Summary
Direct cortical and subcortical stimulation has been claimed to be the gold standard for exploring brain function. In this field, efforts are now being made to move from intraoperative naming-assisted surgical resection towards the use of other language and cognitive tasks. However, before relying on new protocols and new techniques, we need a multi-staged system of evidence (low and high) relating to each step of functional mapping and its clinical validity.
In this article we examine the possibilities and limits of brain mapping with the aid of a visual object naming task and various other tasks used to date. The methodological aspects of intraoperative brain mapping, as well as the clinical and operative settings, were discussed in Part I of this review.
Keywords: cognitive tasks , eloquent areas , language mapping , subcortical tracts
Introduction
In Part I of this two-part review we focused on the background to, rationale for and methodological (clinical and instrumental) aspects of awake surgery and intraoperative brain mapping.
The purpose of this second part is to describe experiences of intraoperative mapping of language and other functions, discussing inclusion and exclusion criteria and the definition of positive sites, as well as their representation and surgical management.
Visual object naming
Visual object naming (VON) is a task designed to assess the presence and degree of anomia, a very common symptom in many aphasic subjects. Its use during awake surgery can be important for achieving reliable language localization ( Ojemann, 1979, 1983; Herholz et al. 1997; Berger et al., 1989). Furthermore, being rapid and easy to administer, it is well suited to the already limiting conditions of awake surgical procedures.
Procedure
The current literature reports that VON is the task most commonly used for mapping the dominant hemisphere, and the procedure described by Ojemann is the one most widely reported on in the context of awake surgery. The procedure is described in varying detail, depending on the study aim. In many cases, although the authors give a step-by-step description of how the VON task was administered during intraoperative mapping, the method is basically a replica of Ojemann’s procedure ( Schwartz et al., 1999; Ojemann and Dodrill, 1985; Peraud et al., 2004). Some authors cite the source and briefly describe the procedure ( Haglund et al., 1994; Ojemann, 1983; Ojemann et al., 1989; Lucas et al., 2004; Duffau et al., 2002a; Roux et al., 2004; Bello et al., 2006; Duffau et al., 2006; Roux et al., 2003; Otani et al., 2005). Occasionally, however, the source is not cited or the methodology described. The extensive use of VON is a sign of its recognized validity ( Bello et al., 2008). The most frequently cited references are Ojemann and Whitaker, 1978; Ojemann 1983, 1991; and Ojemann et al., 1989. Berger (1994) and Berger and Ojeman (1992) performed awake mapping in the same way.
Step-by-step Procedure: Before mapping
According to Ojemann et al. (1989), the aim of intra-operative stimulation mapping is to identify “eloquent” areas where one or more language functions are located. Larger craniotomies are performed with the aim of defining both the areas involved in the surgical resection and those considered “classic” language locations. Here we summarize the main steps followed before language mapping under Ojemann’s protocol (excluding the details on local anesthesia):
Prior to language mapping, the rolandic cortex is identified by stimulation. The sensorimotor cortex is identified by associated evoked motor and sensory responses to stimulation of the tongue, teeth, throat or face.
Language mapping uses the highest current that does not produce an afterdischarge on electrocorticography.
A current range of 1.5–10 mA is delivered by a constant-current stimulator in 4-second trains at 60 Hz across 1-mm bipolar electrodes separated by a 5-mm gap.
The sites selected for stimulation mapping are identified by small tags randomly applied to the cortex (10–20 sites per subject).
With regard to point 1, the area immediately anterior to the face motor cortex is investigated because electrical interferences may render the patient unable to mimic simple orofacial movements (such as protrusion of the tongue), which may also be essential for speech. The purpose of this first step is to distinguish between motor and sensory areas and Broca’s area ( Ojemann, 1983). In 95.5% of cases, Broca’s area was found in sites where a speech arrest was evoked during the task without a simultaneous motor response in the mouth; these sites were usually located in the area lying directly anterior to the face motor cortex (see the “Counting” section below) ( Haglund et al., 1994). In order to exclude disturbances due to muscle participation in speech arrest, Schäffler et al. (1996) looked for “positive” and “negative” symptoms evoked by the inability to perform motor tasks during stimulation, for example, during protrusion and rapidly alternating tongue movements. Bello et al. (2007) identified Broca’s area by asking patients to count and also by electromyography (three electrodes placed on the upper lip, lower lip, and cheek). All the other steps (points 2, 3, 4) have been discussed elsewhere (Part I).
Step-by-step procedure: language mapping
Language function is measured by showing the patient a set of black-and-white slides with a line drawing of a common object such as a bell, a hand, a car or a ring, prompted by a carrier phrase “That is a…” or “This is a…” ( Ojemann, 1979). Later, we describe the batteries of slides used for the VON task.
The slides are displayed for 4 seconds each, and the patient is instructed to name each slide as it appears.
A current is applied as the slide appears on the screen and continued until the next slide appears or until the patient shows disturbances. The sequence proceeds with each slide associated with stimulation followed by at least one “stimulation-free” slide. At least three stimulations are delivered for each small selected cortical site. As regards the distance between tags, it has been shown that the effects of stimulation on naming may be quite localized, changing within a centimeter, even on the same gyrus (Szelenyi et al., 2010; Ilmberger et al. 2001; Ojemann and Whitaker, 1978).
Responses are recorded by manual scoring for immediate feedback to the surgeon, and by audiotape for further analysis ( Ojemann et al., 1989; Ojemann, 1983).
As regards point 1, the carrier phrase is used to discriminate between anomia and speech arrest and/or motor disturbances ( Table I ). Points 1 and 2 do not differ significantly between studies. As regards point 3, some authors start the stimulation immediately before presenting the slides ( Hamberger et al., 2005; Bello et al., 2007). Here, it must be noted that after more than four repetitions the learning effect can limit the test validity ( Ojemann et al., 2002). Point 4 will be described in the “Data recording” section below.
Table I .
Naming: error definitions reported in the literature.
| ERROR | DEFINITION |
|---|---|
|
Articulatory interferences
Omission Anomia |
|
| Speech arrest |
|
| Aphasic arrest |
|
| Perseveration |
|
| Hesitation (or delay) |
|
| Tip-of-the-tongue |
|
| Kinds of paraphasia |
|
| Dysarthria |
|
Language task
Battery
The source of the images used in intraoperative mapping varies across studies and is not often cited. The main batteries (listed below) differ with regard to various parameters (frequency, familiarity, name category). Some authors report the sources used for the line-drawing objects, and they are usually the ones detailed below:
The Boston Naming Test ( Kaplan et al., 1983) comprises 60 line drawings characterized by decreasing frequency and divided by subject category: vegetables, wild animals, tools ( Pouration et al., 2003, 2004; Hamberger et al., 2005, 2007). Some authors use a shorter version made up of 50 items ( Lubrano et al., 2004).
The 260-item Snodgrass and Vanderwart Test ( Snodgrass and Vanderwart, 1980). Typically, subsets of stimuli are selected from this pool to prepare naming/comprehension tasks to tap into the subject’s knowledge of biological categories (e.g., animals, fruit, vegetables), inanimate entities (e.g., tools, vehicles, furniture), and other sets of objects (e.g., musical instruments, body parts). A popular example is the 80-item test constructed by Laiacona et al. (1993) and used in several studies during awake surgery. Also available is a shorter version of the Snodgrass and Vanderwart Test which contains only high-frequency items ( Hamberger et al., 2003).
The DO80 comprises 80 black-and-white pictures controlled for variables such as frequency, familiarity, age of acquisition and level of education ( Metz-Lutz et al., 1992). These stimuli are homogeneous within the different categories and normative data are available ( Metz-Lutz et al., 1992; Mandonnet et al., 2006; Gil Robles et al., 2005; Benzagmout et al., 2007; Matsuda et al., 2012).
The naming subtest from the Aachener Aphasie Test ( Gharabaghi et al., 2006; Peraud et al., 2004).
Other authors use black-and-white line drawings ( Ojemann and Whitaker, 1978; Ojemann, 1983; Ojemann et al. 1989, 2002; Otani et al., 2005).
Item selection
As a standard procedure, an intraoperative test is preliminarily administered to each patient. Ojemann (1979) chose items that patients were able to name very rapidly and easily during preoperative testing. Items that patients were unable to name during preoperative assessment were deleted ( Ojemann et al., 1989; Roux et al., 2003; Hamberger et al., 2005). However, Ojemann does not specify how many items a patient can fail to name without being excluded from an awake surgery protocol. Roux and Lubrano recommend a 10% error cut-off ( Roux et al., 2003; Lubrano et al., 2004). According to Little and Friedman (2004), however, because a preoperative object naming error rate >25% cannot be statistically correlated with performance on intraoperative naming during cortical stimulation, in such instances interference with naming cannot be reliably interpreted as a consequence of stimulation ( Little and Friedman, 2004; Sarubbo et al., 2012 a , b ). By adopting a restrictive cut-off during item selection, the probability of false positives due to language variability is reduced. Furthermore, the authors suggested that patient performance can be improved by training patients to name objects, thereby increasing the reliability of intraoperative language mapping. This strategy must be analyzed more carefully, owing to the practice effect on the distribution of positive naming sites found in a previous study on verb generation ( Ojemann et al., 2002) and the differences between the use of naming and automatic series, e.g. counting ( Petrovich Brennan et al., 2007).
Positive sites
Error definition
Undefined errors can also occur in the absence of stimulation. Without careful preoperative assessment, the intraoperative definition of errors will remain very uncertain. Ojemann found that intraoperative errors varied in the range of 4.6 to 22% ( Ojemann, 1979; Ojemann et al., 1989). Haglund et al. (1994) excluded patients with an average intraoperative baseline (no stimulation) error rate >25%. Both authors agree that the higher the rate of spontaneous error, the lower the test validity. Nevertheless, this finding was not considered in later studies.
Ojemann et al. (1989), using the single sample binomial test to determine whether a site is essential for language, examined response accuracy during the naming task. These authors provide a non-parametric description of how to determine whether a site can be interpreted as essential: “A site was determined to be related to language function if the chance probability of errors evoked at that site was less than 0.05 […] evoking errors during two of three stimulations at a site often achieved that level of statistical significance….” Some authors use other strategies. For instance, Peraud tested all sites at least twice and considered two effective stimulations out of two as acceptable in order to consider a site positive for language function ( Peraud et al., 2004). Others chose three out of three stimulations to ensure that a cortical site is essential (3/3 tries) ( Gatignol et al., 2004; Roux et al., 2004). Hamberger et al. (2005) stimulated at least twice, for both visual and auditory naming, at each site. When one of two attempts was inaccurate, two more trials were run. Sites were considered critical only when at least 75% of responses were inaccurate (3/4 tries).
In conclusion, with a few exceptions, it seems that most surgeons would deem at least two positive responses out of three stimulations necessary for validating a functional site ( Haglund et al., 1994; Schwartz et al., 1999; Roux et al., 2004; Bello et al., 2007).
Again, it should be noted that after four repetitions the learning effect limits the test validity ( Ojemann et al., 2002) (see “Distribution according to individual characteristics”).
Types of errors
The VON task is easy to apply but much more difficult to interpret. We here present two levels of description: the definition of error types ( Table I ) and the patterns of errors reported by the different authors ( Table II ). When classifying types of errors, one must distinguish between speech arrest, anomia, and speech disturbances. Speech arrest has already been mentioned ( Table I ). Anomia refers to the inability to produce the name of an object despite a preserved capacity to speak, as demonstrated when reading the carrier phrase “This is a...” ( Ojemann et al., 1989).
Table II .
Naming: the most common error patterns reported in the literature.
| AUTHORS | ERROR PATTERNS |
|---|---|
| Bello et al., 2007 | • Anomic episodes, phonemic or semantic paraphasia |
| Benzagmout et al., 2007 | • Phonemic, semantic and phonetic paraphasia, slowness with initiation, perseveration |
| Petrovich Brennan et al., 2007 | • Paraphasic errors, speech arrest, hesitation; word finding difficulty, dysarthria, circumlocution, hypophonia (no description) |
| Corina et al., 2005 | • Anomia, delayed, semantic paraphasia |
| Duffau et al., 2002b | • Speech arrest, anomia, dysarthria, spontaneous speech reduction |
| Fontaine et al., 2002 | • Aphasic misnaming: defined as semantic paraphasia and confusion of expressions, aphasic arrest defined as production of carrier phrase followed by failure to name stimulus |
| Hamberger et al., 2005 | • Tip-of-the-tongue: correct response after phonemic cueing, increased naming latency |
| Ojemann and Whitaker, 1978 | • Total arrest of speech, failure to respond |
| Ojemann, 1979 | • A total arrest of speech, anomia |
| Ojemann, 1993 | • Naming errors: correct name after slide changed (after stimulation stopped), substitution (e.g., fork instead of spider) |
| Roux et al., 2004 | • Articulatory interferences, pure reading arrest, paraphasias, ocular movements, others (hesitations and perseverations) |
| Sanai et al., 2008 | • Speech arrest, dysarthria, anomia, alexia, expressive and receptive aphasia. Mild deficit: paraphasias noted but did not influence resection |
| Signorelli et al., 2001 | • Paraphasias, speech arrest, perseveration, anomia and comprehension disorders |
All other disturbances have been variously named and assigned various definitions. At the dawn of awake mapping, Penfield et al. (1937) listed the following as aphasic-like errors: distortion and repetition of syllables and words , confusion of numbers while counting, inability to name with preserved ability to speak, misnaming and perseveration. Later, Van Buren et al. (1978) schematically divided naming responses into four classes: 1) correct; 2) correct but with a significant delay or hesitation ( Table III ); 3) substitution or misnaming; and 4) omission or complete failure to name. The last two (3 and 4) were regarded as errors. Ojemann classified errors as omissions, nonsense words, jargon, and other non-specified errors ( Ojemann et al., 1989).
Table III .
Reading: definition of error types.
| ERROR | DEFINITION |
|---|---|
| Articulatory interferences |
|
| Pure reading arrest |
|
| Alexia |
|
| Aphasia, receptive |
|
| Paraphasia |
|
| Jargon |
|
| Miscellaneous findings |
|
| Ocular movements |
|
The type of error classification used is closely related to a study’s aim; it can vary from simply scoring as an error any deviation from the correct response during stimulation, to a more complex definition of error types ( Bello et al., 2007; Duffau et al., 1999; 2003a ; 2006 ; Benzagmout et al., 2007).
Clinical validation
Ojemann observed that stimulation of a single cortical site can alter a language function such as naming after each stimulation. There is further evidence that partially eloquent sites (two out of three correct responses) are less crucial for language than “100%” eloquent sites ( Ojemann, 1983).
In 1994, Haglund et al. reported a statistically significant correlation between postoperative worsening and resection distance from a positive site. One centimeter has been regarded as a safe critical distance from the positive site ( Trinh et al., 2013; Haglund et al., 1994; Sanai et al., 2008; Bello et al., 2007; Roux et al., 2004; Skirboll et al., 1996). However, later observations cast doubt on this criterion. Resection at a distance <1 cm from a positive cortical area caused postoperative deficits that spontaneously resolved ( Peraud et al., 2004; Kral et al., 2006). Seeck et al. (2006), in a single-case study, observed that resection margins do not necessarily correspond to functional sites. New aphasic symptoms did not occur after surgery, despite the resection of a left temporoparietal area where language functions were impaired or blocked during electrical stimulation. Moderate aggravation of pre-existing word-finding difficulties was observed in the presence of normal spontaneous speech and oral comprehension.
The first resection outcome analysis is usually performed 3–7 days after an operation, and followed by two more at 1 and 3 months post-surgery. Follow-up evaluation is usually performed after 6 months or more ( Sarubbo et al., 2012b; Bello et al., 2008; Hamberger et al., 2005). Hamberger et al. prolonged the single outcome evaluation time to 1 year; in such circumstances, however, it is difficult to establish whether the outcome examined at 1 year is the result of the mapping hypothesis or an unrelated event linked to other processes such as brain plasticity.
Distribution of positive sites
There follows a description of the distribution of language sites identified using the VON task.
Cortical topography
Different techniques for describing cortical topography have been used to indicate positive cortical sites for naming. The presence of variability in the location of sites whose stimulation evoked significant errors in naming was determined by aligning individual patient maps to the rolandic cortex and the sylvian fissure ( Ojemann et al., 1989). The authors divided the brain surface into small squares drawn schematically in relation to surface landmarks. The frontal cortex was divided into the inferior, middle and superior frontal gyri, beginning with the most anterior evoked motor response, which identified the anterior limit of the motor cortex. The inferior frontal gyrus was further divided into inferior and superior zones. The temporoparietal cortex was identified as the area extending from the posterior end of the sylvian fissure to the projection of the foot of the central sulcus onto the fissure. This region was divided into four zones: the superior, middle, and inferior temporal gyri and the parietal operculum.
Haglund et al. (1994) divided the temporal lobe into three sectors: the superior temporal gyrus, middle temporal gyrus, and inferior temporal gyrus. Each of these three sectors was further divided into six zones: one anterior to the projection of the central sulcus; one posterior to the end of the sylvian fissure, and four in between. The same cortical topography system was adopted by various authors ( Malow et al., 1996; Schäffler et al., 1996). Schäffler et al. (1996) and Schwartz et al. (1999), using implanted subdural grids, divided the lateral temporal lobe, inferior frontal lobe and inferior parietal lobe into separate squares (48 one-centimeter squares). Sanai et al. (2008) used the same criteria during intraoperative mapping, while Roux et al. (2011) referred to the standard stereotactic Talairach coordinates.
In addition, the neuronavigator has made identifying the exact location of sites increasingly easier, with a view to creating a common parameter for site definition ( Fig.s 1 , 2 , over).
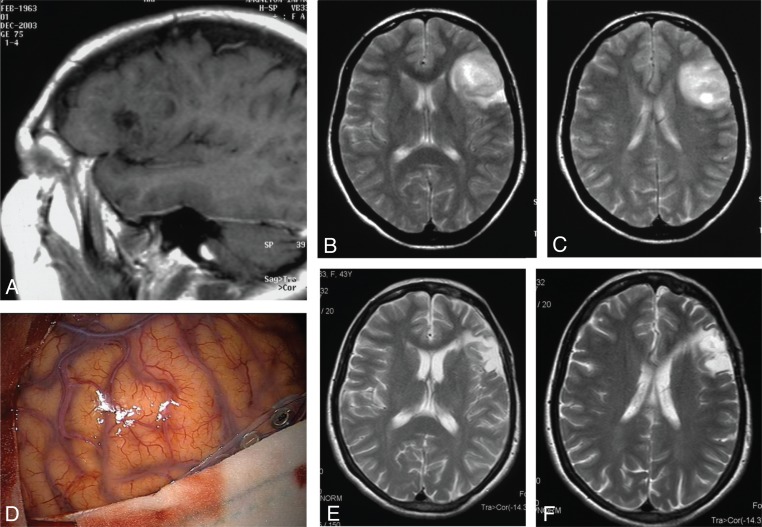
Figure 1 .
Oligodendroglioma of Broca’s area.
(A) Enhanced T1-weighted sagittal MR scans showing a hypointense tumor of the left inferior frontal gyrus. (B,C) T2-weighted axial MR scans highlighting the real extension of the tumor area. (D) Intraoperative picture showing an apparently normal brain surface where it is impossible to identify the tumor borders (in this regard, neurovavigation plays a major role in cortical low-grade glioma demarcation). (E,F) Postoperative T2-weighted axial MR scans showing complete tumor resection.
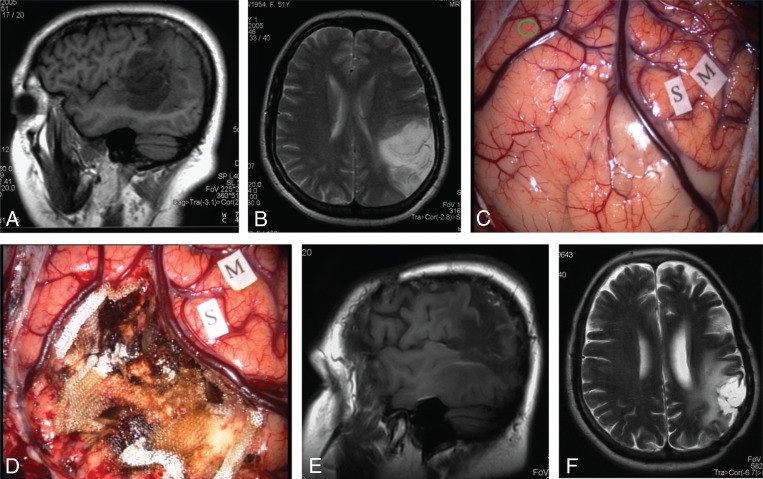
Figure 2 .
Anaplastic astrocytoma of Wernicke’s area.
(A) Enhanced T1-weighted sagittal MR scan showing a hypointense area in the supramarginal gyrus, angular gyrus, and posterior superior temporal gyrus. (B) T2-weighted axial MR scan showing subcortical extension of the tumor. (C) Intraoperative picture showing the tumor area between two veins. S= sensory area, M=motor area, green circle=essential language area. (D) Intraoperative picture at the end of the resection showing complete tumor removal. (E,F) Postoperative enhanced T1-weighted sagittal and T2-weighted axial MR scans showing the operative cavity surrounded by a hyperintense area. The patient is recurrence-free 8 years after surgery.
General distribution
Ojemann et al. (1989 , 2003) attempted to chart the distribution of language functions, reporting interference over a wide area of the left lateral cortex, extending beyond the limits of the classical model. They also documented a substantial variability in individual organization ( Ojemann 1979; Ojemann et al., 1989, 2003). As a rule, at least one area was described in the inferior frontal gyrus and one or more areas in the temporoparietal perisylvian cortex. Taking a different approach to language mapping, Sanai et al. (2008) found a different location for naming. Their resection strategy did not require identification of the stimulation-induced positive sites within the field of exposure; instead, they resected cortical tissue on the basis of negative sites. They found fewer naming sites (distributed predominantly in the superior and middle temporal gyrus) than those classically described in previous language studies of the temporal lobe ( Ojemann, 1990). The authors stated that these data were not due to a site-selection bias, since, despite the use of tailored craniotomies, they were able to map the entire temporal lobe by summing all the cortical stimulations from 186 patients with temporal lobe lesions.
Other studies identified naming locations in specific regions such as the insula, the striatum and opercular region and the basal temporal language area ( Duffau and Fontaine, 2005; Duffau et al., 2005; Gil Robles et al., 2005; Peraud et al., 2004; Lüders et al., 1988, 1991).
Essential areas are commonly localized on one or more cortical surface areas of 1–2 cm 2 and most patients will produce errors following stimulation in any uninterrupted area of cortex >1.5 cm 2 . However, some may need stimulation of uninterrupted areas >2.5 cm 2 in order to show language errors ( Ojemann et al., 1989). These areas may have well-defined boundaries or be surrounded by a small rim of cortex where a single error can be evoked. The terminology referring to these cortical regions is variously descriptive, with authors using, for example, the terms discreteness ( Haglund et al., 1994), nodes ( Schwartz et al., 1999) and shell-core ( Schäffler et al., 1996).
Distribution according to individual characteristics
The location of cortical sites in relation to demographic characteristics [gender, age, preoperative verbal intelligence quotient (VIQ)] and learning effect has been described ( Berger et al., 1989; Haglund et al., 1994; Mateer et al., 1982; Ojemann et al., 1989; Schwartz et al., 1999). Mateer et al. (1982) found gender-related differences in the distribution of naming sites, with males exhibiting a higher proportion of sites in the left anterior temporal cortex than females. Later studies, however, failed to find a correlation between gender and naming site distribution ( Devinsky et al., 2000; Haglund et al., 1994; Schwartz et al., 1999). On average, female patients seem to have a significantly larger number of sites than males (3.1 versus 2.1) ( Haglund et al., 1994).
Ojemann et al. (2003) compared the results of a previous study ( Ojemann et al., 1989) performed in an adult population (117 patients, mean age 30.8 years) with those of a study in a pediatric population (26 patients, <16 years old). The older age group had a higher frequency of naming errors and more positive sites; the pediatric group was more likely to have multiple essential areas in the temporoparietal regions. The location of essential areas in adults does not seem to change over time if further lesions do not occur ( Ojemann, 1991).
Differences in language organization are apparently related to VIQ and primarily involve the temporal lobe. Patients who preoperatively had a lower VIQ score had larger language areas than those with a higher preoperative VIQ score ( Ojemann, 1983; Devinsky et al., 2000).
Naming changes due to stimulation of the superior temporal gyrus were significantly more likely in patients with a lower pre-operative VIQ score, while patients with a high VIQ score made more naming errors when stimulation was applied to the middle temporal gyrus ( Ojemann et al., 1989).
A practice effect due to repetition of the verb generation task can also influence the number of naming sites. Specifically, more sites were found to interfere with novel than with practiced verb generation. It seems that, as the task is performed more efficiently, the cortical area devoted to the task itself decreases; this effect has been found in the frontal, temporal and parietal cortex ( Haglund et al., 1994; Ojemann et al., 2002).
Bilingual patients
Studies in multilingual patients have generated various results depending on the type of spoken language, the tasks applied, and the patients involved. In bilingual speakers, it is not clear whether the two languages are mediated by multiple separate cortical areas or by shared areas. Some studies report that in bilingual patients multiple separate areas of the cortex mediate the different languages ( Kim et al., 1997, Lucas et al., 2004, Ojemann et al., 1989; Roux and Trémoulet, 2002 ; Roux et al., 2003; Walker et al., 2004; Bello et al., 2006). Other investigations have pointed out that common areas of the brain are activated during language tasks ( Illes et al., 1999; Pillai et al., 2003).
Overall, research recommends performing separate intraoperative mapping for each language in which a patient is fluent in order to avoid the potential confounding effect of language switching between trials, since language switching has been shown to induce cortical activation ( Hernandez et al., 2000).
As regards distribution, naming and reading tasks are known to activate similar and/or different areas in the brain ( Lucas et al., 2004; Rapport et al., 1983; Roux and Trémoulet., 2002 ; Walker et al., 2004). In bilinguals, language-specific interference was consistently found in the posterior temporoparietal regions ( Lubrano et al., 2004; Lucas et al., 2004; Pouration et al., 2000; Rapport et al., 1983; Roux et al., 2004; Roux and Trémoulet 2002 ; Walker et al., 2004). Nevertheless, language-specific areas in bilinguals can also be found in the frontal regions ( Bello et al., 2006; Lubrano et al., 2004; Ojemann et al., 2003; Pouration et al., 2000; Roux et al., 2004). These language-specific sites were found regardless of proficiency, age of acquisition and language type. Further studies are needed to elucidate this issue.
Distribution of visual object naming versus other language tasks
Even when surgical resection respects the margins of positive sites, patients may still display a postoperative language deficit, as seen in comprehension or reading ( Petrovich Brennan et al., 2007). Specific sites for different language functions exist and are often found in close relationship to naming sites.
Counting
Counting is used to document the speech arrest response during electrocortical stimulation. Speech arrest often occurs on stimulation of the area directly anterior to the face motor cortex and the posterior part of the superior temporal gyrus, extending towards the inferior parietal lobe ( Schwartz et al., 1999; Haglund et al., 1994). By contrast, Sanai et al. (2008) found speech arrest during counting in 73.9% of frontal lobe sites; none of the patients had a speech arrest during parietal lobe stimulation (nothing was said about the temporal lobe).
Petrovich Brennan et al. (2007) used direct cortical stimulation to compare the sensitivity of the two tasks (VON and counting) most commonly used to identify language areas. The results can be summarized as follows: first, more sites are found during object naming than during counting; second, counting errors belong to specific categories (such as speech arrest or hypophonia). Also, both tasks are affected in some sites, whereas in others only the naming task is disrupted; the reverse pattern never occurs. This suggests that choosing only the naming task may yield false-negative language localizations.
Auditory naming
Naming an object after hearing a definition (auditory naming) involves a location different from that involved in VON ( Hamberger et al., 2005, 2007; Malow et al., 1996). In particular, Malow et al. (1996), using subdural electrodes in patients with temporal lobe epilepsy, found that stimulation of the anterior and posterior temporal cortex elicited errors in auditory naming but not in VON. Hamberger et al. (2007) found a systematic representation of auditory naming sites anterior and close to the visual naming sites in the mid-posterior part of the superior temporal gyrus.
Verb generation
Selective impairment between action naming and retrieving object names, as documented by Snodgrass et al. (1980) and Miceli et al. (1984), has been confirmed in awake surgery ( Ojemann et al., 2002; Corina et al., 2005). Stimulus presentation in action naming is the same as in VON ( Herholz et al., 1997). The data reveal a possible double dissociation between action and object naming. In such cases, the region that causes a disruption of object naming lies anteriorly to that implicated in action naming ( Corina et al., 2005).
Two different maps emerge from the use of verb generation and visual naming tasks: one in the frontal and one in the temporoparietal area. The frontal lobe sites where stimulation interfered with verb generation were always located posteriorly 1 cm or less from the sites responding during object naming. Conversely, close vicinity and overlapping rarely occurred in the temporal and parietal regions ( Ojemann et al., 2002). Furthermore, specific sites of verb generation task impairment were more frequently localized in the posterior (temporal and parietal) regions, while the middle temporal gyrus seemed to be more specifically associated with object naming.
Reading
Areas where stimulation mapping causes changes during reading or VON tasks only, and regions where both functions overlap have been identified in the temporal regions ( Schwartz et al., 1999; Sanai et al., 2008). In particular, areas positive for reading have been found adjacent to naming sites (distance ≤1 cm). Specific sites for reading were often found in the posterior temporal lobe, supramarginal gyrus, and dominant angular gyrus ( Roux et al., 2004). Speech arrest is rarely encountered during interference with reading (21% of cases); this suggests that reading is part of a network that cannot be defined by automatic series.
Distribution in relation to different pathologies
Any brain lesion can, to some extent, modify (displace and reorganize) functional pathways, whose description may therefore be influenced by the disease.
Epilepsy and gliomas
Haglund et al. (1994) investigated differences between brain tumor and epilepsy patients (the same 117 patients reported by Ojemann et al. in their study published in 1989). The brain tumor group had fewer positive naming sites in the superior temporal gyrus than the epilepsy group. The authors suggested that a slow-growing lesion eliminates or alters essential language sites (see also “Primary operation and tumor recurrence”) ( Fig. 3 ).
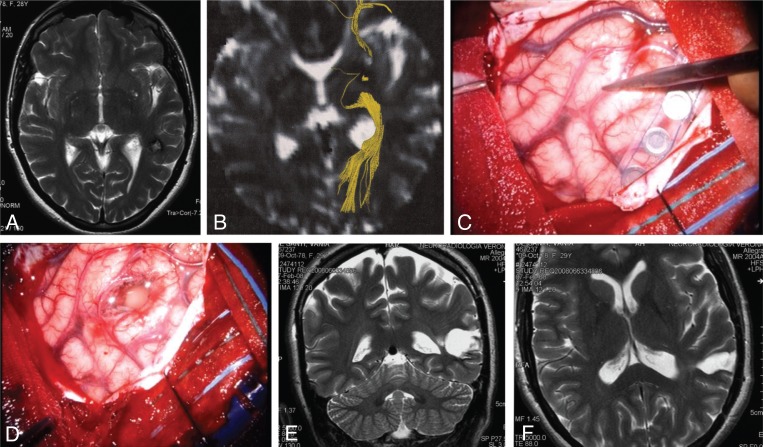
Figure 3 .
Subcortical left temporal cavernous angioma.
The critical surgical issues are: corticectomy in a safe area, safe removal of perilesional gliosis, and sparing of the optic radiations. (A) T2-weighted axial MR scan showing the hypointense subcortical lesion. (B) Diffusion imaging highlighting the optic radiations (yellow). (C,D) Intraoperative pictures showing the site of an essential language area in the middle temporal gyrus (C) and the site of corticectomy in the superior temporal gyrus (D). (E,F) Postoperative T2-weighted coronal and axial MR scans showing the poroencephalic cavity, larger than the size of the cavernous angioma because it encompassed the surrounding gliotic area.
Low- and high-grade gliomas
Although high-grade gliomas are associated with a higher rate of preoperative clinical deficits, the rate of cortical language sites in low- and high-grade gliomas is similar ( Haglund et al., 1994; Bello et al., 2007). These data are consistent with previous research on high-grade gliomas, where individual variability in eloquent sites was found, similarly to low-grade gliomas ( Signorelli et al., 2001).
Primary operation and tumor recurrence
While the coexistence of tumor and function in the same site limits the extent of resection in 10–15% cases, it has been observed that this is not a definitive obstacle. Based on experience of mapping in recurrent tumor, the concept of a “multi-stage surgical approach” has been put forward, in keeping with the observed reshaping of functional tissue that occurs in the interval between the two operations ( Duffau et al., 2002b, Martino et al., 2009; Robles et al., 2008). Martino et al. (2009) analyzed functional and oncological results in a small sample of patients (n=19) re-operated for recurrent low-grade glioma in eloquent areas. They demonstrated the safety and advantages of repeat surgery strategies: only 15% of the patients who had a second operation in the same areas as the first operation for the removal of tissue that could not be removed initially had permanent deficits after the second operation. Robles et al. (2008) reported two cases of language reorganization involving perilesional language areas after the second operation.
Subcortical mapping
Tumor removal starts after cortical mapping is completed. During this second stage, in some preliminary studies, direct stimulation was applied at the subcortical level to identify the deep functional limits of the resection, i.e., the white-matter pathways. The functional pathways were followed from the cortical eloquent sites already mapped to the depth of the resection cavity, with or without image-assisted guidance. The glioma was removed by alternating resection and subcortical stimulation while the patient had to continue naming, the only task used in all studies ( Duffau, 2008).
The superior longitudinal fasciculus (SLF) and its component, the arcuate fasciculus (AF), and the inferior fronto-occipital fasciculus (IFOF) constitute the real surgical limits of resection in most cases, dorsolaterally and ventromesially. They are also known, because of their functional properties (according to the different error types they induce) as the “dorsal phonemic” and the “ventral semantic” stream, respectively ( Martino et al., 2010; Sarubbo et al., 2013; De Benedictis et al., 2012). The AF runs from the posterior part of the superior and middle temporal gyri towards the frontal lobe, deeply to the SLF. This latter component connects the inferior parietal lobule to Broca’s area and the middle frontal gyrus. Anteriorly, the IFOF runs close to the SLF and underneath the head of the caudate nucleus. Particular attention must be paid in order to accurately identify and preserve the deep component of the IFOF, which runs vertically from the temporal lobe to the middle frontal gyrus. The head of the caudate nucleus, which is involved in the inhibitory control of cognition, can be identified by inducing reproducible perseverations that follow direct stimulation of the posterolateral side of the anterior horn of the lateral ventricle, which constitutes another important surgical landmark for the medial extent of resection. At the level of the middle temporal region, the IFOF runs along the roof of the temporal horn, superior and lateral to the optic radiations. Within the posterior temporal region, a clear separation was identified between the fibers of the IFOF (oriented anterior–posterior) and those of the AF (more superficial and oriented superior–inferior) ( De Benedictis et al., 2012; Martino et al., 2013).
Clinical validation
The first subcortical stimulation studies were conducted by Duffau a decade ago ( Duffau et al., 2002a, 2003a). At that time, however, the landmarks for safe surgery within the left frontal and temporal lobes were defined on the basis of the anatomical distribution of functional pathways rather than based on surgical anatomy. Thereafter, the development of subcortical mapping techniques paralleled the development of diffusion tensor imaging (DTI). The surgical procedure was preferably guided by DTI information for subcortical anatomy, whereas gyral anatomy serves for orientation on the cortex ( Bello et al., 2010). Validation of electrical stimulation and the functional role of the subcortical network has followed two protocols: 1) merging pre- and postoperative DTI, relying on the subcortical evoked response alone; and 2) merging intraoperative DTI with postoperative DTI, correlated by comparing images of intraoperatively positive sites ( Leclercq et al., 2010; Bello et al., 2008). In these studies, positive stimulation mapping was concordant with DTI fiber bundles (IFOF, SLF and AF) in 81% and 97% of stimulations. Subcortical stimulation failed to evoke language disturbances in areas when the tract was interrupted inside the tumor mass. This is typical of low-grade gliomas (whereas high-grade gliomas usually displace the tracts) and remains one of the most difficult situations to manage in neurologically intact patients ( Bello et al., 2008).
Interestingly, the surgical landmarks of subcortical pathways have been demonstrated only recently, long after these pathways were identified by electrical stimulation ( Duffau et al., 2002a, 2003a, 2008; Sarubbo et al., 2012a, b; Martino et al., 2010, 2013) ( Fig. 4 ). Initial surgical studies dealt with fiber bundle anatomy, as suggested by the emerging DTI technique. What investigators failed to realize, however, was that this technique is inadequate and substantially different from surgical anatomy which requires consistent landmarks during an operation. This approach considerably limited the reproducibility and validation of sub-cortical mapping for some years ( Duffau et al., 2008).
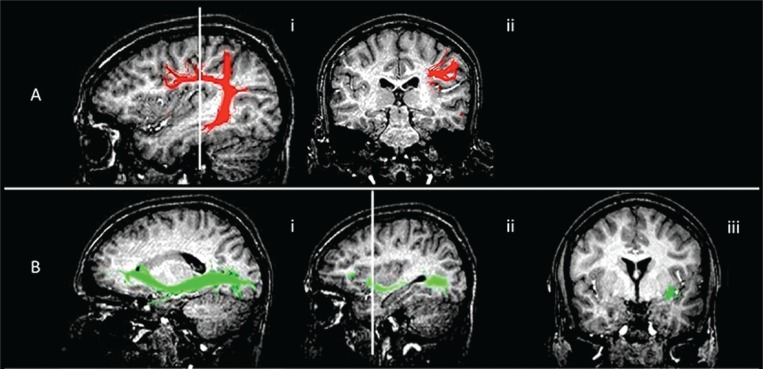
Figure 4 .
Functional imaging showing subcortical surgical landmarks.
MR diffusion tractography anatomy (healthy volunteer). Arcuate fasciculus/superior longitudinal fasciculus and inferior fronto-occipital fasciculus fiber tract reconstruction overlaid on 3D T1-weighted images, showing their relationship with the surgical landmarks.
(A) Arcuate fasciculus. i) sagittal view, anatomy of the tract; ii) coronal view, the tract runs deep in the intersection between the distal sylvian point and the superior sulcus of the insula. This fasciculus runs medially and parallel to the superior longitudinal fasciculus which connects the prefrontal, parietal and temporal cortices.
B) Inferior fronto-occipital fasciculus. i) sagittal view, anatomy of the tract; ii) sagittal reference for the coronal view, at the bottom of the insula, at the level of anterior sulcus, before the ascending part of the tract ; iii) coronal view, at this level the tract is lateral, it then turns medially towards the caudate nucleus.
Other cognitive tasks
Other tasks linked to language (reading, writing, short-term memory) and other functions (calculation, visual, visuospatial) have been used for intraoperative mapping. Some are part of a standard battery ( Skirboll et al., 1996; Schwartz et al., 1999; Sanai et al., 2008; Ojemann, 1983), while others are performed for clinical reasons or, in rare cases, to investigate a specific function. In the rolandic area, the first steps are the same as those described for mapping with VON.
Counting
Counting is considered to be “automatic” speech, just like reciting the months of the year and the days of the week or nursery rhymes and familiar songs. Some authors refer to these tasks as “non-propositional” speech, as opposed to “propositional” speech (the formulation of a message, as in VON), or “over-learned” serial speech ( Petrovich Brennan et al., 2007). Counting is commonly used preoperatively and intraoperatively together with VON, since it is a simple way to elicit continuous and fluent motor speech. While it is not necessary to explain in detail the procedures of the counting task, what must be underlined is that it has an important role in defining “speech arrest” ( Table I ), distinguishing it from the “speech arrest“ caused by motor and sensory areas ( Sanai et al., 2008; Schwartz et al., 1999; Haglund et al., 1993; Lüders et al., 1995).
Comprehension
Some authors have developed tasks to test comprehension during intraoperative mapping. In the Palm Tree and Pyramid Test, the subject is presented with three pictures and asked to point to two conceptually related pictures ( Gatignol et al., 2004). Other commonly used tasks are brief versions of the Token Test and the sentence and word comprehension test ( Bello et al., 2008; Ilmberger et al., 2001; Rutten et al., 2002; Pouration et al., 2002). In general, positive sites are elicited in the posterior areas of the superior temporal lobe. The posterior part of the superior temporal gyrus showed a dissociation between sites where anomia occurred after stimulation and others where comprehension deficits, with preserved ability to name the object, were observed ( Duffau, 2006).
Reading
The reading test, a common component of intraoperative task batteries and subdural electrode stimulation protocols, has been used as a supplementary resource in language mapping ( Chacko et al., 2013; Duffau et al., 2003b; Lesser et al., 1986; Lüders et al., 1988; Ojemann, 1983; Sanai et al., 2008; Schwartz et al., 1999; Skirboll et al., 1996). Some authors investigating reading tasks compared them to other tasks (e.g., writing, VON, counting, calculation and so forth) ( Roux et al., 2003, 2004; Lubrano et al., 2004).
Both sentences and single words are used as stimuli. Ojemann (1979) was the first to map the areas involved in sentence reading. He assessed this function by including it in a sequence of tasks that evaluate short-term memory (see “Working memory”). The “reading” trial – as storage phase or distractor – is instrumental for assessing another function (short-term memory). Roux et al. ( 2003 , 2004 ) and Lubrano et al. (2004) used a set of unrelated sentences previously unrehearsed by patients. Stimulation was applied randomly to the cortex during reading for the entire duration of one of the slides.
Single-word stimuli have been also used; the items were projected sequentially onto a computer screen and the patient was asked to read each one ( Sanai et al., 2008). Often, authors do not specify how they construct sentences or select words. The criteria for defining a site as positive were the same as those used for VON; testing was conducted at least three separate times, and the sites that did not show reproducible language interference were not considered eloquent areas ( Roux et al., 2003, 2004; Sanai et al., 2008). No consensus has been reached to date on the definition of errors or the procedure ( Table III ).
As regards distribution, several foci were found in the posterior middle and superior temporal gyri ( Lesser et al., 1986; Schwartz et al., 1999). Sanai et al. (2008) found reading sites sparsely located in the temporal lobe, mostly in the inferior parietal lobe, 1–2 cm behind the somatosensory cortex.
When reading sites were spared by surgery, transient postoperative dyslexia was found in 26% of the patients, probably due to brain retraction or edema ( Roux et al., 2004). Little is known about patients in whom reading sites overlapping the tumor area were resected.
Writing
Agraphia was first described in the 1860s. Exner (1881) postulated that lesions of the foot of the middle frontal gyrus could cause writing deficits. Writing models differentiate between a lexical system, based on whole-word retrieval involved in writing familiar, regular and irregular words, and a sublexical phoneme-to-grapheme conversion system for writing unfamiliar words and pseudo-words. Few authors have performed awake surgery with the aim of mapping and sparing areas involved in writing ( Lubrano et al., 2004; Roux et al., 2003, 2004).
Patient positioning is important when writing functions are evaluated intraoperatively. The patient is placed supine, and a three-point head fixation device is applied. After being fixed and rotated 30° opposite to the side of the craniotomy, the head and shoulders are raised slightly (10–20°) so that the patient feels comfortable and can see her/his own hands while writing. Patients use the dominant hand and a pencil to write horizontally on an A4 sheet of paper ( Lubrano et al., 2004) laid on a stiff pad. The procedure typically includes sentence dictation at each cortical site and direct stimulation applied for less than 4 seconds while the patient writes ( Roux et al., 2003; Lubrano et al., 2004). Lubrano et al. also asked subjects to copy written material (isolated letters, words, numbers). In the course of the procedure, they excluded from the analysis those sites where interference from hand contraction or eye movements was noted.
In studies that investigated writing disorders, several error types were analyzed without attempting to relate them to an underlying cognitive deficit: dyspraxia and dysphasia; writing arrest; neographism; difficulty in letter formation; omission of words or letters; and deterioration of handwriting ( Lubrano et al., 2004; Roux et al., 2003). Site distribution seems to be extremely localized (within 1 cm) in the angular gyrus ( Roux et al., 2003). Lubrano et al. (2004) identified sites in the superior temporal gyrus and posterior portion of the frontal lobe which are not usually associated with reading or naming. Postoperatively, all patients were able to write normally but some had difficulties with spoken language.
Working memory
Working memory has been studied ever since the beginning of awake procedures. There is evidence documenting a role for the language cortex in short-term memory ( Van Buren et al., 1978; Ojemann and Whitaker, 1978; Ojemann and Mateer, 1979; Ojemann and Dodrill, 1985; Ojemann et al., 1998, 2002, 2003). The procedure for assessing short-term memory is similar across studies. It consists of a set of three slides; stimulation persists for 5 seconds, and each site is stimulated at least three times. Stimulation is applied randomly during each of the three phases and the patient is never cued as to when it occurs. Memory tasks include encoding, storage and retrieval. The three steps are evaluated by means of distinct tasks:
Encoding (or input): picture naming;
Storage: reading a sentence or number counting ( Ojemann and Whitaker, 1978);
Recall of a word presented during the input phase.
Ojemann and Whitaker (1978) added a task variant consisting of four black-and-white slides. The first three are the same as in the previous procedure, whereas the fourth is a recognition task (note that at this point stimulation is never applied in order to make sure that the patient recognizes the object).
During the item selection procedure, trials during which stimulation caused errors or hesitations were excluded. Trials with naming errors were excluded when analyzing memory performance in order to ensure that the information committed to memory had been processed correctly ( Ojemann, 1983).
With reference to the three task phases, the sites identified during the storage phase are more frequently located in the temporal and parietal regions, while those identified in the recall phase are mostly frontal ( Ojemann, 1979).
Brandling-Bennett et al. (2012) reported the case of a patient with a tumor in the posterior third ventricle undergoing awake surgery to test memory during fornix manipulation. The task comprised three steps: 1) presentation of four consecutive line-drawn pictures, which the patient was asked to name and remember; 2) a distractor task (simple calculation); 3) recognition of the four pictures among eight consecutive individual line drawings, four of which were novel. Patient responses (yes/no) for each recognition picture were monitored. Each response was scored as correct or incorrect. All areas were tested twice. Those for which the patient failed to recognize either of the two pictures presented during stimulation were designated as “positive” memory areas; when the patient recognized only one picture, the area was designated as “contributing” to the memory area. Postoperative evaluation revealed no memory deficit in the patient.
Calculation
Numerical processing is a basic function of the human brain and its impairment can cause social and professional problems. Functions related to arithmetic tasks have been investigated infrequently. Very simple tasks have been used: addition (e.g., 29 + 30) ( Roux et al., 2003, 2009), addition and subtraction ( Kurimoto et al., 2006), and multiplication and subtraction ( Yu et al., 2011; Pu et al., 2011; Duffau et al., 2002c). Calculation errors during stimulation were graded as: hesitation; no answer; or incorrect answer. A site was defined as positive by some authors when two or more errors occurred in it for each specific operation ( Yu et al., 2011), and by other authors when three errors out of three stimuli were observed ( Roux et al., 2009). Functional areas involved in simple multiplication (table problems) are located at the posterior end of the sylvian fissure (close to the language sites), while subtraction is more likely to be located in the superior angular gyrus ( Pu et al., 2011). The left hemisphere is involved in calculation, while the right hemisphere is not related to calculation processes. As in other tasks, arithmetic problems solved correctly before an operation were selected as intraoperative stimuli.
Postoperative assessment showed transient calculation deficits only when resection was close to the numerical processing area (<1 cm) ( Pu et al., 2011) while permanent impairment was found in three patients whose calculation areas were not spared ( Roux et al., 2009).
Visuospatial functions
While language has been extensively mapped intraoperatively, spatial functions have received less attention in clinical practice ( Roux et al., 2011; Bartolomeo et al., 2007; Gharabaghi et al., 2006; Thiebaut de Schotten et al., 2005). This is perhaps because spatial functions are not thought to suffer serious consequences after the removal of small portions of brain tissue. Contrary to this idea, however, is the well-known dramatic effect that spatial neglect has on clinical outcome, rehabilitation and quality of life ( Cherney et al., 2001; Gillen et al., 2005; Paolucci et al., 2001). The task most frequently used to detect neglect is line bisection. Patients who present with neglect following a right-hemisphere lesion typically shift the midline towards the right side. Intraoperative line bisection makes it possible to identify functionally important areas, thus preventing the occurrence of postoperative spatial neglect. Recently, Roux et al. (2011) analyzed the behavioral effect induced by stimulation during the line bisection task in 50 patients. Asked to bisect a line while their cortex was electrically stimulated, the patients showed symptoms of neglect (a rightward midline shift) when the stimulation involved the right inferior parietal lobe and the right superior temporal lobe (more frequently positive results: inferior parietal lobule, post-central gyrus, superior temporal gyrus, pre-central gyrus). The fiber tracts found to be involved in midline deviation during subcortical mapping were the superior occipitofrontal fasciculus and the superior longitudinal fasciculus.
Gharabaghi et al. (2006), using a visual search task, studied a patient undergoing tumor resection. The patient was asked to look for L-shaped targets among rotated L-shaped distractors presented on a PC monitor, while different portions of the cortex were stimulated. Electrocortical stimulation of the central portion of the right superior temporal gyrus deteriorated visual search performance to mere guessing, thus suggesting that this area plays a crucial role in exploratory behavior.
Visual pathways
Despite the negative effect that permanent hemianopia can have on quality of life, little attention has been paid to the risk of damaging the visual pathways ( Duffau, 2004; Gras-Combe et al., 2012). A first, unconfirmed study ( Duffau, 2004) analyzed the phosphenes in patients stimulated during awake mapping. One recently developed task for mapping the areas involved in visual functions is a modified version of the picture naming task in which two pictures are placed diagonally on the screen in front of the patient, one in the quadrant to be spared and the other in the opposite quadrant. A red cross at the center of the screen serves as a fixation point. The patient was required to name both pictures during mapping of the regions close to the visual pathways. Transient visual disturbances in the contralateral visual hemifield prevented the patient from naming the picture in the contralateral quadrant but not the one in the ipsilateral quadrant.
Saccadic movements
Eye movement plays a role in complex behaviors such as attention, motivation, inhibition, spatial memory and decisional processes ( Milea et al., 2005). There are few intraoperative functional mapping studies on the cortical structures involved in ocular movements ( Milea et al., 2005). Two kinds of eye movement task have been described: fixation , in which the patient maintains fixation on the target; and saccades , in which the patient performs self-paced continuous, horizontal saccades ( Milea et al., 2002). Eye movements are monitored using electro-oculography with bitemporal electrodes. The current amplitude is usually 1–4 mA. When stimulation was applied during the fixation task, a slow movement (8°/sec) appeared, with an amplitude of 11–15°, while a rapid backward movement occurred at the end of the stimulation (97°/sec; range 15–23°). During the saccade tasks, movement suppression occurred 90 ms after the onset of electrical stimulation. Saccades were recorded again after the end of the electrical pulse. The cortical areas involved in the control of eye movements were identified close to the primary motor cortex of the hand; the frontal eye field was defined as the lateral frontal cortex that elicits rapid eye movements when stimulated, while the dorsolateral prefrontal cortex was involved in programming saccades. These experiments were designed to afford better insight into the neurophysiological mechanisms but they did not influence operative planning.
Data recording
The responses produced by patients during awake mapping are also important for data analysis after surgery. Published data document different levels of response analysis, depending on the goal of mapping. Recording of responses requires a professional technician in the operating room, audiotape and/or video-tape equipment, and blinded or multiple postoperative examiners. Duffau et al. (2006) underline the importance of having a speech therapist in the operating room to analyze accurately and in real-time the functional responses induced by stimulation.
Haglund et al. (1994) audiotaped a patient during language mapping and later had the recording reviewed by a blinded examiner for final analysis of significant language sites.
Another practice is to audio-videotape mapping procedures and record the spoken responses through a microphone placed near the patient’s mouth ( Malow et al., 1996; Peraud et al., 2004; Roux et al., 2003; Van Buren et al, 1978). In this way, movement of the mouth and facial muscles can be followed, as can eye movements that may interfere with reading, which often occur following stimulation of frontal sites ( Milea et al., 2002). A more efficient postoperative evaluation can be achieved by multiple investigators validating the intra-operative data ( Corina et al., 2005; Sanai et al., 2008). In all these studies, a picture of the brain was systematically taken before and during brain mapping to show the explored and positive sites, along with a written record of the findings during stimulation.
Concluding remarks
In conclusion, intraoperative procedures for cortical language mapping have been in use for some time. New procedures for non-language tasks have been or are being tested. Evidence and experimental conditions for their validation, as well as surgical standards for subcortical mapping must be implemented before these latter procedures can be relied upon in clinical practice.
References
- Bartolomeo P , Thiebaut de Schotten M , Duffau H . Mapping of visuospatial functions during brain surgery: a new tool to prevent unilateral spatial neglect . Neurosurgery . 2007 ; 61 : E1340 . doi: 10.1227/01.neu.0000306126.46657.79. [DOI] [PubMed] [Google Scholar]
- Bello L , Acerbi F , Giussani C , et al. Intraoperative language localization in multilingual patients with gliomas . Neurosurgery . 2006 ; 59 : 115 – 125 . doi: 10.1227/01.NEU.0000219241.92246.FB. [DOI] [PubMed] [Google Scholar]
- Bello L , Gallucci M , Fava M , et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas . Neurosurgery . 2007 ; 60 : 67 – 82 . doi: 10.1227/01.NEU.0000249206.58601.DE. [DOI] [PubMed] [Google Scholar]
- Bello L , Gambini A , Castellano A , et al. Motor and language DTI fiber tracking combined with intraoperative sub-cortical mapping for surgical removal of gliomas . Neuroimage . 2008 ; 39 : 369 – 382 . doi: 10.1016/j.neuroimage.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Bello L , Castellano A , Fava E , et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations . Neurosurg Focus . 2010 ; 28 : E6 . doi: 10.3171/2009.12.FOCUS09240. [DOI] [PubMed] [Google Scholar]
- Benzagmout M , Gatignol P , Duffau H . Resection of World Health Organization Grade II gliomas involving Broca’s area: methodological and functional considerations . Neurosurgery . 2007 ; 61 : 741 – 752 . doi: 10.1227/01.NEU.0000298902.69473.77. [DOI] [PubMed] [Google Scholar]
- Berger MS , Kincaid J , Ojemann GA , et al. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors . Neurosurgery . 1989 ; 25 : 786 – 792 . doi: 10.1097/00006123-198911000-00015. [DOI] [PubMed] [Google Scholar]
- Berger MS , Ojemann GA . Intraoperative brain mapping techniques in neuro-oncology . Stereotact Funct Neurosurg . 1992 ; 58 : 153 – 161 . doi: 10.1159/000098989. [DOI] [PubMed] [Google Scholar]
- Berger MS . Lesions in functional (“eloquent”) cortex and subcortical white matter . Clin Neurosurg . 1994 ; 41 : 444 – 463 . [PubMed] [Google Scholar]
- Brandling-Bennett EM , Bookheimer SY , Horsfall JL , et al. A paradigm for awake intraoperative memory mapping during forniceal stimulation . Neurocase . 2012 ; 18 : 26 – 38 . doi: 10.1080/13554794.2010.547509. [DOI] [PubMed] [Google Scholar]
- Chacko AG , Thomas SG , Babu KS , et al. Awake craniotomy and electrophysiological mapping for eloquent area tumours . Clin Neurol Neurosurg . 2013 ; 115 : 329 – 334 . doi: 10.1016/j.clineuro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Cherney LR , Halper AS , Kwasnica CM , et al. Recovery of functional status after right hemisphere stroke: relationship with unilateral neglect . Arch Phys Med Rehabil . 2001 ; 82 : 322 – 328 . doi: 10.1053/apmr.2001.21511. [DOI] [PubMed] [Google Scholar]
- Corina DP , Gibson EK , Martin R , et al. Dissociation of action and object naming: evidence from cortical stimulation mapping . Hum Brain Mapp . 2005 ; 24 : 1 – 10 . doi: 10.1002/hbm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis A , Sarubbo S , Duffau H . Subcortical surgical anatomy of the lateral frontal region: human white matter dissection and correlations with functional insights provided by intraoperative direct brain stimulation: laboratory investigation . J Neurosurg . 2012 ; 117 : 1053 – 1069 . doi: 10.3171/2012.7.JNS12628. [DOI] [PubMed] [Google Scholar]
- Devinsky O , Perrine K , Hirsch J , et al. Relation of cortical language distribution and cognitive function in surgical epilepsy patients . Epilepsia . 2000 ; 41 : 400 – 404 . doi: 10.1111/j.1528-1157.2000.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Sichez JP , et al. Intra-operative direct electrical stimulations of the central nervous system: The Salpêtrière experience with 60 patients . Acta Neurochir (Wien) . 1999 ; 141 : 1157 – 1167 . doi: 10.1007/s007010050413. [DOI] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Sichez N , et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study . Brain . 2002a ; 125 : 199 – 214 . doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- Duffau H , Denvil D , Capelle L . Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy . J Neurol Neurosurg Psychiatry . 2002b ; 72 : 511 – 516 . doi: 10.1136/jnnp.72.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H , Denvil D , Lopes M , et al. Intraoperative mapping of the cortical areas involved in multiplication and subtraction: an electricostimulation study in a patient with a left parietal glioma . J Neurol Neurosurg Psychiatry . 2002c ; 73 : 733 – 738 . doi: 10.1136/jnnp.73.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Denvil D , et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation . J Neurol Neurosurg Psychiatry . 2003a ; 74 : 901 – 907 . doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H , Capelle L , Denvil D , et al. Usefulness of intra-operative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients . J Neurosurg . 2003b ; 98 : 764 – 778 . doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- Duffau H . Peroperative functional mapping using direct electrical stimulations: methodological considerations . Neurochirurgie . 2004 ; 50 : 474 – 483 . doi: 10.1016/s0028-3770(04)98328-2. [DOI] [PubMed] [Google Scholar]
- Duffau H , Fontaine D . Successful resection of a left insular cavernous angioma using neuronavigation and intraoperative language mapping . Acta Neurochir (Wien) . 2005 ; 147 : 295 – 208 . doi: 10.1007/s00701-004-0357-6. [DOI] [PubMed] [Google Scholar]
- Duffau H , Gatignol P , Mandonnet E , et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations . Brain . 2005 ; 128 : 797 – 810 . doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H . New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity – a review . J Neurooncol . 2006 ; 79 : 77 – 115 . doi: 10.1007/s11060-005-9109-6. [DOI] [PubMed] [Google Scholar]
- Duffau H , Taillandier L , Gatignol P , et al. The insular lobe and brain plasticity : lessons from tumor surgery . Clin Neurol Neurosurg . 2006 ; 108 : 543 – 548 . doi: 10.1016/j.clineuro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Duffau H . The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography . Neuropsychologia . 2008 ; 46 : 927 – 934 . doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Duffau H , Peggy Gatignol ST , Mandonnet E , et al. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with grade II glioma in the left dominant hemisphere . J Neurosurg . 2008 ; 109 : 461 – 471 . doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Exner S . Untersuchungen über die Localisation der Functionen in der Grosshirnrinde des Menschen . Vienna : Baumuller ; 1861 . [Google Scholar]
- Fontaine D , Capelle L , Duffau H . Somatotopy of the supplementary motor area: evidence by correlation of the extent of surgical resection with the clinical patterns of deficit . Neurosurgery . 2002 ; 50 : 297 – 305 . doi: 10.1097/00006123-200202000-00011. [DOI] [PubMed] [Google Scholar]
- Gil Robles S , Gatignol P , Capelle L , et al. The role of the dominant striatum in language: a study using intraoperative electrical stimulations . J Neurol Neurosurg Psychiatry . 2005 ; 76 : 940 – 946 . doi: 10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatignol P , Capelle , Le Bihan R , et al. Double dissociation between picture naming and comprehension: an electrostimulation study . Neuroreport . 2004 ; 15 : 191 – 195 . doi: 10.1097/00001756-200401190-00037. [DOI] [PubMed] [Google Scholar]
- Gharabaghi A , Fruhmann Berger M , Tatagiba M , et al. The role of the right superior temporal gyrus in visual search – insights from intraoperative electrical stimulation . Neuropsychologia . 2006 ; 44 : 2578 – 2581 . doi: 10.1016/j.neuropsychologia.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Gillen R , Tennen H , McKee T . Unilateral spatial neglect: relation to rehabilitation outcomes in patients with right hemisphere stroke . Arch Phys Med Rehabil . 2005 ; 86 : 763 – 767 . doi: 10.1016/j.apmr.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Gras-Combe G , Moritz-Gasser S , Herbet G , et al. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways . J Neurosurg . 2012 ; 117 : 466 – 473 . doi: 10.3171/2012.6.JNS111981. [DOI] [PubMed] [Google Scholar]
- Haglund MM , Berger MS , Shamseldin M , et al. Cortical localization of temporal lobe language sites in patients with gliomas . Neurosurgery . 1994 ; 34 : 567 – 576 . doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- Haglund MM , Ojemann GA , Blasdel GG . Optical imaging of bipolar cortical stimulation . J Neurosurg . 1993 ; 78 : 785 – 793 . doi: 10.3171/jns.1993.78.5.0785. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ , Seidel WT , Goodman RR , et al. Temporal lobe stimulation reveals anatomic distinction between auditory naming processes . Neurology . 2003 ; 60 : 1478 – 1483 . doi: 10.1212/01.wnl.0000061489.25675.3e. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ , Seidel WT , McKhann GM , 2nd , et al. Brain stimulation reveals critical auditory naming cortex . Brain . 2005 ; 128 : 2742 – 2749 . doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ , McClelland S , 3rd , McKhann GM , 2nd , et al. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions . Epilepsia . 2007 ; 48 : 531 – 538 . doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Herholz K , Reulen HJ , von Stockhausen HM , et al. Preoperative activation and intraoperative stimulation of language-related areas in patients with glioma . Neurosurgery . 1997 ; 41 : 1253 – 1262 . doi: 10.1097/00006123-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Hernandez AE , Martinez A , Kohnert K . In search of the language switch: an fMRI study of picture naming in Spanish-English bilinguals . Brain Lang . 2000 ; 73 : 421 – 431 . doi: 10.1006/brln.1999.2278. [DOI] [PubMed] [Google Scholar]
- Illes J , Francis WS , Desmond JE , et al. Convergent cortical representation of semantic processing in bilinguals . Brain Lang . 1999 ; 70 : 347 – 363 . doi: 10.1006/brln.1999.2186. [DOI] [PubMed] [Google Scholar]
- Ilmberger J , Eisner W , Schmid U , et al. Performance in picture naming and word comprehension: evidence for common neuronal substrates from intraoperative language mapping . Brain Lang . 2001 ; 76 : 111 – 118 . doi: 10.1006/brln.2000.2415. [DOI] [PubMed] [Google Scholar]
- Kaplan EF , Goodglass H , Weintraub S . The Boston Naming Test . 2nd ed . Philadelphia : Lea & Febigeer ; 1983 . [Google Scholar]
- Kim KH , Relkin NR , Lee KM , et al. Distinct cortical areas associated with native and second languages . Nature . 1997 ; 388 : 171 – 174 . doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Kral T , Kurthen M , Schramm J , et al. Stimulation mapping via implanted grid electrodes prior to surgery for gliomas in highly eloquent cortex . Neurosurgery . 2006 ; 58 ( 1 Suppl ): ONS36 – 43 . doi: 10.1227/01.neu.0000193925.98348.f5. [DOI] [PubMed] [Google Scholar]
- Kurimoto M , Asahi T , Shibata T , et al. Safe removal of glioblastoma near the angular gyrus by awake surgery preserving calculation ability – case report . Neurol Med Chir (Tokyo) . 2006 ; 6 : 46 – 50 . doi: 10.2176/nmc.46.46. [DOI] [PubMed] [Google Scholar]
- Laiacona M , Barbarotto R , Trivelli C , et al. Dissociazioni semantiche intercategoriali: Descrizione di una batteria standardizzata e dati normativi . Archivio di Psicologia, Neurologia, e Psichiatria . 1993 ; 54 : 209 – 248 . [Google Scholar]
- Leclercq D , Duffau H , Delmaire C , et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations . J Neurosurg . 2010 ; 112 : 503 – 511 . doi: 10.3171/2009.8.JNS09558. [DOI] [PubMed] [Google Scholar]
- Lesser RP , Lüders H , Morris HH , et al. Electrical stimulation of Wernicke’s area interferes with comprehension . Neurology . 1986 ; 36 : 658 – 663 . doi: 10.1212/wnl.36.5.658. [DOI] [PubMed] [Google Scholar]
- Little K , Friedman AH . Awake craniotomy for malignant glioma resection . International Congress Series . 2004 ; 1259 : 409 – 414 . [Google Scholar]
- Lubrano V , Roux FE , Démonet JF . Writing specific sites in frontal areas: a cortical stimulation study . J Neurosurg . 2004 ; 101 : 787 – 798 . doi: 10.3171/jns.2004.101.5.0787. [DOI] [PubMed] [Google Scholar]
- Lucas TH , 2nd , McKhann GH , 2nd , Ojemann GA . Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients . J Neurosurg . 2004 ; 101 : 449 – 457 . doi: 10.3171/jns.2004.101.3.0449. [DOI] [PubMed] [Google Scholar]
- Lüders HO , Lesser RP , Dinner DS , et al. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy . Epilepsia . 1988 ; 29 ( Suppl 2 ): S56 – 65 . doi: 10.1111/j.1528-1157.1988.tb05799.x. [DOI] [PubMed] [Google Scholar]
- Lüders HO , Lesser RP , Hahn J , et al. Basal temporal language area . Brain . 1991 ; 114 : 743 – 754 . doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- Lüders HO , Dinner DS , Morris HH , et al. Cortical electrical stimulation in humans. The negative motor areas . Adv Neurol . 1995 ; 67 : 115 – 129 . [PubMed] [Google Scholar]
- Malow BA , Blaxton TA , Sato S , et al. Cortical stimulation elicits regional distinctions in auditory and visual naming . Epilepsia . 1996 ; 37 : 245 – 252 . doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Mandonnet E , Jbabdi S , Taillandier L , et al. Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping . Neuro Oncol . 2006 ; 91 : 63 – 69 . doi: 10.1215/15228517-2006-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J , Taillander L , Moritz-Gasser S , et al. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas . Acta Neurochir (Wien) . 2009 ; 151 : 427 – 436 . doi: 10.1007/s00701-009-0232-6. [DOI] [PubMed] [Google Scholar]
- Martino J , Vergani F , Robles SG , et al. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures . Neurosurgery . 2010 ; 66 ( 3 Suppl Operative ): 4 – 12 . doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- Martino J , De Witt Hamer PC , Berger MS , et al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study . Brain Struct Funct . 2013 ; 218 : 105 – 121 . doi: 10.1007/s00429-012-0386-5. [DOI] [PubMed] [Google Scholar]
- Mateer CA , Polen SB , Ojemann GA , et al. Cortical localization of finger spelling and oral language: a case study . Brain Lang . 1982 ; 17 : 46 – 57 . doi: 10.1016/0093-934x(82)90004-9. [DOI] [PubMed] [Google Scholar]
- Matsuda R , Coello AF , De Benedictis A , et al. Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: surgical technique and functional results: clinical article . J Neurosurg . 2012 ; 117 : 1076 – 1081 . doi: 10.3171/2012.9.JNS12662. [DOI] [PubMed] [Google Scholar]
- Metz-Lutz MN , Wioland F , Brock G . A real-time approach to spoken language processing in aphasia . Brain Lang . 1992 ; 43 : 565 – 582 . doi: 10.1016/0093-934x(92)90083-q. [DOI] [PubMed] [Google Scholar]
- Miceli G , Silveri MC , Villa G , et al. On the basis for the agrammatic’s difficulty in producing main verbs . Cortex . 1984 ; 20 : 207 – 220 . doi: 10.1016/s0010-9452(84)80038-6. [DOI] [PubMed] [Google Scholar]
- Milea D , Lobel E , Lehéricy S , et al. Intraoperative frontal field stimulation elicits ocular deviation and saccade suppression . Neuroreport . 2002 ; 13 : 1359 – 1364 . doi: 10.1097/00001756-200207190-00029. [DOI] [PubMed] [Google Scholar]
- Milea D , Lobel E , Lehéricy S , et al. Cortical mechanisms of saccade generation from execution to decision . Ann N Y Acad Sci . 2005 ; 1039 : 232 – 238 . doi: 10.1196/annals.1325.022. [DOI] [PubMed] [Google Scholar]
- Ojemann GA . Individual variability in cortical localization of language . J Neurosurg . 1979 ; 50 : 164 – 169 . doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- Ojemann GA . Brain organization for language from the perspective of electrical stimulation mapping . Behav Brain Sci . 1983 ; 6 : 189 – 206 . [Google Scholar]
- Ojemann GA . Organization of language cortex derived from investigations during neurosurgery . Semin Neurosci . 1990 ; 2 : 297 – 305 . [Google Scholar]
- Ojemann GA . Cortical organization of language and verbal memory based on intraoperative investigations . Progress in Sensory Physiology . 1991 ; 12 : 192 – 230 . [Google Scholar]
- Ojemann SG , Berger MS , Lettich E , et al. Localisation of language function in children: results of electrical stimulation mapping . J Neurosurg . 2003 ; 98 : 465 – 470 . doi: 10.3171/jns.2003.98.3.0465. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Dodrill CB . Verbal memory deficits after temporal lobectomy for epilepsy. Mechanism and intraoperative prediction . J Neurosurg . 1985 ; 62 : 101 – 107 . doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- Ojemann G , Mateer C . Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems . Science . 1979 ; 205 : 1401 – 1403 . doi: 10.1126/science.472757. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Ojemann J , Lettich E , et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients . J Neurosurg . 1989 ; 71 : 316 – 326 . doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Ojemann SG , Fried I . Lessons from the human brain: neuronal activity related to cognition . The Neuroscientist . 1998 ; 4 : 285 – 300 . [Google Scholar]
- Ojemann JG , Ojemann GA , Lettich E . Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming . J Neurosurg . 2002 ; 97 : 33 – 38 . doi: 10.3171/jns.2002.97.1.0033. [DOI] [PubMed] [Google Scholar]
- Ojemann GA , Whitaker A . The bilingual brain . Arch Neurol . 1978 ; 35 : 409 – 412 . doi: 10.1001/archneur.1978.00500310011002. [DOI] [PubMed] [Google Scholar]
- Otani N , Bjeljac M , Muroi C , et al. Awake surgery for glioma resection in eloquent areas-Zurich’s experience and review . Neurol Med Chir (Tokyo) . 2005 ; 45 : 501 – 511 . doi: 10.2176/nmc.45.501. [DOI] [PubMed] [Google Scholar]
- Paolucci S , Antonucci G , Grasso MG , et al. The role of unilateral spatial neglect in rehabilitation of right brain-damaged ischemic stroke patients: a matched comparison . Arch Phys Med Rehabil . 2001 ; 82 : 743 – 749 . doi: 10.1053/apmr.2001.23191. [DOI] [PubMed] [Google Scholar]
- Penfield W , Boldrey E . Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation . Brain . 1937 ; 60 : 389 – 443 . [Google Scholar]
- Peraud A , Ilmberger J , Reulen HJ . Surgical resection of gliomas WHO grade II and III located in the opercular region . Acta Neurochir (Wien) . 2004 ; 146 : 9 – 18 . doi: 10.1007/s00701-003-0165-4. [DOI] [PubMed] [Google Scholar]
- Petrovich Brennan NM , Whalen S , de Morales Branco D , et al. Object naming is a more sensitive measure of speech localization than number counting: converging evidence from direct cortical stimulation and fMRI . Neuroimage . 2007 ; 37 ( Suppl 1 ): S100 – S108 . doi: 10.1016/j.neuroimage.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Picht T , Kombos T , Gramm H , et al. Multimodal protocol for awake craniotomy in language cortex tumour surgery . Acta Neurochir (Wien) . 2006 ; 148 : 127 – 137 . doi: 10.1007/s00701-005-0706-0. [DOI] [PubMed] [Google Scholar]
- Pillai JI , Araque JM , Allison JD , et al. Functional MRI study of semantic and phonological language processing in bilingual subjects: preliminary findings . Neuroimage . 2003 ; 19 : 565 – 576 . doi: 10.1016/s1053-8119(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Pouration N , Bookheimer SY , O’Farrell AM , et al. Optical imaging of bilingual cortical representations. Case report . J Neurosurg . 2000 ; 93 : 676 – 681 . doi: 10.3171/jns.2000.93.4.0676. [DOI] [PubMed] [Google Scholar]
- Pouration N , Bookheimer SY , Rex DE , et al. Utility of pre-operative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations . J Neurosurg . 2002 ; 97 : 21 – 32 . doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Pouration N , Bookheimer SY , Rubino G , et al. Category-specific naming deficit identified by intraoperative stimulation mapping and postoperative neuropsychological testing. Case report . J Neurosurg . 2003 ; 99 : 170 – 176 . doi: 10.3171/jns.2003.99.1.0170. [DOI] [PubMed] [Google Scholar]
- Pouratian N , Cannestra AF , Bookheimer SY , et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals . J Neurosurg . 2004 ; 101 : 458 – 466 . doi: 10.3171/jns.2004.101.3.0458. [DOI] [PubMed] [Google Scholar]
- Pu S , Li YN , Wu CX , et al. Cortical areas involved in numerical processing: an intraoperative electrostimulation study . Stereotact Funct Neurosurg . 2011 ; 89 : 42 – 47 . doi: 10.1159/000321186. [DOI] [PubMed] [Google Scholar]
- Rapport RL , Tan CT , Whitaker HA . Language function and dysfunction among Chinese- and English-speaking polyglots: cortical stimulation, WADA testing and clinical studies . Brain Lang . 1983 ; 18 : 342 – 366 . doi: 10.1016/0093-934x(83)90024-x. [DOI] [PubMed] [Google Scholar]
- Robles S , Gatignol P , Lehéricy S , et al. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas . J Neurosurg . 2008 ; 109 : 615 – 624 . doi: 10.3171/JNS/2008/109/10/0615. [DOI] [PubMed] [Google Scholar]
- Roux FE , Trémoulet M . Organization of language areas in bilingual patients. A cortical stimulation study . J Neurosurg . 2002 ; 97 : 867 – 864 . doi: 10.3171/jns.2002.97.4.0857. [DOI] [PubMed] [Google Scholar]
- Roux FE , Boetto S , Sacko O , et al. Writing, calculating and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome . J Neurosurg . 2003 ; 99 : 716 – 727 . doi: 10.3171/jns.2003.99.4.0716. [DOI] [PubMed] [Google Scholar]
- Roux FE , Lubrano V , Lauwers-Cances V , et al. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients . Brain . 2004 ; 127 : 1796 – 1810 . doi: 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- Roux FE , Boukhatem L , Draper L , et al. Cortical calculation localization using electrostimulation . J Neurosurg . 2009 ; 110 : 1291 – 1299 . doi: 10.3171/2008.8.JNS17649. [DOI] [PubMed] [Google Scholar]
- Roux F , Dufor O , Lauwers-Cances V , et al. Electrostimulation mapping of spatial neglect . Neurosurgery . 2011 ; 69 : 1218 – 1231 . doi: 10.1227/NEU.0b013e31822aefd2. [DOI] [PubMed] [Google Scholar]
- Rutten GJ , Ramsey NF , van Rijen PC , et al. Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas . Ann Neurol . 2002 ; 51 : 350 – 360 . doi: 10.1002/ana.10117. [DOI] [PubMed] [Google Scholar]
- Sanai N , Mirzadeh Z , Berger MS . Functional outcome after language mapping for glioma resection . N Engl J Med . 2008 ; 358 : 18 – 27 . doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Sarubbo S , Latini F , Sette E , et al. Is the resection of gliomas in Wernicke’s area reliable? : Wernicke’s area resection . Acta Neurochir (Wien) . 2012a ; 154 : 1653 – 1662 . doi: 10.1007/s00701-012-1416-z. [DOI] [PubMed] [Google Scholar]
- Sarubbo S , Le Bars E , Moritz-Gasser S , et al. Complete recovery after surgical resection of left Wernicke’s area in awake patient: a brain stimulation and functional MRI study . Neurosurg Rev . 2012b ; 35 : 287 – 292 . doi: 10.1007/s10143-011-0351-4. [DOI] [PubMed] [Google Scholar]
- Sarubbo S , De Benedictis A , Maldonado IL , et al. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle . Brain Struct Funct . 2013 ; 218 : 21 – 37 . doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Schäffler L , Lüders HO , Beck GJ . Quantitative comparison of language deficits produced by extraoperative electrical stimulation of Broca’s, Wernicke’s and basal temporal language areas . Epilepsia . 1996 ; 37 : 463 – 475 . doi: 10.1111/j.1528-1157.1996.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Schwartz TH , Devinsky O , Doyle W , et al. Function-Specific high-probability “nodes” identified in posterior language cortex . Epilepsia . 1999 ; 40 : 575 – 583 . doi: 10.1111/j.1528-1157.1999.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Seeck M , Pegna AJ , Ortigue S , et al. Speech arrest with stimulation may not reliably predict language deficit after epilepsy surgery . Neurology . 2006 ; 66 : 592 – 594 . doi: 10.1212/01.wnl.0000199254.67398.a7. [DOI] [PubMed] [Google Scholar]
- Signorelli F , Guyotat J , Isnard J , et al. The value of cortical stimulation applied to the surgery of malignant gliomas in language areas . Neurol Sci . 2001 ; 22 : 3 – 10 . doi: 10.1007/s100720170029. [DOI] [PubMed] [Google Scholar]
- Skirboll SS , Ojemann GA , Berger MS , et al. Functional cortex and subcortical white matter located within gliomas . Neurosurgery . 1996 ; 38 : 678 – 684 . [PubMed] [Google Scholar]
- Snodgrass JG , Vanderwart M . A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity . J Exp Psychol Hum Learn . 1980 ; 6 : 174 – 215 . doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Szelényi A , Bello L , Duffau H , et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice . Neurosurg Focus . 2010 ; 28 : E7 . doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M , Urbanski M , Duffau H , et al. Direct evidence for a parietal pathway subserving spatial awareness in humans . Science . 2005 ; 309 : 2226 – 2228 . doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Trinh VT , Fahim DK , Shah K , et al. Subcortical injury is an independent predictor of worsening neurological deficits following awake craniotomy procedures . Neurosurgery . 2013 ; 72 : 160 – 169 . doi: 10.1227/NEU.0b013e31827b9a11. [DOI] [PubMed] [Google Scholar]
- Van Buren JM , Fedio P , Frederick GC . Mechanism and localization of speech in the parietotemporal cortex . Neurosurgery . 1978 ; 2 : 233 – 239 . doi: 10.1227/00006123-197805000-00009. [DOI] [PubMed] [Google Scholar]
- Walker JA , Quiñones-Hinojosa A , Berger MS . Intraoperative speech mapping in 17 bilingual patients undergoing resection of a mass lesion . Neurosurgery . 2004 ; 54 : 113 – 118 . doi: 10.1227/01.neu.0000097270.95721.3b. [DOI] [PubMed] [Google Scholar]
- Yu X , Chen C , Pu S , et al. Dissociation of subtraction and multiplication in the right parietal cortex: evidence from intraoperative cortical electrostimulation . Neuropsychologia . 2011 ; 49 : 2889 – 2895 . doi: 10.1016/j.neuropsychologia.2011.06.015. [DOI] [PubMed] [Google Scholar]