Summary
An open study was conducted with the aim of reporting long-term clinical outcome of endovascular treatment for chronic cerebrospinal venous insufficiency (CCSVI) in patients with multiple sclerosis (MS).
Twenty-nine patients with clinically definite relapsing-remitting MS underwent percutaneous transluminal angioplasty for CCSVI, outside a clinical relapse. All the patients were regularly observed over at least two years before the first endovascular treatment and for at least two years after it (mean post-procedure follow up 30.6±6.1 months). The following clinical outcome measures were used: annual relapse rate and Expanded Disability Status Scale (EDSS) score. All the patients were observed intensively (mean 6 hours) on the day of the endovascular treatment to monitor for possible complications (bleeding, shock, heart attack, death).
We compared the annual relapse rate before and after treatment (in the two years before and the two years after the first endovascular treatment) and the EDSS score recorded two years before versus two years after the treatment.
Overall, 44 endovascular procedures were performed in the 29 patients, without complications. Thirteen of the 29 patients (45%) underwent more than one treatment session because of venous re-stenosis: 11 and two patients underwent two and three endovascular treatments respectively.
The annual relapse rate of MS was significantly lower post-procedure (0.45±0.62 vs 0.76±0.99; p=0.021), although it increased in four patients. The EDSS score two years after treatment was significantly lower compared to the EDSS score recorded at the examination two years before treatment (1.98±0.92 vs 2.27±0.93; p=0.037), although it was higher in four patients. Endovascular treatment of concurrent CCSVI seems to be safe and repeatable and may reduce annual relapse rates and cumulative disability in patients with relapsing-remitting MS. Randomized controlled studies are needed to further assess the clinical effects of endovascular treatment of CCSVI in MS.
Keywords: multiple sclerosis, neurological outcome, venous angioplasty
Introduction
Multiple sclerosis (MS) is the most common chronic disabling disease of the central nervous system (CNS) in young adults, affecting 1 in 1,000 people in Western countries (1). Pathologically, MS is characterized primarily by multifocal inflammation, demyelination and gliosis of the white matter with axonal and neuronal loss as secondary phenomena (2). Clinically, MS manifests itself with acute relapses or subtle progression of focal neurological deficits. Although a link between the venous system and the focal brain lesions typical of MS has been suspected on the basis of pathological evidence since the first half of the nineteenth century (3), recently the existence of this link was strongly questioned (4). In 2009 we and other groups observed a very high prevalence of a condition named chronic cerebrospinal venous insufficiency (CCSVI) in patients with MS (5–8). CCSVI is characterized by venous malformations of the jugular, azygos and lumbar veins leading to a decreased venous outflow from the CNS and the formation of collateral venous channels (5). However this observation was not replicated in other studies (9–12), a discrepancy possibly reflecting differences in techniques, training, learning curves and criteria used for Doppler evaluation of the extra and intracranial veins, which is the optimal screening tool for CSSVI (5). Consequently the relationship between CCSVI and MS remains controversial (3,13–15). There are, as yet, no reports of prospective controlled trials in this field.
Endovascular treatment has been suggested for CCSVI in MS patients as a possible means of improving venous out-flow (16,17). We here report the results of the extended follow up of patients included in a preliminary self-controlled study which evaluated the clinical effects of endovascular treatment for CCSVI in a cohort of relapsing-remitting (RR) MS patients, followed in a single MS center (16).
Materials and methods
Patient population
This open study constituted the long-term clinical follow up of an interventional study approved by the ethics committee of the University of Ferrara in February 2007 (16). The cohort was composed of 45 consecutive patients with clinically definite RR-MS (18) regularly followed both at the neuroimmunological diseases clinic at the Bellaria Hospital in Bologna, Italy, and at the Vascular Diseases Center of the Ferrara University Hospital, where they underwent endovascular treatment for CCSVI until September 2010. The patients underwent routine clinical examinations at the neuroimmunological diseases clinic every three months both before and after endovascular treatment of CCSVI; all the endovascular procedures were performed by the same neurologist (FS) who has 28 years of experience in neuroimmunological diseases. All the patients underwent Doppler US evaluation at the Ferrara University Vascular Diseases Center one month after the first endovascular procedure, and at least every three months thereafter. The diagnosis of CCSVI was based on examination of cerebrospinal venous return with the subject placed on a tilt bed; for this purpose, the extracranial echo-color Doppler (ECD) methodology for investigating the internal jugular veins (IJVs) and vertebral veins (VVs) was combined with transcranial color coded Doppler sonography (TCCS) for studying the deep cerebral veins. As mentioned in previous papers (5,16,17,19), non-invasive diagnosis of CCSVI required fulfillment of at least two of the following five criteria: i) reflux in the IJVs and/or VVs in sitting and supine posture; ii) reflux in the intracranial veins ; iii) high-resolution B-mode evidence of IJV stenoses and\or intraluminal defects; iv) flow not Doppler-detectable in the IJVs and/or VVs; v) no increase in the cross-sectional area of the IJV on transition from upright to supine posture. In all patients with ECD and TCCS features suggesting CSSVI, the diagnosis was confirmed by catheter venography.
Of the original 45 patients recruited for the present study, we excluded six who underwent endovascular treatment during a relapse, nine patients with pre-procedural observation lasting less than two years and one patient with post-procedural observation lasting less than two years.
This yielded a final cohort of 29 patients who were relapse-free and off steroids in the 30 days before endovascular treatment. The clinical features of these patients are summarized in Table 1.
Table I.
Clinical findings in the 29 patients with RR-MS included in the study.
| Gender M/F | 13/16 |
| Age (mean±SD)* | 35.0±6.9 years (range 21–49) |
| Disease duration (mean±SD)* | 87±64.8 months (range 24–192) |
| EDSS (mean±SD)* | 2.36±0.96 (range 1.0–4.5) |
| Pharmacological treatment* | |
| immunomodulating | n=20 |
| immunosuppressive | n=2 |
| Type of CCSVI° | A=5 |
| B=18 | |
| C=6 | |
| Number of endovascular procedures | 1=16 |
| 2=11 | |
| 3=2 |
at the time of the first endovascular treatment;
(A=steno-obstruction of the proximal azygos associated with a closed stenosis of one jugular vein; B=significant stenosis of both jugular veins and the proximal azygos; C=bilateral stenosis of both jugular veins with a normal, but overloaded, azygos system) (5).
Seventeen of the 29 patients were included in a previous report in which the mean post-treatment follow up was 18 months (16). The mean follow up after the first endovascular therapy session in the present series was 30.6±6.1 months.
Thus, the present cohort is composed of patients with minimum pre- and postoperative observations lasting 24 months, with no relapse at the time of the procedure, and who underwent uninterrupted follow up at our neurological department.
During the four years of clinical observation considered in the present study (two years before and two years after the first endovascular treatment session), 20 patients received immunomodulating therapy, either interferon-β-1b (Rebif) (n=16) or glatiramer acetate (Copaxone) (n= 4) and two underwent immunosuppressive treatment with mitoxantrone (Novantrone).
During relapses each patient was treated with a high-dose steroid regimen (methylprednisolone 1g/day iv for 5 days).
Endpoints
For computation of the clinical outcome, the date of the first endovascular treatment session was taken as the temporal reference. As clinical outcome measures for this study we used the annual relapse rate and the EDSS (Expanded Disability Status Scale) score, both of which are established instruments for assessing the efficacy of pharmacological treatments in RR-MS (20).
In particular, relapse was defined as the appearance of new or worsening neurological symptoms, or the reappearance of old neurological symptoms, in both cases excluding subjective sensory disturbances alone, after a 30-day period of stability (21). An event was considered a relapse only when the subject’s symptoms were accompanied by objective neurological changes implying an increase of at least 0.5 in the EDSS score as compared to the previous evaluation.
The EDSS categorizes a person’s level of disability. EDSS scores range from 0 to 10, with higher scores indicating more severe disability.
The EDSS score is based upon neurological testing and examination of functional systems. The functional systems, or areas of the CNS that control bodily functions, can be summarized as follows:
Pyramidal (ability to walk);
Cerebellar (coordination);
Brain stem (speech and swallowing);
Sensory (touch and pain);
Bowel and bladder functions;
Visual;
Mental;
Other (includes any other neurological findings due to MS).
For example EDSS scores from 1.0 to 4.5 refer to people with MS who are fully ambulatory while EDSS scores from 5.0 to 9.5 are defined by the impairment of ambulation.
The EDSS score was recorded every three months, never during a neurological relapse.
PTA of the jugular and azygos veins
Previous papers by our group report the strategies and technical details of the percutaneous transluminal angioplasty used to treat CSVVI in MS (16,17).
Briefly, after catheterization of the vessel harboring the stenosis the patient received 5000 IU of intravenous heparin. Lesions of the azygos vein were treated with 8–10 mm diameter angioplasty balloons between 2 and 6 cm in length, inflated to a maximum pressure of 8 atm. Inflations were held for 30–60 seconds and repeated as many times as required. Lesions of IJVs were treated first with a compliant balloon (10–12 mm in diameter, 2–4 cm in length) which was then inflated to a pressure of 8 atm. Small caliber balloons were selected in cases of severe atresia or segmental hypoplasia (5 mm Cutting balloon, Boston Scientific, USA), followed by subsequent remodeling with an 8 mm compliant balloon. The inflation was maintained for one minute and repeated 2–3 times. In the event of a poor post-procedural outcome, the treatment was repeated with a non-compliant high-pressure balloon (10 mm Blue-max, Boston Scientific, USA; 12–14 mm Atlas, Bard, USA).
Patients were treated with low molecular weight heparin at a dosage in accordance with the individual risk for a duration of three weeks, as previously reported (16,17). Patients in whom we used the Cutting balloon received prophylaxis with fondaparinux 7.5 mg (Arixtra, Glaxo, Brentford, UK) s.c. once a day for three weeks. All patients were assessed for post-operative venous thrombosis by means of vascular ultrasound examination before being discharged.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, Version 16.0). Statistical non-parametric tests (one-tailed Wilcoxon matched pairs test) were adopted to compare the annual relapse rate in the two years before and after the first endovascular treatment and the EDSS scores recorded at the examinations conducted two years before and after treatment. A significant p-value threshold of 0.05 was employed. Finally, a possible significant correlation between changes in annual relapse rate (after treatment minus before treatment) and changes in EDSS score (score two years after minus score two years before treatment) was sought using the Pearson correlation r-coefficient (p<0.05).
Results
Overall, 44 endovascular procedures were carried out in the 29 patients. Sixteen patients underwent a single endovascular treatment, while 11 had two procedures and two underwent three sessions after US Doppler during follow up showed re-stenosis of the previously treated veins. In no case was the second treatment session performed before 18 months had elapsed from the first procedure; seven of the 13 patients who underwent more than one treatment had a clinical neurological relapse; the other six underwent treatment only for a re-stenosis found on ECD. This last group had no clinical relapses during follow up. No major procedural or post-procedural complication associated with the endovascular treatment was observed in any of the patients. Four patients reported transient headache.
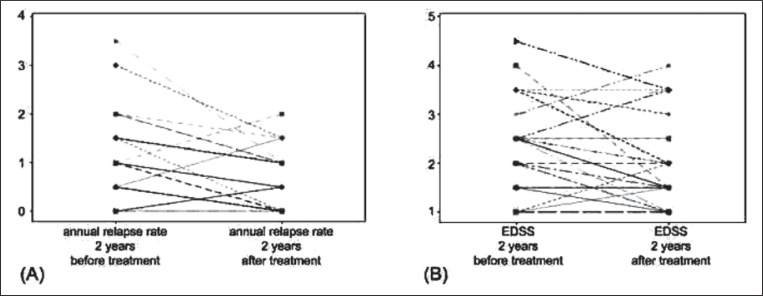
The annual relapse rate was lower (p=0.021) in the period after (mean 0.45±0.62; median 0.5, range 0–3.5) as compared to the period before (mean 0.76±0.99; median 0, range 0–2) treatment, although it increased in four patients (Fig.1). The EDSS score recorded at the examination two years after treatment (mean 1.98±0.92; median 2.5, range 1–4.5) was lower (p=0.037) than the mean EDSS score recorded at the examination two years before treatment (mean 2.27±0.93; median 1.5, range 1–4.0), although it was increased in four patients (Fig.1). The mean EDSS score at the time of the angioplasty was 2.3±0.8.
Figure 1.
Individual changes in annual relapse rate and Expanded Disability Status Scale scores in patients submitted to percutaneous transluminal angioplasty for CCSVI
The figure shows individual variations in the annual relapse rate (A) in the two years of clinical observation before and after the first percutaneous transluminal angioplasty for CCSVI, and in the EDSS score (B) at the examinations performed two years before and two years after the first endovascular treatment in 29 patients with RR-MS. Note that the mean annual relapse rate increased after treatment in four patients (in two patients the lines are superimposed), while the mean EDSS increased in four patients.
No significant correlation was observed between annual relapse rate and EDSS score changes (r=0.13).
Discussion
This preliminary open study confirms that endovascular treatment for CCSVI in RR-MS patients seems to be safe (16,17) and suggests that it might, if used as an adjunct to the usual immunomodulating or immunosuppressive therapy, have sustained beneficial clinical effects, in terms of decreased annual relapse rate and disability.
However, several elements suggest that caution should be exercised in generalizing these results. These include the small sample size, the study design (different from that of a randomized clinical trial), the fact that the study was carried out in two single centers (specializing respectively in neurological assessment and endovascular therapy), the gross clinical measurements used to evaluate the therapeutic effects with the absence of neuroimaging techniques and, especially, the fact that neither the patients nor the clinical assessor were blind to the treatment. However, the self-controlled design is considered a valid tool for preliminary clinical studies (22), while the annual relapse rate and the EDSS, notwithstanding their limitations, which include different definitions of the former (23) and relatively low inter-operator agreement for the EDSS, especially for values <4.5 (24), are accepted clinical outcome measures for new treatments of RR-MS (20) because they are widely used and effectively photograph the clinical situation. Neurological testing and examination of functional systems are especially important at the “less severe” lower end of the scale, when a patient is still ambulatory, yet experiencing some abnormal signs or disability in other areas.
Finally, the regression of the mean error was a potential shortcoming in the design of our study.
The reasons why five of the 29 patients in our series had no benefit in terms of decreased annual relapse rate (4 patients) or EDSS score (4 patients) following endovascular treatment of CCSVI are not clear. Indeed, review of the endovascular treatment revealed that the vascular result aimed at was achieved. However, occasional cases of patients with a paradoxical increase of lesion activity detected on MR imaging following endovascular treatment of CSSVI have been reported (17). Also, the high rate of re-stenosis of the extracranial veins in our sample, namely 45% in the two years after the first endovascular treatment session, is in line with a recent observation reporting a 27% re-stenosis rate after one year follow up in another patient sample (17).
The lack of a correlation between decrease of annual relapse rate and EDSS in our patients seems to suggest that the endovascular treatment has two distinct beneficial effects. Moreover, it is in line with literature findings indicating a dissociation at biological level between recurrent acute focal inflammation and progressive degeneration of the central nervous system (25).
We can only speculate on the mechanisms underlying the beneficial clinical effects of endovascular treatment of CCSVI in our MS patients. A recent perfusion MRI study indicated that hypoperfusion of the brain is associated with the severity of CCSVI in MS patients (26).
Hence it is possible that resolution of the obstruction of the venous outflow through endovascular treatment could improve brain perfusion and prevent the tissue damage associated with disability. Interestingly, global brain hypoperfusion is an aspect of MS that cannot be explained by autoimmunity and/or focal disturbed flow linked with inflammation; by contrast, the hypoperfusion could be related to the presence of CCSVI (27).
The mechanism accounting for the apparent decrease in the relapse rate following endovascular treatment of CCSVI is obscure. However it has been suggested that abnormal venous reflux in the cerebral and spinal veins associated with CSSVI increases the expression of adhesion molecules, particularly intercellular adhesion molecule-1, by the cerebrovascular endothelium (28).
This, in turn, could lead to increased permeability of the blood-brain barrier. Inflamed and activated endothelium could secrete pro-inflammatory cytokines. In these settings, monocytes could transform into antigen-presenting cells and initiate an autoimmune attack against myelin-containing cells. Theoretically, resolution of the venous reflux with endovascular treatment may taper and ultimately extinguish the processes mentioned above and ultimately determine a decrease of the relapse rate.
Although the clinical follow up of our patients was too short to allow inferences about the potentiality of endovascular treatment of CCSVI as a means of preventing secondary progression in patients with RR-MS, this may actually be a consequence of the two beneficial effects mentioned above and constitute a real advance in the therapy of this type of MS. A longer clinical follow up of the present cohort is needed (29).
In conclusion, the present preliminary study showing sustained beneficial clinical effects of endovascular treatment of CCSVI in RR-MS fully supports the design and execution of multicenter randomized controlled trials which include the use of neuroimaging techniques. Conversely it does not justify the use of endovascular treatment of CSSVI in RR-MS patients outside the boundaries of clinical studies.
Acknowledgments
FS and PZ received technical equipment from Esaote Biomedica, and financial support from Fondazione Hi-larescere. The authors thanks Rodolfo Daini, MD, for the English revision.
References
- 1.Sadovnick AD, Ebers GC. Epidemiology of multiple sclerosis: a critical overview. Can J Neurol Sci. 1993;20:17–29. doi: 10.1017/s0317167100047351. [DOI] [PubMed] [Google Scholar]
- 2.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 3.Haacke EM. Chronic cerebral spinal venous insufficiency in multiple sclerosis. Expert Rev Neurother. 2011;11:5–9. doi: 10.1586/ern.10.174. [DOI] [PubMed] [Google Scholar]
- 4.Filippi M, Rocca MA, Barkhof F, et al. Multiple sclerosis and chronic cerebrospinal venous insufficiency: the neuroimaging perspective. AJNR Am J Neuroradiol. 2011;32:424–427. doi: 10.3174/ajnr.A2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:392–399. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simka M, Kostecki J, Zaniewski M, Majewski E, Hartel M. Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis. Int Angiol. 2010;29:109–114. [PubMed] [Google Scholar]
- 7.Al-Omari MH, Rousan LA. Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis. Int Angiol. 2010;29:115–120. [PubMed] [Google Scholar]
- 8.Zivadinov R, Marr K, Cutter G, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology. 2011;12:138–144. doi: 10.1212/WNL.0b013e318212a901. [DOI] [PubMed] [Google Scholar]
- 9.Doepp F, Paul F, Valdueza JM, Schmierer K, Schreiber SJ. No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol. 2010;68:173–183. doi: 10.1002/ana.22085. [DOI] [PubMed] [Google Scholar]
- 10.Sundstrom P, Wåhlin A, Ambarki K, Birgander R, Eklund A, Malm J. Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study. Ann Neurol. 2010;68:255–259. doi: 10.1002/ana.22132. [DOI] [PubMed] [Google Scholar]
- 11.Wattjes MP, van Oosten BW, de Graaf WL, et al. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry. 2011;82:429–435. doi: 10.1136/jnnp.2010.223479. [DOI] [PubMed] [Google Scholar]
- 12.Mayer CA, Pfeilschifter W, Lorenz MW, et al. The perfect crime? CSSVI not leaving a trace in MS. J Neurol Neurosurg Psychiatry. 2011;82:436–440. doi: 10.1136/jnnp.2010.231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan O, Filippi M, Freedman FS, et al. Chronic cerebrospinal venous insufficiency and multiple sclerosis. Ann Neurol. 2010;67:286–290. doi: 10.1002/ana.22001. [DOI] [PubMed] [Google Scholar]
- 14.Van Rensburg SJ, van Toorn R. The controversy of CSSVI and iron in multiple sclerosis: is ferritin the key? Neurology. 2010;75:1581–1582. doi: 10.1212/WNL.0b013e3181fb44f0. [DOI] [PubMed] [Google Scholar]
- 15.Khan O, Tselis A. Chronic cerebrospinal venous insufficiency and multiple sclerosis: science or science fiction? J Neurol Neurosurg Psychiatry. 2011;82:355. doi: 10.1136/jnnp.2010.228098. [DOI] [PubMed] [Google Scholar]
- 16.Zamboni P, Galeotti R, Menegatti E, et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg. 2009;50:1348–1358. doi: 10.1016/j.jvs.2009.07.096. Erratum in: J Vasc Surg 2010;51:1079. [DOI] [PubMed] [Google Scholar]
- 17.Zamboni P, Galeotti R, Weinstock-Guttman B, Kennedy C, Salvi F, Zivadinov R. Venous angioplasty in patients with multiple sclerosis. Results of a pilot study. Eur J Vasc Endovasc Surg. 2012;43:116–122. doi: 10.1016/j.ejvs.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revision to the “Mc Donald criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 19.Zamboni P, Menegatti E, Galeotti R, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci. 2009;282:21–27. doi: 10.1016/j.jns.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Plosker GL. Interferon-‚-1b: a review of its use in multiple sclerosis. CNS Drugs. 2011;25:67–88. doi: 10.2165/11206430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 22.Louis TA, Lavori PW, Bailar JC, III, Polansky M. Crossover and self-controlled designs in clinical research. In: Bailar JC III, Mosteller F, editors. Medical Uses of Statistics. Waltham MA, USA: NEJM Books; 1986. pp. 67–90. [DOI] [PubMed] [Google Scholar]
- 23.Inusah S, Sormani MP, Cofield SS, et al. Assessing changes in relapse rates in multiple sclerosis. Mult Scler. 2010;16:1414–1421. doi: 10.1177/1352458510379246. [DOI] [PubMed] [Google Scholar]
- 24.Amato MP, Grimaud J, Achiti I, et al. European validation of a standardized clinical description of multiple sclerosis. J Neurol. 2004;251:1472–1480. doi: 10.1007/s00415-004-0567-0. [DOI] [PubMed] [Google Scholar]
- 25.Young PJ, Lederer C, Eder K, et al. Relapses and subsequent worsening of disability in relapsing-remitting multiple sclerosis. Neurology. 2006;67:804–808. doi: 10.1212/01.wnl.0000234064.17156.03. [DOI] [PubMed] [Google Scholar]
- 26.Zamboni P, Menegatti E, Weinstock-Guttman B, et al. Hypoperfusion of brain parenchyma is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a cross sectional preliminary report. BMC Med. 2011;9:22. doi: 10.1186/1741-7015-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J. Vascular aspects of multiple sclerosis. Lancet Neurol. 2011;10:657–666. doi: 10.1016/S1474-4422(11)70105-3. [DOI] [PubMed] [Google Scholar]
- 28.Simka M. Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. Curr Neurovasc Res. 2009;6:132–139. doi: 10.2174/156720209788185605. [DOI] [PubMed] [Google Scholar]
- 29.Siskin GP, Haskal ZJ, McLennan G, et al. Development of a research agenda for evaluation of interventional therapies for chronic cerebrospinal venous insufficiency: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2011;22:587–593. doi: 10.1016/j.jvir.2011.03.007. [DOI] [PubMed] [Google Scholar]