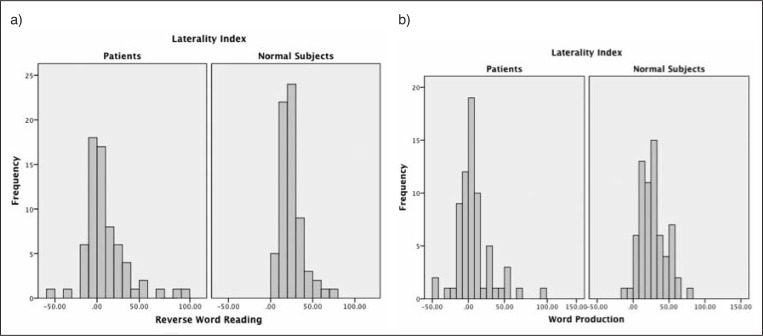
Our results also showed that on the WP task, the patients had an LI of 9.59±24.9, which was significantly different (t=−3.86, p<0.0001) from that recorded in the age-/gender-/handedness-matched healthy subjects (23.21±14.64). On the RWR task, the LI of the patients was also significantly different from that of the controls (6.99±23.37 for patients vs 27.3±18.1 for healthy controls, t=−6.23, p<0.001). Figure 1 shows the distribution of LI in all the study participants.
Figure 1 .
Distribution of LI in reverse word reading (a) and word production (b) tasks.
Factors that might change the laterality index in patients
In the second step of our analyses, we evaluated the probable impact of potential determinants of abnormal language lateralization in the patient population. Repeated measures ANOVA was used to examine the effect of lesion type, lesion location, lesion hemisphere, presenting symptom and patient gender (between-subject factors) and task (two-level within-subjects factor). No statistically significant result was produced by the main effects of the above-mentioned factors on LI (p>0.05); moreover, the interaction of the factors, too, was not significant (p>0.05). The results of the within-subjects analysis showed that type of task did not exert a statistically significant influence on the calculated LI.
Effects of symptom duration and age on mean laterality index in patients
Linear regression showed that the mean LI was not influenced either by patient age or by symptom duration. For the WP task, age and symptom duration did not correlate significantly with the calculated LI (p=0.077 and 0.563 respectively). This was also true for the LI calculated on the RWR task (p=0.09 for age and p=0.61 for symptom duration).
Independent predictors of atypical language dominance
In the last step of our analysis we looked for independent determinants of atypical language dominance. When age, gender, symptom age, presenting symptom and lesion site were inserted into the logistic model, patient age was found to be the only independent predictor of atypical language dominance (B=−0.06; Wald=4.4; p=0.03; odds ratio=0.9). Indeed, the odds ratio for this variable indicates that fewer cases with an abnormal LI might be anticipated in the older population.
Discussion
This study is the first evaluation of the effects of “different” brain lesions on the induction of language lateralization abnormalities in patients with a “variety” of neurological symptoms. There are numerous case series and case reports that focused on epilepsy patients, and yet a large number of patients who need neurosurgical interventions present with symptoms other than seizures. The fact that our findings on the factors that determine abnormal language lateralization differed from those of previous studies conducted in epilepsy patients justifies our approach and underlines the importance of conducting research of this kind.
The low LI values found in our research might stem from several issues. The first is the block-design nature of the task paradigm. The tasks used in our series incorporated a neutral rest condition and hence the activated regions might include other, non-language (e.g. visual, eye movement, etc.) regions. Most of these non-language regions exhibit a bilateral pattern and may reduce the language-related preponderance ( Seghier, 2008). The low LI values found in our study might also be related to our method of calculating the LI. Since we wanted to observe any possible compensatory activation in the entire brain, we had to use a whole-hemisphere ROI rather than a tunnel vision approach. It should be noted that the low threshold (i.e. 10%) we used to define atypical language lateralization has previously been validated by others (Yuan et al., 2002; Holland et al., 2008).
Although our results confirmed a significant difference between the LI of patients with any kind of intracerebral lesion as compared with healthy subjects, they failed to demonstrate a significant impact of symptom duration, lesion type or lesion site. The latter finding is the intriguing peculiarity of our study. It is well-documented that patients with brain insults have a higher incidence of atypical language lateralization than do healthy subjects ( Springer et al.,1999 ; Adcock et al., 2003; Brázdil et al., 2005), but most of these studies only considered epilepsy patients. There exist few studies that included diverse brain lesions. In one of these studies, Ulmer et al. (2004) retrospectively reviewed a total of 85 functional areas within 5 mm of the edge of a potentially resectable lesion in 50 patients. In as many as 27% of these areas, they found a reduced fMRI signal in perilesional eloquent cortex in conjunction with preserved or increased signal in homologous contralateral brain areas. They suggested a possible lesion-induced transhemispheric cortical reorganization to homologous brain regions. Although their study was not confined to language areas, their results are consistent with our findings.
Unlike previous reports in epilepsy patients, our findings did not demonstrate any correlation with symptom duration, which, therefore, was not a significant predictor of language lateralization. Our different findings might be attributed to our diverse patient population, given that epilepsy patients were the target group of all the previous reports. Moreover, the minimum duration of the symptoms in our patient population was 12 months, a period of time that may be sufficient to allow brain plasticity to alter the language dominance. In the present study we insisted on including patients with old brain lesions (i.e. older than a year) since any notable difference in brain function in the presence of a new brain impairment might be a transient finding. Most of the mechanisms involved in brain plasticity, including deletion of damaged neurons through apoptosis, proliferation and pruning of synapses, and activity-dependent refinement of synaptic connections ( Johnston, 2004), are time-consuming events. Indeed, this study set out to evaluate patients with established brain function and not those whose brains are “under construction”.
In our series of patients there was no significant correlation between lesion site and LI. Lesion site in our study means two things: the hemisphere involved and the anatomical site of the lesion. Previous reports consistently showed that atypical language lateralization is almost exclusively expected in left-side lesions. Powell and colleagues (2007) showed that the effect is less noticeable in right temporal lobe epilepsy patients in whom the non-language-dominant hemisphere is affected, than it is in left temporal lobe epilepsy. Although the presenting symptom of the patients in our study was different from those of previous reports, this inconsistency might be due to the small number of patients with a right-hemisphere lesion in our study (nine patients versus 53 with a left-hemisphere lesion). Moreover, none of the lesions in our series of patients caused aphasia, which suggests that none or few of the lateralized language regions were disturbed by a given lesion or that the alteration was at the sub-clinical level (the question of how the LI might behave in the presence of a lesion capable of altering language function at the clinical level remains to be addressed). This explanation may also justify the fact that our data failed to show any statistically significant impact of anatomical site on LI in the patient population. Additionally, the algorithm for calculation of LI in this study was applied to a whole-brain ROI. This approach for LI calculation might cancel out intrahemispheric effects of anatomical site of the lesion on the measured laterality.
One might argue that our null results with regard to the importance of lesion site/hemisphere on LI distribution are “masked” by the huge variability of patients recruited to this study. To address this, we used a logistic model to look for masking/enhancing effects of variables on each other. In our model, patient age was the only variable that showed a statistically significant effect on the development of an abnormal LI after controlling for other variables. The calculated odds ratio showed that age could independently predict atypical LI. This result was adjusted for possible effects of symptom type and lesion site. On the other hand, neither the location of the lesion nor patient age could independently predict abnormal LI. Moreover, the odds ratio for age implied that an older subject is less likely to develop an abnormal LI. From a clinical standpoint this finding is of great importance and may indicate a gradual decline in compensatory capacity as a patient gets older. Further studies are needed to confirm the clinical consequences of this weaker capacity of older patients.
Our study has several limitations. Employing fMRI as a tool for determining language lateralization in patients with intracerebral lesions as a presurgical evaluation may result in misleading or incorrect assumptions. A number previous studies as well as theoretical considerations raise concerns that lesions can affect BOLD-effect generation and detection critically ( Wellmer et al., 2009). This would impair the validity of fMRI, in particular in patients with lesions close to classical cortical language areas. It has been postulated that near cavernomas and arteriovenous malformations, signal loss may result in signal destruction and false-negative BOLD results ( Thickbroom et al., 2004). Holodny and colleagues (2000) suggested that infarctions, brain edema, and cerebrovascular disease with stenoses of intraand extra-cerebral arteries can lead to falsely localized activation or false-negative activation maps. Furthermore, the production of BOLD-contrast enhancement can be altered in the vicinity of space-occupying glial and non-glial tumors ( Holodny et al., 2000; Ruff et al., 2008). Moreover, prior surgery for brain tumors, a finding prevalent in patients with intracranial tumors, is associated with a decrease in the volume of fMRI activation, most probably due to susceptibility artifacts ( Kim et al., 2005).
Despite these several technical considerations with regard to the use of fMRI as a diagnostic tool for determining language lateralization in patients with cerebral lesions, this study nevertheless produced conclusive findings. We found that patients with a variety of cerebral lesions and a diversity of clinical manifestations show an abnormal language lateralization. Moreover, our results showed that in our selected population, there are no significant impacts of symptom duration, side (hemisphere) or anatomical site of the lesion in producing an abnormal LI. Considering all the potential determinants predicting abnormal LI, our study found patient age to be the only independent predictor of atypical language preponderance.
Acknowledgments
This project was financially supported by the Iran National Science Foundation (INSF).
References
- Adcock JE , Wise RG , Oxbury JM , et al. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy . Neuroimage . 2003 ; 18 : 423 – 438 . doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Brázdil M , Chlebus P , Mikl M , et al. Reorganization of language-related neuronal networks in patients with left temporal lobe epilepsy – an fMRI study . Eur JNeurol . 2005 ; 12 : 268 – 275 . doi: 10.1111/j.1468-1331.2004.01127.x. [DOI] [PubMed] [Google Scholar]
- Brázdil M , Zákopcan J , Kuba R , et al. Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions . Epilepsy Behav . 2003 ; 4 : 414 – 419 . doi: 10.1016/s1525-5050(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Duchowny M , Jayakar P , Harvey AS , et al. Language cortex representation: effects of developmental versus acquired pathology . Ann Neurol . 1996 ; 40 : 31 – 38 . doi: 10.1002/ana.410400108. [DOI] [PubMed] [Google Scholar]
- Friston KJ , Holmes AP , Worsley KJ , et al. Statistical parametric maps in functional imaging: a general linear approach . Human Brain Mapp . 1995 ; 2 : 189 – 210 . [Google Scholar]
- Hécaen H . Acquired aphasia in children and the ontogenesis of hemispheric functional specialization . Brain Lang . 1976 ; 3 : 114 – 134 . doi: 10.1016/0093-934x(76)90009-2. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C , Kurthen M , Linke DB , et al. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy . Brain Cogn . 1997 ; 33 : 135 – 150 . doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L , Chiron C , Jambaqué I , et al. Late plasticity for language in a child’s non-dominant hemisphere: a pre- and post-surgery fMRI study . Brain . 2002 ; 125 : 361 – 372 . doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L , Gaillard WD , Mott SH , et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study . Neurology . 1997 ; 48 : 1003 – 1012 . doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Holland SK , Plante E , Weber Byars A , et al. Normal fMRI brain activation patterns in children performing a verb generation task . Neuroimage . 2001 ; 14 : 837 – 843 . doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holodny AI , Schulder M , Liu WC , et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery . AJNR Am J Neuroradiol . 2000 ; 21 : 1415 – 1422 . [PMC free article] [PubMed] [Google Scholar]
- Johnston MV . Clinical disorders of brain plasticity . Brain Dev . 2004 ; 26 : 73 – 80 . doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- Lacadie CM , Fulbright RK , Rajeevan N , et al. More accurate Talairach coordinates for neuroimaging using non-linear registration . Neuroimage . 2008 ; 42 : 717 – 725 . doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois F , Connelly A , Cross JH , et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study . Brain . 2004 ; 127 : 1229 – 1236 . doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Liégeois F , Connelly A , Salmond CH , et al. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy . Neuroimage . 2002 ; 17 : 1861 – 1867 . doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- Kim MJ , Holodny AI , Hou BL , et al. The effect of prior surgery on blood oxygen level-dependent functional MR imaging in the preoperative assessment of brain tumors . AJNR Am J Neuroradiol . 2005 ; 26 : 1980 – 1985 . [PMC free article] [PubMed] [Google Scholar]
- Mahdavi A , Houshmand S , Oghabian MA , et al. Developing optimized fMRI protocol for clinical use: comparison of different language paradigms . J Magn Reson Imaging . 2011 ; 34 : 413 – 419 . doi: 10.1002/jmri.22604. [DOI] [PubMed] [Google Scholar]
- Müller RA , Rothermel R , Behen ME , et al. Language organization in patients with early and late left-hemisphere lesion: a PET study . Neuropsychologia . 1999 ; 37 : 545 – 557 . doi: 10.1016/s0028-3932(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Powell H , Parker GJ , Alexander DC , et al. Abnormalities of language networks in temporal lobe epilepsy . Neuroimage . 2007 ; 36 : 209 – 221 . doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Rasmussen T , Milner B . The role of early left-brain injury in determining lateralization of cerebral speech functions . Ann N Y Acad Sci . 1977 ; 299 : 355 – 369 . doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Rathore C , George A , Kesavadas C , et al. Extent of initial injury determines language lateralization in mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS) . Epilepsia . 2009 ; 50 : 2249 – 2255 . doi: 10.1111/j.1528-1167.2009.02300.x. [DOI] [PubMed] [Google Scholar]
- Rausch R , Walsh GO . Right-hemisphere language dominance in right-handed epileptic patients . Arch Neurol . 1984 ; 41 : 1077 – 1080 . doi: 10.1001/archneur.1984.04050210075018. [DOI] [PubMed] [Google Scholar]
- Ruff I , Petrovich Brennan NM , Peck K , et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery . AJNR Am J Neuroradiol . 2008 ; 29 : 528 – 535 . doi: 10.3174/ajnr.A0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman J , Smith ML , Scott K . The impact of age at seizure onset on the likelihood of atypical language representation in children with intractable epilepsy . Brain Cogn . 2002 ; 48 : 517 – 520 . [PubMed] [Google Scholar]
- Seghier ML . Laterality index in functional MRI: methodological issues . Magn Reson Imaging . 2008 ; 26 : 594 – 601 . doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA , Binder JR , Hammeke TA , et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study . Brain . 1999 ; 122 : 2033 – 2046 . doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW , Byrnes ML , Morris IT , et al. Functional MRI near vascular anomalies: comparison of cavernoma and arteriovenous malformation . J Clin Neurosci . 2004 ; 11 : 845 – 848 . doi: 10.1016/j.jocn.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Ulmer JL , Hacein-Bey L , Mathews VP , et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments . Neurosurgery . 2004 ;55: 569 – 581 . doi: 10.1227/01.neu.0000134384.94749.b2. [DOI] [PubMed] [Google Scholar]
- Wellmer J , Weber B , Urbach H , et al. Cerebral lesions can impair fMRI-based language lateralization . Epilepsia . 2009 ; 50 : 2213 – 2224 . doi: 10.1111/j.1528-1167.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- Woods RP , Dodrill CB , Ojemann GA . Brain injury, handedness, and speech lateralization in a series of amobarbital studies . Ann Neurol . 1988 ; 23 : 510 – 518 . doi: 10.1002/ana.410230514. [DOI] [PubMed] [Google Scholar]
- Yuan W , Szaflarski JP , Schmithorst VJ , et al. fMRI shows atypical language lateralization in pediatric epilepsy patients . Epilepsia . 2006 ; 47 : 593 – 600 . doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]