Summary
Genetic, neuropathological and magnetic resonance imaging findings support the presence of diffuse white matter cytoarchitectural disruption in bipolar disorder. In this study, diffusion-weighted imaging (DWI) was applied to study cortical white matter microstructure organisation in 24 patients with DSM-IV bipolar disorder and 35 matched normal controls.
DWI images were obtained using a 1.5 Tesla scanner and apparent diffusion coefficient (ADC) values were determined over regions of interest placed, bilaterally, in the frontal, temporal, parietal, and occipital white matter.
Significantly increased ADC values were found in bipolar patients with respect to normal controls in the right temporal lobe, left parietal lobe and bilateral occipital lobes. ADC values did not associate significantly with age or with clinical variables (p>0.05).
Diffuse cortical white matter alterations on DWI in bipolar disorder denote widespread disruption of white matter integrity and may be due to altered myelination and/or axonal integrity.
Keywords: mood disorders, cortical white matter, magnetic resonance, neuroscience, neuroimaging, diffusion-weighted imaging
Introduction
Diffusion-weighted imaging (DWI) is a promising magnetic resonance technique able to detect alterations at cellular level beyond the resolution of conventional structural magnetic resonance imaging (MRI) sequences. The DWI technique is sensitive to water molecule diffusion in the local cellular milieu. The degree of white matter axonal and myelination disruption is measured through the rate of water molecule diffusion and can be quantified by the apparent diffusion coefficient (ADC). High ADC measures correspond to relatively unimpeded water diffusion (e.g. ventricles or demyelinated plaques), while low ADC measures reflect preserved myelinated axons (1). In normal white matter, the axons are myelinated and tightly packed in a highly organised extracellular matrix which is largely made up of glial cells and processes. Damage to the axonal membrane, changes in its permeability, reduced integrity of intra-axonal microtubules and axonal de- or dysmyelination have all been suggested to account for pathological increases in ADCs (2,3).
Several lines of investigation strongly support a role of glial abnormalities in the pathophysiology of bipolar disorder. Post mortem reports have shown reduced density of oligodendroglial cells in the frontal cortex and adjacent white matter (4,5) and decreased perineuronal oligodendrocytes in the prefrontal cortex of bipolar patients (6). Also, genetic studies in bipolar disorder have documented alterations in the neuroregulin-1 (NRG-1) gene, which was shown, in studies with mice, to be involved in the regulation of axonal migration and myelination (7–9). Furthermore, abnormal myelin staining of white matter in the dorsolateral prefrontal cortex has been reported in bipolar disorder (10).
Several diffusion imaging studies have shown increased ADCs in cortical white matter in relatively small samples of subjects with bipolar disorder, suggesting diffuse microstructural disruption (11–15), as well as in unaffected first-degree relatives (16). In this DWI study, we set out to explore cortical white matter microstructure by identifying regions of interest (ROIs) distributed over the frontal, temporal, parietal and occipital lobes in a sample of patients with bipolar disorder, expecting to find widespread disruption of white matter organisation.
Materials and methods
Subjects
Twenty-four DSM-IV bipolar disorder patients (mean age 49.64±8.96 years; 11 males, 13 females; all Caucasians) and 35 matched healthy controls (mean age 46.17±8.96 years; 17 males, 18 females; all Caucasians) were enrolled. Clinical diagnoses were confirmed using the Item Group Checklist of the Schedule for Clinical Assessment in Neuropsychiatry (IGC-SCAN) (17). The IGC is a semi-structured standardised checklist encompassing 41 psychopathological item groups (18). For inter-rater analysis, the findings of trained research clinical psychologists, with extensive experience in administering SCAN, were compared with those of a senior investigator; the psychologists achieved similar diagnoses in at least 8 out of 10 IGC-SCAN administrations. The Italian version of the SCAN was drawn up by our group (19). Regular consensus meetings were held to ensure reliability of the IGC-SCAN diagnoses. Patients with other Axis I disorders, alcohol or substance abuse, a history of traumatic head injury with loss of consciousness, epilepsy or other neurological or medical diseases were excluded from the study. Patients were recruited from a community-based mental health register, the South Verona Psychiatric Case Register (PCR) (20) and clinical information was retrieved from psychiatric interviews, the attending psychiatrist, and medical records. Clinical symptoms were assessed using the Hamilton Depression Rating Scale (HAM-D) and the Bech-Rafaelsen Mania Rating Scale (BRMRS) on the day of the MRI session. All the patients had a BRMRS score of less than 6 and a HAM-D score of less than 15, with the exception of two subjects with a mild state of mania (BRMRS scores of 17 and 18 respectively) without psychotic symptoms, and one subject with mild depressive symptoms (HAM-D score of 16). All the patients were receiving psychotropic medications. We calculated the cumulative prescribed daily dose/defined daily dose (PDD/DDD) ratio (21) for psychotropic drugs and for each group of medications (antidepressants, typical and atypical neuroleptics and mood stabilisers; see Table 1). Thirteen patients were using antipsychotics: six patients were on atypical neuroleptics (5 olanzapine, 1 quetiapine), four were on typical neuroleptics (2 clotiapine, 1 haloperidol, 1 fluphenazine) and three patients were using both (respectively, perphenazine+quetiapine, haloperidol+risperidone, quetiapine+zuclopentixol). Control individuals underwent a brief interview, modified from the SCID-IV non-patient version (SCID-NP), in order to exclude any DSM-IV Axis I disorders, a history of psychiatric disorders among first-degree relatives, of alcohol or substance abuse, or of head injury, and current neurological or medical illness, including hypertension and diabetes.
Table 1.
Characteristics of the study sample and controls
| Healthy Controls (n=35) | Bipolar Patients (n=24) | Statistics | p | |
|---|---|---|---|---|
| Age (years) | 46.17 ± 8.96 | 49.67 ± 11.36 | t=−1.32 | 0.19 |
| Males/Females, n | 17/18 | 11/13 | X2=0.04 | 0.84 |
| Right-handed, n | 27 | 23 | Fisher test | 0.07 |
| Ethnicity | Caucasian | Caucasian | – | – |
| Age at onset (years) | – | 35.58 ± 13.43 | ||
| Disease duration (years) | – | 14.16 ± 10.33 | – | – |
| BD I/BD II | – | 15/9 | – | – |
| Number of prior admissions | – | 4.17 ± 4.47 | – | – |
| Lifetime antipsychotic use (years) | – | 12.10 ± 7.50 | – | – |
| PDD/DDD APs |
– | 0.74 ± 0.35 | – | – |
| PDD/DDD Typical APs |
– | 0.42 ± 0.29 | – | – |
| PDD/DDD Atypical APs |
– | 0.74 ± 0.26 | – | – |
| PDD/DDD Mood stabilisers |
– | 0.74 ± 0.30 | – | – |
| PDD/DDD Antidepressants |
– | 1.30 ± 0.64 | – | – |
| HDRS score | – | 6.17 ± 4.55 | – | – |
| BRMRS score | – | 2.54 ± 4.87 | – | – |
| ICV (cm3) | 1437.21 ± 142.39 | 1503.02 ± 196.13 | t=−1.49 | 0.14 |
| Intracranial CSF (cm3) | 359.74 ± 57.49 | 381.97 ± 75.39 | t=−1.28 | 0.20 |
| Intracranial white matter (cm3) | 581.26 ± 54.51 | 565.82 ± 80.54 | t=0.88 | 0.38 |
| Intracranial grey matter (cm3) | 551.26 ± 61.33 | 553.87 ± 86.31 | t=–0.14 | 0.89 |
Abbreviations: BD=bipolar disorder; BRMRS=Bech Rafaelsen Mania Rating Scale; CSF=cerebrospinal fluid; ICV=intracranial volume; PDD/DDD=prescribed daily dose to defined daily dose; APs=antipsychotics; HAM-D=Hamilton Depression Rating Scale.
Members of the control group were enrolled by word of mouth or were recruited voluntarily among hospital or university staff or subjects undergoing MRI for a single previous episode of dizziness, without evidence of central nervous system abnormalities on the scan, as reviewed by the neuroradiologist (R.C.); in all cases, their dizziness was due to benign paroxysmal positional vertigo or to non-toxic labyrinthitis. The control group participants were scanned only after a full medical history and general neurological, otoscopic and physical examinations, and after they had completely recovered from their dizziness. None was taking any medication at the time of participation, including drugs for nausea or vertigo. This research study was approved by the Biomedical Ethics Committee of the Azienda Ospedaliera of Verona. All the subjects gave their signed informed consent, after having understood all the issues involved in their participation in the study.
MRI procedure
MRI scans were acquired with a 1.5T Siemens Magnetom Symphony Maestro Class, Syngo MR 2002B (Siemens, Erlangen, Germany). A standard head coil was used for RF transmission and reception of the MR signal and restraining foam pads were used to minimise head motion. T1-weighted images were first obtained to verify the subject’s head position and the image quality (TR= 450 ms, TE=14 ms, flip angle=90°, FOV=230×230, 18 slices, slice thickness=5 mm, matrix size=384×512). Diagnostic PD/T2-weighted images were then acquired (TR=2500 ms, TE=24/121 ms, flip angle=180°, FOV=230×230, 20 slices, slice thickness= 5 mm, matrix size=410×512) in the axial plane parallel to the anterior-posterior (AC-PC) commissure. Subsequently, diffusion-weighted echo-planar images were acquired in the axial plane parallel to the AC-PC line (TR=3200 ms, TE=94 ms, FOV=230×230, 20 slices, slice thickness=5 mm with 1.5 mm gap, matrix size=128×128; these parameters were the same for b=0, b=1000, and the ADC maps) and in the coronal plane from the frontal to the occipital lobes (TR=5000 ms, TE=94 ms, FOV=230×230, 30 slices, slice thickness=4 mm with 0.4 mm gap, matrix size=128×128; these parameters were the same for b=0, b=1000, and the ADC maps). Specifically, three gradients were acquired in three orthogonal directions.
Image analysis
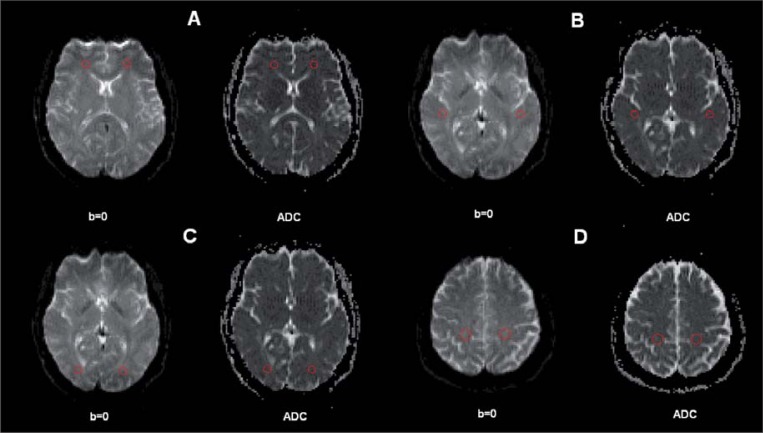
Images were displayed on a commercial Siemens workstation for the post-processing analyses. Circular ROIs standardised at 5 pixels (corresponding to an area of 0.16 cm2) were placed, bilaterally, in the frontal, temporal, parietal, and occipital cortices on the non-diffusion weighted (b=0) echoplanar images, as per our prior method (22) (Fig. 1).The ROIs were automatically transferred to the corresponding maps to obtain the ADC of water molecules. All diffusion data were measured by a trained rater (blind to the study hypotheses, group assignment, and socio-demographic/clinical data) who had achieved high reliability with another rater, established by tracing 10 training scans.
Figure 1.
Regions of interest (ROIs) placed in cortical white matter.
Circular ROIs were automatically transferred from the b=0 echoplanar images to the apparent diffusion coefficient (ADC) maps (A=frontal lobes; B=temporal lobes; C= occipital lobes; D= parietal lobes).
Indeed, the intra-class correlation coefficients (ICCs) were over 0.95. Specifically, 0.99 for the frontal and parietal lobes, bilaterally, and for the right temporal and occipital lobes; 0.98 for the left temporal lobe and 0.97 for the left occipital lobe.
ADC maps were obtained from the diffusion images with b=1000, according to the following equation: −bADC = ln (A(b) / A(0)), where A(b) is the measured echo magnitude, b is the measure of diffusion weighting and A(0) is the echo magnitude without diffusion gradient applied (23). The resulting ADC was expressed in units of 10−5 mm2/second.
Anatomical landmarks
The ROIs were systematically placed in the frontal, temporal, parietal and occipital cortex, as described in our previous work by Andreone et al. (22).
Statistical analysis
SPSS for Windows software, version 18.0 (SPSS Inc., Chicago), was used to perform all statistical analyses, and the two-tailed statistical significance level was set at p<0.05. First, we compared demographic variables using Students’s t-test and Pearson’s chi-square, as appropriate. A general linear model (GLM) for repeated measures (hemisphere and ROI as repeated measures factors) was used to compare the ADCs of cortical white matter microstructure between bipolar patients and control subjects, with age and gender as covariates.
The assumptions that the vector of the measures followed a multivariate normal distribution (Shapiro-Wilk test) and the variance-covariance matrices were circular in form (Mauchley’s test) were verified. A univariate GLM was performed to compare the ADCs of left and right brain structures between bipolar patients and healthy control participants.
Pearson’s and Spearman’s correlation analyses were used to explore possible associations between age and clinical variables and ADC measures. In this case the Bonferroni correction was used for multiple comparisons considering three clusters of variables: chronological age, clinical variables, and scale measures.
Results
The GLM for repeated measures (hemisphere and frontal, temporal, parietal and occipital lobe regions as repeated measures factors and age and gender as covariates) showed significant differences in ADC values between bipolar patients and controls (F=20.39, df=1/55, p<0.001). Compared with the control group, the bipolar patients had significantly greater ADC values for the right temporal lobe (p=0.007), left parietal lobe (p=0.023), and bilateral occipital lobe (p<0.001), with a trend towards increased ADC values for the left frontal lobe (p=0.07) and right parietal lobe (p=0.052). No significant differences were found for the remaining ADC measures (with univariate GLM, age and gender as covariates) (Table 2).
Table 2.
Apparent diffusion coefficients (ADCs) in healthy controls and bipolar patients.
| ADC values (mm2/sec) | Healthy Controls (n=35) | Bipolar Patients (n=24) | Statistics (df=1/55) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | S.D. | Mean | S.D. | F | p | |
| Right frontal lobe | 75.06 | 3.10 | 76.38 | 4.29 | 1.39 | 0.24 |
| Left frontal lobe | 71.43 | 3.19 | 73.44 | 3.68 | 3.33 | 0.07 |
| Right temporal lobe | 75.48 | 3.76 | 78.87 | 4.45 | 7.94 | 0.007 |
| Left temporal lobe | 74.84 | 4.22 | 76.77 | 4.59 | 1.63 | 0.21 |
| Right parietal lobe | 70.52 | 4.15 | 72.71 | 3.19 | 3.95 | 0.052 |
| Left parietal lobe | 72.23 | 3.63 | 74.33 | 3.10 | 5.49 | 0.02 |
| Right occipital lobe | 77.64 | 3.44 | 84.44 | 5.93 | 27.67 | <0.001 |
| Left occipital lobe | 76.73 | 3.46 | 82.71 | 4.12 | 33.50 | <0.001 |
A GLM for repeated measures was performed to compare ADC values (with age and gender as covariates, F=20.39, p<0.001) between bipolar patients and normal controls. The table reports the statistics of the univariate GLM.
The ADC values did not significantly correlate with age in either group (Spearman’s correlation coefficients, p>0.05, after Bonferroni correction) or with clinical features in the bipolar patients (age at disease onset, disease duration, number of hospitalisations, lifetime antipsychotic use, HAM-D score, BRMRS score, PDD/DDD ratio) (Spearman’s correlation coefficients, p>0.05, after Bonferroni correction).
Discussion
In this study, a widespread alteration of the cortical white matter microstructure was found in patients with bipolar disorder, who showed, in particular, significantly higher ADC values in the temporal, parietal and occipital areas. There also emerged a trend towards an increased ADC in the left frontal lobe. Altered white matter microstructure was found in temporal and occipital regions and in the frontal-occipital fasciculus in previous diffusion imaging studies (24,25), suggesting that these anomalies may represent an altered neural substrate in this disorder. In samples of bipolar patients, these same areas have also been described to have altered perfusion parameters (26), and, in a single photon emission tomography study (27), to subtend altered neuropsychological profiles, correlated with pathological blood flow pattern (SPECT).
Bipolar disorder is thus characterised by deficits in neurocognitive functioning (28–31), and abnormal activation of parietal and occipital cortices has been found in bipolar patients during the performance of memory, attention and emotional tests, as documented by PET (32) and functional magnetic resonance reports (fMRI) (33–35). Therefore, parietal and occipital areas may be involved in the pathophysiology of bipolar disorder, particularly in cognition and perception, along with the prefrontal and temporal cortices, the latter mostly accounting for the disruption of emotional processing (36).
Microstructural deficits were not affected by clinical features in this study, supporting the hypothesis that bipolar disorder may be characterised by diffuse cortical white matter alterations. This may represent a quantitative phenotype of the disease, independent of chronicity and treatment, as also argued in the the diffusion tensor imaging literature (37,38). Disrupted connectivity may, ultimately, contribute to cognitive alterations seen in bipolar disorder, such as abnormalities of sustained attention, executive functions, and associative memory (39,40), which however were not explored here. Therefore, further investigations coupling DWI and neuropsychological assessment are warranted in order to better delineate the impact of white matter pathology on cognition in bipolar disorder patients.
It is necessary to consider some potential limitations of this study. First, diffusion tensor sequences were not collected. However, our DWI protocol, being relatively short, may potentially be added to any conventional volumetric scan, providing information on subtle tissue organisation even when MRI fails to detect gross anatomical abnormalities. Second, the group of bipolar patients comprised treated subjects with different clinical states, mostly euthymic or mildly to moderately depressed at the time of the MRI scan. For this reason, a role of psychopathology and psychotropic medications on DWI measures cannot be completely ruled out, even though none of the patients suffered from severe depression or mania. Further studies are needed to clarify whether the abnormalities we found may be characteristic of bipolar disorder in general or of a subgroup of patients (e.g. elderly, chronic, psychotic, rapid cyclers). Third, some of the controls were selected from individuals undergoing MR scanning for dizziness. However, as mentioned, they were fully recovered at the time of imaging and showed no evidence of inflammatory disease or of central nervous system abnormalities on the scan; auditory evoked potentials were not investigated in these subjects.
In conclusion, this study provides evidence of cortical white matter pathology in bipolar disorder, including the temporal, parietal and occipital lobes. Further studies in first-episode drug-free bipolar disorder patients and high-risk subjects are warranted to understand whether these microstructural alterations are present before the onset of the disease or appear subsequently as a result of the illness. Also, future DWI studies are expected to investigate the correlation, over time, of white matter changes with the functional impairment, which often persists during euthymia in bipolar disorder (41).
Acknowledgments
This work was partly supported by grants from the American Psychiatric Institute for Research and Education (APIRE Young Minds in Psychiatry Award), from the Italian Ministry for Education, University and Research (PRIN n. 2005068874), from Veneto StartCup 2007 to Dr Brambilla, and from the Regione Veneto, Italy (159/03, DGRV n. 4087).
References
- 1.Helenius J, Soinne L, Perkiö J, et al. Diffusion-weighted MR imaging in normal human brains in various age groups. AJNR Am J Neuroradiol. 2002;23:194–199. [PMC free article] [PubMed] [Google Scholar]
- 2.Beaulieu C, Allen PS. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MRI of the nervous system. Magn Reson Med. 1994;32:579–583. doi: 10.1002/mrm.1910320506. [DOI] [PubMed] [Google Scholar]
- 3.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 4.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol. 2007;10:537–545. doi: 10.1017/S1461145707007626. [DOI] [PubMed] [Google Scholar]
- 6.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Georgieva L, Dimitrova A, Ivanov D, et al. Support for neuregulin 1 as a susceptibility gene for bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:419–427. doi: 10.1016/j.biopsych.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh AM, Moorhead TW, Job D, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- 9.Thomson PA, Christoforou A, Morris SW, et al. Association of Neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol Psychiatry. 2007;12:94–104. doi: 10.1038/sj.mp.4001889. [DOI] [PubMed] [Google Scholar]
- 10.Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007;151:179–188. doi: 10.1016/j.psychres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Beyer JL, Taylor WD, MacFall JR, et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30:2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 12.Regenold WT, D’Agostino CA, Ramesh N, Hasnain M, Roys S, Gullapalli RP. Diffusion-weighted magnetic resonance imaging of white matter in bipolar disorder: a pilot study. Bipolar Disord. 2006;8:188–195. doi: 10.1111/j.1399-5618.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 13.Macritchie KA, Lloyd AJ, Bastin ME, et al. White matter microstructural abnormalities in euthymic bipolar disorder. Br J Psychiatry. 2010;196:52–58. doi: 10.1192/bjp.bp.108.058586. [DOI] [PubMed] [Google Scholar]
- 14.Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37:1096–1098. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- 15.Sexton CE, MacKay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66:814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Chaddock CA, Barker GJ, Marshall N, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Schedules for Clinical Assessment in Neuropsychiatry. Geneva: WHO; 1992. [Google Scholar]
- 18.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Schede di valutazione clinica in neuropsichiatria. SCAN 2.1. In: Tansella M, Nardini M, editors. SCAN-Schedules for Clinical Assessment in Neuropsychiatry. Rome: Il Pensiero Scientifico Editore; 1996. Glossary. [Google Scholar]
- 20.Amaddeo F, Tansella M. Information systems for mental health. Epidemiol Psichiatr Soc. 2009;18:1–4. [PubMed] [Google Scholar]
- 21.Nosè M, Barbui C. A simple approach to manage dosages in drug-epidemiology research. Epidemiol Psichiatr Soc. 2008;17:186–187. doi: 10.1017/s1121189x00001263. [DOI] [PubMed] [Google Scholar]
- 22.Andreone N, Tansella M, Cerini R, et al. Cortical white-matter microstructure in schizophrenia. Diffusion imaging study. Br J Psychiatry. 2007;191:113–119. doi: 10.1192/bjp.bp.105.020990. [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 24.Bruno S, Cercignani M, Ron MA. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord. 2008;10:460–468. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 25.Haznedar MM, Roversi F, Pallanti S, et al. Fronto-thalamostriatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal N, Bellani M, Perlini C, et al. Increased fronto-temporal perfusion in bipolar disorder. J Affect Disord. 2008;110:106–114. doi: 10.1016/j.jad.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Benabarre A, Vieta E, Martínez-Arán A, et al. Neuropsychological disturbances and cerebral blood flow in bipolar disorder. Aust N Z J Psychiatry. 2005;39:227–234. doi: 10.1080/j.1440-1614.2004.01558.x. [DOI] [PubMed] [Google Scholar]
- 28.Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disord. 2005;7:216–235. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain SR, Sahakian BJ. The neuropsychology of mood disorders. Curr Psychiatry Rep. 2006;8:458–463. doi: 10.1007/s11920-006-0051-x. [DOI] [PubMed] [Google Scholar]
- 30.Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- 31.Murphy FC, Sahakian BJ. Neuropsychology of bipolar disorder. Br J Psychiatry. 2001;178(Suppl 41):S120–127. [PubMed] [Google Scholar]
- 32.Berns GS, Martin M, Proper SM. Limbic hyperreactivity in bipolar II disorder. Am J Psychiatry. 2002;159:304–306. doi: 10.1176/appi.ajp.159.2.304. [DOI] [PubMed] [Google Scholar]
- 33.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 34.Marchand WR, Lee JN, Thatcher GW, et al. A functional MRI study of a paced motor activation task to evaluate frontal-subcortical circuit function in bipolar depression. Psychiatry Res. 2007;155:221–230. doi: 10.1016/j.pscychresns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Surguladze SA, Marshall N, Schulze K, et al. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 37.Brambilla P, Bellani M, Yeh PH, Soares JC. Myelination in bipolar patients and the effects of mood stabilizers on brain anatomy. Curr Pharm Des. 2009;15:2632–2636. doi: 10.2174/138161209788957519. [DOI] [PubMed] [Google Scholar]
- 38.Bellani M, Brambilla P. Diffusion imaging studies of white matter integrity in bipolar disorder. Epidemiol Psychiatr Sci. 2011;20:137–140. doi: 10.1017/s2045796011000229. [DOI] [PubMed] [Google Scholar]
- 39.Brambilla P, Cerruti S, Bellani M, et al. Shared impairment in associative learning in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1093–1099. doi: 10.1016/j.pnpbp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Brambilla P, Macdonald AW, 3rd, Sassi RB, et al. Context processing performance in bipolar disorder patients. Bipolar Disord. 2007;9:230–237. doi: 10.1111/j.1399-5618.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosa AR, Reinares M, Amann B, et al. Six-month functional outcome of a bipolar disorder cohort in the context of a specialized-care program. Bipolar Disorders. 2011;13:679–686. doi: 10.1111/j.1399-5618.2011.00964.x. [DOI] [PubMed] [Google Scholar]