Summary
Ring chromosome 20 [r(20)] syndrome is a chromosomal disorder characterized by epilepsy and intellectual disability. Distinctive electroclinical features and wakefulness EEG patterns have been described. The EEG features of sleep have not yet been evaluated.
We studied the pattern of sleep in six patients aged 2–59 years who underwent at least one polysomnographic recording.
Their sleep pattern evolution is described as deterioration ranging from normal to destructured NREM/REM sleep. NREM sleep alterations were observed from childhood and were more evident in adulthood. EEG abnormalities detected during wakefulness persisted, with morphological changes, during sleep. During NREM sleep all the subjects presented high amplitude delta sequences with a sharply contoured or notched appearance, prevalent over frontal regions. The theta rhythm of wakefulness was seen to persist during REM sleep.
Ring chromosome 20 syndrome shows sleep alterations that seem to be age-related. A potential role of cortical and thalamocortical dysfunction is discussed.
Keywords: electroencephalography , epilepsy , ring chromosome 20 syndrome , sleep
Introduction
Ring chromosome 20 [r(20)] syndrome is a well-defined chromosomal disorder that is characterized by mild to moderate intellectual disability and epilepsy, in the absence of significant diagnostic dysmorphic features. Psychomotor development may be normal or mildly delayed until epilepsy onset. The mechanism underlying the seizure disorder in [r(20)] syndrome remains unknown.
Epilepsy onset usually occurs in childhood. Seizures show a peculiarity that could be helpful for diagnosis: they comprise terror and hallucinations that are wrongly attributed to psychiatric disorders, and should instead be considered ictal phenomena ( Canevini et al., 1998; Augustijn et al., 2001, Ville et al., 2006).
Frequently, seizures occur both in sleep and in wakefulness. Nocturnal seizures can present as recurrent episodes of frontal lobe seizures with motor signs, but they can also be subtle nocturnal seizures (SNSs), i.e. associated with minimal or moderate motor signs ( Augustijn et al., 2001).
In some cases seizures can be controlled by antiepileptic drug (AED) therapy, but in most cases seizures soon become drug resistant.
In most adolescents and adults reported, the striking epileptic characteristic is the occurrence of long-lasting episodes of non-convulsive status epilepticus (NCSE), consisting of a prolonged state of confusion of varying severity ( Inoue et al., 1997).
Distinctive electroclinical features have been described in the syndrome: i) bursts or long trains of theta waves, with a peak at 5 Hz and a sharply countoured or notched appearance, predominant over temporal regions, and intermingled with normal or nearly normal background activity ( Canevini et al., 1998); ii) runs of long-lasting bilateral paroxysmal high-voltage slow waves with occasional spikes over the frontal lobes, described as NCSE ( Inoue et al., 1997); iii) highly characteristic bursts of diffuse but frontally dominant high-voltage fast activity, associated with SNSs ( Augustijn et al., 2001).
Sleep disturbances have not been reported and, to date, no polysomnographic (PSG) studies have been carried out in patients with [r(20)] syndrome.
Prompted by a personal observation of severe destructuring of nocturnal sleep in an adult patient with [r(20)] syndrome, we reviewed sleep EEG recordings previously carried out in [r(20)] syndrome patients to study epilepsy rather than analyze sleep features. These patients’ polysomnographic recordings were reviewed in order to i) analyze sleep architecture; ii) assess any age-related changes in sleep structure and sleep EEG abnormalities; and iii) evaluate the relationship between sleep and the paroxysmal EEG pattern in children, adolescents and adults with [r(20)] syndrome.
Materials and methods
Subjects
Six subjects with [r(20)] syndrome (2 males and 4 females, mean age 24.5 years, range 31 months - 59 years) were included in this study. Patients 4, 5 and 6 have already been described in a previous paper ( Canevini et al., 1998). Comprehensive evaluation of the patients included brain MRI, multiple video-PSG recordings, and intelligence quotient (IQ). Ictal semiology was described in accordance with the report of the ILAE task force on classification and terminology ( Blume et al., 2001).
The clinical diagnosis was confirmed by cytogenetic analysis that revealed [r(20)] mosaicism in all the subjects; no epilepsy-related microdeletions or subtelomeric deletions were detected.
Methods
Each patient underwent at least one in-lab video-PSG study (synchronous recording of image and signals using the PSG System Plus, Micromed, Treviso, Italy). These studies were performed during diurnal or nocturnal sleep, or both. The EEG was acquired with full-scalp positioning of leads according to the international 10–20 system: Fp1, Fp2, F3, F4, F7, F8, Fz, Cz, C3, C4, P3, P4, Pz, T3, T4, T5, T6, O1, O2, common reference; two channels were dedicated to a bipolar horizontal electrooculogram and a chin electromyogram. The display system used allowed rearrangement of EEG traces into various montages.
After a preliminary visual analysis, PSG recordings were scored according to the AASM criteria for sleep staging by two neurologists trained in video-PSG scoring and specialized in sleep disorders ( Iber et al., 2007).
Karyotype analysis was performed on blood lymphocytes by standard procedures. For each analysis at least 100 mitoses were studied ( Zou et al., 2006).
Intelligence scales were selected individually for the patients according to best practice standards. In accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSMIV-TR), we classified patients’ IQs as follows: normal: IQ > 85; borderline intellectual functioning (BIF): IQ from 84 to 71; mild mental retardation: IQ from 70 to 55 (DSMIV-TR).
Results
Sixteen PSG recordings (7 nocturnal/9 diurnal sleep) were evaluated.
All patients except one (patient 1, two years old, who received a prenatal diagnosis and was affected by mild growth retardation) presented epileptic seizures and were on AED treatment. Mean age at epilepsy onset was 10.4 years. Two patients (patients 3 and 5) had drug-resistant focal epilepsy. Four out of five subjects presented seizure occurrence both during wakefulness and during sleep. One patient (patient 6) reported seizures only during wakefulness. Patients 3 and 5 had presented NCSE during their lifetime. Seizures during sleep were recorded in two patients (patients 2 and 3).
IQ was classified as normal in two subjects, and BIF in three. One subject was affected by mild mental retardation.
The clinical data and PSG features of each subject are summarized in table I .
Table I .
Clinical data and polysomnographic features of all the patients.
| Subject n. | Sex/age | Age at Epilepsy onset | Drug-resistant epilepsy/NCSE | IQ | Seizure type acc. to Blume (2001) | Seizure circadianity | [r(20)] mosaicism |
|---|---|---|---|---|---|---|---|
| 1 | f/31 m | n.a. | n.a. | normal | n.a. | n.a. | 29 |
| 2 | f/8 y | 6 y | −/− | BIF | hyperkinetic | WS | 33 |
| 3 | f/13 y | 9 y | +/+ | BIF | experiential hyperkinetic | WS | 53 |
| 4 | m/34 y | 21 y | −/− | normal | generalized tonic-clonic | WS | 13 |
| 5 | m/30 y | 5 y | +/+ | mild mental retardation | experiential autonomic | WS | 83 |
| 6 | f/59 y | 11 y | −/− | BIF | dyscognitive | W | 12 |
|
| |||||||
| Subject n. | EEG (wakefulness) | PSG features | IEAs during sleep | ||||
|
| |||||||
| 1 | normal | Reduction of NREM physiological sleep hallmarks. | NREM: bursts of delta activity (2 to 3 Hz) of high amplitude with a sharply contoured or notched appearance, predominant over frontotemporal regions bilaterally. REM: no IEAs. | ||||
| 2 |
Bursts or long trains of theta activity (peak frequency 5Hz) with sharply contoured or notched appearance, predominantly over left frontotemporal regions. Synchronous/asynchronous slow spike-and-wave complexes prevalent over frontal regions. |
Preserved cyclic alternation of NREM/REM sleep. Reduction of NREM physiological sleep hallmarks. SWS recorded. | NREM: bursts or long trains of delta activity (2 to 3 Hz) of high amplitude and with a sharply contoured or notched appearance, predominant over frontotemporal regions bilaterally. REM: no IEAs. | ||||
| 3 | Bilateral, synchronous/asynchronous slow spike-wave complexes with left frontal predominance. | Reduction of NREM physiological sleep hallmarks. No SWS recorded. No REM sleep recorded. | NREM: persistence of synchronous/asynchronous slow spike-wave complexes with left frontal predominance. | ||||
| 4 | Bursts or long trains of theta activity (peak frequency 5Hz) with sharply contoured or notched appearance, predominantly over frontotemporal regions. | Preserved cyclic alternation of NREM/REM sleep. Reduction of NREM physiological sleep hallmarks. No SWS recorded. No saw tooths. | NREM: bursts or long trains of theta activity, progressively intermingling with or substituted by delta frequencies (2 to 3 Hz) of high amplitude and with a sharply contoured or notched appearance, predominant over frontotemporal regions bilaterally. REM: no IEAs. | ||||
| 5 | Bursts or long trains of theta activity (peak frequency 5Hz) with sharply contoured or notched appearance, predominantly over frontotemporal regions. Sequences of bi/triphasic sharp waves, bilateral/diffuse, prevalent over anterior region. | Preserved cyclic alternation of NREM/REM sleep. Absence of NREM physiological sleep hallmarks. No SWS recorded. No saw tooths. | NREM: persistence of theta activity over anterior regions, intermingled with delta frequencies, with a sharply contoured appearance, bilateral and diffuse with anterior predominance of amplitude. REM: sequences of theta activity with a notched appearance, predominantly over frontotemporal regions. Diffuse, biphasic sharp-waves, isolated or grouped in short sequences alternating with periods of marked depression of EEG signal. Global, marked deterioration of PSG features during follow-up. | ||||
| 6 | Bursts or long trains of theta activity, 5–7 Hz, with sharply contoured or notched appearance, predominantly over mid-temporal regions | Preserved cyclic alternation of NREM/REM sleep. Absence of NREM physiological sleep hallmarks. No SWS recorded. No saw tooths. | NREM: persistence of theta activity over anterior regions, intermingled with delta frequencies, with a sharply contoured appearance, bilateral and diffuse with anterior predominance of amplitude. REM: sequences of theta activity with a notched appearance, predominantly over frontotemporal regions. Diffuse, biphasic sharp waves, isolated or grouped in short sequences. | ||||
Abbreviations: m=months; y=years; n.a.=not applicable; NCSE=non-convulsive status epilepticus; BIF=borderline intellectual functioning; WS=wakefulness and sleep; W=wakefulness; SWS=slow-wave sleep; IEAs=interictal epileptiform abnormalities.
Polysomnographic features
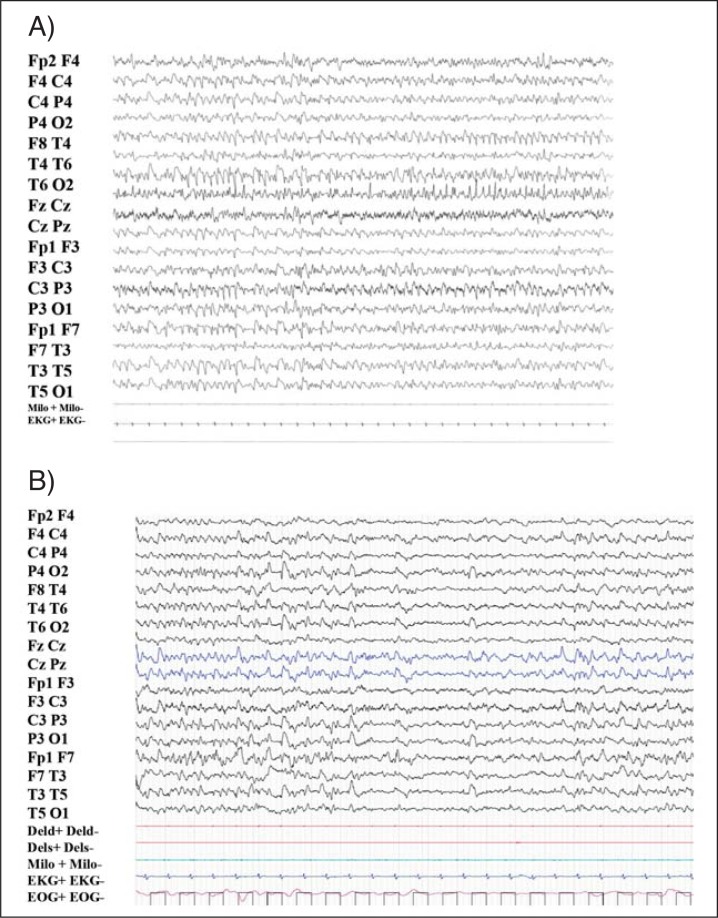
Cyclic alternation of NREM/REM sleep was maintained in all the subjects, except patient 3, probably because the high number of seizures recorded on nocturnal polysomnography in this patient interfered with the sleep macrostructure. Adult patients showed variable degrees of sleep alteration, ranging from severe reduction of sleep spindles and K-complexes (patient 4) to the absence of sleep-specific patterns (patients 3, 5 and 6). REM sleep was found to have normal polygraphic features. A normal sleep pattern was found in the two youngest patients, except for a mild reduction of sleep spindles and K-complexes. All the PSG recordings showed a high number of awakenings. In one case (patient 5), deterioration of sleep structure with progressive loss of sleep spindles and K-complexes was found to be associated with reduction in the amount of slow-wave sleep during a 14-year follow-up period. This patient’s sleep pattern was very similar to his mother’s (patient 6) and consisted of periods of normal REM sleep alternating with periods of NREM sleep lacking the distinctive features of the NREM sleep stages ( Fig. 1 , over).
Figure 1 .
NREM sleep.
NREM sleep recorded in patients 6 (A) and 5 (B) (mother and son), at age 45 and 30 years respectively. The spindles and K-complexes appear markedly reduced. Subject 5, during a 14-year follow-up showed a deterioration of sleep pattern with progressive reduction of the physiological sleep pattern, the spindles and K-complexes becoming similar to those of his mother. 20-sec epoch.

Modulation of interictal epileptiform abnormalities and of the peculiar EEG pattern during sleep
Interictal epileptiform abnormalities (IEAs) detected during wakefulness persisted, with morphological changes, during sleep.
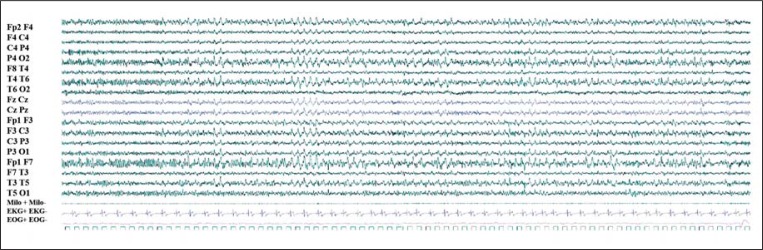
Bursts of theta/delta activity along with sharp waves, bilateral and diffuse, prominent over the anterior regions, occurred at sleep onset and persisted into REM sleep in two subjects (patients 4 and 5) ( Fig. 2 , over).
Figure 2 .
EEG pattern during wakefulness-sleep transition in adult patients.
Detected in patients 4 and 5, the pattern consisted of persistence of theta activity over anterior regions, intermingled with delta frequencies with a sharply contoured appearance, bilateral and diffuse, with anterior predominance of amplitude. The pattern persisted during NREM sleep. 80-sec epoch.
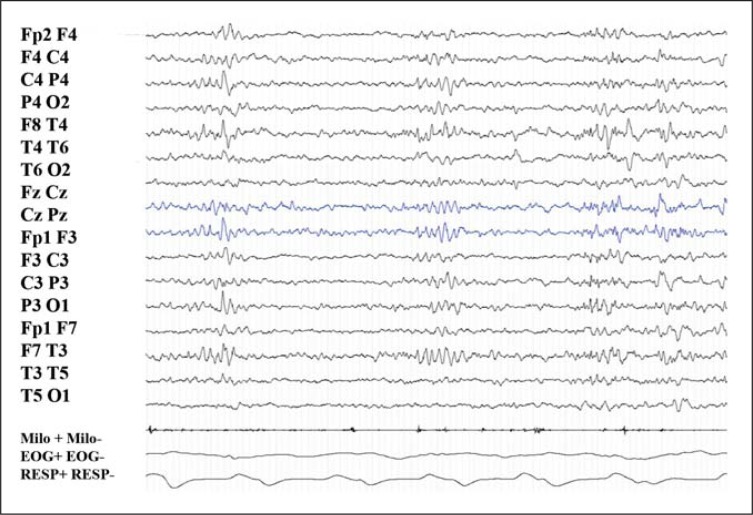
During NREM sleep all the patients showed the presence of high-amplitude delta sequences with a sharply contoured or notched appearance, prevalent over the frontal regions; these often occurred bilaterally, were synchronous or asynchronous, and in most cases showed interhemispheric amplitude asymmetry. This EEG pattern was present in all patients, however, it was particularly evident in patient 2 (8 years old), prominent in adult patients, and less evident in the youngest subject ( Fig. 3 , over).
Figure 3 .
Peculiar EEG pattern during NREM sleep, in childhood.
Peculiar EEG pattern (bilateral high-amplitude delta sequences with sharply contoured or notched appearance, prevalent over frontal regions) in patient 1 recorded at 31 months of age. Previous PSG recordings were normal and failed to demonstrate the presence of this peculiar EEG pattern. 20-sec epoch.
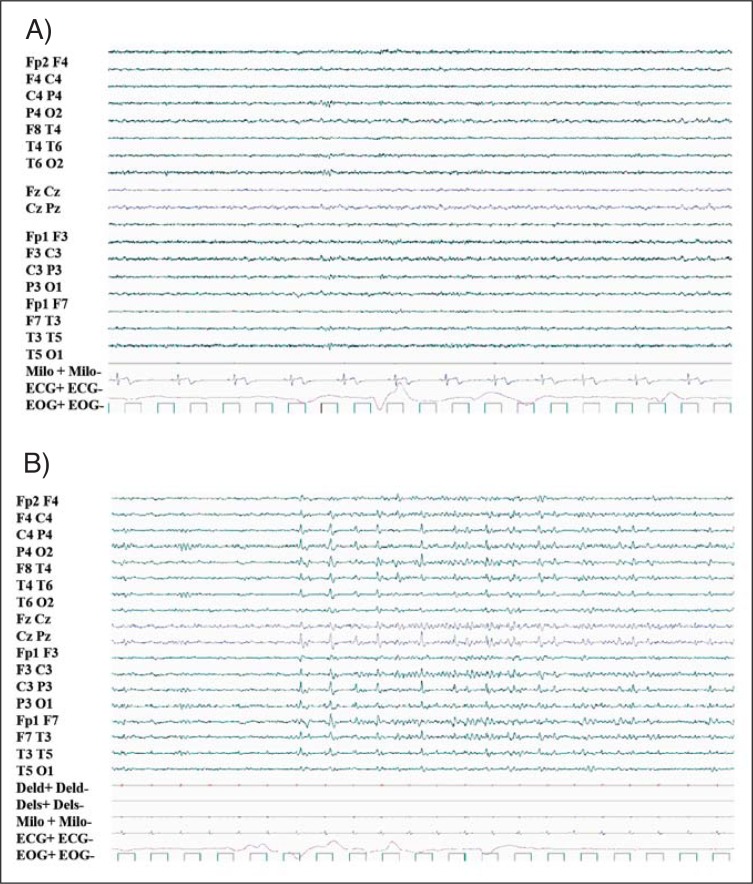
Interictal epileptiform abnormalities detected during wakefulness persisted during REM sleep ( Fig. 4 , over). Periods of diffuse marked depression of the EEG signal were detected during NREM and REM sleep in one subject (patient 5).
Figure 4 .
REM sleep.
REM sleep with normal PSG features and persistence of IEAs detected during wakefulness. A) patient 4, B) patient 5. 20-sec epoch.

Discussion
This study suggests that patients with [r(20)] syndrome show a peculiar sleep pattern, characterized by age-related changes in sleep architecture from infancy into adulthood.
Major abnormalities in sleep patterns, often associated with epilepsy and sleep disorders, are also seen in other genetically determined syndromes, such as Angelman and Rett syndrome ( Pelc et al., 2008). Although no sleep disorders have been reported in [r(20)] syndrome, it is known that seizures in these patients could occur mostly during sleep, especially in childhood ( Inoue et al., 1997).
Little is known about sleep EEG activity and paroxysmal alterations of this activity in [r(20)] syndrome: Augustijn et al. (2001) reported six children with [r(20)] syndrome and SNSs, describing the occurrence of polyspike and wave discharges during NREM sleep, associated with the appearance of 2–5 minute periods with a burst-suppression like pattern in slow-wave sleep, and the disappearance of epileptiform activity during REM ( Augustijn et al., 2001).
Ville et al. (2006) found physiological sleep patterns and different, recognizable sleep stages in three out of five patients with [r(20)] syndrome, aged 12–24 years. During sleep recording, all the patients showed bifrontal slow spike-and-wave discharges, in two cases less diffuse and continuous than during wakefulness ( Ville et al., 2006).
In the present series of patients, identification of the different NREM sleep stages was difficult, despite maintenance of the cyclic alternation of NREM/REM sleep and of the REM sleep PSG features. In fact, hallmarks of normal sleep (K-complexes and spindles) were absent or difficult to detect, especially in the older patients. The other main limiting factor was the substantial amount of IEAs emerging at sleep onset that persisted in NREM sleep. The possible role of epileptic phenomena, both ictal and interictal, in the disruption of sleep continuity in this group of patients is supported by the high number of awakenings documented in all the subjects. The first-night effect is not a likely explanation for such a high number of awakenings. Even though signs and symptoms of sleep disorders were absent, the lack of respiratory/limb movement PSG data makes it impossible to exclude the presence of sleep breathing disorders and periodic leg movements during sleep.
Sleep breathing disorders and periodic leg movements are known to be common and potentially reciprocally facilitating manifestations in epileptic syndromes ( Manni et al., 2003), namely in genetically determined conditions associated with intellectual disability such as Angelman syndrome ( Miano et al., 2005).
The differences found between the children and adults suggest a progressive deterioration of sleep structure in the course of the disease that cannot be explained by age alone. This hypothesis is strongly supported by the history of patient 5 who, during a follow-up period lasting fourteen years, showed a progressive deterioration of his sleep pattern. His sleep features in adolescence became similar to those of his mother (subject 6) in adulthood.
The particular EEG pattern ( Canevini et al., 1998) previously described in wakefulness recordings and consisting of bursts or long trains of theta waves, with a peak at 5 Hz and a sharply countoured or notched appearance, was found to persist during REM sleep.
During NREM sleep, bursts or sequences of high-amplitude delta activity with a sharply countoured or notched appearance were evident, prevalent over the anterior regions of both hemispheres. This pattern was more pronounced in the adult cases.
The mechanism underlying sleep deterioration in these patients might be related to dysregulation of the circuits involving the basal ganglia, which have been shown to be impaired in patients with [r(20)] syndrome ( Young et al., 2007; Lee et al., 2007). In particular, patients with [r(20)] syndrome showed a significant reduction of dopamine uptake in the caudate and putamen ( Biraben et al., 2004). This finding has been correlated with the inability of the basal ganglia circuit to interrupt the seizures, which results in the long-lasting NCSE typical of the syndrome. Recent evidence has indicated that dopamine can also play an important role in modulating sleep ( Lee et al., 2007). The caudate nucleus was found to be positively correlated with activity in the pre-frontal cortex and the thalamus in sleep modulation ( Alexander et al., 1986). Since a basal ganglia impairment has been found in patients with [r(20)] syndrome, this could explain the progressive loss of physiological sleep structure in these patients.
It might be hypothesized that sleep deterioration in [r(20)] syndrome results from the above-mentioned mechanisms of sleep dysregulation in combination with abundant epileptic phenomena (IEAs and seizures).
The above-described sleep alterations do not seem to be related to the percentage of [r(20)] chromosome mosaicism or to behavioral problems associated with intellectual disability (present in only one subject).
This study has some important limitations: the retrospective design of the study, the lack of an adaptation night, and the lack of respiratory/limb movement PSG data.
Nevertheless, our analysis of sleep patterns in these subjects with [r(20)] syndrome revealed the following peculiar features:
EEG sleep patterns characterized (across all the studied ages) by high-amplitude delta sequences with a sharply contoured or notched appearance, prevalent over the frontal regions;
loss of recognizable NREM sleep stages, which seemed to be progressive and age-dependent.
This report on the sleep characteristics of [r(20)] syndrome opens up new avenues for further insight into this rare disease. PSG investigations are a useful tool for diagnosing and following up [r(20)] patients.
References
- Alexander GE , DeLong MR , Strick PL . Parallel organization of functionally segregated circuits linking basal ganglia and cortex . Annu Rev Neurosci . 1986 ; 9 : 357 – 381 . doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Augustijn PB , Parra J , Wouters CH , et al. Ring chromosome 20 epilepsy syndrome in children: electroclinical features . Neurology . 2001 ; 57 : 1108 – 1111 . doi: 10.1212/wnl.57.6.1108. [DOI] [PubMed] [Google Scholar]
- Biraben A , Semah F , Ribeiro MJ , et al. PET evidence for a role of the basal ganglia in patients with ring chromosome 20 epilepsy . Neurology . 2004 ; 63 : 73 – 77 . doi: 10.1212/01.wnl.0000132840.40838.13. [DOI] [PubMed] [Google Scholar]
- Blume WT , Lüders HO , Mizrahi E , et al. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology . Epilepsia . 2001 ; 42 : 1212 – 1218 . doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- Canevini MP , Sgrò V , Zuffardi O , et al. Chromosome 20 ring: a chromosomal disorder associated with a particular electroclinical pattern . Epilepsia . 1998 ; 39 : 942 – 951 . doi: 10.1111/j.1528-1157.1998.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Iber C , Ancoli-Israel S , Chesson A , et al. The AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical Specifications . 1st ed . Westchester, IL : American Academy of Sleep Medicine ; 2007 . [Google Scholar]
- Inoue Y , Fujiwara T , Matsuda K , et al. Ring chromosome 20 and nonconvulsive status epilepticus: a new epileptic syndrome . Brain . 1997 ; 120 : 939 – 953 . doi: 10.1093/brain/120.6.939. [DOI] [PubMed] [Google Scholar]
- Lee BF , Chiu NT , Kuang Yang Y , et al. The relation between striatal dopamine D2/D3 receptor availability and sleep quality in healthy adults . Nucl Med Commun . 2007 ; 28 : 401 – 406 . doi: 10.1097/MNM.0b013e3280bad8b6. [DOI] [PubMed] [Google Scholar]
- Manni R , Terzaghi M , Arbasino C , et al. Obstructive sleep apnea in a clinical series of adult epilepsy patients: frequency and features of the comorbidity . Epilepsia . 2003 ; 44 : 836 – 840 . doi: 10.1046/j.1528-1157.2003.55702.x. [DOI] [PubMed] [Google Scholar]
- Miano S , Bruni O , Elia M , et al. Sleep breathing and periodic leg movement pattern in Angelman syndrome: a polysomnographic study . Clin Neurophysiol . 2005 ; 116 : 2685 – 2692 . doi: 10.1016/j.clinph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Pelc K , Cheron G , Boyd SG , et al. Are there distinctive sleep problems in Angelman syndrome? . Sleep Med . 2008 ; 9 : 434 – 441 . doi: 10.1016/j.sleep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Ville D , Kaminska A , Bahi-Buisson N , et al. Early pattern of epilepsy in the ring chromosome 20 syndrome . Epilepsia . 2006 ; 47 : 543 – 549 . doi: 10.1111/j.1528-1167.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Young D , Nagarjan L , de Klerk N , et al. Sleep problems in Rett syndrome . Brain Dev . 2007 ; 29 : 609 – 616 . doi: 10.1016/j.braindev.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YS , Van Dyke DL , Thorland EC , et al. Mosaic ring 20 with no detectable deletion by FISH analysis: characteristic seizure disorder and literature review . Am J Med Genet A . 2006 ; 140 : 1696 – 706 . doi: 10.1002/ajmg.a.31332. [DOI] [PubMed] [Google Scholar]