Summary
The network of interactions between mitral and granule cells in the olfactory bulb is a critical step in the processing of odor information underlying the neural basis of smell perception. We are building the first computational model in 3 dimensions of this network in order to analyze the rules for connectivity and function within it.
The initial results indicate that this network can be modeled to simulate experimental results on the activation of the olfactory bulb by natural odorants, providing a much more powerful approach for 3D simulation of brain neurons and microcircuits.
Keywords: computational modeling , lateral inhibition , mitral cells , olfactory glomeruli , tufted cells
The neural basis of odor recognition may have a significant impact in terms not only of efforts to provide a better explanation of physiological and behavioral processes at brain system level, but also of industrial applications (in the setting of the development of odor-sensitive devices and the fragrance industry). While experimental findings have given important clues regarding the activation patterns of the cells involved in odor recognition, the underlying network mechanisms are, as with other brain systems, still unknown. This lack of information on the olfactory system is due mainly to the fact that the circuits carrying out sensory processing in this system are usually investigated experimentally in single cells or in small, randomly selected sets of cells. The functional effects of network-wide processes, in relation to the patterns of glomeruli activated by different odors, thus remain relatively unknown.
In an attempt to help solve this problem we have used a bottom-up approach involving simulations performed according to a general strategy for realistic modeling of neuronal networks, which can be integrated into the more general framework that will be developed within the Human Brain Project (see D’Angelo et al. in this issue, for a basic description of computational modeling techniques and theory). This approach required the development, in a fully integrated NEURON+Python parallel environment, of programming patterns that are generic for use in other brain regions. For the olfactory bulb system, we started with accurate models at the single neuron level ( Shen et al., 1999), before building simple microcircuit models, in order to gain insight into interactions between individual mitral and granule cells ( Migliore and Shepherd, 2008; Migliore et al., 2010; McTavish et al., 2012), and a large-scale 1D model ( Yu et al. 2013), in order to analyze how the spatiotemporal dynamics of lateral inhibition can contribute to producing a reliable olfactory code. We were able to demonstrate that lateral inhibition can modulate the evolving dynamics of the olfactory bulb network, generating mitral and granule cell responses that account for critical experimental findings. However, a realistic representation of dendritic field coverage of mitral cells in the external plexiform layer, and its functional consequences, essentially requires a full 3D representation. Towards this goal, we are implementing a general, expandable, and high-performance computational structure, supercomputer-oriented, which allows us to run different series of simulations using a variety of experimental constraints and input data.
We first devised general rules, applicable to other brain systems, so as to be able, subsequently, to extend our experience with the first 3D model of the olfactory bulb for processing natural odorants. Experimentally ( Vincis et al., 2012), it was found that natural odors activate a broad range of glomeruli (dense representation).
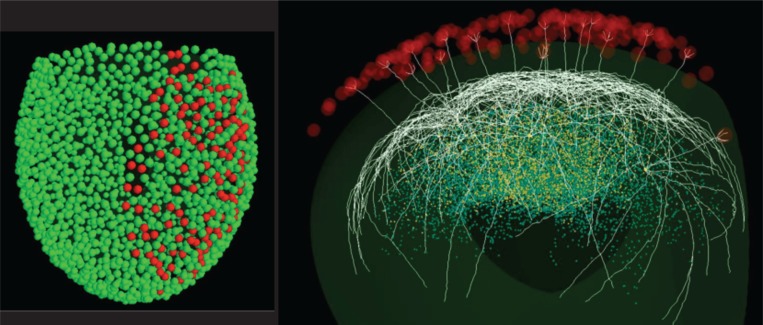
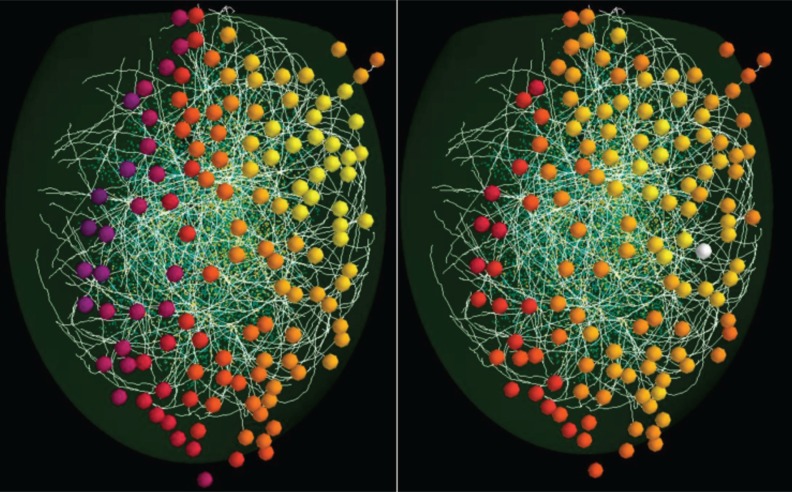
Using an algorithm that considers the statistical distribution for a few morphological properties derived from fully reconstructed mitral cells ( Igarashi et al., 2012), it is possible, through a specific procedure, to generate the full population of mitral cells present in the entire olfactory bulb. In this case, we used 635 cells, five for each of the 127 glomeruli for which we have experimental data. Figure 1 (over) shows how the model is organized. Active (red) and inactive (green) glomeruli above the surface are positioned to reproduce the ellipsoidal shape of an olfactory bulb, together with 18 mitral cells projecting to different glomeruli ( Fig. 1, right ) and connected with a population of granule cells (circles below the mitral cell lateral dendrites). The synthetic cells are characterized by an intrinsic variability of their parameters within the experimental range, and their properties are statistically indistinguishable from those of the real neurons. Approximately 40,000 simplified (e.g. using a soma and a single radial dendrite) granule cells were generated, randomly inserted into the network, and connected with mitral cell lateral dendrites using a collision detection algorithm. Active properties and synaptic properties, constrained by experimental findings, were taken from our previous models (Yu et al., 2012). We are now in the process of testing how the activity elicited in each of the 127 glomeruli by 19 natural odorants ( Vincis et al., 2012) drives the self-organization of the network under different conditions. As can be seen from the differential activation of the glomeruli [represented with different colors in Fig. 2 , compare with Fig. 2a in Vincis et al., (2012) ], different odors exhibit a considerable overlap in glomerular activation. The underlying network can thus be expected to show quite extensive interaction not only during odor training but also during each sniff. The major questions that we would like to investigate with this model are thus related to how and under what conditions the network self-organization occurring during odor learning can disambiguate the firing activity of the mitral cells in such a way as to form a reliable odor code.
Figure 1 .
(left) Model of 1800 glomeruli in the olfactory bulb, stimulated by natural odors (red) or unstimulated (green); (right) 3D representation of 18 typical synthetic mitral cells (white) connected to different glomeruli (red) by their apical dendrites. The cloud of dots below the mitral cell lateral dendrites represents granule cell bodies; granule cells connected to more than one mitral cell are shown in yellow.
Figure 2 .
Model of glomerular activation in response to “banana” (left) and “mint” (right) odorants.
The model can be expanded to incorporate models of additional experimentally identified mechanisms as they become available, and it can be transformed into a facility for modeling and investigating the functions of other brain systems, according to the general approach that will be developed within the Human Brain Project. This is a crucial step towards simulating the entire human brain and, in pursuit of this ultimate goal, we are using the above method and approach to begin the implementation of a full 3D Purkinje-granule cell cerebellar network.
Acknowledgments
We are grateful for support from the SenseLab Project (National Institute of Deafness and Other Communication Disorders) and grant NS11613 from NINDS which supports NEURON development.
References
- D’Angelo E , Solinas S , Garrido J , et al. Realistic modeling of neurons and networks: towards brain simulation . Funct Neurol . 2013 ; 28 : 153 – 166 . [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM , Ieki N , An M , et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex . J Neurosci . 2012 ; 32 : 7970 – 7985 . doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish TS , Migliore M , Shepherd GM , et al. Mitral cell spike synchrony modulated by dendrodendritic synapse location . Front Comput Neurosci . 2012 ; 6 : 3 . doi: 10.3389/fncom.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M , Hines ML , McTavish TS , et al. Functional roles of distributed synaptic clusters in the mitral-granule cell network of the olfactory bulb . Front Integr Neurosci . 2010 ; 4 : 122 . doi: 10.3389/fnint.2010.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M , Shepherd GM . Dendritic action potentials connect distributed dendrodendritic microcircuits . J Comput Neurosci . 2008 ; 24 : 207 – 221 . doi: 10.1007/s10827-007-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen GY , Chen WR , Midtgaard J , et al. Computational analysis of action potential initiation in mitral cell soma and dendrites based on dual patch recordings . J Neurophysiol . 1999 ; 82 : 3006 – 3020 . doi: 10.1152/jn.1999.82.6.3006. [DOI] [PubMed] [Google Scholar]
- Vincis R , Gschwend O , Bhaukaurally K , et al. Dense representation of natural odorants in the mouse olfactory bulb . Nat Neurosci . 2012 ; 15 : 537 – 539 . doi: 10.1038/nn.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y , McTavish TS , Hines ML , et al. Sparse distributed representation of odors in a large-scale olfactory bulb circuit . PLoS Comput Biol. 2013 ;9:e1003014. doi: 10.1371/journal.pcbi.1003014. [DOI] [PMC free article] [PubMed] [Google Scholar]