Summary
The aim of this study was to investigate clinical predictors of, and rates of conversion to, dementia syndrome in a case series of patients with amnestic mild cognitive impairment (aMCI). Two hundred and eight aMCI subjects were followed over a six-year period. A lower Mini Mental State Examination score was a significant predictor of dementia, and mild cognitive impairment patients with behavioral and psychiatric symptoms showed a faster conversion rate.
Keywords: dementia, memory, mild cognitive impairment
Introduction
The main classification systems describing predementia cognitive decline generally incorporate subjective, objective and functional dimensions in varying ways and to different degrees. Of these systems, the classification criteria for mild cognitive impairment (MCI) have received the most attention (1).
The term MCI describes cognitive impairment in elderly persons that is not sufficiently severe to qualify for a diagnosis of dementia. MCI can present with a variety of symptoms; it is termed amnestic MCI (aMCI) when memory loss is the predominant symptom, and it is frequently a prodromal state of Alzheimer’s disease (AD) (2).
Patients with MCI who may develop AD have been studied using neuropsychological tests, neuroimaging (both structural and functional), cerebrospinal fluid analysis, and other biomarkers, both in isolation and in combination (3–5).
Efforts to identify clinical predictors of progression to AD in patients with MCI have generally focused on demographic factors, neurocognitive performance and biological factors (such as apolipoprotein epsilon 4 allele). Fleisher et al. studied all these variables and concluded that progression from MCI to AD was best determined using ApoE status and four common, easily administered cognitive measures: the Symbol Digit Modalities Test, the Delayed 10-Word List Recall, the New York University Paragraph Recall Test (Delayed) and the ADAS-cog total score (6).
Neuropsychiatric disturbances are common among MCI patients (7). Recent studies have investigated and underlined their relationship with cognitive status, and their role as possible predictors of evolution to dementia (8,9). In this report, the contributions of clinical, cognitive and neuropsychiatric variables were analyzed in a consecutive series of aMCI patients, to detect the contribution of different risk categories to the development of dementia and to investigate the rate of conversion in the dementia subgroup.
Materials and Methods
In this retrospective investigation we considered a case series of subjects who fulfilled diagnostic criteria for aMCI (2) and were followed for six years. The participants were recruited among outpatients attending the Laboratory of Neuropsychology and the Alzheimer’s Assessment Unit (UVA) at the Circolo Hospital and Macchi Foundation in Varese and at the IRCCS C. Mondino National Neurological Institute Foundation in Pavia, Italy.
Of a total of 235 consecutive patients, observed between 2000 and 2010, 27 subjects were lost to follow up (nine died and 18 dropped out because they changed medical practitioners or refused to be included in the study).
After their first examination, the aMCI subjects were evaluated every 12 months to identify patients who had converted to dementia. We considered the baseline picture in the search for an association between clinical variables and conversion to dementia, as defined by the current clinical criteria (10–14).
At the baseline examination, all patients underwent a standard neurological examination, laboratory tests (routine blood and thyroid work up, B12 and folate levels, Venereal Disease Research Laboratory test), and neuroimaging investigation (brain computed tomography, CT). The use of psychiatric drugs that may affect cognition or motor functions (such as antipsychotics or anticholinergics) was excluded. The presence/absence of temporal atrophy was determined by a categorical variable (radial width of the temporal horn) using CT-based cut-off values (15). A validated CT-based weighted rating scale sensitive enough to detect different degrees of subcortical ischemic vascular disease (SIVD) was also applied. This rating scale is able to capture different degrees of SIVD associated with mild cognitive deterioration (16).
Mild parkinsonian signs were evaluated looking for the presence of changes in axial function, rigidity and tremor (17).
Affective symptoms were assessed using the Geriatric Depression Scale (GDS) (18). GDS scores were used to exclude patients with severe depression (score >17) and as a between-groups control variable (score 0–10 = absent, score 11–16 = mild-moderate depression). The Neuropsychiatric Inventory (NPI) (19) and its subscales were used for the evaluation of behavioral and psychological symptoms of dementia (BPSDs). Domain scores of 4 or more are indicative of clinical significance and are used as entry criteria for treatment trials of dementia-associated neuropsychiatric symptoms (20).
All the patients underwent a standardized baseline neuropsychological battery, designed to assess the cognitive functions that are generally involved in dementia and to select cases with aMCI. The tests administered included: the Mini Mental State Examination (MMSE), the Trail Making Test, parts A and B (TMT), and the Mental Deterioration Battery (MDB) (21–23). The MDB is comprised of eight tests: four evaluate the processing of verbal material and four the processing of visuospatial material. The verbal tests are: immediate (IR) and delayed recall (DR) of Rey’s 15 words, word fluency (FAS) and phrase construction. The visuospatial tests are: Raven’s 47 progressive colored matrices, immediate visual memory (IVM) and copying of drawings, freehand (CD FH) and with landmarks (CD WL). The neuropsychological evaluation was repeated every 12 months to detect patients who had converted to dementia (impairment of general cognitive and functional abilities).
Statistical analysis
Standardized and validated Italian language versions of all the above tests are available. The patients’ cognitive performance was evaluated against normative data for the Italian population. Raw scores from each test were corrected for age, sex, and educational level and transformed into corrected scores, in accordance with Italian standardization studies.
Descriptive statistics, comparison of means (ANOVA) and a non-parametric test (chi-square test) were used to analyze differences between the groups and to identify subjects who had or had not converted to dementia. Survival analyses (Kaplan-Meier and Cox Regression) were used to detect the effects of clinical variables on conversion to dementia. The predictive variables for aMCI subjects developing cognitive decline within and after two years were also analyzed using a logistic regression model (SPSS version 16.0, SPSS Inc., Chicago, IL, USA). The critical value for statistical significance was set at 0.05.
Results
Two hundred and eight cases were followed up for six years. Their mean age was 73.6±6.9 years (range 52–84); 84 (40.4%) were males and 124 (59.6%) were females. Their educational level was 7.5±3.6 years (range 2–18). Sixty-one subjects (29.3%) had a known family history of dementia.
In the follow up we considered the first point (examination) at which the patient was found to have progressed to a diagnosis of dementia. After six years, 143/208 (68.8%) of the aMCI patients had converted to a definite diagnosis of dementia. The mean time for conversion was 18.2 months (range 6–72 months). Over the course of the six-year study, the overall rate of progression from aMCI to AD was 11.5% per year. However, 80.7% of the cases converted within three years, albeit with a decreasing trend from the first year to the third year of observation (Fig.1). As expected, a diagnosis of AD was established in 93.0% of the converted cases, whereas non-Alzheimer dementias (vascular/degenerative) were observed in the remaining 7.0%. During the follow-up period, the condition of 37 subjects (17.8%) remained unchanged, whereas 28 cases (13.4%) reverted to normality. Like the conversion to dementia, the return to normality was especially observable in the first three years of observation.
Figure 1.
Amnestic mild cognitive impairment: annual rate of conversion to dementia (143/208 cases)
A lower baseline MMSE score (25 to 27) was a significant predictor of conversion to overt dementia. Other factors distinguishing the converted patients (at the first examination in which they were positive for dementia) from the non-converted group were folate/B12 deficiency, temporal atrophy and the presence of mild to moderate subcortical ischemic vascular lesions (CT-based weighted rating scale). Atrial fibrillation (AF) was more common among the non-converted subjects. Age, level of education and sex were not significantly related to the progression from MCI to AD (Table 1).
Table 1.
Clinical and neuropsychological characteristics of the groups of aMCI patients who converted and did not convert to dementia
| Variables | Converted n 143 (68.8%) | Not converted n 65 (31.2%) | Significance (p) |
|---|---|---|---|
| Age (M±SD) | 73.9±7.9 | 72.7±8.1 | n.s. |
| Sex (F/M) | 89/54 | 35/30 | n.s. |
| Educational level, yrs (M±SD) | 7.4±3.7 | 7.7±3.5 | n.s. |
| Family history of dementia | 38 (26.6%) | 23 (35.4%) | n.s. |
| MMSE score (M±SD) | 25.4±1.5 | 26.1±1.7 | 0.005 |
| BPSDs (NPI score ≥4) | 72 (50.3%) | 28 (43.1%) | n.s. |
| NPI total score (M±SD) | 21.0±8.3 | 19.1±7.5 | n.s. |
| Depression (GDS score 11–16) | 57 (39.9%) | 30 (46.2%) | n.s. |
| GDS total score (M±SD) | 9.7±5.4 | 10.6±7.0 | n.s. |
| CT – SIVD | 68 (47.6%) | 22 (33.8%) | 0.04 |
| Temporal atrophy | 69 (48.3%) | 16 (24.6%) | 0.01 |
| Diabetes | 17 (11.9%) | 12 (18.5%) | n.s. |
| Hypertension | 81 (56.6%) | 39 (60%) | n.s. |
| Myocardial infarction | 16 (11.2%) | 11 (16.9%) | n.s. |
| Atrial fibrillation | 7 (4.9%) | 9 (13.8%) | 0.03 |
| Hypercholesterolemia | 55 (38.5%) | 21 (32.3%) | n.s. |
| Hypothyroidism | 17 (11.9%) | 12 (18.5%) | n.s. |
| B12/folate deficiency | 13 (9.1%) | 1 (1.5%) | 0.03 |
| RBD | 7 (4.9%) | 3 (4.6%) | n.s. |
| Mild parkinsonian signs | 7 (4.9%) | 0 | n.s. |
Abbreviations: BPSDs=behavioral and psychological symptoms of dementia; NPI=Neuropsychiatric Inventory; GDS=Geriatric Depression Scale; CT – SIVD=subcortical ischemic vascular disease, identified on CT scan; RBD=REM sleep behavior disorder
The results of the neuropsychological tests (TMT and MDB) are detailed in Table 2. In accordance with the selection criteria, the population showed a selective deficit of episodic memory. No significant differences were detected, at the baseline evaluation, between the converted and non-converted groups.
Table 2.
Results of baseline neuropsychological tests of the groups of aMCI patients who converted and did not convert to dementia
| Neuropsychological | Converted n 143 (68.8%) M±SD | Not converted n 65 (31.2%) M±SD | Significance (p) |
|---|---|---|---|
| TMT B-A | 111±27 | 101±25 | n.s. |
| Rey - Immediate recall | 26.5±4.0 | 27.1±4.1 | n.s |
| Rey - Delayed recall | 3.4±2.1 | 3.7±2.3 | n.s. |
| Verbal fluency | 25.4±5.5 | 26.2±4.9 | n.s |
| Phrase construction | 17.9±3.1 | 18.5±3.1 | n.s. |
| Immediate visual memory | 17.9±2.7 | 17.8±2.5 | n.s |
| Raven’s matrices 47 | 25.8±3.7 | 25.1±3.8 | n.s |
| Copying drawings FH | 9.3±1.2 | 9.4±1.0 | n.s |
| Copying drawings WL | 67.2±1.5 | 67.5±1.4 | n.s. |
Abbreviations: TMT B-A=Trail Making Test, difference part B – part A; FH=freehand; WL=with landmarks
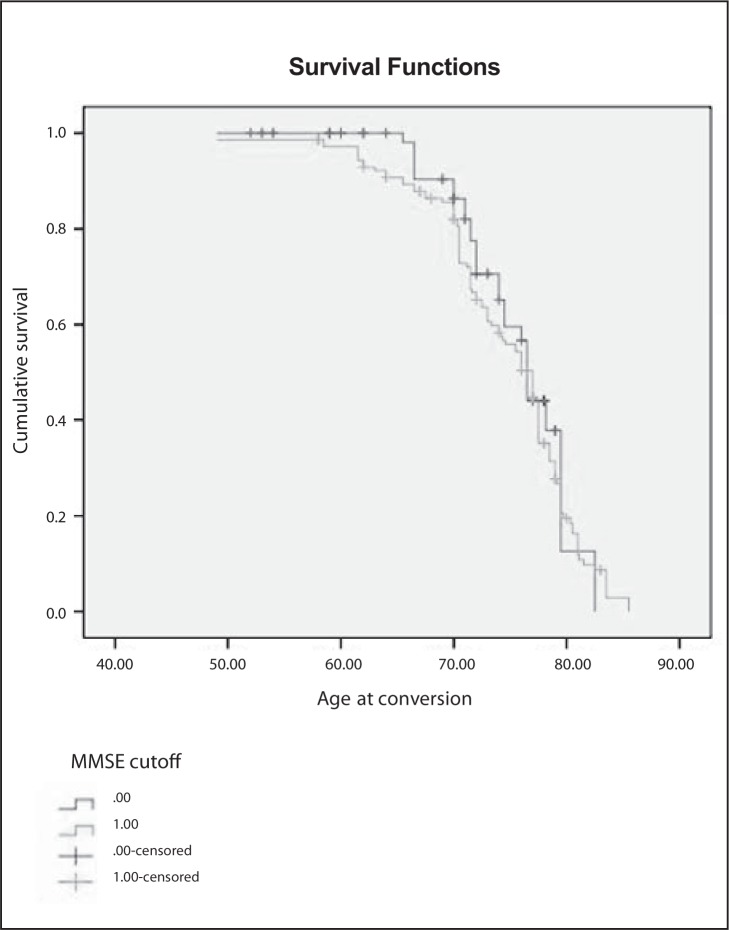
The effect of MMSE score on conversion is reported as Kaplan-Meier survival estimates in figure 2, over. A Cox regression analysis was performed for all the variables reported in Table 1. Significant factors were MMSE score (p<0.02), B12/folate deficiency, temporal atrophy and the presence of SIVD (each p<0.05).
Figure 2.
Effect of MMSE score on conversion to dementia (0:score 28–30, 1:score 25–27)
The predictive variables for aMCI subjects developing cognitive decline within versus after two years were also analyzed using a logistic regression model. All the variables reported in Table 1 were included. The logistic regression analysis showed that aMCI patients with BPSDs (NPI score ≥4) had a faster conversion rate to dementia (p<0.05).
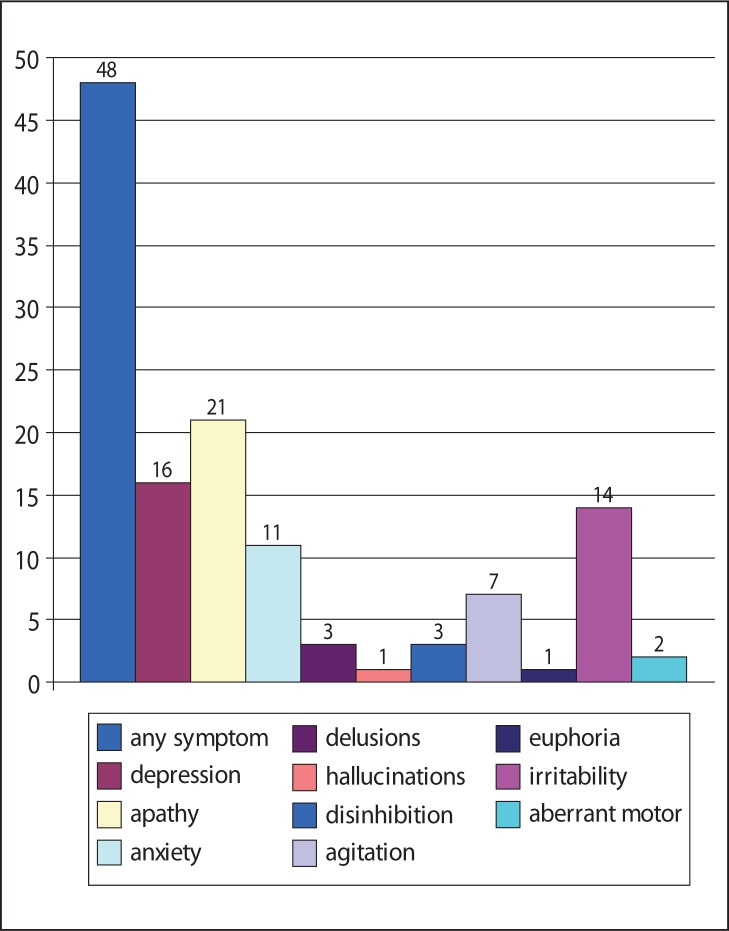
At the baseline evaluation, BPSDs were found, overall, in 100 cases (48%). The frequency of each NPI symptom is shown in figure 3 (over). A subsequent logistic regression analysis of single NPI items revealed statistical significance (p<0.05) only for apathy.
Figure 3.
Overall percentages of different neuropsychiatric symptoms (NPI score ≥4) in amnesic MCI patients
Discussion
Mild cognitive impairment is a heterogeneous condition that frequently converts to AD, but not all patients progress to dementia. The prospect, over the next 15 years, of new disease-modifying therapies for AD has reinforced the need to detect the disease in its earliest stages.
In our aMCI population the main factor associated with conversion to dementia, albeit with a very rapid descending trend from the first to the third year of observation, was the presence of lower cognitive scores on the MMSE. The annual rate of conversion, calculated over a six-year period, was 11.5%, which is in line with values reported previously in the literature. A lower MMSE score could indicate the presence of subtle deficits across many cognitive domains.
On the other hand, a group of 28 cases (13.4% of the entire population) reverted to normality. This percentage is similar to the rate observed in the Cache County Memory Study for cases with clinical dementia rating equal to 0.5. However, several differences should be taken into account, including the different neuropsychological tests administered and the relative rarity of cases with the amnestic form of MCI in the Cache Study (24).
Another factor found to be associated with conversion was the presence of B12/folate deficiency. Impaired vitamin B12 metabolism may be one of many factors contributing to the development of cognitive impairment and dementia and may modulate the course of the disease. The mechanisms by which this occurs are not completely understood and may include elevated concentrations of homocysteine, or a role of vitamins in maintenance of the integrity of the blood-brain barrier and reduced methylation capacity (25).
In the presence of a cognitive decline a neuroimaging examination is generally requested in order to exclude causes of reversible or vascular dementia (26). A further aim of our study was to highlight the importance of CT in detecting the signs of degenerative (temporal atrophy) or vascular (SIVD) abnormalities. Our analysis (Cox regression) confirmed, with slight significance (p<0.05), the capacity of CT to identify non-converting patients.
Vascular risk factors, such as diabetes mellitus and hypertension, are associated with both the occurrence of and the progression to AD. A recent study showed that vascular risk factors (including hypertension, diabetes mellitus, hypercholesterolemia and cerebrovascular disease) promote conversion from MCI to AD (27). In our patients vascular risk factors did not differ between the two groups, with the exception of mild to moderate SIVD, which was significantly increased in the converted group. Our findings, on the effect of AF, run counter to evidence suggesting a predictive role for AF (28). In our opinion, this discrepancy could be related to differences in the selection of the patients enrolled, with other authors including not only pure aMCI patients, but also those with multi-domain and non-amnestic MCI.
Neuropsychiatric disturbances are seen in 35–75% of MCI patients (29). Recent studies have investigated and highlighted their relationship with cognitive status and their role as possible predictors of the evolution to dementia (6,7). In our study, the presence of BPSDs was an important factor, among aMCI patients, for a faster rate of conversion. In particular, apathy was the NPI item most frequently observed in these patients.
Apathy has an important impact on MCI and should be considered a mixed cognitive/psychiatric disturbance related to ongoing AD neurodegeneration, being associated with an increased neurofibrillary tangle burden in the anterior cingulate cortex (7).
Many biomarkers, related to neurodegenerative processes, have recently been proposed for detecting AD in its early stages. The possible application of these biomarkers in clinical practice raises a series of questions regarding differences in their sensitivity/specificity and in the access that Alzheimer’s disease assessment units have to advanced technology. According to the new definition proposed by Dubois et al. (26), if MCI is associated with positive biomarkers or imaging (i.e. suggestive of pathological changes present in AD), then the MCI diagnosis is encompassed in the AD one.
There is still a need to further validate the currently existing biomarkers in large unselected samples and to avoid the pitfalls of workup bias and circular diagnostic processes (30). For these reasons, a better definition of clinical predictors of dementia would be useful for the identification of prodromal dementia in naturalistic and unselected AD populations. Considerable work is, in fact, needed to validate biomarker-based criteria and to standardize biomarker analysis for use in community settings. The core criteria for a diagnosis of MCI are clinical. Recent recommendations from the National Institute on Aging stress that there are no clear demarcations between normal aging and MCI or between MCI and dementia, and that clinical judgment must be used to make these distinctions (31).
References
- 1.Chong MS, Sahadevan S. Preclinical Alzheimer’s disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 3.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo B, Siepi D, Sabalich I, Tranfaglia C, Parnetti L. Cerebrospinal fluid neuron-specific enolase: a further marker of Alzheimer’s disease? Funct Neurol. 2008;23:93–96. [PubMed] [Google Scholar]
- 5.Mauri M, Sinforiani E, Bono G, et al. Interaction between Apolipoprotein epsilon 4 and traumatic brain injury in patients with Alzheimer’s disease and mild cognitive impairment. Funct Neurol. 2006;21:223–228. [PubMed] [Google Scholar]
- 6.Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 7.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D, Coen R, Kilroy D, et al. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int J Geriatric Psychiatry. 2011;26:166–172. doi: 10.1002/gps.2509. [DOI] [PubMed] [Google Scholar]
- 9.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. Journal of Alzheimers Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J. Neural Transm Suppl. 2000;59:23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- 14.McKeith I, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 15.Rossi ER, Joachim C, Smith AD, Frisoni GB. The CT-based radial width of the temporal horn: pathological validation in AD without cerebrovascular disease. Int J Geriat Psychiatry. 2004;19:570–574. doi: 10.1002/gps.1132. [DOI] [PubMed] [Google Scholar]
- 16.Geroldi C, Galluzzi S, Testa C, Zanetti O, Frisoni GB. Validation study of a CT-based weighted rating scale for subcortical ischemic vascular disease in patients with mild cognitive deterioration. Eur Neurol. 2003;49:193–209. doi: 10.1159/000070183. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol. 2004;61:1273–1276. doi: 10.1001/archneur.61.8.1273. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. Am J Geriatr Psychiatry. 2001;9:346–360. [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 23.Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 24.Mayeux R, Reitz C, Brickman AM, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment – Part 1. Alzheimers Dement. 2011;7:15–34. doi: 10.1016/j.jalz.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eussen SJ, de Groot LC, Joosten LW, et al. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr. 2006;84:361–370. doi: 10.1093/ajcn/84.1.361. [DOI] [PubMed] [Google Scholar]
- 26.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 28.Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment. Arch Gerontol Geriat. 2007;44(Suppl 1):155–165. doi: 10.1016/j.archger.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 30.Oksengard AR, Cavallin L, Axelsson R, et al. Lack of accuracy for the proposed ‘Dubois criteria’ in Alzheimer’s disease: a validation study from the Swedish brain power initiative. Dement Geriatr Cogn Disord. 2010;30:374–380. doi: 10.1159/000321121. [DOI] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]