Summary
Awake surgery requires coordinated teamwork and communication between the surgeon and the anesthesiologist, as he monitors the patient, the neuroradiologist as he interprets the images for intraoperative confirmation, and the neuropsychologist and neurophysiologist as they evaluate in real-time the patient’s responses to commands and questions. To improve comparison across published studies on clinical assessment and operative settings in awake surgery, we reviewed the literature, focusing on methodological differences and aims. In complex, interdisciplinary medical care, such differences can affect the outcome and the cost-benefit ratio of the treatment. Standardization of intraoperative mapping and related controversies will be discussed in Part II.
Keywords: brain tumors , complications , language assessment , neuropsychological assessment , surgical treatment
Introduction
With the advent of new anesthetic agents and the refinement of surgical techniques, awake surgery coupled with cortical mapping continues to push forward the frontiers of neurosurgery, aided by advances in imaging technologies, including functional magnetic resonance imaging (fMRI), magnetoencephalography, event-related potentials, electroencephalography (EEG), positron-emission tomography, transcranial magnetic stimulation and optical imaging ( Simos et al., 1999; Rutten et al., 1999; Ruge et al., 1999; Pouration et al., 2002 ; Bookheimer et al., 1997; Papanicolau et al., 1999 ; Nariai et al., 2005; Najib et al., 2011). New developments in information technology and image-guided surgery have prompted researchers to compare non-invasive and invasive mapping in the awake patient ( Rutten et al., 2002a; Hill et al., 2000; Kamada et al., 2007). But despite its rapid evolution, the basic technical principles of electrocortical mapping have remained essentially the same since Wilder Penfield’s groundbreaking studies in the first half of the 20 th century. His technique remains the gold standard for language mapping ( Fitzgerald et al., 1997 ; Pouration et al., 2004 ; Wiedemayer et al., 2004), wherein task disruption during cortical stimulation is taken to indicate that the underlying cortical area is essential for task performance. What has changed is the increasing feasibility of in vivo brain mapping, which is both safe and acceptable for the patient, and the greater variety of selective tasks ( Sielbergerd et al., 1992 ; Bulsara et al., 2005; Serletis and Bernstein, 2007).
From the basic sciences to clinical applications, new avenues of inquiry have been opened up by translational research and cooperation between neuroscientists and neurosurgeons. The connection between brain location and function is now viewed in the context of a complex anatomo-functional scenario that encompasses local cytoarchitectural variability, multimodal pathways and dynamic compensatory mechanisms, all extending far beyond the original notion of lateralization of brain function ( Duffau et al., 2002, 2006; Duffau 2005a; Faugeras et al., 2004). This perspective differs significantly from that of the isolated pioneers of Penfield’s time, thus precluding comparison between then and now. Today’s sophisticated instruments and multidisciplinary teams are complementary aspects of the same innovation that ushered in the new translational era ( Vigneau et al., 2006; Tharin and Golby, 2007). Through closer cooperation between scientists and clinicians, we can refine our methods of collecting data before, during and after awake surgery, as well as improve the criteria for selecting, defining and classifying parameters of interest, thereby reducing the risk of drawing misleading conclusions.
Objectives
This two-part article reviews the indications for intra-operative brain mapping, the role played by each specialist on the team, and the quality of the published evidence. It goes on to discuss how these components fit together in clinical practice.
Methods of the review
Full-text articles were retrieved independently by two authors and then, using a pre-established format, submitted for data extraction and summary to the other authors according to their relative areas of expertise. In a first step, the selected articles were discussed by all the authors to create a knowledge basis and to define a common methodology and terminology, given the authors’ diverse professional backgrounds. For practical purposes, this study was divided into two parts. Part I focuses on:
the feasibility and efficacy of awake surgery;
anesthesia management;
electrical simulation characteristics;
clinical settings and outcome assessment.
Papers were included for review only if their endpoints were both intraoperative mapping and awake surgery.
In Part II of this article ( Talacchi et al., 2013), devoted to language and cognitive mapping, we focus on:
- the potential and limitations of intraoperative cognitive mapping;
- the representation and reproducibility of language and non-language functions.
The studies included in this part of the review report on cognitive end-points as measured by neurophysiological techniques applied to clinical research.
Rationale for the clinical review
Building on Penfield’s pioneering work, Ojemann, during the 1970s and 1980s, developed reliable concepts for cortical language mapping; his protocol remained a milestone for future studies ( Ojemann, 1979, Ojemann et al., 1989). In awake patients, the choice of visual object naming tasks, as suggested by Penfield’s clinical observations, was initially supported by findings that anomia is the most sensitive clinical deficit ( Saetti et al., 1999). This was later confirmed by intraoperative ( Haglund et al., 1994) and clinical studies in epilepsy and tumor surgery ( Haglund et al., 1994; Ojemann and Dodrill, 1985; Sanai et al., 2008).
While the initial assumption was that no electrically identified areas should be removed if postsurgical language complications were to be avoided, it was later increasingly assumed that postsurgical language deficits would not occur following resection of the cortical areas that did not generate language deficits after electrical stimulation ( Sanai et al., 2008). This indirect message is gaining acceptance, although most studies lack comprehensive pre- and postoperative clinical assessment and objective determination of cognitive complications.
Moreover, the original assumption that resection of an essential language area will result in postoperative aphasia has not been definitively confirmed to date ( Peraud et al., 2004; Seeck et al., 2006), nor has the assumption that sparing positive sites for a naming task will necessarily preserve other language functions ( Whittle et al., 2003, 2005; Petrovich Brennan et al., 2007; Hamberger et al., 2005). As we continue to move from an intraoperative naming-assisted surgical resection to other language and cognitive tasks and from cortical to subcortical stimulation, the need has emerged for a critical appraisal of current methods, classification schemes and definitions.
The aims of tumor surgery and epilepsy surgery differ: minimizing neurological sequelae is only one aspect of treatment that can be tailored to the features of a lesion, as documented by clinical and instrumental studies. What essentially distinguishes cancer from epilepsy are the presenting symptoms and impairment. Improvement of preoperative clinical impairment and radical tumor resection are the end-points of tumor surgery, while improvement of preoperative performance is the end-point of epilepsy treatment ( Buckner et al., 2001; Hamberger, 2007).
In glioma surgery, for example, increased indications for tumor removal, a higher rate of radical tumor resection, and a lower rate of postoperative impairments have all been recognized ( Duffau, 2005b), but there is a need for better quality evidence confirming the clinical advantages.
Furthermore, while cortical mapping was originally applied to epilepsy surgery where resection is limited to the cortex, its indications were later extended to tumor surgery involving the white matter. With the advent of subcortical neurofunctional imaging techniques, the question as to whether and how these differences imply different clinical and operative settings has recently been raised. There are mixed situations between extremes. Low-grade gliomas benefit most from awake surgery. They pose a considerable challenge in that they share characteristics of both epilepsy and tumors, with a long history that could influence neurofunctional anatomy in patients with a normal neurological examination ( Duffau et al., 2005b; Duffau 2005b, 2006a, b , 2007). In this review, we will focus on brain tumor surgery in different clinical situations.
Feasibility and efficacy of awake surgery
Awake surgery procedures pose a series of challenges, namely the need for: integration of different types of knowledge; coordination of a multidisciplinary team of specialists; cooperation in different settings (operating room, ward, outpatient clinic); application of surgical and research protocols; and technical adjustments to make research comparable. The requisites for awake surgery vary and it therefore includes a great variety of resources selected case-by-case: it ranges from a minimalistic approach that reduces hospitalization and discomfort for the patient, with or without cognitive mapping, to a more complex multidisciplinary approach involving specialists in neurophysiology, cognition and rehabilitation ( Ebel et al., 2000; Blanshard et al., 2001). The feasibility of awake surgery has been studied in comparison with general anesthesia, albeit without an economic or time-cost analysis of treatment.
Absolute contraindications to anesthesia in awake surgery are obstructive sleep apnea and difficult intubation ( Picht et al., 2006).
Duration of surgery: Gupta et al. (2007) reported a shorter mean operating time in the general anesthesia group than in the awake surgery group (182 vs 196 min; p<0.05), as did Keifer et al. (2005) and Taylor and Bernstein (1999) (195 and 209 min, respectively). Bello et al. (2007) reported much longer operating times (mean: 345 min, longest: 405 min, and mean awake time; 105 min). Whittle et al. (2005) reported a mean awake time of 62 min (range: 10–105 min).
Intraoperative medical complications are classified as: anesthetic (inadequate or excessive sedation, pain, nausea, vomiting); respiratory (oxygen saturation [SpO 2 ] <90%, increased CO 2 , hypoventilation <8 breaths/min, airway obstruction); hemodynamic (hyper- or hypotension, tachy- or bradycardia); and neurological (convulsions, brain swelling, new neurological deficit) ( Sarang and Dinsmore, 2003; Keifer et al., 2005). Skucas and Artru (2006) focused on medical complications, including airway problems, hypoxemia, hypertension, hypotension, tachycardia, brady-cardia, hypercapnia, seizures, nausea, poor patient cooperation, brain swelling and local anesthetic toxicity. In their review of the literature, they found that hyper- and hypotension are frequent in awake surgery (11 and 56%, respectively). In their study involving 332 patients, they observed that airway problems are infrequent: only 2% of patients developed hypoxemia (SpO 2 <90%) and only 1.8% required intubation or placement of a respiratory device. Respiratory problems occurred more frequently in obese patients and those with asthma or chronic obstructive pulmonary disease. Interestingly, whereas they noted that intractable seizures occurred in only 3% of patients, rates of up to 16% were reported by other authors ( Serletis and Bernstein, 2007; Taylor and Bernstein, 1999; Bello et al., 2007; Petrovich Brennan et al., 2007). The use of propofol to reduce intraoperative seizures has been recommended ( Gignac et al., 1993; Herrick et al., 1997; Danks et al., 1998, Huncke et al., 1998; Berkenstadt et al., 2001; Sarang and Dinsmore, 2003). Patient agitation and lack of compliance were reported among the exclusion criteria.
Blood loss: Gupta et al. (2007) observed that there is less blood loss in awake surgery than in general anesthesia (266 vs 365 ml; p<0.05).
Local postoperative complications: Taylor and Bernstein (1999) found a 2.5% rate of wound complications and postoperative hematoma, similar to that reported in a large 1995 study on 1427 elective supratentorial craniotomies.
Complaints of discomfort include minor disturbances in 25% ( Otani et al., 2005) and 28% of cases ( Danks et al., 1998), anxiety in 29% ( Whittle et al., 2003), fear in 15% ( Whittle et al., 2005), fatigue in 40% ( Bello et al., 2007), and significant discomfort in 20% ( Danks et al., 1998).
Mean postoperative hospital stay and intensive care unit (ICU) admission were not found to be significant factors ( Gupta et al., 2007). Awake craniotomy was associated with low morbidity and mortality and reduced the need for ICU admission and total hospital stay. It minimized invasive intraoperative monitoring, lowering the incidence of infectious complications. There is evidence that appropriate monitoring can help in the prevention and treatment of secondary damage during and after a neurosurgical procedure and that, because it measures the exact level of sedation without risk, monitoring can offer greater safety and comfort ( Taylor and Bernstein, 1999; Serletis and Bernstein, 2007; Blanshard et al., 2001).
Patient age: there is general consensus that patients must be older than 11 years of age ( Berger et al., 1989). Establishing local anesthesia as a valid alternative to general anesthesia could eventually extend the indications of cognitive mapping and research, regardless of location and clinical presentation.
The efficacy of awake surgery has been compared with an alternative treatment modality using implanted grid electrodes, a two-stage in vivo mapping procedure done prior to resection. Early and recent reports described no additional complications due to second craniotomy and highlighted the advantages of having a comprehensive assessment of multiple cognitive tasks and epileptic activity in order to accurately define their topographical relationships. Referral centers for epilepsy surgery ( Kral et al., 2006) continue to apply this well-known methodology (two-stage procedure). The disadvantages are the imprecision of cortical mapping and the need for a second operation ( Duffau et al., 2003, 2005a; Duffau 2007). fMRI alone has been shown to be inadequate for predicting essential language sites ( Giussani et al., 2011).
Preoperative evaluation
Multimodal imaging
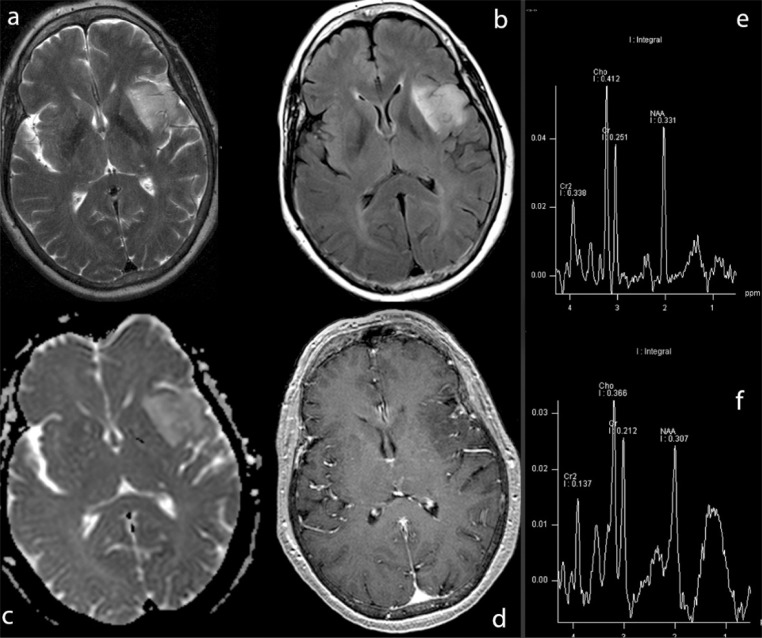
In neurosurgery, fMRI is generally used to assess the risk of postoperative functional deficits and to identify brain regions involved in various functions (i.e., sensorimotor, tactile, language, vision and hearing), especially in lesions located in close proximity to the eloquent cortex ( Haberg et al., 2004; Petrella et al., 2006). Sunaert (2006) identified three main goals of presurgical fMRI: 1) to estimate the risk of eventual neurological deficits by measuring the distance between the margin of planned tumor resection and eloquent/essential functional areas ( Haberg et al., 2004); 2) to select patients for intraoperative cortical stimulation ( Petrella et al., 2006); 3) to provide guidance for functional neuronavigation based on preoperatively acquired structural information ( Rasmussen et al., 2007). Functional data can be obtained from high-resolution magnetic resonance (MR) morphological images acquired during the same session, enhancing the possibility of identifying functional foci within specific anatomical structures. The most common applications of presurgical fMRI are sensorimotor and language mapping ( Sunaert 2006; Stippich et al., 2007). The fMRI signal of motor paradigms is robust and the tasks are feasible and easily repeated. Much more complex is the mapping of cortical eloquent areas, as the function itself implies higher cortical involvement. The diagnostic objectives include mapping of the speech centers (Broca’s and Wernicke’s areas) and determination of the speech dominant hemisphere. fMRI targets defined areas activated by specific stimuli, making the choice of tasks very important. The drawback is the lack of general agreement within the scientific community on standardization of task design, i.e., which is the best task or how many tasks should be used during an MR procedure, especially when evaluating higher cognitive functions. Overall sensitivity and specificity of fMRI in cerebral lesions is 83% and 82%, respectively, while the sensitivity and specificity of fMRI are 88% and 87%, respectively, for hand motor function alone, and 80% and 78%, respectively, for language ( Bizzi et al., 2008). The lower rate of patient sensitivity for language as compared to sensorimotor areas presumably reflects tumor-related receptive and expressive aphasias, as well as related cognitive loss or mechanisms of compensation. Sensitivity (65%) was lower and specificity (93%) higher in WHO grade IV as compared to grade II (sensitivity, 93%; specificity, 79%) and III (sensitivity, 93%; specificity, 76%) gliomas ( Bizzi et al., 2008). In presurgical planning, functional evaluation of verbal capacities is especially useful for determining hemispheric dominance because of the good correlation between fMRI and the Wada test ( Binder et al., 1996; Knecht et al., 2000). Even though the amobarbital test is still the clinical gold standard for the assessment of language dominance, this technique is disputed on methodological and practical grounds. On the other hand, calculation of the lateralization index with fMRI is a means of defining, safely and non-invasively, the localization of the functional areas related to the tumor. Most studies have calculated a lateralization index to quantify the proportion of activation in both hemispheres ( Rutten et al., 2002b); the lateralization index varies, ranging from −100 (all activation in the right hemisphere) to 100 (all activation in the left hemisphere). A cutoff value of the index is then chosen to determine whether patients have typical or atypical language dominance. Unfortunately, there is no consensus on an optimal fMRI protocol or cutoff values for the lateralization index due to the variability in the indexes reported across fMRI studies ( Gaillard et al., 2004; Kamada et al., 2006). Nor is there complete agreement between fMRI protocols and the Wada test to date. Therefore, combining multiple fMRI language tasks is currently the best strategy and yields reproducible and reliable results ( Rutten et al., 2002b). When atypical language dominance is suspected, activation maps should be inspected for possible mixed dominance, as frontal and temporoparietal areas can be located in different hemispheres ( Kamada et al., 2006). Further advantages may be obtained by integrating fMRI with other imaging modalities such as diffusion tensor imaging (DTI) and magnetic resonance spectroscopy (MRS) ( Fig.s 1A , B ). DTI and fiber tractography are two MR techniques based on the concept of anisotropic water diffusion in myelinated fibers that allow three-dimensional reconstruction and visualization of white-matter tracts. Tractography potentially solves the problem of determining the extent to which infiltration of abnormal tissue can help the surgeon to minimize residual tumor volume, i.e., it facilitates preoperative planning by showing whether a tumor is compressing, abutting, or infiltrating the contiguous white-matter tracts ( Lu et al., 2004; Bello et al., 2008, 2010; Bizzi et al., 2012). The power of this information in many clinical situations is such that 3D maps are already routinely being integrated with neurosurgical navigation systems. The technique is also attracting interest as a useful tool for postoperative follow-up ( Coenen et al., 2001; Field et al., 2004; Mori et al., 2002; Clark et al., 2003). DTI provides information about the integrity, displacement and/or interruption of white-matter tracts in and around a tumor due to edema or tumor infiltration ( Clark et al., 2003; Lu et al., 2003; Yamada et al., 2003). However, the heterogeneity of brain tumors in the context of complex environments (e.g., edema, mass effects) and the inherent heterogeneity of diffusion anisotropy in normal white matter reduce the overall specificity of DTI measures. Yet although it may be difficult to separate edematous from infiltrated tracts, DTI-based tractography is fairly reliable for determining whether the mass is displacing or interrupting a tract. False-negative results can be found in regions with T2-signal hyperintensity and elevated diffusivity ( Young and Knopp, 2006). In presurgical sensorimotor planning, standardization is highest for the corticospinal and thalamocortical tracts, whereas in language mapping the superior longitudinal fasciculus, the arcuate fasciculus, and the inferior fronto-occipital fasciculus are the white-matter bundles that more often need to be validated with intraoperative electrical stimulation. New acquisition schemes and more sophisticated software models are being developed to extract finer anatomical information from each voxel. Although attractive in its simplicity, the diffusion tensor model has been shown to be inadequate in the many brain regions that contain so-called “crossing fibers” ( Frank, 2001; Tuch, 2004; Wedeen et al., 2005), i.e. co-localization of two or more differently oriented fiber bundles within the same voxel. The term “crossing fibers” is itself somewhat misleading, as it includes any situation where multiple fiber orientations contribute to the signal measured for the same imaging voxel. This applies to configurations that may not initially have been thought of as “crossing fibers”, e.g. fiber bundles that “brush” past each other within the same imaging voxel, or even curving or “fanning” fibers. Crossing fibers are endemic to diffusion-weighted imaging (DWI), due to its coarse resolution (2 to 3 mm) as compared to the white-matter structures of interest [even the pyramidal tracts are only 3 mm thick in subcortical regions ( Ebeling and Reulen, 1992)]. Indeed, recent studies have shown that a significant proportion of the white matter contains crossing fibers, with the most recent estimating that multiple fiber orientations can be detected in over 90% of white-matter voxels ( Behrens et al., 2007). These effects have an obvious impact on the diffusion tensor and any measures derived from it. These are the reasons for the growing interest in using higher-order models to capture more fully the information that DWI can provide. Several new DTI algorithms currently being tested and implemented in clinical settings may reveal the very intricate interactions between microstructure and signal and the sheer complexity of the white matter itself. DTI still provides a unique and non-invasive means of probing tissue microstructure in vivo and is by far the most promising tool for studying white matter and its organization in living humans. When combined with functional brain mapping, DTI provides an efficient tool for comprehensive, non-invasive, functional anatomy mapping of the human brain ( Bello et al., 2008). In glioma surgery, the approach to diffuse subcortical gliomas and the decision to resect the infiltrated brain tissue surrounding the tumor core are the cornerstones of the modern aggressive surgical strategy. This is the rationale for obtaining knowledge of brain functions at the tumor margin in individual cases. MRS and DTI have been advocated as promising tools for delineating the extent of tumor infiltration ( Price et al., 2003; Stadlbauer et al., 2004, 2006). High-resolution spectroscopic imaging can aid in pretreatment grading and characterization of intra-axial lesions, especially when routine MR sequences do not provide accurate differential diagnosis ( Sibtain et al., 2007; Galanaud et al., 2006) ( Fig.1A ).
Figure 1A .
Infiltrative tumor of the left insula. Integrated neuroimaging.
The lesion, which exhibits a hyperintense signal on T2 (a) and FLAIR (b) images, shows no enhancement after Gd-DTPA administration (d). The DWI pattern (c) is slightly hetereogenous, with medium-low apparent diffusion coefficient values (trend towards high cellularity). 2D CSI MR spectroscopy (e, long TE sequence; f, short TE sequence) shows marked choline elevation, NAA reduction, mild increase in myo-inositol and creatine, evidence of lipids, data consistent with proliferative lesion. Histology: mixed oligo-astrocytoma (WHO grade II–III).
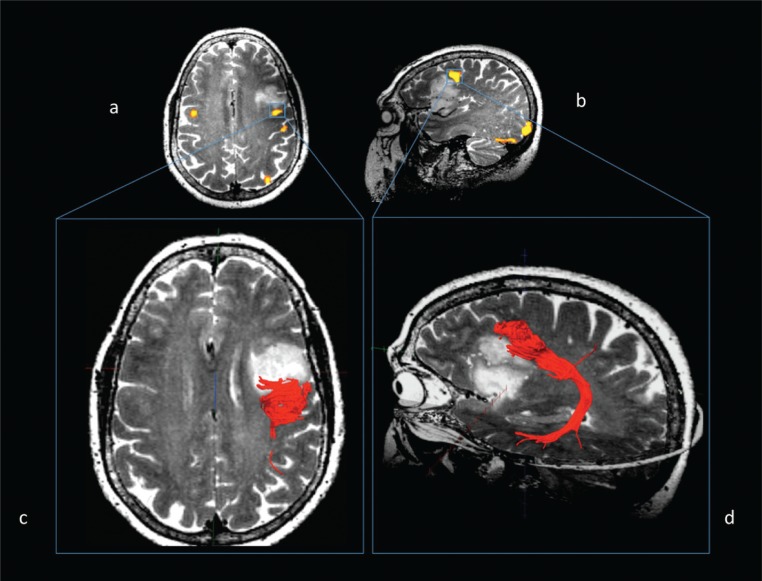
Figure 1B .
Same case. Functional connectivity: fMRI (a, b) and MR diffusion tractography (c, d).
Left arcuate fasciculus reconstruction using fMRI clusters of activation in the dorsolateral prefrontal cortex as seeding points, evoked during a word generation task and overlaid on T2-weighted images (a, axial view; b, sagittal view). Fibers of the left arcuate fasciculus (in red) overlaid on axial (c) and sagittal (d) T2-weighted images, although strictly adjacent to the posterior margin of the lesion, are dislocated but not infiltrated by the tumor.
Clinical evaluation
Together with structural and functional imaging, the presenting symptoms and physical examination also help to guide the surgical strategy. Disturbances in language-related functions, whether transient or progressive, functional or organic, predispose to higher operative risk than location itself ( Peraud et al., 2004; Benzagmout et al., 2007). The standard assessments for dominance are the Edinburgh Handedness Inventory, Wada test and/or fMRI with a verb generation task ( Duffau et al., 2003).
The second step in patient assessment is neurological examination to reveal disturbances in speech and cognition. However, neurological examination does not yield reliable or sufficient information about the type of dysphasia or for specific classification of mild impairments. This is an important issue since 26 to 55% of patients with mild-to-moderate deficits undergo awake surgery for mapping ( Bello et al., 2007; Sanai et al., 2008; Skirboll et al., 1996).
While there is general consensus that mapping requires that patients have no significant disorder which would affect their performance of the task during the operation, some authors give a clearer meaning to preoperative assessment, showing that sensitive tasks can maximize testing efficiency. They state that clinical syndromes and standardized batteries have failed to characterize subtle deficits and/or selective deficits and that task sensitivity can be enhanced through the choice of appropriate individualized tasks ( Petrovich Brennan et al., 2007; Bello et al., 2007; Whittle et al., 2003). The clinical objective is to recognize preserved functions or subprocesses in order to preserve them intraoperatively ( Petrovich Brennan et al., 2007; Pouratian et al., 2003). This research can be pursued with a group of cognition experts who can support operative planning by administering personalized tests and tasks in a given patient. Ultimately, preoperative clinical assessment serves 1) to detect subtle impairment, 2) to assess postoperative results, 3) to guide intraoperative mapping, and 4) to determine eligibility for awake surgery.
Language assessment
Generally, pre-operative evaluation is limited to the naming task ( Ojemann et al., 1989; Haglund et al., 1994; Hamberger et al., 2005) (see Part II). Further assessment of specific functions investigates: spontaneous speech, language fluency, object naming, written/oral comprehension, reading, dictation, and repetition (these assessments constitutite the baseline battery for French authors). Additional tests include: writing sentences and words ( Sanai et al., 2008); oral controlled association by phonetic cue and semantic cue; famous face naming; action picture naming; and transcoding tasks ( Bello et al., 2007).
However, there is a clear discrepancy between the availability of sophisticated tests and the lack of detailed quantification of test results. Some studies analyzed only submaximal scores ( Benzagmout et al., 2007), some recorded a simple yes or no answer ( Peraud et al., 2004; Duffau et al., 2003; Signorelli et al., 2001), while others authors classified only severe deficits ( Sanai et al., 2008); none differentiated selective scoring according to individual patients or groups of patients. The original test battery was rarely reported, and when cited it was the Token test, Aachener Aphasie Test or Boston Diagnostic Aphasia Examination ( Peraud et al., 2004; Bello et al., 2007; Petrovich Brennan et al., 2007).
In addition, preoperative evaluation may not match postoperative evaluation, with a predictable loss of information useful for prognosis and interpretation of clinical results. The consistent use of test batteries would allow investigation of language functions, parameters of interest, test quality and criteria to define abnormality. In some studies, detailed tests were performed only preoperatively ( Gupta et al., 2007; Lubrano et al., 2004; Roux et al., 2003; Bello et al., 2007) or only postoperatively ( Picht et al., 2006; Haglund et al., 1994; Pouratian et al., 2003) and impairments were variably categorized.
It is still controversial whether preoperative impairment is a positive or negative prognostic factor. In a group of patients with similar pre- and intraoperative findings, Duffau et al. (2003) , according to the postoperative course, distinguished between patients with tumor-infiltrating brain areas and patients with tumor-compressing brain areas, since postoperative deterioration was thought to occur in severely infiltrated brains. A worse outcome in patients with severe premorbid conditions is a common observation ( Whittle et al., 2003; Bello et al., 2007; Gupta et al. 2007). In contrast, Haglund et al. (1994) found a higher rate of improvement than of worsening (67 vs 22%) in impaired patients. Postoperative deterioration remains a challenge for the team, because inadequate mapping may be the result of the quality or type of the intraoperative task or of the neurophysiological parameters selected.
Multilingual patients
A subject is usually defined as multilingual when he or she uses more than two languages or dialects in his everyday life ( Fabbro, 2001; Kim et al., 1997). Subjects can be categorized as early multilingual (when the second or third language is acquired during childhood) or late multilingual (when other languages are learned in adulthood) ( Kim et al., 1997). Depending on the level of fluency, subjects can be further subdivided in classes of low or high proficiency ( Fabbro, 2001; Kim et al., 1997).
A standardized or complete examination in the preoperative phase is infrequently described, but in all studies the patients were evaluated for their naming ability in each language in which they were proficient. In some studies, other tests were carried out as well. The recommendation is that, during a brain mapping procedure, neurosurgeons studying language organization with electrostimulation in bilinguals/multilinguals test all languages in which the subjects are fluent ( Ojemann et al., 1979b ; Roux and Trémoulet, 2002 ; Roux et al., 2004; Lucas et al., 2004; Walker et al., 2004; Bello et al., 2006).
Cognition and quality of life
When surgery for intractable epilepsy is performed on the basis only of thorough assessment by a team of professionals (neuropsychologist, speech therapist, neurologist) who are not ordinarily part of a neurosurgical team, it is necessary to consult the neuropsychologist in order to ascertain which function, besides language, is served by a brain region that may be surgically removed. Yet, the neuropsychologist’s role is seldom defined in relation to brain tumor treatment.
Tumors in the dominant hemisphere may profoundly affect cognitive function well beyond language function. Although some deficits are related to tumor site, typically in low-grade glioma patients, a wider spectrum of deficits, often not limited to a single cognitive domain, is encountered in high-grade glioma ( Tucha et al., 2000; Yoshi et al., 2008 ). This makes the assessment battery crucial for global evaluation and longitudinal study ( Table I ) ( Talacchi et al., 2011).
Table I .
Classification of patient alertness during the operation (modified from Chernik et al., 1990).
| Score | Responsiveness | Speech | Facial expression | Eyes |
|---|---|---|---|---|
| 5 (alert) | Responds readily to voice with normal tone | Normal | Normal | Clear, no ptosis |
| 4 | Responds slowly to voice with normal tone | Mild slowing | Mild relaxation | Mild ptosis (less than half the eye) |
| 3 | Responds after calling loudly or repeatedly | Prominent slowing or slurring | Marked relaxation (slack jaw) | Marked ptosis (half the eye or more) |
| 2 | Responds after mild prodding or shaking | Few recognizable words | - | - |
| 1 | Does not respond to mild prodding or shaking | - | - | - |
| 0 | Does not respond to pain | - | - | - |
Scant attention has been paid to the impact that primary brain tumors can have on quality of life ( Taphoorn et al., 1992, 2005; Giovagnoli and Boiardi, 1994; Weitzner et al., 1996; Weitzner and Meyers, 1997; Buckner et al., 2001). Contrary to what is seen in other cancer patients when the burden of the disease is assessed, in brain tumor patients a decrease in cognitive and emotional functioning may result from cerebral disease. Subclinical symptoms, personality changes, and mood disturbances may prove to be as burdensome to patients, or more so, than certain focal neurological deficits ( Giovagnoli et al., 2005). As these often go unrecognized on self-assessment, it is necessary to seek the expert opinion of specialists with neuropsychological experience ( Påhlson et al., 2003 ; Taphoorn and Klein, 2004).
Inclusion criteria
Pathology
The proximity of critical pathways can pose a significant challenge to standard operative strategies. The concept of the eloquent area is evolving and may potentially be extended to all measurable functions. Possible causes of brain damage include: the trajectory in subcortical tumors; abnormal anatomy in recurrent tumors; distorted anatomy due to the tumor; low-grade glioma; irregular tumors; the periphery in high-grade glioma; the gliotic rim in cavernous angioma; epilepsy and temporary arterial occlusion ( Matsuda et al., 2012). Not all these categories are mentioned in the literature when the tumor is characterized. Nevertheless, they are all known to be crucial factors in surgical outcome, and knowledge of the eloquent cortex may help the surgeon to avoid clinical consequences.
The aims of the surgical strategy, particularly in surgery for glioma, may be linked to orientation (trajectory, abnormal anatomy, distorted anatomy), which is not usually histology-dependent, and removal (low grade, irregular margins, periphery). However, whether different surgical strategies require different types of assessment and intraoperative mapping strategies is far from established.
Exclusion criteria
Eligibility
Studies in patients undergoing awake craniotomy have reported that the primary cause of anxiety is the fear of pain ( Santini et al., 2012). Although awake craniotomy is generally considered to be well tolerated, complications such as emotional distress and agitation are reported and lead to loss of control, the need for more sedation, and failure of the mapping project. Once the patient has been given a detailed description of the procedure and provided his fully informed consent, the decision to operate will depend on whether he can be reasonably expected to be cooperative. Failure rates due to agitation vary from 2 to 8%, but are not systematically reported ( Sahjpaul, 2000).
Language ability
Since the aim of awake language mapping is to preserve speech, preoperative normal function is the reference parameter. Detailed preoperative language examinations address this issue. Because criteria and cut-off values for surgical inclusion are rarely given, the role of a detailed evaluation in symptomatic patients is often unclear. Some authors stated that patients are excluded if the preoperative error rate is >25%, due to the subsequent difficulty of deciding whether an intraoperative error was evoked or not ( Signorelli et al., 2001; Little and Friedman, 2004). Haglund et al. (1994) , without conducting preoperative assessment, excluded from their study patients who had an intraoperative error rate >25% without stimulation interference (see Part II).
In tumor series, the proportion of eligible patients with mild-moderate deficits is quite high (26–55%) ( Bello et al., 2007; Sanai et al., 2008; Skirboll et al., 1996), while the proportion of excluded patients varies considerably (5–30%) ( Sanai et al., 2008; Pouratian et al., 2003; Bello et al., 2007) and is rarely reported. Roux et al. (2003) and Lubrano et al. (2004) excluded from surgical procedures all cases with a Boston Naming score <90%, but they were alone in using a high cutoff to define the functional criterion for exclusion.
Operative setting
Anesthesia management
Procedures that identify and map specific brain areas are becoming increasingly complex. The anesthesiologist is responsible for inducing states of analgesia that do not interfere with patient comfort or electrophysiological monitoring, while still ensuring cardiorespiratory stability. During surgical procedures involving Broca’s and Wernicke’s areas, verbal contact is essential and should be maintained. A good anesthetic technique entails analgesia, anesthesia or sedation and respiratory and hemodynamic control without interfering with electrocorticographic and neuropsychological testing ( Frost and Booij, 2007), but there is no general consensus on the best anesthesia approach.
Current techniques include continuous sedation ( Sarang and Dinsmore, 2003) with fast-acting agents and local anesthesia of the scalp. Airway management remains a concern due to the risk of aspiration or oversedation (SpO 2 <90%) because patients continue to breathe spontaneously. Propofol, fentanyl, remifentanyl and midazolam are commonly used. Propofol can affect EEG monitoring ( Herrick et al., 1997), but intravenous drugs are, nevertheless, preferable since the ideal anesthetic for neurosurgery (rapid onset, easily controllable duration of action, no effect on the cardiovascular or respiratory system, no nausea or vomiting and no interference with neurological and neurophysiological evaluation) does not yet exist. The level of sedation is fundamental, since oversedation results in an uncooperative patient and respiratory depression, while undersedation makes the patient uncomfortable.
Local anesthesia
During sedation, blockage of the auricolotemporal, zygomaticotemporal, supraorbital, supratrochlear, lesser occipital and greater occipital nerves is mandatory to allow painless skin incision. Among the long-acting agents, ropivacaine and levobupivacaine seem preferable owing to their safe action on the heart. Costello et al. (2005) reported safe dosages of up to 4.5 mg/kg for ropivacaine and up to 2.5 mg/kg for levobupivacaine.
Anesthetics
Propofol has a rapid onset of action and is quickly removed from the bloodstream by redistribution and metabolism; this means that the level of anesthesia or sedation can change rapidly. Nevertheless, propofol can lead to respiratory depression. It should also be noted that propofol interacts with gamma-aminobutyric acid receptors, leading, at low dosages, to central nervous system hyperactivity with movements mimicking tonic-clonic seizures. Propofol also has a neuroprotective action, probably mediated by its antioxidant properties which may play a role in apoptosis, ischemia-reperfusion injury, and inflammation-induced neuronal injury.
Narcotics: remifentanyl seems to be the most appropriate narcotic during awake surgery because of its rapid onset, rapid half-life and lack of accumulation even after prolonged infusion. Remifentanyl can lead to muscle rigidity, postoperative shivering, a low risk of postoperative agitation and seizures ( Grønlykke et al., 2008), and bradycardia.
In conclusion, various different anesthesia protocols and drugs can be used in intraoperative mapping, but the two basic factors for obtaining an optimal result are good patient selection and good communication between the awake surgery team members.
Surgical procedure and strategy
Intraoperative mapping (electrical stimulation, cognitive tasks, and response) is described in Part II of this article. Here we discuss the choice of tasks in preoperative assessment, operative tools and strategy, in short, how these are used in a clinical situation.
The intraoperative microscope and the ultrasonic aspirator are elements essential to an accurate surgical technique. Patient positioning is dictated by the craniotomy site. But because patient comfort is another important factor, the patient is positioned while awake. Temporo-occipital and temporoparietal craniotomy are quite posterior, but the patient can be positioned more comfortably lying on his side and supported by a soft pillow and mattress. The patient is positioned so that he is accessible to the anesthesiologist and neuropsychologist or speech therapist, and can receive and respond to commands during cognitive testing.
Initially, wide craniotomy was performed to expose the classical areas and to confirm the negative sites surrounding the lesion by mapping the positive areas ( Ojemann et al., 1989). With increasing reliance on mapping, craniotomy has been gradually reduced to the size needed to approach the lesion ( Sanai et al., 2008).
Precise intraoperative description of mapping sites leads to greater accuracy in describing results. With image-assisted surgery, probabilistic location ( Sanai et al., 2008) can be replaced by exact location ( Reithmeier et al., 2003). The neuronavigator can support different aims. It can define the cortical edges of lesions, particularly in low-grade gliomas which are difficult to differentiate from normal cortex ( Benzagmout et al., 2007), and it can establish the site of corticectomy and the trajectory in the approach to subcortical lesions.
Before removing tumor or tumor-infiltrated brain tissue, it should be remembered that neurological functions can also be found in the same areas: at the tumor edge in high-grade glioma and within the tumor in low-grade glioma ( Ojemann et al., 1996; Bello et al., 2006). In structural and functional mapping, determination of the tumor periphery in an extraoperative setting with MRS and MRI is increasingly being supplemented by its use in an intraoperative setting. While ultrasound is the instrument of choice after brain shifting, intraoperative MRI has great appeal for structural definition as well as for functional information, validating connectivity as determined on preoperative DTI ( D’Andrea et al., 2012). Optical spectroscopic imaging, optical coherence tomography, and 5-amino-levulinic acid fluorescence are innovative intraoperative techniques that detect the tumor periphery when visual inspection is not sufficient to distinguish normal from infiltrated brain tissue ( Sobottka et al., 2008; Stummer et al., 2008; Giese et al., 2008).
When these procedures are combined, the surgical strategy clearly becomes critically important. In this regard, some authors reported that the real advantage of mapping, with or without resection-enhancing intra-operative techniques, is the extent of the tumor resection ( Schucht et al., 2012; Talacchi et al., 2010; De Benedictis et al., 2010; Ius et al., 2012).
Duffau proposed an alternative strategy to tumor “visual monitoring”: “All resections were pursued until eloquent subcortical pathways were encountered around the surgical cavity. Thus, there was no margin left around the cortico-subcortical eloquent areas.” ( Duffau, 2005a). However, there are not enough data to validate this strategy to date, and it should be reconsidered only after feasibility, reproducibility and safety studies have been performed in clinical settings (bottom-up processing of evidence).
One major limitation in clinical comparative inference is that multiple cortical or subcortical sites are manipulated during an operation, making it impossible to relate an event to the manipulation of a specific site. In other words, improvement in surgical strategy is driven by numerous methodological issues. Taken together, choices regarding patient positioning, surgical technique, tumor definition, comparison between clinical and intraoperative information, functional studies, and intraoperative tools will lead to a good result. By contrast, considering only one or few functional variables may be confounding and misleading in decision making, or even disappointing when looking at a study design that links aims, methods and results. This is why research studies today should be validated in a clinical setting, taking into account surgical complications, which are critical to expand our current knowledge ( Sawaya et al., 1998). This, in turn, is why the challenge of awake surgery and cognitive mapping ultimately resides in the medical team’s ability to pursue clinical objectives by uniting their professional knowledge.
Electrical stimulation
Electrical parameters and neurophysiological effects
At first sight, the principles of cortical stimulation for language mapping appear to be well established, with the classical 50–60 Hz (high frequency) bipolar Penfield technique the one generally employed for historical reasons. However, detailed analysis reveals inconsistencies between cortical stimulation protocols ( Pouratian et al., 2004). Because electrophysiological parameters affect the results of stimulation, localization of function varies across studies depending on which stimulation parameters and mapping strategies are chosen.
Electrophysiological stimulation of the cortex relies on several different neurophysiological parameters which, in turn, can influence the final effect of mapping. The use of monopolar or bipolar stimulation is one of these variables. The vast majority of authors use bipolar stimulation with either a probe or two adjacent electrodes attached to a strip or grid. The inter-electrode distance is usually 5–10 mm and the electrode diameter varies up to four-fold (1 to 4 mm), which – when other parameters are kept constant – can influence the charge density applied to the cortex. Bipolar stimulation is thought to produce a higher, more focal current density than monopolar stimulation and to facilitate the excitation of neural cells parallel to the bipolar axis ( Nathan et al., 1993; Haglund et al., 1994; Schekutiev and Schmid, 1996; Manola et al., 2007). However, the actual dispersion of current in bipolar cortical stimulation and the related risk of activating distant cortical sites have never been systematically studied, and the lack of selectivity, particularly at higher intensities, may be a real drawback. While monopolar and bipolar cortical stimulation have similar sensitivity for mapping the motor cortex, bipolar stimulation is the only technique currently available for intraoperative mapping and monitoring of the speech-related cortex ( Kombos and Süss, 2009 ).
Although high-frequency stimulation (50–60 Hz) of eloquent areas is the most widespread technique, there is some evidence that low-frequency stimulation may also be effective: lowering the stimulation frequency decreases the probability of inducing afterdischarges without significantly compromising mapping efficacy ( Chen et al., 1997; Zangaladze et al., 2008; Hoshino et al., 2005). These preliminary observations warrant further investigation in the intra-operative setting; nonetheless, it would be advisable to start cortical mapping at a lower frequency first.
The short-train technique (5–7 stimuli, 0.5 ms duration, ISI 4.1 ms = 250 Hz, at a train repetition rate of 1 or 2 Hz) is recommended for mapping the motor cortex and subcortical motor pathways; however, it cannot be used for language and cognitive mapping because the train duration is too short (about 20 ms) to significantly inhibit the cognitive function being tested. Furthermore, it is unclear to what extent other parameters such as polarity (monophasic, alternating or biphasic square wave pulses), duration of single stimuli (0.2–1 ms), and train stimulation (1–8 sec) can influence the mapping of eloquent areas.
Mapping strategies are among the other main variables that may affect the results of stimulation. Two different theories subtend the choice of strategy. Some authors apply the concept that thresholds (the minimum stimulation current needed to induce functional changes) vary across the exposed cortex depending on the task being assessed and the location being mapped. This is in keeping with the observation that afterdischarge thresholds can vary significantly not only across a population but also at different cortical sites in the same subject ( Lesser et al., 1984; Pouratian et al., 2004). Accordingly, maximizing the stimulation currents at each cortical site is attempted to ensure the absence of eloquent function ( Woolsey et al., 1979; Lesser et al., 1984; Pouratian et al., 2002 a , b ). But in so doing, aftercharge thresholds in the adjacent cortices are often exceeded, increasing the risk of distal activation due to current spreading to adjacent sites.
Other authors ( Van Buren et al., 1978; Berger et al., 1989; Ojemann et al., 1989) keep stimulation intensity constant while mapping the entire cortex and set the threshold just below the lowest current observed to induce afterdischarges. With this strategy, the risk of inducing afterdischarges (which may invalidate the results) and clinical seizures is minimized but eloquent cortical sites may not be identified.
Side effects: seizures
The occurrence of intraoperative seizures induced by cortical stimulation using the 60-Hz technique is reported in up to 24–27% of cases ( Sartorius and Wright, 1997; Burke et al., 1999); whether this risk is higher in patients with symptomatic epilepsy than in those with asymptomatic epilepsy remains debated ( Szelenyi et al., 2010 ). Most such seizures can be controlled by irrigating the cortex with cold Ringer’s solution ( Sartorius and Berger, 1998), potentially obviating the need to administer antiepileptic drugs which could increase thresholds in electrical mapping. Overall, seizure occurrence may affect the mapping strategy and reduce mapping reliability to some extent. Electrocorticography (EcoG) with a 4–8 electrode strip placed on the exposed cortex adjacent to the stimulated regions helps to continuously monitor the patient for epileptic seizures and afterdischarge activity (spikes or sharp waves within 5 seconds of stimulus termination) so that language errors due to subclinical seizure activity can be recognized and correct stimulation verified by recording stimulation artifacts. The trial results are automatically excluded if afterdischarges in response to stimulation are observed. The use of EcoG and the choice of appropriate neurophysiological parameters can aid in minimizing the risk of intraoperative clinical seizures.
Subcortical mapping
As with the literature on cortical motor mapping, most of the studies on subcortical mapping report the range of stimulation intensities but fail to give a detailed analysis of subcortical thresholds ( Keles et al., 2004; Duffau et al., 2003; Bello et al., 2007; Henry et al., 2004). Interestingly, many authors state that for sub-cortical mapping they use the same current intensity to elicit either a cortical sensory or a motor response. However, there is, as yet, no clear explanation for this neurophysiological strategy. The problem of current spreading with increasing intensity and the different impedance of gray and white matter would suggest that a mere translation of the cortical threshold to a subcortical level may not be the most appropriate approach. Instead, the correspondence between anatomical information, as determined by DTI, and neurophysiological data with subcortical mapping should be validated according to detailed threshold information rather than the less specific “positive sub-cortical mapping sites”.
Standardization of intraoperative neurophysiological techniques should be based, above all, on correlations between intraoperative findings and postoperative outcomes of the functions tested. From this perspective, some attempts have been made with regard to motor function by establishing preliminary criteria for the interpretation of motor evoked potentials in brain surgery ( Neuloh and Schramm, 2004; Kombos et al., 2001).
Currently, there are no evidence-based criteria to inform guidelines or substantiate the need for neuro-physiological mapping; however, the usefulness of these techniques has been demonstrated by the countless patients in whom the risk of postoperative language deficits was minimized thanks to the use of intraoperative neurophysiology.
Postoperative evaluation
Postoperative settings vary considerably. A comparative neurological examination is usually performed between the pre- and postoperative phase, often including language evaluation as well as MRI, with different timings (immediate or delayed), but rarely accounting for complications or a wider battery of neuropsychological evaluations ( Vives and Piepmeier, 1999). Interestingly, the perioperative period, conventionally defined as 30 days after surgery, was extended to 3–4 months in some studies or even up to 12 months, which is ordinarily the duration set for evaluating permanent deficits ( Sawaya et al., 1998; Duffau et al., 1999, 2003).
While awake surgery is claimed to decrease postoperative morbidity in eloquent areas, immediate postoperative evaluations showed a surprisingly high rate of deterioration of functions, usually >50%, which can be explained by a surgeon’s confidence when working with eloquent areas, as demonstrated by progressive improvement within a few weeks. At 3 months after surgery, the improvement rate usually decreases to <20% ( Duffau et al., 2003; Meyer et al., 2001; Bello et al., 2007).
The degree of deterioration varies widely depending on the clinical scale on which it is measured, often arbitrarily set at one level with high and low cutoff scores, which leads to gross differences in recording deficits ( Signorelli et al., 2001; Meyer et al., 2001; Sanai et al., 2008) or at two levels (mild, moderate-severe) ( Haglund et al., 1994; Bello et al., 2007), or at three levels (mild, moderate, severe) ( Duffau et al., 2003). The quality of deficits is rarely defined, and usually only receptive, expressive or mixed language ( Haglund et al., 1994; Sanai et al., 2008) deficits are mentioned, which provides a simplified evaluation compared with the preoperative evaluation. A few authors ( Roux et al., 2003; Lubrano et al., 2004; Bello et al., 2007) used specific preoperative test categories for postoperative site-by-site evaluation. To date it is unclear whether we are using redundant preoperative tests or an excessively restricted postoperative evaluation. The intraoperative results of patients with and without deficits have never been analyzed separately, but it must be taken into account that reporting by subjects with cognitive disorders is less reliable for cognitive mapping.
Experience with motor pathway mapping has shown the risk of relying on a single function while we are operating on a wide anatomical area. False-negative sites are task-specific, largely function-specific, and can produce complications (i.e., visual field defects, Sanai et al., 2008).
The Glioma Outcome Project classified complications as systemic, local, and neurological ( Chang et al., 2003). This is the benchmark, or the minimum standardized outcome set, against which the surgical series can be defined as operations harboring greater risk. In situations where negative sites are task-related, cognitive examination is advisable to check for false-negative results ( Talacchi et al., 2012). However, complications are occasionally reported ( Sanai et al., 2008; Peraud et al., 2004, Lacroix et al., 2001) and patients, in spite of possible additional impairments, are seldom evaluated with neuropsychological tools even though such tools have been shown to be effective for studying cognitive functions in the immediate postoperative period ( Talacchi et al., 2011, 2012).
Clinical assessment is also a measure of the study population and outcome. In research settings where functional assessment is more detailed and complex ( Duffau et al., 2002, 2003; Bello et al., 2007), a clinical framework tailored ad hoc should be in place to demonstrate the safety and efficacy of experimental work ( safety net ).
Similarly, neuroimaging is the method of choice to assess oncological outcome and to verify clinical observations, excluding additional lesions adjacent to or distant from the edge of the resected cavity. Timing and sequence are important. Obtaining an MRI scan within 48 hours of an operation allows for early determination of oncological status and alterations in the blood-brain barrier, reliable interpretation of contrast enhancement, and the absence of a paramagnetic effect from hemoglobin degradation products. This 48-hour range is considered the best timing for MRI evaluation ( Albert et al., 1994). Some authors advocate DWI to detect ischemic damage, which helps in the interpretation of vascular events as sequelae of the operation ( Sanai et al., 2008; Trinh et al., 2012). FLAIR images are the optimal sequence for low-grade gliomas, and contrast-enhanced T1-weighted images for high-grade gliomas ( Meyer et al., 2001; van den Bent et al., 2011).
Objective classification of tumor remnants requires volume measurements ( Keles et al. 2006). However, these are rarely reported, which makes it difficult to establish the possible advantage of cognitive mapping for maximizing removal ( Skrap et al., 2012).
Few studies reported data about postoperative examinations in bilingual/multilingual patients. The literature supports the fundamental hypothesis that these patients have common but dedicated areas for their languages ( Kim et al., 1997; Lucas et al., 2004; Ojemann et al., 1989; Roux and Trémoulet, 2002 ; Roux et al., 2003; Walker et al., 2004; Bello et al., 2006).
Concluding remarks
In conclusion, we found that in the majority of studies using neurophysiological and imaging-assisted surgery the quality of evidence for the benefits of mapping is scarce (mostly evidence class III, some evidence class II studies) and mainly based on historical control studies, retrospective analyses and expert opinion. Because of the variety of functions that can be tested and sites identified as relevant in language tasks, a clear terminology and consistency between pre-, intra- and post-operative testing is needed before the appropriateness of these techniques can be validated ( Zhang et al., 2012). Meanwhile, systematic adjustment for likely confounding procedures may be achieved through a careful comprehensive clinical approach which enhances safety but is demanding. In this context, more data are needed about non-language functions and quality of life.
With this review we have provided an overview of the methodological controversies in awake surgery with the aim of encouraging surgeons and neuroscientists to collaborate in this fascinating setting.
References
- Albert FK , Forsting M , Sartor K , et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis . Neurosurgery . 1994 ; 34 : 45 – 60 . doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Behrens TE , Berg HJ , Jbabdi S , et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? . Neuroimage . 2007 ; 34 : 144 – 155 . doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L , Acerbi F , Giussani C , et al. Intraoperative language localization in multilingual patients with gliomas . Neurosurgery . 2006 ; 59 : 115 – 125 . doi: 10.1227/01.NEU.0000219241.92246.FB. [DOI] [PubMed] [Google Scholar]
- Bello L , Gallucci M , Fava M , et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas . Neurosurgery . 2007 ; 60 : 67 – 82 . doi: 10.1227/01.NEU.0000249206.58601.DE. [DOI] [PubMed] [Google Scholar]
- Bello L , Gambini A , Castellano A , et al. Motor and language DTI fiber tracking combined with intraoperative sub-cortical mapping for surgical removal of gliomas . Neuroimage . 2008 ; 39 : 369 – 382 . doi: 10.1016/j.neuroimage.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Bello L , Castellano A , Fava E , et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations . Neurosurg Focus . 2010 ; 28 : E6 . doi: 10.3171/2009.12.FOCUS09240. [DOI] [PubMed] [Google Scholar]
- Benzagmout M , Gatignol P , Duffau H . Resection of World Health Organization grade II gliomas involving Broca’s area: methodological and functional considerations . Neurosurgery . 2007 ; 61 : 741 – 752 . doi: 10.1227/01.NEU.0000298902.69473.77. [DOI] [PubMed] [Google Scholar]
- Berger MS , Kincaid J , Ojemann GA , et al. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors . Neurosurgery . 1989 ; 25 : 786 – 792 . doi: 10.1097/00006123-198911000-00015. [DOI] [PubMed] [Google Scholar]
- Berkenstadt H , Perel A , Hadani M , et al. Monitored anesthesia care using remifentanil and propofol for awake craniotomy . J Neurosurg Anesthesiol . 2001 ; 13 : 246 – 249 . doi: 10.1097/00008506-200107000-00013. [DOI] [PubMed] [Google Scholar]
- Binder JR , Swanson SJ , Hammeke TA , et al. Determination of language dominance using functional MRI: a comparison with the Wada test . Neurology . 1996 ; 46 : 978 – 984 . doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Bizzi A , Blasi V , Falini A , et al. Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping . Radiology . 2008 ; 248 : 579 – 589 . doi: 10.1148/radiol.2482071214. [DOI] [PubMed] [Google Scholar]
- Bizzi A , Nava S , Ferrè F , et al. Aphasia induced by gliomas growing in the ventrolateral frontal region: assessment with diffusion MR tractography, functional MR imaging and neuropsychology . Cortex . 2012 ; 48 : 255 – 272 . doi: 10.1016/j.cortex.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Blanshard HJ , Chunh F , Mannien PH , et al. Awake craniotomy for removal of intracranial tumor: considerations for early discharge . Anesth Analg . 2001 ; 92 : 89 – 94 . doi: 10.1097/00000539-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY , Zeffiro TA , Blaxton T , et al. A direct comparison of PET activation and electrocortical stimulation mapping for language localization . Neurology . 1997 ; 48 : 1056 – 1065 . doi: 10.1212/wnl.48.4.1056. [DOI] [PubMed] [Google Scholar]
- Buckner JC , Schomberg PJ , McGinnis WL , et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma . Cancer . 2001 ; 92 : 420 – 433 . doi: 10.1002/1097-0142(20010715)92:2<420::aid-cncr1338>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bulsara KR , Johnson J , Villavicencio AT . Improvements in brain tumor surgery: the modern history of awake craniotomies. Neurosurg Focus. 2005 ;18:e5. doi: 10.3171/foc.2005.18.4.6. [DOI] [PubMed] [Google Scholar]
- Burke D , Nuwer MR , Daube J , et al. Intraoperative monitoring. The International Federation of Clinical Neurophysiology . Electroencephalogr Clin Neurophysiol Suppl. 1999 ; 52 : 133 – 148 . [PubMed] [Google Scholar]
- Chang SM , Parney IF , McDermott M , et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project . J Neurosurg . 2003 ; 98 : 1175 – 1181 . doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- Chen R , Classen J , Gerloff C , et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation . Neurology . 1997 ; 48 : 1398 – 1403 . doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chernik DA , Gillings D , Laine H , et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam . J Clin Psychopharmacol . 1990 ; 10 : 244 – 251 . [PubMed] [Google Scholar]
- Clark CA , Barrick TR , Murphy MM , et al. White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? . Neuroimage . 2003 ; 20 : 1601 – 1608 . doi: 10.1016/j.neuroimage.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Coenen VA , Krings T , Mayfrank L , et al. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note . Neurosurgery . 2001 ; 49 : 86 – 93 . doi: 10.1097/00006123-200107000-00013. [DOI] [PubMed] [Google Scholar]
- Costello TG , Cormack JR , Mather LE , et al. Plasma levobupivacaine concentrations following scalp block in patients undergoing awake craniotomy . Br J Anaesth . 2005 ; 94 : 848 – 851 . doi: 10.1093/bja/aei135. [DOI] [PubMed] [Google Scholar]
- Danks RA , Rogers M , Aglio LS , et al. Patient tolerance of craniotomy performed with the patient under local anesthesia and monitored conscious sedation . Neurosurgery . 1998 ; 42 : 28 – 34 . doi: 10.1097/00006123-199801000-00006. [DOI] [PubMed] [Google Scholar]
- D’Andrea G , Angelini A , Romano A , et al. Intraoperative DTI and brain mapping for surgery of neoplasm of the motor cortex and the corticospinal tract: our protocol and series in BrainSUITE . Neurosurg Rev . 2012 ; 35 : 401 – 412 . doi: 10.1007/s10143-012-0373-6. [DOI] [PubMed] [Google Scholar]
- De Benedictis A , Moritz-Gasser S , Duffau H . Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas . Neurosurgery . 2010 ; 66 : 1074 – 1084 . doi: 10.1227/01.NEU.0000369514.74284.78. [DOI] [PubMed] [Google Scholar]
- Duffau H . Intraoperative cortico-subcortical stimulations in surgery of low-grade gliomas . Expert Rev Neurother . 2005a ; 5 : 473 – 485 . doi: 10.1586/14737175.5.4.473. [DOI] [PubMed] [Google Scholar]
- Duffau H . Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity . Lancet Neurol . 2005b ; 4 : 476 – 486 . doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- Duffau H . From intraoperative electrical cortico-subcortical cerebral mapping to research on brain’s function and plasticity . Rivista Medica . 2006a ;1–2: 15 – 21 . [Google Scholar]
- Duffau H . New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity . J Neurooncol . 2006b ; 79 : 77 – 115 . doi: 10.1007/s11060-005-9109-6. [DOI] [PubMed] [Google Scholar]
- Duffau H . Contribution of cortical and subcortical electrostimulation in brain glioma surgery: methodological and functional considerations . Neurophysiol Clin . 2007 ; 37 : 373 – 382 . doi: 10.1016/j.neucli.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Sichez J-P , et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpêtrière experience with 60 patients . Acta Neurochir (Wien) 1999 ; 141 : 1157 – 1167 . doi: 10.1007/s007010050413. [DOI] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Sichez N , et al. Intraoperative mapping of the subcortical language pathways using direct stimulation . Brain . 2002 ; 125 : 199 – 214 . doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Denvil D , et al. Usefulness of intra-operative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients . J Neurosurg . 2003 ; 98 : 764 – 778 . doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- Duffau H , Gatignol P , Mandonnet E , et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations . Brain . 2005a ; 128 : 797 – 810 . doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H , Lopes M , Arthuis F , et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution . J Neurol Neurosurg Psychiatry . 2005b ; 76 : 845 – 851 . doi: 10.1136/jnnp.2004.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H , Taillandier L , Gatignol P , et al. The insular lobe and brain plasticity: lessons from tumor surgery . Clin Neurol Neurosurg . 2006 ; 108 : 543 – 548 . doi: 10.1016/j.clineuro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Duffau H , Gatignol P , Moritz-Gasser S , et al. Is the left uncinate fasciculus essential for language? A cerebral stimulation study . J Neurol . 2009 ; 256 : 382 – 389 . doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Ebel H , Ebel M , Schillinger G , et al. Surgery of intrinsic cerebral neoplasms in eloquent areas under local anesthesia . Minim Invasive Neurosurg . 2000 ; 43 : 192 – 196 . doi: 10.1055/s-2000-11372. [DOI] [PubMed] [Google Scholar]
- Ebeling U , Reulen HJ . Subcortical topography and proportions of the pyramidal tract. Acta Neurochir (Wien) 1992 ; 118 : 164 – 171 . doi: 10.1007/BF01401303. [DOI] [PubMed] [Google Scholar]
- Fabbro F . The bilingual brain: bilingual aphasia . Brain Lang . 2001 ; 79 : 201 – 210 . doi: 10.1006/brln.2001.2480. [DOI] [PubMed] [Google Scholar]
- Faugeras O , Adde G , Charpiat G , et al. Variational, geometric, and statistical methods for modeling brain anatomy and function. Neuroimage. 2004 ;23(Suppl 1): S46 – 55 . doi: 10.1016/j.neuroimage.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Field AS , Alexander AL , Wu YC , et al. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor . J Magn Reson Imag . 2004 ; 20 : 555 – 562 . doi: 10.1002/jmri.20169. [DOI] [PubMed] [Google Scholar]
- FitzGerald DB , Cosgrove GR , Ronner S , et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation . AJNR Am J Neuroradiol . 1997 ; 18 : 1529 – 1539 . [PMC free article] [PubMed] [Google Scholar]
- Frank LR . Anisotropy in high angular resolution diffusion-weighted MRI . Magn Reson Med . 2001 ; 45 : 935 – 939 . doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- Frost EA , Booij LH . Anesthesia in the patient for awake craniotomy . Curr Opin Anaesthesiol . 2007 ; 20 : 331 – 335 . doi: 10.1097/ACO.0b013e328136c56f. [DOI] [PubMed] [Google Scholar]
- Galanaud D , Nicoli F , Chinot O , et al. Noninvasive diagnostic assessment of brain tumors using combined in vivo MR imaging and spectroscopy . Magn Reson Med . 2006 ; 55 : 1236 – 1245 . doi: 10.1002/mrm.20886. [DOI] [PubMed] [Google Scholar]
- Gaillard WD , Balsamo L , Xu B , et al. fMRI language task panel improves determination of language dominance . Neurology . 2004 ; 63 : 1403 – 1408 . doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Giese A , Kantelhardt SR , Oelckers S , et al. Certified prototype of an OCT-integrated operating microscope for intraoperative optical tissue analysis . 59th Annual Meeting of the German Society of Neurosurgery; 2008 . Meeting abstract (DI.01.01) http://www.egms.de/static/en/meetings/dgnc2008/08dgnc140.shtml. [Google Scholar]
- Gignac E , Manninen PH , Gelb AW . Comparison of fentanyl, sufentanil and alfentanil during awake craniotomy for epilepsy . Can J Anaesth . 1993 ; 40 : 421 – 424 . doi: 10.1007/BF03009510. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR , Boiardi A . Cognitive impairment and quality of life in long-term survivors of malignant brain tumors . Ital J Neurol Sci . 1994 ; 15 : 481 – 488 . doi: 10.1007/BF02334609. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR , Silvani A , Colombo E , et al. Facets and determinants of quality of life in patients with recurrent high grade glioma . J Neurol Neurosurg Psychiatry . 2005 ; 76 : 562 – 568 . doi: 10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani C , Riva M , Gallucci M , et al. Anatomical correlates for category-specific naming of living and non-living things . Neuroimage . 2011 ; 56 : 323 – 329 . doi: 10.1016/j.neuroimage.2011.01.080. [DOI] [PubMed] [Google Scholar]
- Grønlykke L , Knudsen ML , Høgenhaven H , et al. Remifentanil-induced spike activity as a diagnostic tool in epilepsy surgery . Acta Neurol Scand . 2008 ; 117 : 90 – 93 . doi: 10.1111/j.1600-0404.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- Gupta DK , Chandra PS , Ojha BK , et al. Awake craniotomy versus surgery under general anesthesia for resection of intrinsic lesions of eloquent cortex – a prospective randomized study . Clin Neurol Neurosurg . 2007 ; 109 : 335 – 343 . doi: 10.1016/j.clineuro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Haberg A , Kvistad KA , Unsgard G , et al. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome . Neurosurgery . 2004 ; 54 : 902 – 914 . doi: 10.1227/01.neu.0000114510.05922.f8. [DOI] [PubMed] [Google Scholar]
- Haglund MM , Berger MS , Shamseldin M , et al. Cortical localization of temporal lobe language sites in patients with gliomas . Neurosurgery . 1994 ; 34 : 567 – 576 . doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ , Seidel WT , Mckhann GM . Brain stimulation reveals critical auditory naming cortex . Brain . 2005 ; 128 : 2742 – 2749 . doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ , McClelland S , 3rd , McKhann GM , 2nd , et al. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions . Epilepsia . 2007 ; 48 : 531 – 538 . doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Henry RG , Berman JI , Nagarajan SS , et al. Subcortical pathways serving cortical language sites: initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping . Neuroimage . 2004 ; 21 : 616 – 622 . doi: 10.1016/j.neuroimage.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick IA , Craen RA , Gelb AW , et al. Propofol sedation during awake craniotomy for seizures: patient-controlled administration versus neurolept analgesia . Anesth Analg . 1997 ; 84 : 1285 – 1291 . doi: 10.1097/00000539-199706000-00021. [DOI] [PubMed] [Google Scholar]
- Hill DL , Smith AD , Simmons A , et al. Sources of error in comparing functional magnetic resonance imaging and invasive electrophysiological recordings . J Neurosurg . 2000 ; 93 : 214 – 223 . doi: 10.3171/jns.2000.93.2.0214. [DOI] [PubMed] [Google Scholar]
- Hoshino T , Sakatani K , Katayama Y , et al. Application of multichannel near-infrared spectroscopic topography to physiological monitoring of the cortex during cortical mapping: technical case report . Surg Neurol . 2005 ; 64 : 272 – 275 . doi: 10.1016/j.surneu.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Huncke K , Van de Wiele B , Fried I , et al. The asleep-awake-asleep anesthetic technique for intraoperative language mapping . Neurosurgery . 1998 ; 42 : 1312 – 1316 . doi: 10.1097/00006123-199806000-00069. [DOI] [PubMed] [Google Scholar]
- Ius T , Isola M , Budai R , et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients . J Neurosurg . 2012 ; 117 : 1039 – 1052 . doi: 10.3171/2012.8.JNS12393. [DOI] [PubMed] [Google Scholar]
- Kamada K , Takeuchi F , Kuriki S , et al. Dissociated expressive and receptive language functions on magnetoencephalography, functional magnetic resonance imaging, and amobarbital studies. Case report and review of the literature . J Neurosurg . 2006 ; 104 : 598 – 607 . doi: 10.3171/jns.2006.104.4.598. [DOI] [PubMed] [Google Scholar]
- Kamada K , Todo T , Masutani Y , et al. Visualization of the frontotemporal language fibers by tractography combined with functional magnetic resonance imaging and magnetoencephalography . J Neurosurg . 2007 ; 106 : 90 – 98 . doi: 10.3171/jns.2007.106.1.90. [DOI] [PubMed] [Google Scholar]
- Keifer JC , Dentchev D , Little K , et al. A retrospective analysis of a remifentanil/propofol general anesthetic for craniotomy before awake functional brain mapping . Anesth Analg . 2005 ; 101 : 502 – 508 . doi: 10.1213/01.ANE.0000160533.51420.44. [DOI] [PubMed] [Google Scholar]
- Keles GE , Lundin DA , Lamborn KR , et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients . J Neurosurg . 2004 ; 100 : 369 – 375 . doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- Keles GE , Chang EF , Lamborn KR . Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with anaplastic astrocytoma . J Neurosurg . 2006 ; 105 : 34 – 40 . doi: 10.3171/jns.2006.105.1.34. [DOI] [PubMed] [Google Scholar]
- Kim KH , Relkin NR , Lee KM , et al. Distinct cortical areas associated with native and second languages . Nature . 1997 ; 338 : 171 – 174 . doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Knecht S , Drager B , Deppe M , et al. Handedness and hemispheric language dominance in healthy humans . Brain . 2000 ; 123 : 2512 – 2518 . doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kombos T , Süss O . Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: a review . Neurosurg Focus . 2009 ; 27 : E3 . doi: 10.3171/2009.8.FOCUS09141. [DOI] [PubMed] [Google Scholar]
- Kombos T , Suess O , Ciklatekerlio O , et al. Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex . J Neurosurg . 2001 ; 95 : 608 – 614 . doi: 10.3171/jns.2001.95.4.0608. [DOI] [PubMed] [Google Scholar]
- Kral T , Kurthen M , Schramm J , et al. Stimulation mapping via implanted grid electrodes prior to surgery for gliomas in highly eloquent cortex . Neurosurgery. 2006 ;58(1 Suppl): ONS36 – 43 . doi: 10.1227/01.neu.0000193925.98348.f5. [DOI] [PubMed] [Google Scholar]
- Lacroix M , Abi-Said D , Fourney DR , et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent or resection, and survival . J Neurosurg . 2001 ; 95 : 190 – 198 . doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- Lesser RP , Lüders H , Klem G , et al. Cortical after discharge and functional response thresholds: results of extraoperative testing . Epilepsia . 1984 ; 25 : 615 – 621 . doi: 10.1111/j.1528-1157.1984.tb03471.x. [DOI] [PubMed] [Google Scholar]
- Little K , Friedman AH . Awake craniotomy for malignant glioma resection . International Congress Series . 2004 ; 1259 : 409 – 414 . [Google Scholar]
- Lu S , Ahn D , Johnson G , et al. Peritumoral diffusion tensor imaging of high grade gliomas and metastatic brain tumors . AJNR Am J Neuroradiol . 2003 ; 24 : 937 – 941 . [PMC free article] [PubMed] [Google Scholar]
- Lu S , Ahn D , Johnson G , et al. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritu-moral edema: introduction of the tumor infiltration index . Radiology . 2004 ; 232 : 221 – 228 . doi: 10.1148/radiol.2321030653. [DOI] [PubMed] [Google Scholar]
- Lubrano V , Roux FE , Démonet JF . Writing specific sites in frontal areas: a cortical stimulation study . J Neurosurg . 2004 ; 101 : 787 – 798 . doi: 10.3171/jns.2004.101.5.0787. [DOI] [PubMed] [Google Scholar]
- Lucas TH , 2nd , McKhann GM , 2nd , Ojemann GA . Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients . J Neurosurg . 2004 ; 101 : 449 – 457 . doi: 10.3171/jns.2004.101.3.0449. [DOI] [PubMed] [Google Scholar]
- Manola L , Holsheimer J , Veltink P , et al. Anodal vs cathodal stimulation of motor cortex: a modeling study . Clin Neurophysiol . 2007 ; 118 : 464 – 474 . doi: 10.1016/j.clinph.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Matsuda R , Coello AF , De Benedictis A , et al. Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: surgical technique and functional results: clinical article . J Neurosurg . 2012 ; 117 : 1076 – 1081 . doi: 10.3171/2012.9.JNS12662. [DOI] [PubMed] [Google Scholar]
- Meyer FB , Bates LM , Goerss SJ , et al. Awake craniotomy for aggressive resection of primary gliomas located in eloquent brain . Mayo Clin Proc . 2001 ; 76 : 677 – 687 . doi: 10.4065/76.7.677. [DOI] [PubMed] [Google Scholar]
- Mori S , Frederiksen K , van Zijl PC , et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging . Ann Neurol . 2002 ; 51 : 377 – 380 . doi: 10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- Najib U , Bashir S , Edwards D , et al. Transcranial brain stimulation: clinical applications and future directions . Neurosurg Clin N Am . 2011 ; 22 : 233 – 251 . doi: 10.1016/j.nec.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai T , Sato K , Hirakawa K , et al. Imaging of somato-topic representation of sensory cortex with intrinsic optical signals as guides for brain tumor surgery . J Neurosurg . 2005 ; 103 : 414 – 423 . doi: 10.3171/jns.2005.103.3.0414. [DOI] [PubMed] [Google Scholar]
- Nathan SS , Lesser RP , Gordon B , et al. Electrical stimulation of the human cerebral cortex. Theoretical approach . Adv Neurol . 1993 ; 63 : 61 – 85 . [PubMed] [Google Scholar]
- Neuloh G , Schramm J . Motor evoked potential monitoring for the surgery of brain tumours and vascular malformations . Adv Tech Stand Neurosurg . 2004 ; 29 : 171 – 228 . doi: 10.1007/978-3-7091-0558-0_5. [DOI] [PubMed] [Google Scholar]
- Ojemann GA . Individual variability in cortical localization of language . J Neurosurg . 1979 ; 50 : 164 – 169 . doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Mateer C . Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems . Science . 1979 ; 205 : 1401 – 1403 . doi: 10.1126/science.472757. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Dodrill CB . Verbal memory deficits after temporal lobectomy for epilepsy. Mechanism and intraoperative prediction . J Neurosurg . 1985 ; 62 : 101 – 107 . doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Ojemann J , Lettich E , et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients . J Neurosurg . 1989 ; 71 : 316 – 326 . doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ojemann JG , Miller JW , Silbergeld DL . Preserved function in brain invaded by tumor . Neurosurgery . 1996 ; 39 : 253 – 258 . doi: 10.1097/00006123-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Otani N , Bjeljac M , Muroi C , et al. Awake surgery for glioma resection in eloquent areas - Zurich’s experience and review . Neurol Med Chir (Tokyo) 2005 ; 45 : 501 – 511 . doi: 10.2176/nmc.45.501. [DOI] [PubMed] [Google Scholar]
- Pålson A , Ek L , Ahlström G , et al. Pitfalls in the assessment of disability in individuals with low-grade gliomas . J Neurooncol . 2003 ; 65 : 149 – 158 . doi: 10.1023/b:neon.0000003727.09448.dd. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC , Simos PG , Breier JI , et al. Magnetoencephalographic mapping of the language-specific cortex . J Neurosurg . 1999 ; 90 : 85 – 93 . doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- Peraud A , Ilmberger J , Reulen HJ . Surgical resection of gliomas WHO grade II and III located in the opercular region . Acta Neurochir (Wien) 2004 ; 146 : 9 – 18 . doi: 10.1007/s00701-003-0165-4. [DOI] [PubMed] [Google Scholar]
- Petrella JR , Shah LM , Harris KM , et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors . Radiology . 2006 ; 240 : 793 – 802 . doi: 10.1148/radiol.2403051153. [DOI] [PubMed] [Google Scholar]
- Petrovich Brennan NM , Whalen S , De Morales Branco , et al. Object naming is a more sensitive measure of speech localization than number counting: converging evidence from direct cortical stimulation and fMRI . Neuroimage. 2007 ;37(Suppl 1): S100 – S108 . doi: 10.1016/j.neuroimage.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Picht T , Kombos T , Gramm H , et al. Multimodal protocol for awake craniotomy in language cortex tumour surgery . Acta Neurochir (Wien) 2006 ; 148 : 127 – 137 . doi: 10.1007/s00701-005-0706-0. [DOI] [PubMed] [Google Scholar]
- Pouratian N , Cannestra AF , Martin NA , et al. Intraoperative optical intrinsic signal imaging: a clinical tool for functional brain mapping . Neurosurg Focus. 2002a ;13:e1. doi: 10.3171/foc.2002.13.4.2. [DOI] [PubMed] [Google Scholar]
- Pouratian N , Bookheimer SY , Rex DE , et al. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations . J Neurosurg . 2002b ; 97 : 21 – 32 . doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Pouratian N , Bookheimer SY , Rubino G , et al. Category-specific naming deficit identified by intraoperative stimulation mapping and postoperative neuropsychological testing . J Neurosurg . 2003 ; 99 : 170 – 176 . doi: 10.3171/jns.2003.99.1.0170. [DOI] [PubMed] [Google Scholar]
- Pouratian N , Cannestra AF , Bookheimer SY , et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals . J Neurosurg . 2004 ; 101 : 458 – 466 . doi: 10.3171/jns.2004.101.3.0458. [DOI] [PubMed] [Google Scholar]
- Price SJ , Burnet NG , Donovan T , et al. Diffusion tensor imaging of brain tumors at 3T: a potential tool for assesing whait matter tract invasion? . Clin Radiol . 2003 ; 58 : 455 – 462 . doi: 10.1016/s0009-9260(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen IA , Jr , Lindseth F , Rygh OM , et al. Functional neuronavigation combined G. with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data . Acta Neurochir (Wien) 2007 ; 149 : 365 – 378 . doi: 10.1007/s00701-006-1110-0. [DOI] [PubMed] [Google Scholar]
- Reithmeier T , Krammer M , Gumprecht HH , et al. Neuronavigation combined with electrophysiological monitoring for surgery of lesions in eloquent brain areas in 42 cases: a retrospective comparison of the neurological outcome and the quality of resection with a control group with similar lesions . Minim Invas Neurosurg . 2003 ; 46 : 65 – 71 . doi: 10.1055/s-2003-39334. [DOI] [PubMed] [Google Scholar]
- Roux FE , Trémoulet M . Organization of language areas in bilingual patients: a cortical stimulation study . J Neurosurg . 2002 ; 97 : 857 – 864 . doi: 10.3171/jns.2002.97.4.0857. [DOI] [PubMed] [Google Scholar]
- Roux FE , Boetto S , Sacko O , et al. Writing, calculation and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome . J Neurosurg . 2003 ; 99 : 716 – 727 . doi: 10.3171/jns.2003.99.4.0716. [DOI] [PubMed] [Google Scholar]
- Roux FE , Lubrano V , Lauwers-Cances V , et al. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients . Brain . 2004 ; 127 : 1796 – 1810 . doi: 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- Ruge MI , Victor J , Hosain S , et al. Concordance between functional magnetic resonance imaging and intra-operative language mapping . Stereotact Funct Neurosurg . 1999 ; 72 : 95 – 102 . doi: 10.1159/000029706. [DOI] [PubMed] [Google Scholar]
- Rutten GJ , Van Rijen PC , Van Veelen CW , et al. Language area localization with three-dimensional functional magnetic resonance imaging matches intrasulcal electrostimulation in Broca’s area . Ann Neurol . 1999 ; 46 : 405 – 408 . doi: 10.1002/1531-8249(199909)46:3<405::aid-ana17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rutten GJ , Ramsey NF , van Rijen PC , et al. Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas . Ann Neurol . 2002a ; 51 : 350 – 360 . doi: 10.1002/ana.10117. [DOI] [PubMed] [Google Scholar]
- Rutten GJ , Ramsey NF , van Rijen PC , et al. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test . Neuroimage . 2002b ; 17 : 447 – 460 . doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- Saetti MC , Marangolo P , De Renzi E , et al. The nature of the disorder underlying the inability to retrieve proper names . Cortex . 1999 ; 35 : 675 – 685 . doi: 10.1016/s0010-9452(08)70827-x. [DOI] [PubMed] [Google Scholar]
- Sahjpaul RL . Awake craniotomy: controversies, indications and techniques in the surgical treatment of temporal lobe epilepsy . Can J Neurol Sci. 2000 ;(Suppl 1):s55– 63 . doi: 10.1017/s0317167100000676. discussion S92–96 . [DOI] [PubMed] [Google Scholar]
- Sanai N , Mirzadeh Z , Berger MS . Functional outcome after language mapping for glioma resection . N Engl J Med . 2008 ; 358 : 18 – 27 . doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Santini B , Talacchi A , Casagrande F . Eligibility criteria and psychological profiles in patient candidates for awake craniotomy: a pilot study . J Neurosurg Anesthesiol . 2012 ; 24 : 209 – 216 . doi: 10.1097/ANA.0b013e3182464aec. [DOI] [PubMed] [Google Scholar]
- Sarang A , Dinsmore J . Anaesthesia for awake craniotomy - evolution of a technique that facilitates awake neurological testing . Br J Anaesth . 2003 ; 90 : 161 – 165 . doi: 10.1093/bja/aeg037. [DOI] [PubMed] [Google Scholar]
- Sartorius CJ , Wright G . Intraoperative brain mapping in a community setting--technical considerations . Surg Neurol . 1997 ; 47 : 380 – 388 . doi: 10.1016/s0090-3019(96)00340-0. [DOI] [PubMed] [Google Scholar]
- Sartorius CJ , Berger MS . Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer’s lactate to the cortex. Technical note . J Neurosurg . 1998 ; 88 : 349 – 351 . doi: 10.3171/jns.1998.88.2.0349. [DOI] [PubMed] [Google Scholar]
- Sawaya R , Hammoud M , Schoppa D , et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors . Neurosurgery . 1998 ; 42 : 1044 – 1056 . doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- Schekutiev G , Schmid UD . Coaxial insulated bipolar electrode for monopolar and bipolar mapping of neural tissue: technical note with emphasis on the principles of intra-operative stimulation . Acta Neurochir (Wien) 1996 ; 138 : 470 – 474 . doi: 10.1007/BF01420311. [DOI] [PubMed] [Google Scholar]
- Schucht P , Beck J , Abu-Isa J , et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intra-operative fluorescence imaging and brain mapping . Neurosurgery . 2012 ; 71 : 927 – 935 . doi: 10.1227/NEU.0b013e31826d1e6b. [DOI] [PubMed] [Google Scholar]
- Seeck M , Pegna AJ , Ortigue S , et al. Speech arrest with stimulation may not reliably predict language deficit after epilepsy surgery . Neurology . 2006 ; 66 : 592 – 594 . doi: 10.1212/01.wnl.0000199254.67398.a7. [DOI] [PubMed] [Google Scholar]
- Serletis D , Bernstein M . Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors . J Neurosurg . 2007 ; 107 : 1 – 6 . doi: 10.3171/JNS-07/07/0001. [DOI] [PubMed] [Google Scholar]
- Sibtain NA , Howe FA , Saunders DE . The clinical value of proton magnetic resonance spectroscopy in adult brain tumours . Clin Radiol . 2007 ; 62 : 109 – 119 . doi: 10.1016/j.crad.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Signorelli F , Guyotat J , Isnard J , et al. The value of cortical stimulation applied to the surgery of malignant gliomas in language areas . Neurol Sci . 2001 ; 22 : 3 – 10 . doi: 10.1007/s100720170029. [DOI] [PubMed] [Google Scholar]
- Silbergeld DL , Mueller WM , Colley PS , et al. Use of propofol (Diprivan) for awake craniotomies: technical note . Surg Neurol . 1992 ; 38 : 271 – 272 . doi: 10.1016/0090-3019(92)90038-o. [DOI] [PubMed] [Google Scholar]
- Simos PG , Papanicolaou AC , Breier JI , et al. Localization of language-specific cortex by using magnetic source imaging and electrical stimulation mapping . J Neurosurg . 1999 ; 91 : 787 – 796 . doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- Skirboll SS , Ojemann GA , Berger MS , et al. Functional cortex and subcortical white matter located within gliomas . Neurosurgery . 1996 ; 38 : 678 – 684 . [PubMed] [Google Scholar]
- Skrap M , Mondani M , Tomasino B , et al. Surgery of insular nonenhancing gliomas: volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases . Neurosurgery . 2012 ; 70 : 1081 – 1094 . doi: 10.1227/NEU.0b013e31823f5be5. [DOI] [PubMed] [Google Scholar]
- Skucas AP , Artru AA . Anesthetic complications of awake craniotomies for epilepsy surgery . Anesth Analg . 2006 ; 102 : 882 – 887 . doi: 10.1213/01.ane.0000196721.49780.85. [DOI] [PubMed] [Google Scholar]
- Sobottka SB , Krafft C , Geiger K , et al. Identification and classification of gliomas by optical spectroscopic imaging techniques. 59th Annual Meeting of the German Society of Neurosurgery; 2008 . Meeting Abstract (DI.01.05). http://www.egms.de/static/de/meetings/dgnc2008/08dgnc144.shtml. [Google Scholar]
- Stadlbauer A , Moser E , Gruber S , et al. Improved delineation of brain tumors: an automated method for segmentation based on pathologic changes of 1HMRSI metabolites in gliomas . Neuroimage . 2004 ; 23 : 454 – 461 . doi: 10.1016/j.neuroimage.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A , Gruber S , Nimscki C , et al. Preoperative grading of gliomas using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging . Radiology . 2006 ; 238 : 958 – 969 . doi: 10.1148/radiol.2382041896. [DOI] [PubMed] [Google Scholar]
- Stippich C , Rapps N , Dreyhaupt J , et al. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients . Radiology . 2007 ; 243 : 828 – 836 . doi: 10.1148/radiol.2433060068. [DOI] [PubMed] [Google Scholar]
- Stummer W , Schackert G , Pichlmeier U , et al. The impact of resection on the treatment of glioblastoma multi-forme: survival comparison with the RTOG recursive partitioning analysis of ALA glioma study patients . 59th Annual Meeting of the German Society of Neurosurgery; 2008 . http://www.egms.de/static/en/meetings/dgnc2008/08dgnc036.shtml. [Google Scholar]
- Sunaert S . Presurgical planning for tumor resectioning . J Magn Reson Imaging . 2006 ; 23 : 887 – 905 . doi: 10.1002/jmri.20582. [DOI] [PubMed] [Google Scholar]
- Szelényi A , Bello L , Duffau H , et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice . Neurosurg Focus . 2010 ; 28 : E7 . doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- Talacchi A , Turazzi S , Locatelli F , et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series . J Neurooncol . 2010 ; 100 : 417 – 426 . doi: 10.1007/s11060-010-0193-x. [DOI] [PubMed] [Google Scholar]
- Talacchi A , Santini B , Savazzi S , et al. Cognitive effects of tumour and surgical treatment in glioma patients . J Neurooncol . 2011 ; 103 : 541 – 549 . doi: 10.1007/s11060-010-0417-0. [DOI] [PubMed] [Google Scholar]
- Talacchi A , d’Avella D , Denaro L , et al. Cognitive outcome as part and parcel of clinical outcome in brain tumor surgery . J Neurooncol . 2012 ; 108 : 327 – 332 . doi: 10.1007/s11060-012-0818-3. [DOI] [PubMed] [Google Scholar]
- Talacchi A , Santini B , Casartelli M , et al. Awake surgery between art and science. Part II: language and cognitive mapping . Funct Neurol . 2013 ; 28 : 223 – 239 . doi: 10.11138/FNeur/2013.28.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taphoorn MJ , Heimans JJ , Snoek FJ , et al. Assessment of quality of life in patients treated for low-grade glioma: a preliminary report . J Neurol Neurosurg Psychiatry . 1992 ; 55 : 372 – 376 . doi: 10.1136/jnnp.55.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taphoorn MJ , Klein M . Cognitive status in adult patients with brain tumours . Lancet Neurol . 2004 ; 3 : 159 – 168 . doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- Taphoorn MJB , Stupp R , Coens C , et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial . Lancet Oncol . 2005 ; 5 : 934 – 944 . doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- Taylor MD , Bernstein M . Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intaaxial tumors: a prospective trial of 200 cases . J Neurosurg . 1999 ; 90 : 35 – 41 . doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- Tharin S , Golby A . Functional brain mapping and its applications to neurosurgery . Neurosurgery. 2007 ;60(4 Suppl 2): 185 – 202 . doi: 10.1227/01.NEU.0000255386.95464.52. [DOI] [PubMed] [Google Scholar]
- Trinh VT , Fahim DK , Shah K , et al. Subcortical injury is an independent predictor of worsening neurological deficits following awake craniotomy procedures . Neurosurgery . 2013 ; 72 : 160 – 169 . doi: 10.1227/NEU.0b013e31827b9a11. [DOI] [PubMed] [Google Scholar]
- Tuch DS . Q-ball imaging . Magn Reson Med . 2004 ; 52 : 1358 – 1372 . doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- Tucha O , Smeley C , Preier M , et al. Cognitive deficits before treatment among patients with brain tumors . Neurosurgery . 2000 ; 47 : 324 – 333 . doi: 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- Van Buren JM , Fedio P , Frederick GC . Mechanism and localization of speech in the parietotemporal cortex . Neurosurgery . 1978 ; 2 : 233 – 239 . doi: 10.1227/00006123-197805000-00009. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ , Wefel JS , Schiff D , et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas . Lancet Oncol . 2011 ; 12 : 583 – 593 . doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- Vigneau M , Beaucousin V , Hervé PY , et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing . Neuroimage . 2006 ; 30 : 1414 – 1432 . doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vives KP , Piepmeier JM . Complications and expected outcome of glioma surgery . J Neurooncol . 1999 ; 42 : 289 – 302 . doi: 10.1023/a:1006163328765. [DOI] [PubMed] [Google Scholar]
- Walker JA , Quinones-Hinojosa A , Berger MS . Intraoperative speech mapping in 17 bilingual patients undergoing resection of a mass lesion . Neurosurgery . 2004 ; 54 : 113 – 118 . doi: 10.1227/01.neu.0000097270.95721.3b. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ , Hagmann P , Tseng WY , et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging . Magn Reson Med . 2005 ; 54 : 1377 – 1386 . doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Weitzner MA , Meyers CA . Cognitive functioning and quality of life in malignant glioma patients: a review of the literature . Psychooncology . 1997 ; 6 : 169 – 177 . doi: 10.1002/(SICI)1099-1611(199709)6:3<169::AID-PON269>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Weitzner MA , Meyers CA , Byrne K . Psychosocial functioning and quality of life in patients with primary brain tumours . J Neurosurg . 1996 ; 84 : 29 – 34 . doi: 10.3171/jns.1996.84.1.0029. [DOI] [PubMed] [Google Scholar]
- Whittle IR , Borthwick S , Haq N . Brain dysfunction following “awake” craniotomy, brain mapping and resection of glioma . Br J Neurosurg . 2003 ; 17 : 130 – 137 . doi: 10.1080/0268869031000108873. [DOI] [PubMed] [Google Scholar]
- Whittle IR , Midgley S , Georges H , et al. Patient perceptions of “awake” brain tumour surgery . Acta Neurochir (Wien) 2005 ; 147 : 275 – 277 . doi: 10.1007/s00701-004-0445-7. [DOI] [PubMed] [Google Scholar]
- Wiedemayer H , Sandalcioglu IE , Armbruster W , et al. False negative findings in intraoperative SEP monitoring: analysis of 658 consecutive neurosurgical cases and review of published reports . J Neurol Neurosurg Psychiatry . 2004 ; 75 : 280 – 286 . [PMC free article] [PubMed] [Google Scholar]
- Woolsey CN , Erickson TC , Gilson WE . Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation . J Neurosurg . 1979 ; 51 : 476 – 506 . doi: 10.3171/jns.1979.51.4.0476. [DOI] [PubMed] [Google Scholar]
- Yamada K , Kizu O , Mori S , et al. Brain fiber tracking with clinically feasible diffusion-tensor MR imaging: initial experience . Radiology . 2003 ; 227 : 295 – 301 . doi: 10.1148/radiol.2271020313. [DOI] [PubMed] [Google Scholar]
- Yoshii Y , Tominaga D , Sugimoto K , et al. Cognitive function of patients with brain tumor in pre- and postoperative stage . Surg Neurol . 2008 ; 69 : 51 – 61 . doi: 10.1016/j.surneu.2007.07.064. [DOI] [PubMed] [Google Scholar]
- Young RJ , Knopp EA . Brain MRI: tumor evaluation . J Magn Reson Imaging . 2006 ; 24 : 709 – 724 . doi: 10.1002/jmri.20704. [DOI] [PubMed] [Google Scholar]
- Zangaladze A , Sharan A , Evans J , et al. The effectiveness of low-frequency stimulation for mapping cortical function . Epilepsia . 2008 ; 49 : 481 – 487 . doi: 10.1111/j.1528-1167.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- Zhang J , Wu J-S , Lu J-F , et al. Awake language mapping for cerebral glioma surgery. Cochrane Database Syst Rev. 2012 ;4: CD009791 . doi: 10.1002/14651858.CD009791. [DOI] [Google Scholar]