Summary
More and more neuroimaging studies are using in vivo proton magnetic resonance spectroscopy (1H-MRS) to explore correlates of response to therapy in major depressive disorder (MDD). Their aim is to further understanding of the effects of neurotransmitter changes in areas involved in MDD and the mechanisms underlying a good treatment response.
We set out to summarise the literature from the past fifteen years on biochemical correlates of treatment response in MDD patients, reflected in pre- and post-therapy changes in 1H-MRS measurements.
Our literature search identified fifteen articles reporting 1H-MRS studies in MDD treatment; no study used 1P-MRS.
Despite the wide diversity of 1H-MRS methods applied, brain regions studied, and metabolite changes found, there emerged strong evidence of a correlation between changes in neurometabolite concentrations, in particular glutamate, N-acetylaspartate and choline, and a good treatment response to pharmacotherapy or antidepressant stimulation techniques.
Keywords: ECT, major depression disorder, MRI, pharmacological therapy, psychiatric disorders, spectroscopy, 1H-MRS
Introduction
Major depressive disorder (MDD), also known as unipolar depression, is one of the most frequently diagnosed psychiatric disorders. It is a major illness in primary care settings because of its devastating impact on social and work life. Currently, MDD is considered to be the consequence of a malfunction of multiple circuits that connect the limbic system with the prefrontal cortex, the brainstem, and the hypothalamus. Treatment may be behavioural, pharmacological, or, for patients whose depression is refractory to antidepressant drugs or who are severely depressed, involve antidepressant stimulation techniques such as electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS). Neurological mechanisms underlying MDD response are still not clear and objective outcomes that can be used to predict responses to therapy are lacking.
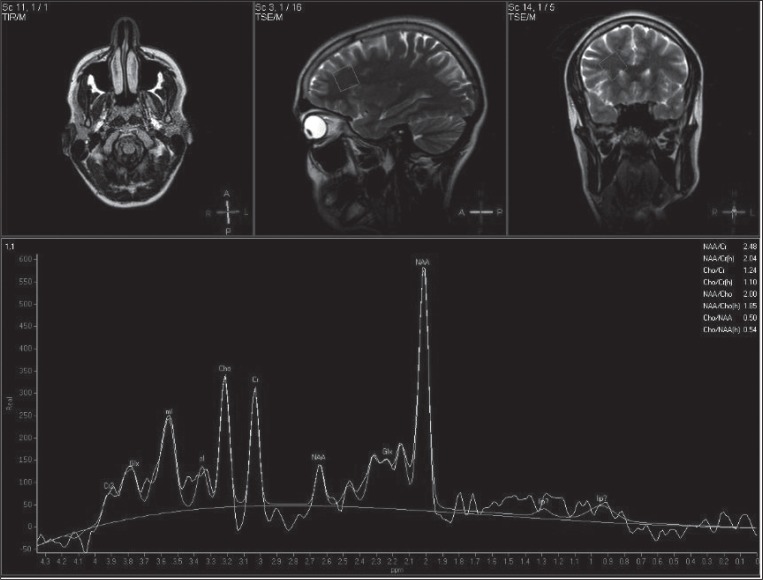
Advanced neuroimaging research has recently offered clues about the mechanisms that possibly underlie the response to therapy in many diseases. In particular, proton magnetic resonance spectroscopy (1H-MRS) measures in vivo the levels of brain metabolites. Since 1H-MRS makes it possible to track disease progression and response to treatment, it has important applications in the daily clinical management of neurodegenerative disease, stroke and tumours (1–3). Recently a growing number of studies have focused on possible applications of 1H-MRS in psychiatric disorders, including unipolar MDD. The metabolites usually assessed using 1H-MRS are: N-acetylaspartate (NAA, 2.05 ppm; a putative marker of neuronal functionality), total creatine (tCr) and creatine phosphate 3.04 and 3.9 ppm; involved in energy metabolism), choline-containing compounds (Cho, 3.22 ppm; involved in membrane synthesis and degradation), glutamate (Glu; excitatory neurotransmitter), myoinositol (3.56 ppm; astrocyte marker) and lactate (doublet at 1.33 ppm; final product of anaerobic glycolysis) (see Fig. 1, over). We set out to review studies that evaluated unipolar MDD treatment efficacy through spectroscopic neuroimaging in order to look for objective outcomes that may predict therapeutic response, and assess the possibility of defining a standard spectroscopy protocol.
Figure 1.
Original and fitted 1H-MRS spectra (TE= 25 ms) originating from frontal deep white matter in a 35-year-old healthy male subject. NAA=N-acetylaspartate, Glx=glutamate, glutamine, Cr=creatine, Cho=choline-containing compounds and mI=myo-Inositol. Voxel size was 2×2×2 cm3.
Materials and methods
In July 2011, we searched the PubMed database for clinical articles dealing with neuroimaging of treated MDD published since 1994. In order to limit the results to relevant articles, the strategy combined, without language restriction, the MeSH term 31P and 1H Magnetic Resonance Spectroscopy AND [the major MeSH term major depressive disorders / drug therapy (SH) OR the major MeSH term major depressive disorders / therapy (SH)*]. All the manuscripts selected were then hand-searched for further relevant publications. In order to qualify for inclusion in this review, studies had to have: i) been an original paper in a peer-reviewed journal; ii) included a group of subjects with unipolar MDD; iii) studied subjects using 1H-MRS; iv) studied subjects before and after an antidepressant treatment protocol; and v) provided explicit subject inclusion criteria.
Results
The search identified fifteen articles (4–18) in which 1H-MRS was used to assess MDD treatment while no paper regarding 31P-MRS was found. The papers found are summarised in Tables 1–3 which focus respectively on the technical aspects of the 1H-MRS applied and on the clinical-metabolic results obtained. Two papers (6,10) focusing on complications in MDD therapy were also previously discussed in a paper already published (19). All the papers selected report longitudinal studies evaluating different typologies of MDD treatment by single voxel 1H-MRS: three using behavioural approaches (1 cognitive behavioural therapy, CBT and 2 sleep deprivation, SD), five using pharmacological and seven antidepressant stimulation techniques, six using ECT and one using rTMS. Most of the studies (10) were performed on a 1.5 T scanner, while two more recent ones (15,17) were performed on a 3T scanner. The studies by Sanacora’s group (3) focused on γ-aminobutyric acid (GABA), using a J-edited sequence on a 2.1T MRI scanner. All the other 12 papers reviewed report data on NAA, Cho and Cr peaks. Moreover, six articles studied glutamine (Gln) and/or Glu (9,11,12,15–17). Different brain regions were evaluated: cingulate cortex, prefrontal cortex, parieto-occipital cortex, hippocampus and amygdalar region, medial frontal cortex, and basal ganglia.
Table 1.
Spectroscopy data
| Authors | Scanner | Type | H-MRS | TE (ms) | Voxel volume (cm3) | Markers Type | Region of interest | |
|---|---|---|---|---|---|---|---|---|
| Sequence | TR (ms) | |||||||
| Bernier D 200918 | 1.5T GE scanner | Single voxel | ___ | 2000 | 30 | 1.6×1.6×1.6 (DPFA) 1.3×1.3×1.3 (PONS) |
Cho, Cr, NAA | Left DPFA and pons |
| Block W 200917 | 3T Gyroscan Achieva Philips | Single voxel | PRESS | 2000 | 140 and 30 | 2.8×1.7×1.3 | NAA, Cr, Cho, ml, Gln, Glx and Gln/Glx and Gln/Cr | Hippocampus body,parts of its head, of the amygdala and of parahippocampal gyrus |
| Murck H 200916 | 1.5T Signa Horizon | Single voxel | PRESS | 2000 and 4000 | 35 | 0.58×0.58×0.58 (DLPFC) and 1.75×1.75×1.75 (POC) | Cho, Cr, NAA, Glx,Gln | DLPFC and POC |
| Luborzewski A 200715 | 3T Medspec Bruker Biospin | Single voxel | PRESS | 3000 | 80 | 2×2×2 (DLPFC); 2.5×4×2 (ACC) | Cho, Cr, NAA, Glu, Gln | DLPFC and ACC |
| Gonul AS 200614 | 1.5T Magnetom Vision, Siemens | Single voxel | PRESS | 1500 | 135 | 2×2×2 | NAA, Cr, Cho, ml and NAA/Cr, Cho/Cr | Medial frontal cortex |
| Sanacora G 200613 | 2.1T Oxford Magnet, Bruker Avance | Single voxel (2 subspectra of 128 scans) | J-editing pulse sequence | 3390 | 80 | 3.9×1.5×3.5 | GABA | Midline of occipital cortex |
| Michael N 200312 | 1.5T Magnetom Sp, Siemens | Single voxel (128 scans) | STEAM | 2500 | 20 | 3.375 | Cho, Cr, NAA, Glx | Left amygdalar region |
| Michael N 200311 | 1.5T Magnetom Sp, Siemens | Single voxel (128 scans) | STEAM | 2500 | 20 | 3.375 | Cho, Cr, NAA, Glx | DLPFC |
| Obergriesser T 200310 | 1.5T Siemens | Single-voxel | PRESS | 1800 | 135 | ___ | Cho, Cr, NAA | Left and right hippocampus |
| Pfleiderer B 20039 | 1.5T Magnetom Sp, Siemens | Single voxel | STEAM | 2500 | 20 | 3.375 | Glx, Cho, Cr, NAA | Left anterior (pregenual) cingulum |
| Sanacora G 20038 | 2.1T Oxford Magnet, Bruker Avance | Single voxel (2 subspectra of 128 scans) | J-editing pulse sequence | 3390 | 68 | 1.5×3×3 | GABA | Midline of occipital cortex |
| Sanacora G 20027 | 2.1T Oxford Magnet, Bruker Avance | Single voxel (2 subspectra of 128 scans) | J-editing pulse sequence | 3390 | 68 | 1.5×3×3 | GABA | Midline of occipital cortex |
| Ende G 20006 | 1.5T Siemens Vision | Single-voxel | PRESS | 1800 | 135 | ___ | Cho, Cr, NAA | Left and right hippocampus |
| Sonawalla SB 19995 | 1.5T Signa General Electric Medical System | Single voxel | STEAM | 2000 | 30 | 8 | Cho/Cr and NAA/Cr | Head of left caudate and putamen |
| Charles HC 19944 | 1.5T GE Signa | Single voxel | STEAM | 2000 | ___ | 3 | NAA/Cr, NAA/Cho, Cho/Cr | Basal ganglia and thalamus |
Abbreviations: NAA=N-acetylaspartate; Cr=creatine; Cho=choline-containing compounds; m=myoinositol; Glu=glutamate; Glx=glutamate and glutamine; Gln=Glutamine; GABA=γ-aminobutyric acid; ACC=anterior cingulate cortex; DLPFC=left dorsolateral prefrontal cortex; DPFA=dorsal prefrontal area; POC=parieto-occipital cortex; MDD=major depressive disorder.
Table 3.
Unipolar MDD patients
| Authors | Subjects | Males:Females | Age (years) | Diagnostic criteria |
|---|---|---|---|---|
| Bernier D 200918 | 12 | 0:12 | 21–30, mean 25 | DSM-IV, HAMD, HDI and POMS |
| Block W 200917 | 11 | 7:4 | mean 36 (SD=10) | DSM-IV, BDI |
| Murck H 200916 | 13 | 8:5 | 31–66; mean 45.5 (SD=11.8) | HAMD |
| Luborzewski A 200715 | 17 | 15:2 | 28–61, mean 45 (SD=11) | HAMD, MADRS, BDI and CORE |
| Gonul AS 200614 | 20 | 3:17 | mean 32.1 (SD=9.2) | DSM-IV and HAMD |
| Sanacora G 200613 | 8 | – | – | DSM-IV and HAMD |
| Michael N 200312 | 13 treatment-resistant | 4:9 | mean 59.7 (SD=15.2) | DSM-IV |
| Michael N 200311 | 12 treatment-resistant | 4:8 | mean 63.4 (SD=10.6) | DSM-IV |
| Obergriesser T 200310 | 12 | 4:8 | mean 63.81 (SD=14.34) | DSM-IV and HAMD |
| Pfleiderer B 20039 | 17 | 5:12 | mean 61.0 (SD=11.2) | DSM-IV |
| Sanacora G 20038 | 8 | 5:3 | mean 46.0 (SD=5.3) | DSM-IV and HAMD |
| Sanacora G 20027 | 11 | 7:4 | mean 39.2 (SD=8.5) | DSM-IV and HAMD |
| Ende G 20006 | 17 | 7:10 | mean 61.28 (SD=13.42) | DSM-IV and HAMD |
| Sonawalla SB 19995 | Drug response group (n=8) and placebo pattern response/non response group (n=7) | 23:18 | 19–56 (mean 38.9) | DSM-III R and HAMD |
| Charles HC 19944 | 7 | – | 63–76 (mean 71.14) | DSM-III R |
Abbreviations: MDD=major depressive disorder; SD=standard deviation; DSM-IV=Diagnostic and Statistical Manual of Mental Disorder, fourth edition; HAMD=Hamilton Depression Rating Scale; HDI=Hamilton Depression Inventory; MADRS=Montgomery Asberg Depression Rating Scale; BDI=Beck Depression Inventory; CORE=CORE measurement of psychomotor activity; POMS=Profile of Mood States
These articles differed widely in the 1H-MRS methods applied and the metabolite changes found. Regardless of the 1H-MRS method applied, metabolite quantification was reported in two ways: i) relative to a reference peak, either water content or Cr (4,5,17), which is assumed to be stable (with the limit that this assumption is not verifiable and results are not comparable among different scanners); or ii) in an absolute way (6–16,18).
Discussion
Technical aspects
Proton magnetic resonance spectroscopy (1H-MRS) is an advanced MR technique that allows non-invasive in vivo evaluation of metabolite concentrations and characterisation of biochemical changes in the brain, providing data complementary to the structural and functional information furnished by conventional and non-conventional MRI techniques. In 1H-MRS, protons are distinguished by their molecular environment, given that the resonance frequency of hydrogen depends, to a small extent, on the electronic structure of its surroundings (chemical shift).
Quantifying and interpreting metabolic information from brain 1H-MRS is complicated by a number of variables, such as inadequate water suppression and field homogeneity, partial voluming effects, temperature dependence, chemical shift artefacts (CSAs) and irregular volume of interest (VOI) profile, all of which can affect the apparent concentration determination.
Currently, the 1H-MRS methods usually implemented are single-voxel and multi-voxel acquisitions. In single-voxel techniques, the VOI is typically a cuboid defined by the intersection of three mutually orthogonal slabs, either through a 90° pulse followed by two 180° refocusing pulses (PRESS) or through a sequence of three 90° pulses (STEAM), the last having intrinsically 50% less signal than PRESS. The shorter TEs used in STEAM and recently implemented in PRESS present the drawback of residual eddy currents, but they show smaller T2 losses and higher signal-to-noise ratios (SNRs). Multi-voxel 1H-MRS uses a grid made up of multiple single voxels and is thus able to cover a broader region of interest, but this is at the expense of a long acquisition time and a lower SNR. The most common acquisition protocol in this case is chemical shift imaging.
In order to resolve metabolites such as GABA, which are overlapped by the proton resonances of Cr, Glu and NAA, J-edited 1H-MRS has been proposed (7,8,13) as an alternative to conventional 1H-MRS. Glu, often a mixture of Glu, Gln and GABA, designated glutamine/glutamate complex or “Glx”, having a complex spin system, usually gives a poor resolution spectrum in conventional 1H-MRS at 1.5T. If conventional 1H-MRS is applied, an adequate peak database collected on the ad-hoc phantom is required in order to perform a satisfactory fit. If J-edited 1H-MRS is used, quantification is much simpler due to its capability to resolve the metabolites of interest, but an ad-hoc sequence must still be implemented on the scanner, starting from the 3D image-selected in vivo spectroscopy sequence for voxel localisation, and this requires a freedom in sequence handling and implementation that goes beyond that of conventional clinical MRI scanners. Differences in results between studies may be attributable simply to the use of different methodologies that, connected to lower magnetic field strengths, obtain poor resolution of the Glu spectrum. Hence the identification of a dedicated consensus method for quantification of Glu seems crucial.
These technical aspects raise the problem of spectra comparability, which depends on several factors: the scanner, the localisation pulse and the scanning parameters used. In particular, for meaningful data comparison, T1 and T2 corrections are necessary, together with compensation for voxel localisation, partial voluming effects and CSAs, an aspect neglected in some previous papers (4). Having said that, a direct comparison between spectra at the same TE, but with different localisation sequences, is indeed possible in principle, as long as the 50% signal discrepancy between PRESS and STEAM is the only further factor to be taken into account. On the other hand, a direct comparison between different acquisition methods (single- vs multi-voxel) or different methods of quantification (absolute vs relative) is not possible.
It is thus clear that a consensus standard protocol could feasibly be defined, but that such a protocol would have to make provision for a highly complex and non-automatic data analysis. As a general rule, longitudinal relative variations are the results most easily compared, regardless of the scan protocol and quantification method used; furthermore, metabolite ratios give a poorer indication of the real metabolite variation.
The use of a higher magnetic field (3T and more) improves the quality of spectroscopy data, reducing the partial spectral overlap, making it possible to study, for example, the different resonance components of Glx (17).
Study limitations
Articles discussed in this paper differ widely in the 1H-MRS methods applied, the brain regions studied and the metabolite changes found. Moreover, all of them analyse a small number of patients; this is mainly due to difficulties in MDD patient recruitment (restricted inclusion criteria) and in the management of these patients with regard to treatment side effects, non-compliance or refusal to perform a second 1H-MRS scan.
Data reported in the papers reviewed are also obtained using different MRI scan and 1H-MRS acquisition sequences at different magnetic field strengths (most of them at 1.5T), while results concerning Glx, for example, should be validated with higher field strengths to differentiate between different resonance compounds.
Different post-analysis softwares, some manufacturer-supplied, were applied to obtain the quantification of each metabolite peak. This may rapresent a limit in the synthesis of all the results, particularly as regards the absolute quantification of metabolites, while it is a less important factor in the comparison of trends and variation of metabolite concentrations making use of the same methodology between two time points (baseline and post treatment).
Finally, the use of a control group of healthy subjects using antidepressants is not ethically correct, thus the possibility of nonspecific metabolic variation with the use of antidepressants in healthy tissue is still to be demonstrated.
Clinical-metabolic aspects
As shown, available articles on MDD therapy and 1H-MRS are scarce and use different technical approaches. Despite this, there emerges, in MDD, strong evidence of a correlation between changes in neurometabolite concentrations and responses to different treatment approaches.
The first literature available on 1H-MRS in MDD, which dates back to the mid-1990s, focused on study of the basal ganglia, following speculation that an overactive cholinergic system may be involved in the pathophysiology of MDD (20). In particular, three studies (4,5,21) demonstrated the feasibility of applying localised 1H-MRS to patients with MDD and control subjects as a noninvasive means of detecting cytosolic Cho-containing compounds in the brain (22,23), mainly glycerophosphocholine and phosphocholine (24), which are responsible for more than 50% of Cho resonance. Instead, free Cho, acetylcholine, and cytidine diphosphate choline are present at much lower concentrations and make smaller contributions to in vivo 1H-MRS Cho resonance. Charles et al. found that Cho/Cr ratios fell to control levels in MDD patients treated with nafazodone for 2–3 months (4), while Sonawalla et al. found that these ratios showed a 20% increase after 8 weeks of fluoxetine treatment in the true drug response patients versus a 12% decrease in the placebo pattern response/no response group (5). However, these studies were limited by the small numbers of patients investigated; furthermore, the authors simply described the association between response to nafadozone/fluoxetine therapy in MDD and metabolite changes without advancing any hypothesis regarding the underlying mechanism.
Other authors, conducting basal ganglia and cortical area-specific neuroimaging studies, analysed absolute Cho compound changes in different treatments for depression (25,26) and hypothesised that a specific role is played, in MDD, by areas such as the anterior cingulate cortex (ACC) and the left dorsolateral prefrontal cortex (DLPFC). Luborzewki et al. found increased tCho levels in the DLPFC in MDD responders to rTMS, and suggested that they could reflect acute metabolic effects of this treatment (15). The same hypothesis was advanced by Bernier and colleagues (18), who observed in an entire group of MDD women (responders and non responders included) increased tCho levels in the prefrontal cortex after sleep restriction; they also reported a possible correlation between elevated baseline level of Cho measured in the pons and mood improvement after therapy (SD).
Further investigations of the DLPFC and other frontal regions over a longer period of time should provide further insight into the functional relevance of the tCho fraction in the treatment of MDD.
In more recent articles, attention switches to the possible involvement of Glu, the main excitatory transmitter in the human brain, and its related metabolite GABA, in the pathogenesis of MDD. In particular, the breakthrough discovery of an impairment of GABAergic function in MDD patients, as shown by data indicating low GABA concentrations in their plasma and in cerebrospinal fluid (27), prompted interest in investigating the possible effect of MDD treatment on GABA levels. In various studies, Sanacora et al. evaluated GABA concentrations in the occipital cortex, where reduced levels had previously been observed in conditions such as alcohol addiction, frequently associated with high rates of depression (28). They used a localised difference J-editing 1H-MRS protocol (29), not easily replicable, to evaluate and then compare different kinds of MDD treatments, pharmacological (selective serotonin reuptake inhibitors, SSRIs), electroconvulsive (ECT) and cognitive-behavioural (CBT). The significantly increased GABA concentrations observed in the occipital cortex after a course of SSRI treatment or ECT suggested that normalisation of abnormally low cortical GABA concentrations may provide the basis for a common approach to the treatment of MDD (30). Although the mechanism of increased presynaptic GABAergic transmission remains unclear, the observed effects of the SSRI treatments could, in part, be explained by the direct action of serotonin (5-HT) on the GABAergic neurons. Thus, elevation of cortical GABA concentrations might be a common effect of both ECT and SSRIs; alternatively, it may be that reduction of GABA is a state-dependent marker of depression for some individuals that reverses with resolution of the episode. The fact that occipital GABA levels did not rise after CBT (13) seems to point to differences in the mechanisms of action of antidepressant treatments and to suggest that the rise in GABA observed following SSRI treatments and ECT is probably related to specific effects of these treatment modalities on the GABAergic system (7,8), and not simply a state-dependent marker of remission from depression. This hypothesis is further supported by findings of increased cortical GABA after acute administration of an SSRI to healthy subjects (31), increased activity of Glu synthetase after SD (32), reduced GABA in the prefrontal cortex of acutely depressed subjects and normal prefrontal GABA levels in recovered subjects with depression (33,34). Moreover, recent data from Murck and colleagues seem to support this hypothesis: an increase of Gln (the main precursor for GABA synthesis) in patients responding to SD stimulates GABAergic neurotransmission in the dorsolateral prefrontal cortex.
It is also interesting to note that although no significant correlations were found between GABA course and clinical improvement, the subjects with the lowest pre-treatment GABA concentrations showed the largest increases after SSRI treatment. This may simply indicate a ceiling effect on occipital cortex GABA concentrations (7). Alternatively, it may mean that there exist different subgroups of depressed patients, i.e., one group with abnormally low baseline GABA concentrations that exhibit large increases after treatment and a second with relatively normal baseline concentrations that show little change after treatment. The work of Munck et al., too, seems to highlight the importance of differentiating patients with unipolar MDD, in particular with regard to gender and vegetative melancholic features, which may be indices of good response to therapy. The clinical implications of these findings still need to be investigated. Using a novel, dedicated method for the quantification of Glu with a 3T scanner, Luborzewski et al. provided more detailed insight into the differential role of Glu both in the pathophysiology of MDD and in the mechanism underlying the antidepressant action of rTMS (15). Indeed, rTMS of the ACC and DLPFC, as an alternative antidepressant intervention, has been investigated in MDD patients and responders showed lower baseline concentrations of Glu in the DLPFC, which increased after successful rTMS. These data on Glx seem to indicate that in MDD metabolic state-dependent changes within the left DLPFC involve the Glu system and can be reversed in a dose-dependent manner by rTMS. A negative correlation was found between mean changes in Glx and baseline levels, leading the authors to speculate that low Glx levels in the left DLPFC may predict response to rTMS treatment. These findings are in line with earlier studies in which a left prefrontal hypometabolism was able to predict outcome of rTMS treatment (35,36). No alteration was observed in the ACC either in the pre-treatment phase or after rTMS. Other authors demonstrated that reduced Glx levels in the left DLPFC (12) and ACC (9) could successfully be reversed by ECT. Considering the proposed critical role of the ACC in cognition and motor control, and, in this context, the close functional and anatomical connectivity between the ACC and the DLPFC, metabolic changes in this region seem to be highly likely in MDD.
Some limitations of these studies derive from the need to replicate these findings in healthy volunteers in order to control for individual differences in metabolite levels; this is especially important as Glu levels in the DLPFC have not yet been thoroughly investigated in high magnetic field spectroscopy.
At the same time, other studies have instead focused on increased NAA concentrations after successful ECT or SSRI treatment, suggesting that they may indicate a neurotrophic effect of these therapies on the amygdala region (11), but not the hippocampus after ECT (6). Therefore, these therapies may play a positive role in restoring neuronal integrity. Although reports on functional and structural changes of the amygdala in depressive disorders are conflicting (37–39), they nevertheless indicate a possible involvement of these regions in the pathogenesis of MDD (11). The amygdala may be a suitable region for detecting neurotrophic effects by 1H-MRS, since it is a highly neuroplastic brain area (40) and fills the voxel mainly with grey matter (41). This may well provide a neuroanatomical explanation as to why Ende et al. (6) were unable to demonstrate an NAA increase after ECT in the bilateral hippocampus. The absence of an NAA signal reduction after ECT implies that no hippocampal atrophy, neuronal damage or cell death is induced by ECT, although this question is still debated (42,43).
ECT-induced structural changes, namely, mossy fibre/synaptic sprouting (44) or neurogenesis (45), have previously been described in animal experiments. Furthermore, several authors (44,46) have studied the effect of ECT on the expression of growth factors in the human brain, as well as alterations in the function and structure of certain populations of neurons. Duman and Vaidya hypothesised that ECT, via regulation of neurotrophic factors, reverses the atrophy of stress-vulnerable neurons or protects these neurons from further damage (47). ECT may contribute to increased NAA levels by improving glial cell function and Glu metabolism; this hypothesis is supported by the evidence of reduced Glx level, in particular the level of Gln in relation to the other Glx compounds (usually resolved together), in the hippocampus of MDD patients (17) and a parallel significant increase in Glx after successful ECT (10). Similar results in unipolar depressives were observed for the left ACC (9), a region closely interconnected with the amygdala (40).
A recent study by Gonul et al. (14) also demonstrated increased NAA/Cr values in MDD patients responding to SSRI or serotonin norepinephrine reuptake inhibitor treatment compared to pre-treatment values in the left medial prefrontal cortex, where functional and structural abnormalities have consistently been reported in MDD patients (26). No significant difference was observed between the post-treatment NAA/Cr values of patients and those of controls. Moreover, Block et al. also describe an association between treatment response to SSRI and tricyclic antidepressants and increased NAA and Cho levels in the hippocampus independently of the severity of the disease. These results suggest that there might be a loss of neuronal integrity in the left medial frontal cortex of MDD patients, mainly in the left ACC, also because abnormalities are known to be lateralised (48). Antidepressant treatment with its neurotrophic effects might play a positive role in restoring neuronal integrity. These findings are contradictory to those of Pfleiderer et al. (9), although differences in the treatment modalities, 1H-MRS methodology and voxel placement may account for this. Neither Pfleiderer nor Gonul were able to detect any effect of treatment (antidepressant or ECT) on Cho values in the medial frontal cortex. This might be because the medial frontal cortex is not as active as the hippocampus in terms of neuronal plasticity and neurogenesis (6), which allows us to speculate that during antidepressant treatment, the viability of neurons is increased without any significant change in membrane turnover in the medial frontal cortex. These results seems to express a possible loss of neuronal integrity in these regions, a neuronal state that can be restored through the neurotrophic effect of MDD treatments.
Concluding remarks
In conclusion, although this review presents limitations deriving from the small number of papers published and the fact that these studies explored different brain areas using different 1H-MRS protocols, we have shown that 1H-MRS shows good potential for enhancing our knowledge of the mechanisms underlying MDD treatments and for helping in the evaluation of treatment efficacy.
Much more experimental data coming from similar methodologies and making use of other advanced MRI techniques, such as functional MRI and tractography, are needed to support and help explain a powerful and reproducible correlation between treatment response and neurometabolite changes as reflected by 1H-MRS measurements. Higher magnetic fields are also warranted in order to have a more precise objective instrument for identifying spectroscopic pre-treatment indices of good response to therapy, particularly in view of the growing proportion of patients with treatment-resistant depression (49).
Table 2.
Study protocol
| Authors | Objective | Treatment | Protocol timing | Antidepressant response | Responders to treatment | Main findings |
|---|---|---|---|---|---|---|
| Bernier D 200918 | To evaluate neurochemical correlates of sleep restriction | Partial SD | 1H-MRS on the baseline day and the postsleep restriction day | Improvement in HDI total score and at least a 30% improvement in HDI mood score | 5 out of 11 | “Baseline pontine Cho levels distinguished subsequent responders from nonresponders; SD caused a 20.1% decrease in pontine tCr and 11.3% increase in prefrontal Cho in both groups, MDD and healthy controls” |
| Block W 200917 | To study metabolic changes to identify correlates and predictors of treatment response | SSRI (5 citalopram) TCAs (6 nortriptyline) | 1H-MRS at baseline and after 8 weeks | – | Significant decrease in BDI score | “Significant reduction of Glx/Cr and Gln/Cr in the patient group; Gln/Glx ratio showed a trend towards significant reduction; individual effect of treatment correlated with an increase in the NAA and Cho absolute concentrations; low baseline NAA and Cho levels predicted positive treatment effects; no difference in any clinical or metabolic measure, either at baseline or at follow-up between the two treatment groups” |
| Murck H 200916 | To address whether Glx and Gln level changes are related to clinical improvement | Total SD | 1H-MRS pre and post 24h of total SD | – | Significant decrease in HAMD score | “TSD led to an increase in Glx and Gln in the DLPFC only of male patients; Gln increase in patients with vegetative melanchonia; no change in POC” |
| Luborzewski A 200715 | To evaluate neurochemical brain alterations before and after 10 days of high-frequency rTMS of the left DLPFC | 10 sessions (2 weeks) of rTMS of the left DLPFC in naive subjects | 1H-MRS carried out before and after 10 days of rTMS | – | 6 out of 17 | “In the DLPFC, lower Glu and Cho in responders before treatment; after TMS,mean individual Glu increased in responders and decreased in non-responders; Cho increased significantly in responders. No neurochemical alterations in the ACC were detected after rTMS” |
| Gonul AS 200614 | To test the effect of antidepressant treatment on metabolite levels in the left medial frontal cortex | 7 with SSRIs (4 fluoxetine; 3 paroxetine),13 with SNRI (venlafaxine) | 1H-MRS at baseline and after response to treatment | ≥50% decrement of HAMD initial score | 70% (14) | “Pre-treatment NAA/Cr values lower than those of healthy subjects; MDD treatment has significant effect on NAA/Cr; after therapy NAA/Cr values increased significantly in responders (14/20)” |
| Sanacora G 200613 | To test the effect of CBT on occipital cortex GABA vs SSRIs and ECT | CBT | Pre and post 12-week course of CBT | – | Significant decrease in HAMD score | “Clinical response not correlated with pre-CBT GABA levels or post-CBT GABA change; CBT shows a different effect on occipital GABA compared to ECT and SSRI” |
| Michael N 200312 | To evaluate neurotrophic changes during ECT in the left amygdala region | ECT | Pre and post ECT monotherapy;non responders underwent a third 1H-MRS after finally responding to combined ECT/pharmacotherapy | >60% reduction of MADRS baseline score | 10 out of 13 | “Significant Glx trend towards a reduction in patients with unipolar depression; successful ECT was accompanied by increased NAA and Glx levels compared to baseline in all patients; similar increase in NAA was observed in 5/14 non-responders after they had finally responded to the combined ECT/pharmacotherapy” |
| Michael N 200311 | To evaluate metabolic DLPFC changes before and after ECT | ECT | 2–3 ECT × week; 4/12with an insufficient response re-evaluated after a ECT + pharmacotherapy | >60% reduction of MADRS baseline score | 8 out of 12 | “Responders (8/12) showed a marked Glx increase, no longer different from that of controls; it correlated with improvement of MADRS scores; a third measurement revealed a Glx increase after recovery in 2/4 non-responders; Glx was significantly reduced (approximately 67%) prior to ECT in all patients” |
| Obergriesser T 200310 | Follow up of the quantitative changes in Cho and NAA signals in the hippocampal region of patients who remitted from MDD after a course of ECT | ECT | Patients previously studied with 1H-MRS pre- and post-ECT were re-evaluated after a mean of 20±8.6 months after the last ECT | – | 10 out of 12 | “No changes in hippocampal NAA signal; the initially significant increase in the Cho signal reversed to values close to pre-ECT levels” |
| Pfleiderer B 20039 | To evaluate the effects of succesful ECT on Glx levels in the anterior cingulum | ECT | 1H-MRS within 24–48 h of the effective ECT (responders) or after the last ECT (non responders) | >60% reduction of MADRS baseline score | 10 out of 17 | “Marked increase of Glx in responders compared to baseline levels, no longer different from those of controls; stable Glx levels after remission; ECT non-responders did not show marked Glx increase but after clinical response to ECT/venlaflaxine revealed a twofold Glx increase” |
| Sanacora G 20038 | To evaluate occipital cortex GABA increase after ECT | ECT (7/8 bilateral) | Before and at least 1 day after completion of final ECT session | – | “25% complete; 62.5% partial; 12.5% no” response | “Post-ECT GABA significantly increased and higher than pre-ECT concentration (7/8 pts); in 4/8 more than 85% over their pre-ECT levels; still no significant correlation between GABA change and clinical response” |
| Sanacora G 20027 | To evaluate alteration of occipital cortex GABA after SSRI | SSRIs (8 fluoxetine, 3 citalopram) | 1H-MRS before and after a minimum of 5 weeks of SSRI | – | 7 out of 11 had >50% reduction in HAMD score | “Occipital cortex GABA significantly higher after treatment (9/11); no significant correlation between pretreatment and change in occipital cortex GABA and clinical improvement” |
| Ende G 20006 | “Monitoring of quantitative changes in the NAA and Cho signals in the hippocampal region” | ECT | At least 2 datasets were acquired from each patient: one before ECT started and a second after 5 or more ECT treatments, within 30 hours to 10 days of ECT | – | “All patients showed clinical amelioration of depression after ECT (>50% reduction in HAM-D score)” | “No changes in the hippocampal NAA signal after ECT; significant mean increase of Cho-containing compounds” |
| Sonawalla SB 19995 | To assess the relationship between true drug response to fluoxetine and Cho/Cr in the basal ganglia | SSRI (fluoxetine) | Patients underwent 1H-MRS before and after an 8-week open trial with fluoxetine | Improvement on CGI | 8 out of 15 | “Statistically significant difference in Cho/Cr from baseline to week 8between true drug response group (increased by 20%) and placebo pattern response/non-response group (decreased by 12%); no significantly different change in NAA/Cr after 8 weeks of treatment” |
| Charles HC 19944 | To test Cho in basal ganglia and thalamus before and after recovery | SARI (nefazodone) | 1H-MRS before and 2–3 months after therapy commenced | – | – | “Cho/Cr pre-therapy levels were elevated (compared to those of normal subjects); they fell significantly from pre to post-therapy (reaching control levels)” |
Abbreviations: NAA=N-acetylaspartate; Cr=creatine; tCr=creatine-plus-phosphocreatine; Cho=choline-containing compound; Glu=glutamate; Glx=glutamate and glutamine; GABA=γ-aminobutyric acid; ACC=anterior cingulate cortex; DLPFC=left dorsolateral prefrontal cortex; POC=parieto-occipital cortex; SARIs=serotonin antagonist reuptake inhibitors; SNRIs=serotonin norepinephrine re-uptake inhibitors; SSRIs=serotonin reuptake inhibitors; TCAs=triclyclic antidepressants; ECT=electroconvulsive therapy; CBT=cognitive behavioural therapy; rTMS=repetitive transcranial magnetic stimulation; SD=sleep deprivation; MDD=major depressive disorder; DS=discontinuation syndrome; HAMD=Hamilton depression rating scale; HDI=Hamilton Depression Inventory; MADRS=Montgomery Asberg Depression Rating Scale; CGI=Clinical Global Impression.
References
- 1.Eliassen JC, Boespflug EL, Lamy M, Allendorfer J, Chu WJ, Szaflarski JP. Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: a review. Top Stroke Rehabil. 2008;15:427–450. doi: 10.1310/tsr1505-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith HR, Stewart CC, den Hollander JA. Proton magnetic resonance spectroscopy in dementias and mild cognitive impairment. Int Rev Neurobiol. 2009;84:105–31. doi: 10.1016/S0074-7742(09)00406-1. [DOI] [PubMed] [Google Scholar]
- 3.Law M. Advanced imaging techniques in brain tumors. Cancer Imaging. 2009;9:S4–9. doi: 10.1102/1470-7330.2009.9002. Spec No A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Payne M, Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1121–1127. doi: 10.1016/0278-5846(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 5.Sonawalla SB, Renshaw PF, Moore CM, et al. Compounds containing cytosolic choline in the basal ganglia: a potential biological marker of true drug response to fluoxetine. Am J Psychiatry. 1999;156:1638–1640. doi: 10.1176/ajp.156.10.1638. [DOI] [PubMed] [Google Scholar]
- 6.Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57:937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 7.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin re-uptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 8.Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 9.Pfleiderer B, Michael N, Erfurth A, et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 10.Obergriesser T, Ende G, Braus DF, Henn FA. Long-term follow-up of magnetic resonance-detectable choline signal changes in the hippocampus of patients treated with electroconvulsive therapy. J Clin Psychiatry. 2003;64:775–780. doi: 10.4088/jcp.v64n0706. [DOI] [PubMed] [Google Scholar]
- 11.Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- 12.Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 13.Sanacora G, Fenton LR, Fasula MK, et al. Cortical gamma-aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry. 2006;59:284–286. doi: 10.1016/j.biopsych.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Gonul AS, Kitis O, Ozan E, et al. The effect of antidepressant treatment on N-acetyl aspartate levels of medial frontal cortex in drug-free depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:120–125. doi: 10.1016/j.pnpbp.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Luborzewski A, Schubert F, Seifert F, et al. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. J Psychiatr Res. 2007;41:606–615. doi: 10.1016/j.jpsychires.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Murck H, Schubert MI, Schmid D, Schussler P, Steiger A, Auer DP. The glutamatergic system and its relation to the clinical effect of therapeutic-sleep deprivation in depression - an MR spectroscopy study. J Psychiatr Res. 2009;43:175–180. doi: 10.1016/j.jpsychires.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Block W, Traber F, von Widdern O, et al. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- 18.Bernier D, Barthxa R, Devarajan S, Macmaster FP, Schmidt MH, Rusak B. Effects of overnight sleep restriction on brain chemistry and mood in women with unipolar depression and healthy controls. J Psychiatry Neurosci. 2009;34:352–360. [PMC free article] [PubMed] [Google Scholar]
- 19.Caverzasi E, Pichiecchio A, Calligaro A, et al. Complications in major depressive disorder therapy: a review of magnetic resonance spectroscopy studies. Funct Neurol. 2008;23:129–132. [PubMed] [Google Scholar]
- 20.Janowsky DS, el-Yousef MK, Davis J-M, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 21.Renshaw PF, Lafer B, Babb SM, et al. Basal ganglia choline levels in depression and response to fluoxetine treatment: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;41:837–843. doi: 10.1016/S0006-3223(96)00256-9. [DOI] [PubMed] [Google Scholar]
- 22.Petroff OA. Biological 1H NMR spectroscopy. Comp Biochem Physiol B. 1988;90:249–260. doi: 10.1016/0305-0491(88)90069-7. [DOI] [PubMed] [Google Scholar]
- 23.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hänicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9:79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- 24.Klein J, Gonzalez R, Köppen A, Löffelholz K. Free choline and choline metabolites in rat brain and body fluids: sensitive determination and implications for choline supply to the brain. Neurochem Int. 1993;22:293–300. doi: 10.1016/0197-0186(93)90058-d. [DOI] [PubMed] [Google Scholar]
- 25.Ende G, Demirakca T, Tost H. The biochemistry of dysfunctional emotions: proton MR spectroscopic findings in major depressive disorder. Prog Brain Res. 2006;156:481–501. doi: 10.1016/S0079-6123(06)56027-3. [DOI] [PubMed] [Google Scholar]
- 26.Dougherty DD, Rauch SL. Brain correlates of antidepressant treatment outcome from neuroimaging studies in depression. Psychiatr Clin North Am. 2007;30:91–103. doi: 10.1016/j.psc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 28.Behar KL, Rothman DL, Petersen KF, et al. Preliminary evidence of reduced cortical GABA levels in localized 1H-NMR spectra of alcohol dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- 29.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical γ-aminobutyric acid levels in depressed patients by proton magnetic spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 31.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 32.Bettendorff L, Sallanon-Moulin M, Touret M, Wins P, Margineanu I, Schoffeniels E. Paradoxical sleep deprivation increases the content of glutamate and glutamine in rat cerebral cortex. Sleep. 1996;19:65–71. doi: 10.1093/sleep/19.1.65. [DOI] [PubMed] [Google Scholar]
- 33.Hasler G, Neumeister A, van der Veen JW, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 35.Kimbrell TA, Little JT, Dunn RT, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 36.Eschweiler GW, Wegerer C, Schlotter W, et al. Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res. 2000;99:161–172. doi: 10.1016/s0925-4927(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 37.Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroantomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 38.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2436–2441. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 39.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 40.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 41.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 42.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashtari M, Greenwald BS, Kramer-Ginsberg E, et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 44.Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- 45.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 46.Gombos Z, Spiller A, Cottrell GA, Racine RJ, McIntyre Burnham W. Mossy fiber sprouting induced by repeated electroconvulsive shock seizures. Brain Res. 1999;844:28–33. doi: 10.1016/s0006-8993(99)01924-1. [DOI] [PubMed] [Google Scholar]
- 47.Duman RS, Vaidya VA. Molecular and cellular actions of chronic electroconvulsive seizures. J ECT. 1998;14:181–193. [PubMed] [Google Scholar]
- 48.Drevets WC, Price JL, Simpson JR, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SM. Electroconvulsive therapy, brain-derived neurotrophic factor, and possible neurorestorative benefit of the clinical application of electroconvulsive therapy. J ECT. 2008;24:160–165. doi: 10.1097/YCT.0b013e3181571ad0. [DOI] [PubMed] [Google Scholar]