Summary
Magnetic resonance imaging (MRI) provides an in vivo assessment of cortical and subcortical regions affected in Parkinson’s disease (PD). This review summarizes the most important conventional and non-conventional MRI techniques applied in this field.
Standard neuroimaging techniques have played a marginal role in the diagnosis and follow-up of PD, essentially being used only to discriminate atypical syndromes from PD, to exclude secondary causes such as vascular lesions, and to confirm the absence of specific imaging features found in atypical parkinsonisms. However, non-conventional MRI techniques, i.e. new neuroimaging approaches such as magnetic resonance spectroscopy, diffusion tensor imaging, and functional MRI, may allow the detection of structural, functional and metabolic changes useful not only for differential diagnosis, but also for early diagnosis and outcome and treatment monitoring in PD. In addition, we illustrate the advantages of high-field MRI over lower magnetic fields, highlighting the great potential of advanced neuroimaging techniques.
Keywords: diffusion tensor imaging, functional magnetic resonance imaging, high-field MRI, magnetic resonance spectroscopy, Parkinson’s disease, volumetric MRI
Introduction
Parkinson’s disease (PD) is a degenerative neurological disorder that is characterized by progressive loss of dopaminergic neurons in the substantia nigra (SN). Idiopathic PD has an incidence of about 85% and is more common than the familial, autosomal hereditary form, which has an incidence of up to 15% (Weintraub et al., 2008). An increasing prevalence of PD can be detected in advanced age: 1% among 60-year-olds and 3% among 80-year-olds (Di Napoli et al., 2007). The pathological process leading to PD begins decades before the typical motor symptoms appear, and by the time the diagnosis is made, about 70% to 80% of striatal dopamine (DA) (Bohnen et al., 2006) and at least one-third of SN neurons (Greffard et al., 2006) and striatal dopaminergic fibers (Marek and Jennings, 2009) are already lost. The four primary symptoms of PD are rigidity, bradykinesia, postural instability and tremor, and they are characteristically asymmetric at onset. PD usually affects people over the age of 50 years. Early symptoms of PD are subtle and occur gradually. As the disease progresses, the tremor may begin to interfere with daily activities. Other symptoms may include depression and other emotional changes. Dementia is twice as prevalent in PD patients as in age-matched controls (Jellinger et al., 2002). Until recently, the diagnosis of PD was based essentially on clinical criteria, such as the findings of akinetic rigid syndrome usually with asymmetric onset, resting tremor, and a sustained, good response to levodopa (Schapira, 2006). Standard neuroimaging techniques have played a marginal role in the diagnosis and follow-up of PD and definitive diagnosis is confirmed by postmortem examination of patients’ brain tissue (Taylor and Counsell, 2006). The value of conventional magnetic resonance imaging (MRI) is still not clear, because the technique is not sensitive enough to detect the anatomical and functional changes induced by PD, especially in the very early stages and in dubious cases. Indeed, in these cases, the MR examination may be negative and the diagnosis based only on neurological evaluation and assessment of therapeutic response. Structural MRI may give normal findings in patients with idiopathic PD, even those with a long disease duration. Standard MRI is essentially used to distinguish atypical syndromes from PD, to exclude secondary causes such as vascular lesions, and to confirm the absence of specific imaging features found in atypical parkinsonisms (Schrag et al., 2000). However, new neuroimaging approaches may make it possible to study more specifically the progression of nigral degeneration. In particular, neuroimaging techniques, such as MR spectroscopy (MRS), diffusion tensor imaging (DTI), and functional MRI (fMRI) have improved the quantification of neuronal damage and offer the possibility of directly measuring the brain’s status and activity.
One of the main advantages of high-field MRI is its high spatial resolution and consequent greater and more accurate anatomical definition. In addition, while magnetic susceptibility artifacts due to the iron selectively deposited in the SN are enhanced by higher magnetic fields, quantification of iron deposits, using the relaxometry technique, greatly benefits from such fields.
The following review aims to provide a brief summary of investigations relating to the early diagnosis of PD and the quantification of the pathological process of the disease, focusing on the use of advanced MRI techniques such as MRS, DTI, and fMRI, recently introduced into the assessment of PD patients.
A literature search was conducted in the online databases of PubMed, ISI Web of Knowledge, DIMDI and COCHRANE, as well as in specialized databases and journals (Tables I and II).
Table I.
Conventional MRI studies in PD patients
| Reference | Imaging method | Magnetic field | Main findings |
|---|---|---|---|
| Pujol et al., 1992 | Conventional | 1.5 T | T2-weighted images showed significant reduction of the width of the SN pars compacta that correlated strongly with PD patients’ motor performance. |
| Oikawa et al., 2002 | Conventional | 1.5 T | SN volume loss was not found in PD patients by using proton density-weighted spin echo or inversion-recovery images. |
| Adachi et al., 1999 | Conventional | 1.5 T | SN size was not reduced in PD patients, but in patients with secondary parkinsonism, as shown using multishot diffusion-weighted imaging. |
| Gorell et al., 1995 | Conventional | 1.5 T | Increased iron content was found within the SN in PD patients by measuring T2 and T2* relaxation times and it was significantly different from that found in the control group. |
| Martin, 2009 | Conventional | 1.5 T | This study showed the presence of lateral SN pars compacta abnormalities in untreated patients with early PD, consistent with increased iron content and corresponding to the known distribution of neuronal loss. |
| Graham et al., 2000 | Conventional | 1.5 T | Elevated iron content was found in the SN of PD patients, and reduced iron levels were found in the putamen; reduction in this region was positively correlated with disease duration. |
| Zijlmans, 2010 | Conventional | 1.5 T | Lacunar infarctions located in unilateral or bilateral basal ganglia and white matter of the frontal lobe were found in patients with vascular parkinsonism. Instead, in PD patients, ischemic changes are not detectable. |
| Gama et al., 2010 | Conventional | 1.5 T | Dimensions and cutoff values obtained from routine MRI could differentiate between PD, PSP and MSA with good sensitivity, specificity and accuracy. |
| Quattrone et al., 2008 | Conventional | 1.5 T | The MR parkinsonism index may help to distinguish patients with PSP from those with PD and MSA-P on an individual basis. |
Abbreviations: MRI=magnetic resonance imaging; SN=substantia nigra; PD=Parkinson’s disease; PSP=progressive supranuclear palsy; MSA=multiple system atrophy; MSA-P=Parkinson variant of multiple system atrophy
Table II.
Studies using advanced neuroimaging techniques in PD patients
| Reference | Imaging method | Magnetic field | Main findings |
|---|---|---|---|
| Martin et al., 2009 | MRI-based volumetric analysis | 1.5 T | This study reported reduced white matter volume in the right temporal lobe of untreated PD patients compared with controls. |
| Geng et al., 2006 | MRI-based volumetric analysis | 3 T | The putamen volume was significantly decreased in patients with early PD and advanced PD compared with controls. The putamen atrophy was correlated with the severity of clinical findings. |
| Schuff, 2009 | MRI-based volumetric analysis | 1.5 T – 3 T and higher | High-field MRI, compared to lower field MRI, could lead to stronger and more robust findings of brain atrophy in the basal ganglia of PD patients, even in the early stages of disease. |
| Sabatini et al., 2000 | fMRI | 1.5 T | The frontal hypoactivation observed in PD patients was restricted to the rostral part of the SMA and to the dorsolateral prefrontal cortex. |
| Péran et al., 2006 | fMRI | 3 T | The identification of activated cortical areas during fMRI could allow tracking of corticostriatal circuit fibers and quantification of their possible depletion in PD. |
| Péran et al., 2009 | fMRI | 3 T | A comparison between GenA and ON revealed slight differences in brain activation located above all in the premotor and prefrontal cortices, particularly as regards GenAMBO > ONMMO. |
| Gattellaro et al., 2009 | DTI | 1.5 T | Widespread microstructural abnormalities were found, even in the early stages of PD, bilaterally in the genu of the corpus callosum, in the superior longitudinal fasciculus, and in the cingulum, but they did not differentiate PD patients from control subjects. |
| Zhan et al., 2012 | DTI | 3 T | Regional and specific alterations in microscopic integrity of white matter and basal ganglia were found in PD subjects, especially in the precentral gyrus, SN, putamen, posterior striatum, frontal lobe, and the SMA. |
| Menke et al., 2009 | DTI | 3 T | The combined use of DESPOT1 and high-field DTI provided a useful tool for more accurate identification and segmentation of the SN based on its connectivity, to differentiate PD patients from control subjects. |
| Heerschap et al., 1993 | 1H MRS | 1.5 T | NAA/Cr was reduced in the SN of PD patients compared with controls. |
| Choe et al., 1998 | 1H MRS | 1.5 T | NAA/Cr was increased in the SN of PD patients compared with controls. |
| Guevara et al., 2010 | 1H MRS | 1.5 T | NAA concentrations in the basal ganglia in MSA and PSP were different compared with PD. |
| Abe et al., 2000 | 1H MRS | 1.5 T | Reduction of the NAA/Cr ratio in the putamen correlated well with the severity of parkinsonism. |
| Lucetti et al., 2007 | 1H MRS | 1.5 T | Cortical NAA/Cr and Cho/Cr ratios could be impaired in de novo PD patients and dopaminergic therapy able to improve motor function could restore the Cho/Cr ratio in the motor cortex. |
| Hattingen et al., 2009 | 1H MRS, 31P MRS | 3 T | This study showed metabolic abnormalities consistent with mitochondrial dysfunction. |
| Griffith et al., 2008 | 1H MRS | 3 T | The Glu/Cr ratio was reduced in PD patients compared with controls, a finding not reported in previous studies performed at 1.5 T. |
Abbreviations: PD=Parkinson’s disease; MSA=multiple system atrophy; PSP=progressive supranuclear palsy; MRI=magnetic resonance imaging; fMRI=functional magnetic resonance imaging; DTI=diffusion tensor imaging; 1H MRS=proton magnetic resonance spectroscopy; 31P MRS=phosphorus magnetic resonance spectroscopy; DESPOT1=driven-equilibrium single-pulse observation of T1; SN=substantia nigra; SMA=supplementary motor area; NAA/Cr=N-acetylaspartate/creatine; Cho/Cr=choline/creatine; Glu/Cr=glutamate/creatine; GenA=generation of action verbs; ON=Object Naming; GenAMBO=generation of action verbs with manipulable biological objects; ONMMO=object naming with man-made objects
Conventional magnetic resonance imaging
Over the past decade various studies set out to investigate nigral degeneration in PD using conventional MRI. Several authors used T2-weighted MR images (Pujol et al., 1992), proton density-weighted spin echo images, inversion-recovery images (Oikawa et al., 2002), or multishot diffusion-weighted imaging (Adachi et al., 1999) in order to determine the area of the SN for group comparisons, and reported contradictory results. Other groups exploited the paramagnetic effect of increased nigral iron content in PD patients (Dexter et al., 1989), measuring T2 and T2* relaxation times (Gorell et al., 1995) and finding significant differences between PD patients and control subjects. Iron promotes important metabolic processes in the brain, which may ultimately result in neuronal death (Barja, 2004).
A recent study, using a 1.5 T MRI scanner, reported increased iron content in the SN pars compacta in PD subjects. The most marked changes were found in severe disease, which suggests that measurement of regional iron content in the SN may provide an indication of the severity of the disease (Martin, 2009). It is also possible to evaluate brain iron deposition in vivo using high-field strength spin-echo T2-weighted MRI (Graham et al., 2000). However, technical limitations of the scanners, due to the intrinsic characteristics and small size of the anatomical structures involved in the disease, make MR examination particularly difficult in PD. Indeed, given the small size of the SN – in normal individuals, it usually measures just a few square millimeters –, high spatial resolution is crucial for its precise quantification.
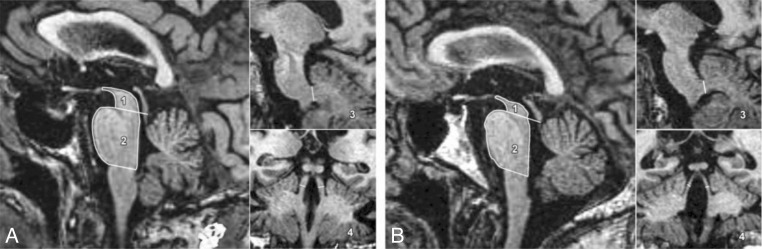
Moreover, conventional MRI may, when it gives positive findings, support a clinical diagnosis of vascular parkinsonism and may also help to distinguish this condition from PD and from other parkinsonisms. For example, there are obvious imaging changes in patients with vascular parkinsonism, mainly manifested as lacunar infarction located in unilateral or bilateral basal ganglia and in the white matter of the frontal lobe. Furthermore, ischemic changes have been seen in the cerebral peduncle and lateral cerebral ventricle. Conversely, ischemic changes are not detectable in PD (Zijlmans, 2010). Other studies have evaluated the diagnostic value of conventional MRI in the differential diagnosis of PD, progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). A recent study showed differences between PD, PSP and MSA in the morphometry of the pons area, and middle and superior cerebellar peduncles. Dimensions and cutoff values of these brain structures obtained from routine MRI may differentiate among PD, PSP and MSA with good sensitivity, specificity and accuracy (Gama et al., 2010). In another study, an MR parkinsonism index was calculated on the basis of the pons area-midbrain area ratio (P/M) and the middle cerebellar peduncles width-superior cerebellar peduncles width ratio (MCP/SCP), for the purpose of differentiating PSP from PD and the Parkinson variant of multiple system atrophy (MSA-P) (Fig. 1). The authors also reported the values of brain structure measurements in PSP, MSA-P, PD patients and normal controls (Table III). The study results showed that the MR parkinsonism index [(P/M) (MCP/SCP)] may help to distinguish patients with PSP from those with PD and MSA-P on an individual basis (Quattrone et al., 2008). Indeed, the MR parkinsonism index value was greater in patients with PSP than in patients with PD, MSA and normal controls.
Figure 1.
Volumetric MRI evidence of brain areas.
Sagittal and coronal T1-weighted volumetric spoiled gradient-echo MR images (15.2/6.8; section thickness, 0.6 mm; frequency- and phase-encoding matrix, 256×256; flip angle, 15°) show midbrain area (1), pons area (2), width of middle cerebellar peduncles (MCP) (3), and width of superior cerebellar peduncles (SCP) (4) in (A) a control participant and (B) a patient with progressive supranuclear palsy (PSP). Images show marked atrophy of both midbrain and SCP in the PSP patient in comparison with the healthy control participant. In the patient with PSP, values were as follows: midbrain area, 60 mm2; pons area, 502 mm2; MCP width, 8.15 mm; and SCP width, 1.70 mm. In the control participant, values were as follows: midbrain area, 108 mm2; pons area, 478 mm2; MCP width, 10.05 mm; and SCP width, 4.10 mm.
From: Quattrone et al., 2008 (used with permission)
Table III.
Measurements for brain structures in patients with PSP, MSA-P, and PD and control participants
| Group | Midbrain area (mm2) | Pons area (mm2) | SCP width (mm) | MCP width (mm) |
|---|---|---|---|---|
| PSP patients (n = 33) | 63 (38–94) | 406 (230–516) | 2.4 (1.6–3.3) | 7.0 (5.1–9.4) |
| MSA-P patients (n = 19) | 110 (63–132) | 343 (244–492) | 2.9 (1.9–3.9) | 5.9 (3.4–7.7) |
| PD patients (n = 108) | 119 (70–163) | 471 (372–615) | 3.7 (3.0–4.8) | 8.7 (8.1–10.2) |
| Control participants (n = 50) | 122 (94–168) | 469 (386–622) | 3.8 (3.1–4.5) | 8.8 (8.1–10.5) |
Note. – Values are medians, and numbers in parentheses are ranges. For all measurements, p<.001 (Kruskal-Wallis test).
From: Quattrone et al., 2008 (used with permission)
Finally, a few studies using high-field conventional MRI are reported; several studies using 1.5 T MRI scanners have already given important results, especially in the in vivo evaluation of brain iron deposition and in differential diagnosis (Table I). However, highfield MRI may provide a new and better method for investigating neurological diseases that result in alteration of brain iron levels in specific areas of the human brain; thus, high-field conventional MRI should be better investigated. Recent developments in MRI technology have led to improved contrast and resolution and are opening up new possibilities for the study of human brain anatomy and functioning. In particular, advanced neuroimaging techniques, sensitized to magnetic susceptibility contrast, provide several advantages especially at high field, allowing the visualization of brain structures that have been difficult to detect using conventional technology.
High-field advanced neuroimaging techniques
Non-conventional MRI techniques could be used to support clinical diagnoses of various motor disturbances, given that structural abnormalities of the pyramidal tract, basal ganglia and cerebellum are easily observed and can be measured using advanced neuroimaging techniques. High magnetic fields can benefit the non-conventional MRI techniques in particular (Péran et al., 2006). This is especially true of fMRI, which benefits from the higher signal-to-noise ratio and increased blood oxygen level-dependent (BOLD) contrast associated with high fields. High-field fMRI studies provide high-resolution images that can be rapidly acquired and with good temporal resolution. Moreover, high-field DTI studies are better able to detect microstructural changes, particularly in white matter, while high-field MRS provides an increased signal-to-noise ratio of the artifact-free spectra, enhanced precision and sensitivity in the quantification of neurochemical metabolites, and increased spectral resolution.
MRI-based volumetric analysis
MRI-based volumetric analysis using 1.5 T scans has been used to identify focal cortical abnormalities in early PD. A recent study reported reduced white matter volume in the right temporal lobe of 26 untreated PD patients compared with 14 controls, in particular in the anterior right fusiform gyrus and superior temporal gyrus. All the patients fulfilled standard criteria for a clinical diagnosis of PD (the UPDRS motor score was 15.8±6.9; the disease duration was 3.0±1.7 years) and none were treated with levodopa or dopamine agonists. These results suggest that right anterior temporal lobe changes occur in untreated PD patients. The earliest changes may occur in subcortical white matter rather than the temporal cortex (Fig. 2, over) (Martin et al., 2009). No similar studies are reported in the literature.
Figure 2.
Voxel-based morphometry of gray and subcortical white matter.
Regions where control volumes are greater than patient volumes (FDR corrected p<0.05). The clusters are located in the white matter (WM) nearest to the right fusiform gyrus and the right superior temporal gyrus. A glass brain schematic is shown in (A). The clusters are overlaid on the average WM image for all the subjects in the study, shown as axial sections (B) and coronal sections (C). The color bar indicates the value of the t-statistic.
From: Martin et al., 2009 (used with permission)
Volumetric studies with 3 T MRI have clearly documented morphological changes in the SN and basal ganglia nuclei. In particular, the whole-brain volume and the volumes of the caudate, putamen, globus pallidus, and SN were calculated on three-dimensional reconstructed images, with the aim of evaluating the ability of high-field MRI-based volumetric analysis to detect morphological changes in these cerebral regions and to investigate the relationship between severity of clinical findings and degree of morphological change. A recent study reported a significantly decreased putamen volume in patients with early PD and advanced PD compared with normal controls. Thus, high-field MRI-based volumetric measurement is a sensitive method for the in vivo assessment of morphological changes in PD. The putamen atrophy was found to be correlated with the severity of clinical findings. This finding could prove valuable for the early diagnosis of PD as the volumetric measurement of the putamen could potentially be a useful indicator of PD in the early stage of the disease (Geng et al., 2006).
MRI volumetric studies performed using 1.5 T scanners allowed the detection of structural alterations in the basal ganglia but significant volume loss became obvious only in the advanced disease stages. Moreover, MRI studies measuring the width of the SN demonstrated loss of signal in a lateral to medial gradient in PD, correlating with conventional clinical measures of disease severity, but only in a small number of patients. Thus, these results show that morphological changes in PD exist, and, in particular, that the greater sensitivity and higher tissue contrast of MRI at higher magnetic fields may, compared with MRI at lower magnetic fields, lead to stronger and more robust findings of brain atrophy in PD (Schuff, 2006).
Functional magnetic resonance imaging
Functional imaging techniques are useful tools for the in vivo assessment of functional connectivity in PD. In activation studies using fMRI, the BOLD signal is detected as an indirect marker of neuronal activity. Functional imaging techniques have been applied to infer the potential role of inflammation and other factors in the etiopathogenesis of PD, as well as to study compensatory and regulatory mechanisms in early PD and subclinical disease in genetic forms of PD. Functional imaging can provide meaningful insights into mechanisms underlying various aspects of motor and non-motor dysfunction in PD and the role of striatal dopaminergic transmission in behavioral processes beyond motor control (Nandhagopal et al., 2008).
Functional MRI, compared with positron emission tomography (PET), is characterized by higher spatial and temporal resolution, better availability and absence of radiating isotopes. Measurement of BOLD fMRI signal increases allows the functional networks in PD and their pharmacological modulation to be analyzed with high spatial and temporal resolution in comparison with PET (Sabatini et al., 2000). Therefore, high-field MRI makes it easier to study cortical activation in PD. Moreover, fMRI data can be correlated to structural studies at the level of the mesencephalon and basal ganglia. The identification of activated cortical areas during fMRI may make it possible to track corticostriatal circuit fibers and to quantify their possible depletion in PD (Péran et al., 2006).
Functional MRI with a 1.5 T scanner has been used in in akinetic PD patients and healthy subjects to study cortical activation changes induced by performance of a complex sequential motor task. Compared with the normal subjects, the PD patients exhibited a relatively decreased fMRI signal, specifically in the rostral part of the supplementary motor area (SMA) and in the right dorsolateral prefrontal cortex. These fMRI data confirm that the frontal hypoactivation observed in PD patients is restricted to these areas (Sabatini et al., 2000).
The increased and more accurate high-field fMRI BOLD signal allows better investigation of cortical activation patterns not only during complex motor tasks but also during complex cognitive tasks.
A recent study, for example, because of PD patients’ difficulty in producing verbs in a word generation situation, investigated the neuronal substrates of action-related word production, using an fMRI paradigm with a 3 T scanner. Comparison of the distribution of brain activities during two tasks, the object naming (ON) and the generation of action-verbs (GenA), revealed slight differences located above all in the premotor and prefrontal cortices (Péran et al., 2009). In the ON task, the main activations were observed bilaterally in the frontal cortex, superior temporal cortex, supplementary motor area, inferior occipital cortex, fusiform gyrus, lingual gyrus and cerebellum. A very similar activation pattern was observed for the GenA task: the only differences were greater activation in the fronto-temporal cortices and in the left inferior and superior parietal cortex (Fig. 3).
Figure 3.
Functional magnetic activation maps after object naming and action verb generation.
Activation maps (p<.05 at cluster level) in each condition (blue: manipulable biological objects; orange: man-made objects). A: object naming, B: generation of action verbs.
From: Péran et al., 2009 (used with permission)
Diffusion tensor imaging
Diffusion tensor imaging offers a unique window onto the connectivity changes, extending beyond the basal ganglia, that accompany the cognitive symptoms of PD. The main purpose of this imaging technique is to assess the microstructural cerebral white matter damage occurring in idiopathic PD. Several DTI studies, using 1.5 T scanners, demonstrated that widespread microstructural damage to frontal and parietal white matter is already present in the early stages of PD (Gattellaro et al., 2009).
In particular, a recent study provided evidence for widespread microstructural abnormalities extending beyond the basal ganglia in PD patients without dementia, occurring bilaterally in the genu of the corpus callosum, in the superior longitudinal fasciculus, and in the cingulum. Nevertheless, the abnormalities observed in this study did not differentiate PD patients from control subjects (Gattellaro et al., 2009).
Diffusion tensor imaging studies using high-field MRI have shown this advanced technique to be capable of detecting regional and specific alterations in the microscopic integrity of the white matter and basal ganglia known to be involved in PD pathology. An association between diffusion abnormality, PD severity and parkinsonism subtype was hypothesized. In particular, in a very recent study, reduced fractional anisotropy was found in PD subjects in regions related to the precentral gyrus, SN, putamen, posterior striatum, frontal lobe, and SMA. Reduced fractional anisotropy in the SN correlated with increased rating scale motor scores (Fig. 4, over). Significant spatial correlations between fractional anisotropy alterations in the putamen and other PD-affected regions were also found in the context of PD subtypes fractional anisotropy index analysis. Thus, microstructural alterations detected with high-field DTI might be considered potential biomarkers for PD (Zhan et al., 2012).
Figure 4.
Regional alterations detected by diffusion tensor imaging.
Significant voxelwise correlations (p<0.05, corrected) between decreased fractional anisotropy and increased total Unified Parkinson’s Disease Rating Scale scores were detected in the white matter at the level of the substantia nigra.
From: Zhan et al., 2012 (used with permission)
Moreover, a recent study demonstrated that combination of the driven-equilibrium single-pulse observation of T1 (DESPOT1) quantitative imaging method with high-field DTI provides images allowing easy identification of the SN and is a useful tool for accurate segmentation of the SN based on its connectivity. Specifically, the DESPOT1 method allows a clear visualization of the SN as a whole. Volumetric comparisons between PD patients and healthy subjects revealed significantly smaller volumes in patients for both the left and the right sides when the whole SN was considered. This model that combines SN volumetry and SN connectivity with the thalamus improved the sensitivity to 100% and specificity to 80% for PD patients. Thus, DESPOT1 and high-field DTI, in combination, provide a useful set of markers for differentiating PD patients from healthy subjects (Menke et al., 2009).
Magnetic resonance spectroscopy
Proton MRS (1H MRS) is a useful non-invasive method for studying central nervous system pathologies that allows in vivo investigation of cerebral metabolites. The main metabolite signals detected by 1H MRS include N-acetylaspartate (NAA; an amino acid contained almost exclusively within neurons), choline (Cho; a metabolite involved in phospholipid membrane synthesis), myo-Inositol (mI; a metabolite contained in glial cells), and creatine (Cr; a cellular energy buffering system). These metabolites provide information that cannot be derived from structural MRI. NAA is a putative marker of neuronal integrity. Cr is a key energy metabolite and, therefore, a possible indicator of defective energy metabolism. Altered neuronal membrane synthesis and degradation can result in changes in Cho. Finally, several processes, including gliosis, observed in neurodegenerative disorders are suspected to manifest as changes in mI (O’Neill et al., 2002). Brain regions where these markers might be found include the SN, basal ganglia (putamen, caudate, globus pallidus, and thalamus), and motor and prefrontal association cortices, and changes in these metabolites might also be visible in the early stages of PD. Several quantitative MRS studies with PD patients, using 1.5 T scanners, have yielded ambiguous results. Compared with controls, one 1H MRS study demonstrated reduced NAA/Cr in the SN of PD patients (Heerschap et al., 1993), while another study found increased NAA/Cr in the same region (Choe et al., 1998).
A recent study, using a 1.5 T scanner, confirmed the hypothesis that NAA concentrations in the basal ganglia in MSA and PSP would show differences compared with PD, specifically in the pallidum, putamen and lentiform nucleus. These findings support the hypothesis that MRS can potentially quantify basal ganglia cellular pathology in MSA and PSP and differentiate these diseases from PD (Guevara et al., 2010).
Moreover, another study demonstrated a reduced NAA/Cr ratio in the putamen that correlated well with the severity of parkinsonism and it was remarked that 1H MRS may also be useful in monitoring patients with various types of parkinsonism (Abe et al., 2000).
1H MRS shows considerable potential as a tool for monitoring pharmacological therapy in PD patients. A study investigating neurochemical and metabolic changes in the motor cortex in a group of de novo PD patients before and after six months of dopamine agonist treatment reported lower Cho/Cr and NAA/Cr ratio values in the motor cortex of PD patients compared with controls. After six months’ therapy, the PD patients showed improved motor performances and increased Cho/Cr ratios in the motor cortex. Thus, this study demonstrated that cortical NAA/Cr and Cho/Cr ratios may be impaired in de novo PD patients and that dopaminergic therapy able to improve motor function may restore the Cho/Cr ratio in the motor cortex (Fig. 5) (Lucetti et al., 2007).
Figure 5.
Brain magnetic resonance spectroscopy images of pre- and post-pergolide treatment.
Each spectrum – (A) of a patient at baseline; (B) of the same patient after 6 mo of pergolide therapy – shows the peaks corresponding to the main brain metabolites N-acetylaspartate (NAA), choline (Cho), myo-Inositol (mI), and phosphocreatine/creatine (Cr).
From: Lucetti et al., 2007 (used with permission)
Despite the existence of some in vitro data, the measurement of cerebral mitochondrial dysfunction in PD patients is challenging. 1H and 31P MRS are powerful non-invasive techniques that allow in vivo evaluation of lactate, a marker of anaerobic glycolysis, and high-energy phosphates, such as adenosine triphosphate and phosphocreatine, directly reflecting mitochondrial function. There is strong in vivo1H and 31P MRS evidence showing that mitochondrial dysfunction of mesostriatal neurons is a central and persistent phenomenon in the pathogenetic cascade of PD in the early stage of the disease. Indeed, several 1H and 31P MRS studies demonstrate metabolic abnormalities consistent with mitochondrial dysfunction, and recent MRS data reveal abnormally elevated lactate levels in PD patients (Hattingen et al., 2009).
Moreover, a recent high-field 1H MRS study documented changes in the metabolite glutamate (Glu) that have not been reported in previous studies performed at 1.5 T. Glu plays a key role in long-term potentiation and is important for learning and memory. In particular, this study showed that the Glu/Cr ratio was reduced in PD patients compared with controls (t=2.54; p=0.019), whereas no differences were observed in NAA/Cr or Cho/Cr ratios. These findings suggest that a reduction in Glu occurs in the cerebral cortex of PD patients (Griffith et al., 2008).
Concluding remarks
A PD diagnosis based on clinical evaluation may be influenced by examiner variability and potential incorrect diagnoses can impact negatively on the outcomes of therapeutic interventions and clinical trials. Thus, the development of neuroimaging techniques is critical to confirm the diagnosis and to assess disease progression and pharmacological treatment. MRI is a non-invasive objective method of assessing in vivo the structure and function of cortical and subcortical regions affected in PD. Nevertheless, using 1.5 T magnetic field, structural and functional alterations in basal ganglia become obvious only in the advanced disease stages.
Recent advances in high-field MRI technology (3 T and higher), compared with lower field MRI, provide increased signal sensitivity and higher tissue contrast intrinsic to the brain, and thus offer new opportunities for assessing brain alterations in PD. High-field MRI may allow greater detection of abnormalities in cortical and subcortical brain structure volume, white matter regional and specific microstructural alterations, and metabolic brain changes even in the early stages of the disease, and may differentiate PD from other parkinsonisms with increased sensitivity and specificity. Indeed, the advanced neuroimaging techniques, such as fMRI, DTI and MRS, greatly benefit from highfield MRI, providing imaging markers for the early diagnosis of PD, predicting the rate of disease progression, assessing and monitoring neuroprotective and disease-modifying pharmacological interventions, and also allowing a more accurate differential diagnosis and outcome evaluation in parkinsonisms.
References
- Abe K, Terakawa H, Takanashi M, et al. Proton magnetic resonance spectroscopy of patients with parkinsonism. Brain Res Bull. 2000;52:589–595. doi: 10.1016/s0361-9230(00)00321-x. [DOI] [PubMed] [Google Scholar]
- Adachi M, Hosoya T, Haku T, et al. Evaluation of thesubstantia nigra in patients with Parkinsonian syndrome accomplished using multishot diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 1999;20:1500–1506. [PMC free article] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Choe BY, Park JW, Lee KS, et al. Neuronal laterality in Parkinson’s disease with unilateral symptom by in vivo 1H magnetic resonance spectroscopy. Invest Radiol. 1998;33:450–455. doi: 10.1097/00004424-199808000-00005. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Wells FR, Lees AJ, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Shah IM, Stewart DA. Molecular pathways and genetic aspects of Parkinson’s disease: from bench to bedside. Expert Rev Neurother. 2007;7:1693–1729. doi: 10.1586/14737175.7.12.1693. [DOI] [PubMed] [Google Scholar]
- Gama RL, Távora DF, Bomfim RC, et al. Morphometry MRI in the differential diagnosis of parkinsonian syndromes. Arq Neuropsiquiatr. 2010;68:333–338. doi: 10.1590/s0004-282x2010000300001. [DOI] [PubMed] [Google Scholar]
- Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery. 2006;58:256–262. doi: 10.1227/01.NEU.0000194845.19462.7B. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Ordidge RJ, Brown GG, et al. Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology. 1995;45:1138–1143. doi: 10.1212/wnl.45.6.1138. [DOI] [PubMed] [Google Scholar]
- Graham JM, Paley MN, Grünewald RA, et al. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain. 2000;123:2423–2431. doi: 10.1093/brain/123.12.2423. [DOI] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet AM, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;2006;63:584–588. doi: 10.1001/archneur.63.4.584. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Okonkwo OC, O’Brien T, et al. Reduced brain glutamate in patients with Parkinson’s disease. NMR Biomed. 2008;21:381–387. doi: 10.1002/nbm.1203. [DOI] [PubMed] [Google Scholar]
- Guevara CA, Blain CR, Stahl D, et al. Quantitative magnetic resonance spectroscopic imaging in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Eur J Neurol. 2010;17:1193–1202. doi: 10.1111/j.1468-1331.2010.03010.x. [DOI] [PubMed] [Google Scholar]
- Hattingen E, Magerkurth J, Pilatus U, et al. Phosphorus and proton magnetic resonance spectroscopy demon-stratesmitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009;132:3285–3297. doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- Heerschap A, Zijlmans J, de Koster A, et al. Metabolite levels at three brain locations in parkinsonism as viewed by proton MRS. SMRM, 12th Annual Meeting; New York. 1993. p. 234. [Google Scholar]
- Jellinger KA, Seppi K, Wenning GK, et al. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm. 2002;109:329–339. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- Lucetti C, Del Dotto P, Gambaccini G, et al. Influences of dopaminergic treatment on motor cortex in Parkinson disease: a MRI/MRS study. Mov Disord. 2007;22:2170–2175. doi: 10.1002/mds.21576. [DOI] [PubMed] [Google Scholar]
- Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology. 2009;72:S21–S26. doi: 10.1212/WNL.0b013e318198df97. [DOI] [PubMed] [Google Scholar]
- Martin WR. Quantitative estimation of regional brain iron with magnetic resonance imaging. Parkinsonism Relat Disord. 2009;15:S215–218. doi: 10.1016/S1353-8020(09)70818-1. [DOI] [PubMed] [Google Scholar]
- Martin WR, Wieler M, Gee M, et al. Temporal lobe changes in early, untreated Parkinson’s disease. Mov Disord. 2009;24:1949–1954. doi: 10.1002/mds.22680. [DOI] [PubMed] [Google Scholar]
- Menke RA, Scholz J, Miller KL, et al. MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. Neuroimage. 2009;47:435–441. doi: 10.1016/j.neuroimage.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, McKeown MJ, Jon Stoessl A. Functional imaging in Parkinson disease. Neurology. 2008;70:1478–1488. doi: 10.1212/01.wnl.0000310432.92489.90. [DOI] [PubMed] [Google Scholar]
- Oikawa H, Sasaki M, Tamakawa Y, et al. The substantia nigra in Parkinson disease: proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. AJNR Am J Neuroradiol. 2002;23:1747–1756. [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Schuff N, Marks WJ, Jr, et al. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Mov Disord. 2002;17:917–927. doi: 10.1002/mds.10214. [DOI] [PubMed] [Google Scholar]
- Péran P, Luccichenti G, Cherubini A, et al. High-field neuroimaging in Parkinson’s disease. In: Salvolini U, Scarabino T, editors. High Field Brain MRI, Use in Clinical Practice. Berlin, Heidelberg: Springer-Verlag; 2006. pp. 194–200. [Google Scholar]
- Péran P, Cardebat D, Cherubini A, et al. Object naming and action-verb generation in Parkinson’s disease: a fMRI study. Cortex. 2009;45:960–971. doi: 10.1016/j.cortex.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Pujol J, Junqué C, Vendrell P, et al. Reduction of the substantia nigra width and motor decline in aging and Parkinson’s disease. Arch Neurol. 1992;49:1119–1122. doi: 10.1001/archneur.1992.00530350033015. [DOI] [PubMed] [Google Scholar]
- Quattrone A, Nicoletti G, Messina D, et al. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology. 2008;246:214–221. doi: 10.1148/radiol.2453061703. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Etiology of Parkinson’s disease. Neurology. 2006;66:S10–23. doi: 10.1212/wnl.66.10_suppl_4.s10. [DOI] [PubMed] [Google Scholar]
- Schrag A, Good CD, Miskiel K, et al. Differentation of atypical parkinsonian syndrome with routine MRI. Neurology. 2000;54:697–702. doi: 10.1212/wnl.54.3.697. [DOI] [PubMed] [Google Scholar]
- Schuff N. Potential role of high-field MRI for studies in Parkinson’s disease. Mov Disord. 2006;24:S684–S690. doi: 10.1002/mds.22647. [DOI] [PubMed] [Google Scholar]
- Taylor KC, Counsell C. Is it Parkinson’s disease, and if not, what is it? Pract Neurol. 2006;6:154–165. [Google Scholar]
- Weintraub D, Comella CL, Horn S. Parkinson’s disease-part 1: pathophysiology, symptoms, burden, diagnosis and assessment. Am J Manag Care. 2008;14:S40–48. [PubMed] [Google Scholar]
- Zhan W, Kang GA, Glass GA, et al. Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov Disord. 2012;27:90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans JC. The role of imaging in the diagnosis of vascular parkinsonism. Neuroimaging Clin N Am. 2010;20:69–76. doi: 10.1016/j.nic.2009.08.006. [DOI] [PubMed] [Google Scholar]