Summary
Transcranial magnetic stimulation (TMS) is a technique developed to non-invasively investigate the integrity of human motor corticospinal tracts. Over the last three decades, the use of stimulation paradigms including single-pulse TMS, paired-pulse TMS, repetitive TMS, and integration with EEG and functional imaging have been developed to facilitate measurement of cortical excitability. Through the use of these protocols, TMS has evolved into an excellent tool for measuring cortical excitability. TMS has high sensitivity in detecting subtle changes in cortical excitability, and therefore it is also a good measure of disturbances associated with brain disorders. In this review, we appraise the current literature on cortical excitability studies using TMS in neurological disorders. We begin with a brief overview of current TMS measures and then show how these have added to our understanding of the underlying mechanisms of brain disorders.
Keywords: cortical excitability, neurological disorders, transcranial magnetic stimulation
Introduction
Cortical excitability
The nervous system is a complex cellular network composed of as many as 10 billion neurons and 60 trillion synapses that mediate interneuronal communication. Each neuron can be regarded as a component in a complex system of highly specialised, distinct neural circuits. Every aspect of behaviour, from primitive reflexes to abstract thinking and emotion, relies on the precision of the computational processes performed by these circuits, which in turn is critically dependent on healthy excitatory and inhibitory systems (1). These systems are facilitated by the interaction of neurotransmitters and cellular receptors to determine the level of neuronal excitability (excited or inhibited) either directly by controlling flow of ions through ion channels or through a complex cascade of intracellular interactions via secondary messengers. Excitation is mainly facilitated by the action of glutamate on N-methyl-d-aspartate (NMDA), and non-NMDA receptors, while inhibition is mainly mediated by the action of gamma-aminobutyric acid (GABA) on GABAA and GABAB receptors. The patterns of interneuronal connections and communication are not irrevocably fixed; they show variability and can be reorganized. Normally this plays a critical role in growth and development, and in learning and memory (2), however abnormal reorganization of brain circuits can also result in disturbed function and manifest as various neurological disorders (3–5).
Neurological disorders are associated with a high degree of disability, marked psychosocial problems and in some cases death. Despite the rapid advances made in the field, leading to improved control of many neurological disorders previously considered untreatable, a significant fraction remain difficult to manage. One of the main challenges hindering the development of more optimized therapeutic options for these patients is that the pathophysiological basis of many neurological disorders remains obscure. This is because the hypotheses developed to explain these disease rely on animal experimental studies. There is thus a huge need for a safe and non-invasive measure of neuronal functions in vivo, in order to achieve a better understanding of how they are altered in neurological disorders.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a non-invasive, painless tool which can be used in humans to measure parameters of cortical excitability in vivo(6–8). TMS stimuli, delivered through a coil to selected scalp locations overlying the primary motor cortex, mainly activate pyramidal neurons transsynaptically. This produces indirect waves descending along the corticospinal fibres. Applied over the motor cortex this discharge can produce a twitch in a corresponding muscle. This muscle activity, referred to as a motor evoked potential (MEP), can be recorded on electromyography (EMG) from many muscles, including the small muscles of the hand (9). Similarly, stimulation over the occipital cortex leads to the perception of ‘phosphenes’ (flashes of light) which are reported by the subject under stimulation.
TMS evokes action potentials in a local population of neurons (6). Highly excitable neurons need to be stimulated less than depressed neurons in order to elicit muscle activity, or to induce the perception of phosphenes. Measurements made using TMS are dependent on small networks of interneurons (excitatory and inhibitory) and their synaptic interactions with each other (6). Thus, in the motor cortex, the stimulus required to produce a typical MEP reflects the global excitability/conductivity of cortical interneurons, fast corticospinal pathways, as well as spinal motoneurons (10). Varying the intensity of stimulation and using different stimulation paradigms can help probe these circuits separately, providing a number of different measures of cortical excitability. Hence TMS is uniquely able to obtain information about the state of excitability of neuronal circuits in vivo in the human brain, and has the potential to link information obtained experimentally (cellular, synaptic, small local networks) with clinical observations. This makes it an excellent tool for studying the pathophysiology underlying many neurological disorders (7,8).
Measures of cortical excitability probed using TMS
TMS was initially used in evaluation of the integrity of the corticospinal tract in humans through conductivity studies (11). It was then progressively applied to the measurement of the excitatory and inhibitory properties of the primary motor cortex itself. There are several physiological protocols utilizing the two broad classes of TMS paradigms: single- or paired-pulse TMS and repetitive TMS (rTMS). The stimulation paradigms used in neurological disorders to date and their pathophysiological significance are summarized in table 1 and figure 1 (over). These parameters have disclosed various defects in cortical excitability associated with these disorders as discussed below.
Table 1.
Summary of TMS paradigms used to date in neurological disorders with their pathophysiological significance.
| Measure | Parameter | Definition | Pathophysiology |
|---|---|---|---|
| A. Membrane excitability | Motor threshold | Minimum level of a given stimulus required to produce a defined response (12). | Membrane excitability of cortical interneurons and reflects conductivity of ion (predominantly sodium) channels (13–15). |
| B. Corticospinal projections | MEP recruitment curves | Stimulus/response curves obtained by recording the size of MEP produced with TMS at a single site using a range of intensities. | Reflects changes in the GABA-ergic and monoaminergic systems as well as sodium and calcium channel properties (16). |
| C. Intracortical circuits | I. Cortical silent period | Period of electromyographic silence that occurs after the MEP when TMS is delivered to the motor cortex during a forceful muscle contraction. | Later part most likely mediated by GABAB inhibitory mechanisms (17–21). |
| II. Paired-pulse paradigms are used to investigate the cellular mechanisms underlying different forms of intracortical inhibition and facilitation (22). They involve a conditioning stimulus which precedes a test stimulus by a number of interstimulus intervals. | |||
| 1. Intracortical inhibition and facilitation |
|
||
| 2. Transcallosal inhibition | The relationship between the two motor cortices can be studied by paired-pulse TMS at both motor cortices (33,34). | Inter-hemispheric inhibition thought to be mediated through excitatory axons that cross the corpus callosum to act on local inhibitory (mainly GABAB-mediated) neurons in the contralateral motor cortex (35). | |
| 3. Short-latency afferent inhibition | Afferent sensory input through stimulation of the median nerve at the wrist or cutaneous fibres at the index finger can modify the excitability of the motor cortex with a complex time course (36). | Thought to be regulated by muscarinic and cholinergic cerebral circuits (36,37). | |
| D. Neuroplasticity changes | RepetitiveTMS | Can be used to induce sustained changes in excitability (synaptic efficacy) that significantly outlast the stimulation period. Low-frequency stimulation results in depression of the target brain area, while high-frequency stimulation induces facilitation of the region (38). Theta burst stimulation is a high-frequency stimulation paradigm that can produce either inhibitory (if applied continuously) or facilitatory (if applied intermittently) effects (39). |
Effects are due to mechanisms similar to the long-term potentiation and long-term depression effects elicited in animal models by low- and high-frequency electrical stimulation, respectively (38). These effects are thought to be predominantly mediated by NMDA receptors (39,40) as well as by modulation of GABA receptor functions (41). |
| E. Corticocortical connectivity | I. TMS and EEG | TMS-evoked surface potentials from any cortical region can be recorded with scalp EEG electrodes and used to estimate regional excitability of the extra-motor cortex (42,43). This increases spatial benefits and also the very high temporal resolution of EEG makes it possible to detect differential effects of brain disturbance on TMS-induced responses. |
|
| II. TMS and fMRI | Combining TMS and fMRI makes it possible to exploit both the good spatial resolution (can identify changes that occur in both cortical and subcortical structures) and the good temporal resolution of TMS. Such data can provide information on connectivity patterns. These patterns reflect the propagation of activity in the stimulated area to distal areas via neural connections (44). |
||
Abbreviations: EEG=electroencephaolography; GABA=gamma (γ)-aminobutyric acid; ISI=interstimulus interval; MEP=motor evoked potential; NMDA=N-methyl-d-aspartate; TMS=transcranial magnetic stimulation.
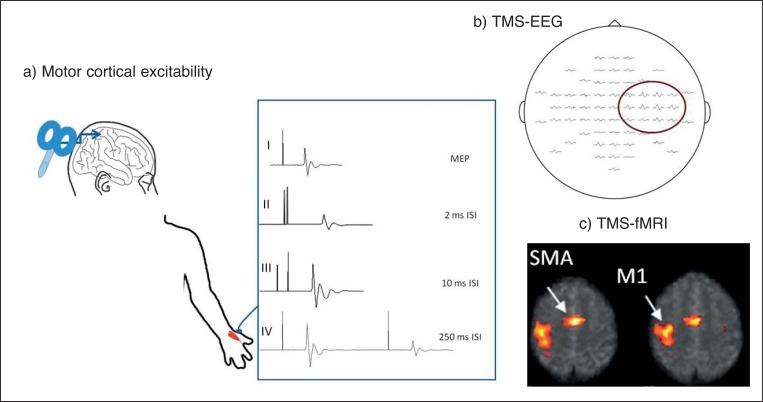
Figure 1.
Schematic representation of the different parameters measured using TMS.
a) Motor cortical excitability showing (I) motor evoked potential (MEP) recorded with a single pulse. Latency is measured from stimulus artefact to initial deflection in baseline; amplitude is measured peak to peak. Examples of (II) short-interval intracortical inhibition at the 2 ms interstimulus interval (ISI), (III) intracortical facilitation at the 10 ms ISI and (IV) long-interval intracortical inhibition at the 250 ms ISI.
b) Averaged EEG responses evoked by TMS. The signals are arranged according to the layout of the electrodes. The amplitudes of the responses are highest in the vicinity of the stimulated site (highlighted) and attenuate with increasing distance from stimulation.
c) Sample of a connectivity map obtained after rTMS of the right motor area on fMRI. SPMs are thresholded for corrected clusters (p<0.05 corrected for multiple comparisons) and are superimposed on the mean EPI image. M1=motor area; SMA=supplementary motor area.
Safety
The only absolute contraindication for TMS/rTMS is the presence of metallic hardware (such as cochlear implants, an internal pulse generator or medication pumps) in close contact with the discharging coil. In such instances there is a risk of inducing malfunction of such implanted devices (45). Single- and paired-pulse TMS are generally considered to be safe even in patients with epilepsy (46), where the crude risk of a TMS-associated seizure ranges from 0.0 to 2.8% for single-pulse TMS and from 0.0 to 3.6% for paired-pulse TMS. With respect to rTMS, the current safety guidelines stipulate that in high risk patients the risk/benefit ratio should be weighed for the patient before each study (45). These include patients with conditions like epilepsy or stroke and those receiving medications that lower seizure threshold.
Cortical excitability in neurological disorders
Epilepsy
The epilepsies are a complex group of syndromes characterized by episodic brain dysfunction manifesting as the occurrence of recurrent seizures (47). Epilepsy syndromes can be broadly classified into two main types: generalized, which mainly include idiopathic generalized epilepsy (IGE), and focal. IGE, as a group, is believed to have a strong underlying genetic basis (48), while focal epilepsies are mostly considered to be due to an underlying focal pathology, such as hippocampal sclerosis or an area of cortical dysgenesis (48), although a genetic basis is thought to underlie some focal epilepsy syndromes (49). Regardless of the type or cause, the proposed underlying mechanism for the epileptic process (based on animal and experimental data) is that it is mediated by a disturbance in the neuronal excitatory/inhibitory balance leading to the formation of hyperexcitable seizure networks (50). How this disturbance comes about (increased excitation, decreased inhibition or both) remains elusive. From this perspective, TMS studies in epilepsy have been very helpful. Results of TMS studies in epilepsy are summarized in table 2 (over). While findings vary somewhat between studies, and likely reflect subject and methodology differences, predominantly in terms of medication and timing of studies, overall, cortical hyperexcitability resulting from defective inhibitory mechanisms seems to be a common feature in most types of epilepsy. It also seems that the alterations occurring within intracortical inhibitory circuits depend on the type of epilepsy, the underlying aetiology, and the site of the epileptic focus. Furthermore, these changes have been found to vary with menstrual cycle (51,52), time of day (53), sleep (54) and sleep deprivation (55,56), suggesting that neuromodulatory transmitters and hormones act at the level of local neuronal network interactions. Alterations in cortical excitability have also been observed for 24 (57) and even up to 48 hours (58) before and after (59) seizures, providing direct evidence of prolonged peri-ictal changes within intracortical circuits.
Table 2.
Summary of interictal TMS findings in patients with epilepsy.
| TMS measure | Generalized epilepsy | Focal epilepsy |
|---|---|---|
| Motor threshold |
|
|
| Cortical silent period |
|
|
| Intracortical inhibition and facilitation |
|
|
| TMS-EEG |
|
Abbreviations: EEG=electroencephaolography; IGE=idiopathic generalized epilepsy; ICF=intracortical facilitation; LICI=long-interval intracortical inhibition; SICI=short-interval intracortical inhibition; TMS=transcranial magnetic stimulation.
Cortical excitability changes associated with epilepsy are also influenced by treatment, with reports of reduction of the baseline hyperexcitability after starting antiepileptic medication (60–65). One study also noted that reduction in cortical excitability to normal or near normal values only occurs in patients who become seizure-free, but not those who continue to have seizures (66). Effects were similar irrespective of the specific antiepileptic drug used. This suggests that despite what is known about the mechanisms of action of each drug, i.e. whether it works on specific channels or receptors, a common effect of anticonvulsants possibly occurs at the level of interneuronal interactions, and these interactions are complex. Patients who continued to have seizures showed evidence of progressive changes in hyperexcitability onset (67). These changes possibly reflect altered receptor properties or disturbed receptor interactions within the inhibitory intracortical circuits, which could be the result of the seizures or may reflect the course of other undefined epiphenomena contributing to the development of pharmaco-resistance. This was reversed with successful epilepsy surgery (68–70), treatment with vagal nerve stimulation (71), continuous anterior thalamic deep brain stimulation (72), and after multiple subpial transection in a single patient with unilateral cortical dysgenesis (73).
Stroke
Ipsilateral MEPs are rarely recorded in healthy subjects at rest. It is thus intriguing that one of the early findings in stroke patients was the presence of ipsilateral MEPs in the paretic limb (95–98). This finding also seemed to correlate with other measures of increased excitability in the unaffected hemisphere (99–101). Cortical silent period (CSP) duration was prolonged in the affected hemisphere after subcortical stroke (102,103), except in patients with post-stroke movement disorder or epilepsy (104). In contrast, short interval intracortical inhibition (SICI) was reduced in the affected hemisphere in the acute phase of a motor cortical stroke (103,105–107) and remained decreased thereafter, regardless of functional recovery. SICI was also reduced in parietal-motor circuits in the intact hemisphere in patients with neglect following stroke compared to patients without neglect and normal controls (108). Also in the unaffected hemisphere, SICI was usually initially reduced, but subsequently returned to normal values (109,110) or even became enhanced compared to the affected hemisphere in patients with good recovery (106,111). This suggests that in the early phases following a stroke, increased intracortical inhibition leads to reduced activity in the unaffected hemisphere, resulting in increased activity of the affected hemisphere, thereby promoting recovery. Further support for this comes from reports of loss of transcallosal inhibition from the affected to the unaffected hemisphere in acute cortical stroke (107,109,112), which would increase the excitability of the unaffected hemisphere. There are also reports of increased transcallosal inhibition from the unaffected to the affected hemisphere just before movement onset in the paretic limb in patients with chronic stroke (113,114). A recent study used dynamic causal modelling to assess effective connectivity in the motor system before and after rTMS of the contralesional motor area in stroke patients (115). The authors reported reduced transcallosal connectivity between homologous parts of the motor area during motor task performance and enhanced intrinsic connectivity between the motor area in the affected hemisphere and the supplementary motor area. These changes in connectivity were accompanied by, and possibly responsible for, an improvement in motor performance, providing evidence of cerebral reorganization following stroke. Thus, the changes in cortical excitability following stroke appear to occur bilaterally, although the pattern seems to correlate with lesion location and stage of recovery.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder of the motor neurons that results in progressive paresis of limb, bulbar and respiratory muscles (116). The mechanisms underlying motor neuron degeneration in ALS remain elusive, although cortical hyperexcitability has been proposed as a possible mechanism (117). One of the hypotheses linked to this concept is a “dying forward” hypothesis whereby corticomotoneurons mediate anterograde degeneration of anterior horn cells via glutamate-mediated excitotoxicity (118). Many TMS studies reported an increased motor threshold (MT) in patients with ALS (119–128). Some found that MEPs could not even be elicited in some of their patients. Conversely, many other studies found a decreased MT (129–132). The reason for this discrepancy is not entirely clear. Because some investigators (133), but not others (134), have observed that MT correlates with disease duration and increases with disease progression (122,124,135,136), it has been suggested that a normal (or even reduced) MT early in the course of illness is consistent with an early phase of cortical hyperexcitability and glutamate-induced excitatory neurotoxicity (130,133). In support of this, several studies report reduction in the duration of the CSP (122,124,136–139), particularly early in the course of the illness (136,137). In addition, decreased SICI has also been demonstrated in ALS using conventional paired-pulse TMS (139–142) as well as using the threshold tracking paired-pulse paradigm. This paradigm in particular consistently showed reduced SICI across different levels of conditioning stimuli (143–146). The change was more prominent in patients with less severe symptoms studied early on but was also seen in advanced cases (143). It was also frequently accompanied by reduced MT, increased MEP recruitment, and shortened CSP (143,145). These findings all indicate increased cortical excitability. The authors postulated that SICI reduction in ALS represents degeneration of inhibitory intracortical circuits, combined with excessive excitation of high-threshold excitatory pathways (146). Interestingly, similar TMS paradigms were all found to be normal in spinobulbar muscular atrophy (Kennedy’s disease), a disorder that clinically may “mimic” ALS, suggesting a lack of significant cortical involvement in this disease (144).
Dementia
Dementia is defined as a serious loss of cognitive ability in a previously unimpaired person, beyond what might be expected from normal aging. Alzheimer’s disease (AD) is the most common form of dementia (147). One of the prevailing theories regarding the cause of AD-related brain degeneration is the amyloid hypothesis, which suggests that accumulation of beta amyloid peptides triggers neuron degeneration through disruption of calcium ion homeostasis (148). The MT was reported to decrease in patients with AD (149) and also a slight and variable reduction in SICI has been observed in some studies (149–151), suggesting hyperexcitability, which in turn suggests reduced inhibition. This seems to be contradicted by the reportedly normal SICI in another study (152), however, TMS-evoked P30 amplitude was reduced in AD subjects in a combined TMS-EEG study (153). This reduction was prominent in the temporo-parietal area, ipsilateral to the stimulation side as well as in the contralateral fronto-central cortex corresponding to the sensorimotor area. P30 is also thought to reflect GABAA mediated activity (154), and thus reduction in its amplitude would support defective inhibition. TMS studies also suggest a cholinergic deficit in AD given that the abnormality most commonly reported in these patients is found in short latency afferent inhibition (SAI) (149,150,155). This is supported by the report of abnormal SAI in dementia with Lewy bodies (a form of dementia that responds to cholinergic medications) (156) and normal SAI in frontotemporal dementia (157), a noncholinergic form of dementia.
Migraine
Migraine is a common medical disorder that has multiple phenotypes with complex and poorly understood underlying mechanisms (158,159). Many hypotheses exist. Among these, disturbances in neurotransmitters, especially calcitonin gene-related peptide and serotonin (160), channelopathies (familial hemiplegic migraine) (161), and cortical spreading depression with subsequent release of inflammatory mediators (162) are the most widely accepted, however, the nature of the underlying pathogenesis remains to be clarified. An increased MT was reported in some migraine studies (163–167) but not in others (168,169), a discrepancy that could be due to differences within the cohorts studied. In patients with migraine with or without aura, shortened CSP was found in the hand muscles (170) as well as facial muscles (171) suggesting defective inhibition. But other studies found a normal CSP (163,167,168,172) and one study even found a prolonged CSP in patients with chronic migraine (172). In patients with migraine with or without aura, SICI tested between attacks was normal in one study (163) and ICF was increased in another (165), whereas in another group of migraineurs with aura SICI was decreased with normal intracortical facilitation (ICF) (173). Long-interval intracortical inhibition (LICI) was investigated in only one study that compared patients with and without aura to controls and patients with new-onset epilepsy (174). The authors found that the pattern of reduced LICI in migraine was very similar to that seen in epilepsy, although of much smaller magnitude. This provides more evidence supporting an overlap between the two paroxysmal disorders. Nevertheless, there is a marked variability in the overall results, which seems to suggest that motor cortical excitability measures are highly dependent on the type of migraine studied and on the stage of illness. In view of this and because of the high prevalence of visual symptoms associated with migraine, many authors chose to study occipital cortical excitability instead. Some of these studies found evidence of occipital cortical hyperexcitability, in particular in migraine with aura, as suggested by reduced threshold for occipital TMS to induce phosphenes in migraineurs (168,175–178) using single or paired pulses. However, other authors found that this threshold was increased in the interictal period (169) or showed increased variability over time (179). Therefore, while there is some evidence to support motor and visual hyperexcitability probably due to decreased inhibition, this remains to be confirmed.
Movement disorders
Dystonia
Dystonia is a disorder characterized by sustained muscle contractions causing twisting and repetitive movements or abnormal postures. The disorder may be genetically determined (primary dystonia) or caused by other factors such as birth-related or other physical trauma, infection, or reaction to pharmaceutical drugs, particularly neuroleptics. It can be localized to a certain group of muscles or can be generalized (180). The precise mechanism is not known, although primary dystonia is suspected to be caused by decreased activity within GABA-ergic circuits including those in the basal ganglia and Purkinje neurons (181). An interplay with environmental conditions is also thought to play a role especially in focal dystonia brought on after trauma, or induced by certain movements or drugs.
A shortened CSP was observed in patients with dystonia involving hand (182) and facial muscles (183). This did not normalize with botulinum toxin injection (184). The CSP was even shorter when the dystonia affected upper and lower facial muscles concurrently than when it affected either alone (183). These findings are consistent with cortical hyperexcitability due to decreased GABA-ergic inhibition. Further evidence of decreased inhibition comes from the reports of reduction in both task-specific (185) and rest (186,187) SICI in patients with upper limb dystonia. SICI was also decreased in DOPA-responsive dystonia (188) and in asymptomatic carriers of the DYT1 gene mutation (one of the genetic abnormalities associated with dystonia) (189). There was also a report of decreased active LICI in patients with writer’s cramp (190), although in another study increased LICI was found using a slightly different paradigm in patients with different types of dystonia (191). Intracortical facilitation on the other hand was found to be normal or slightly increased in patients with dystonia (192). Moreover, decreased SICI was not site-specific and was observed on both the affected and unaffected sides in patients with focal arm dystonia (186), as well as in hand muscles in patients with cervical dystonia (193) and blepharospasm (192). This suggests that the disturbance of inhibitory circuits and the resultant hyperexcitability is not restricted to the circuits clinically affected by the disorder. Support for this comes from evidence of enhanced TMS-induced plasticity changes in focal dystonia even in patients who show no dystonic symptoms in the hand on paired associative studies (194,195) and in patients compared to controls in a continuous theta burst stimulation study (196). There is evidence that this abnormality in SICI is transiently restored with botulinum toxin injection in the dystonic muscles (187), but this was not found in another study in patients with pure writer’s cramp (197).
Parkinsonian Disorders
Parkinsonism refers to a group of neurological disorders chararacterized by tremor, hypokinesia, rigidity, and postural instability (198). While the neurodegenerative condition Parkinson’s disease (PD) is the most common cause of parkinsonism, a wide range of other aetiologies can lead to a similar set of symptoms (199). PD is a degenerative disorder that results from the death of dopaminergic cells in the substantia nigra (midbrain) for an unknown reason. One of the theories proposed to explain the symptoms associated with PD relates to the brainstem-basal ganglia-motor systems (200). Dopaminergic circuits are important for the action of the basal ganglia, which normally exert a constant inhibitory influence on the motor systems. This prevents the motor systems from becoming active at inappropriate times. When a decision is made to perform a particular action, inhibition of the required motor system is reduced, thereby releasing it for activation. Dopamine acts to facilitate this release of inhibition, and thus the net effect of dopamine depletion is to produce hypokinesia. Progressive supra-nuclear palsy is also a degenerative disorder which in its early stages can be mistaken for PD, however later on patients develop difficulty swallowing, ophthalmoparesis especially with vertical gaze, and dementia (201). Multiple system atrophy is another degenerative disorder associated with parkinsonian symptoms together with disturbances in balance and autonomic functions (202). Decreased MT and increased MEP recruitment were found in early- and late-stage patients with PD (203). When these patients were re-studied after proper therapy, the MT was found to have increased in early-stage patients but still remained lower than in normal controls. The CSP was also found to be shorter in patients with PD (203,204) especially at high stimulation intensities (205,206); while this finding supports disturbances within inhibitory circuits, it could also reflect defective dopaminergic circuits, as the CSP was shown to become prolonged following dopaminergic medications (207) as well as surgical lesions of the internal globus pallidus (208,209). In addition, the CSP became longer in early-stage patients after therapy (203) and was found to be more prolonged in patients with PD studied while on dopaminergic medications, compared to controls (210,211). Trains of subthreshold 5-Hz rTMS over the primary motor hand area resulted in prolongation of the CSP (206). This effect could be influenced by dopaminergic circuits as rTMS of the motor area has been shown to induce dopamine release in the striate nucleus (212). Furthermore, the fact that the absence of this effect was not seen in controls suggests that PD patients may be particularly susceptible to modulatory effects of rTMS on intracortical inhibition. Paired-pulse TMS also provides evidence of defective intracortical inhibition. SICI decreased when measured during rest, showing a subsequent improvement with dopaminergic medications (210), deep brain stimulation of the subthalamic nucleus (213) or low-frequency rTMS over the motor cortex (214). Of note, active SICI remained unchanged (211,215), which is interesting considering the static nature of tremor associated with PD. LICI was reported to increase, with subsequent normalization following dopaminergic medications in one study (215). Conversely, decreased LICI, which also normalized in response to dopaminergic medications, was reported in another (216). Some studies observed decreased ICF in advanced PD patients denoting hypo-activity within excitatory circuits (214,217,218). These data suggest impairment of intracortical inhibitory and perhaps even faciltatory circuits. The pattern of this, however, seems to be strongly modulated by the dopaminergic system.
There is also a suggestion of changes in cholinergic circuits, whose pattern varies between the different pakinsonian disorders. In PD, SAI was found to be normal in patients off medications but administration of dopaminergic medication led to reduced SAI (219). In a study that included PD patients with dementia, SAI was found to be increased whereas patients with progressive supra-nuclear palsy showed normal SAI (220). In contrast, patients with multiple system atrophy with parkinsonian features showed reduced SAI (221).
Huntington disease
Huntington disease (HD) is a neurodegenerative genetic disorder that affects muscle coordination and leads to cognitive decline and dementia. It is the most common genetic cause of chorea. Damage mainly occurs in the striatum, but as the disease progresses, other areas of the brain are also significantly affected (222). One of the proposed mechanisms underlying this is increased excitatory output. The CSP was slightly shortened in one study on patients with HD (223) and found to be prolonged and variable in two others (224,225). This difference may be partly explained by the clinical form of HD (224) and the technique used to collect CSP traces (225). The results of the small number of studies that used paired-pulse TMS in HD were also inconsistent: whereas some studies found reduced SICI and LICI (224,226), others did not find any abnormalities (227,228). ICF was found to be normal or slightly increased in patients with HD (226). Thus the limited data available point to disturbances within excitatory circuits leading to minimal hyperexcitability; however this needs to be confirmed in further studies and larger cohorts.
Tourette syndrome
Tourette syndrome is a childhood-onset neuropsychiatric disorder characterized by involuntary movements or vocalizations known as tics. Tics are typically reduced during task performance and concentration. Genetic and environmental factors play a role in the aetiology but the exact causes and pathophysiology are unknown. Cortical disinhibition has been proposed as a possible mechanism specifically within basal ganglia-thalamo-cortical circuits (229). The MT was reportedly similar in patients and in controls while in the resting state, whereas MEP recruitment was found to be more gradual in patients compared to controls (230). With pre-activation, similar recruitment of MEPs and CSP were found in patients and controls. This suggests that the distribution of excitability in the corticospinal system in patients at rest is different to that in healthy individuals (230). In addition, SICI was reduced with no difference in MEP amplitude and ICF at rest, while there was a subsequent increase in MEP amplitude in the pre-movement phase (231). SICI was reduced in these patients in the early phase of movement preparation (similar to rest) followed by a transition towards more inhibition. Subsequently modulation of SICI was comparable to controls, while MEP recruitment was reduced in later phases of movement preparation. These data suggest defective inhibition in Tourette syndrome. It also appears that early during movement preparation, patients start from an abnormally decreased level of SICI and show a subsequent modulation of inhibitory activity to become similar to healthy controls. This suggests that reduced cortical inhibition is one of the factors contributing to the difficulty that patients have in suppressing involuntary tics. Then, during motor performance, motor cortical excitability most likely underlies top-down control from higher motor areas and the prefrontal cortex, which overrides these abnormal subcortical inputs to guarantee adequate behavioural performance (231). SAI was also reduced in patients suggesting impaired activity within cholinergic circuits. This is supported by the report of a single dose of nicotine abolishing the difference between patients and controls in SICI and SAI, with no effect on MT (232).
Tremor
Essential tremor (ET) is a slowly progressive neurological disorder whose most recognizable feature is a tremor of the arms that is apparent during voluntary movements (233). The underlying mechanism is not clear but there is some suggestion that it may be related to defective inhibition particularly in cerebellar cells (234). SICI was reduced in patients with ET and this correlated with motor hyperactivity (235). On the other hand, patients with primary writing tremor were found to have normal SICI and LICI (236). The CSP was normal in all types of tremor, but shortened in cortical myoclonus (82,237). Thus while further studies are needed, there seems to be some evidence supporting defective inhibition in ET but not other forms of tremor.
Cerebellar diseases
While the cerebellum does not serve to initiate most movement, it does interact with areas of the brain that do (238). In doing so, the cerebellum promotes the synchronicity and accuracy of movement required for purposeful motor activity. The main clinical features of cerebellar disorders include incoordination and imbalance. The MT was increased in the contralateral motor cortex in patients with cerebellar damage (239–241). SICI was found to be either normal (242) or increased together with reduced ICF (243–246) in cerebellar ataxia. Interestingly, reduced ICF was also found in patients with inherited spinocerebellar ataxia, specifically types 2 and 3 (244), suggesting a role for genetic properties in influencing the pattern of change in cortical excitability. Increased SICI and reduced ICF were also observed in patients with cerebellar stroke of the superior or the inferior cerebellar artery territories (247). In addition, the CSP was found to be prolonged in patients with cerebellar disease (248–250). Taken together, these results suggest that cerebellar diseases are associated with excessive inhibition and possibly also defective excitation within intracortical motor circuits resulting in reduction in motor cortical excitability.
Concluding remarks
A variety of TMS methods are now available to study cortical excitability changes associated with various neurological disorders. While the yield of these methods is much greater in disorders such as epilepsy, ALS, dystonia and stroke, than in others, TMS has provided some important and insightful in vivo inferences on the mechanisms underlying many neurological disorders. It is likely that studies including more homogenous cohorts and implementing more rigorous study designs and standardized stimulation paradigms will overcome the controversial findings in some of the reports and thus provide more conclusive inferences into even more neurological disorders. Neurophysiological interactions within complex interconnected neuronal networks will also be amenable to further testing with the integration of TMS with EEG and neuroimaging techniques. This holds great promise for addressing more research questions and eventually for the translation of this knowledge into clinical practice.
Acknowledgments
The authors wish to thank Dr Danny Flanagan for his help and continual support during the preparation of this manuscript.
References
- 1.Robinson D. Implications of neural networks for how we think about brain function. Behav Brain Sci. 1992;13:644–655. [Google Scholar]
- 2.Wood AG, Saling MM, O’Shea MF, Jackson GD, Berkovic SF. Reorganization of verbal memory and language: a case of dissociation. J Int Neuropsychol Soc. 1999;5:69–74. doi: 10.1017/s1355617799511090. [DOI] [PubMed] [Google Scholar]
- 3.Romcy-Pereira RN, Garcia-Cairasco N. Hippocampal cell proliferation and epileptogenesis after audiogenic kindling are not accompanied by mossy fiber sprouting or Fluoro-Jade staining. Neuroscience. 2003;119:533–546. doi: 10.1016/s0306-4522(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 4.Routbort MJ, Bausch SB, McNamara JO. Seizures, cell death, and mossy fiber sprouting in kainic acid-treated organotypic hippocampal cultures. Neuroscience. 1999;94:755–765. doi: 10.1016/s0306-4522(99)00358-9. [DOI] [PubMed] [Google Scholar]
- 5.Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 6.Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- 7.Fatemi-Ardekani A. Transcranial magnetic stimulation: physics, electrophysiology, and applications. Crit Rev Biomed Eng. 2008;36:375–412. doi: 10.1615/critrevbiomedeng.v36.i5-6.30. [DOI] [PubMed] [Google Scholar]
- 8.Lefaucheur JP. (Transcranial magnetic stimulation: applications in neurology) Rev Neurol (Paris) 2005;161:1121–1130. doi: 10.1016/s0035-3787(05)85182-3. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- 10.Cracco RQ, Cracco JB, Maccabee PJ, Amassian VE. Cerebral function revealed by transcranial magnetic stimulation. J Neurosci Methods. 1999;86:209–219. doi: 10.1016/s0165-0270(98)00167-8. [DOI] [PubMed] [Google Scholar]
- 11.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 12.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 13.Rossini PM, Berardelli A, Deuschl G, et al. Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1999;52:171–185. [PubMed] [Google Scholar]
- 14.Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of vigabatrin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:124–127. doi: 10.1016/s0924-980x(96)96607-2. [DOI] [PubMed] [Google Scholar]
- 15.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–937. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- 17.Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- 18.Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JL, Gandevia SC. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve. 2001;24:18–29. doi: 10.1002/1097-4598(200101)24:1<18::aid-mus2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Triggs WJ, Cros D, Macdonell RA, Chiappa KH, Fang J, Day BJ. Cortical and spinal motor excitability during the transcranial magnetic stimulation silent period in humans. Brain Res. 1993;628:39–48. doi: 10.1016/0006-8993(93)90935-g. [DOI] [PubMed] [Google Scholar]
- 21.Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]
- 22.Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boroojerdi B. Pharmacologic influences on TMS effects. J Clin Neurophysiol. 2002;19:255–271. doi: 10.1097/00004691-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziemann U. Pharmacology of TMS. Suppl Clin Neurophysiol. 2003;56:226–231. [PubMed] [Google Scholar]
- 26.Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Braun Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 27.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 29.Fedi M, Berkovic SF, Macdonell RA, Curatolo JM, Marini C, Reutens DC. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex. 2008;18:664–669. doi: 10.1093/cercor/bhm100. [DOI] [PubMed] [Google Scholar]
- 30.Florian J, Muller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- 32.Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- 33.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol. 2007;118:308–316. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Mariorenzi R, Zarola F, Caramia MD, Paradiso C, Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalogr Clin Neurophysiol. 1991;81:90–101. doi: 10.1016/0168-5597(91)90002-f. [DOI] [PubMed] [Google Scholar]
- 37.Delwaide PJ, Olivier E. Conditioning transcranial cortical stimulation (TCCS) by exteroceptive stimulation in parkinsonian patients. Adv Neurol. 1990;53:175–181. [PubMed] [Google Scholar]
- 38.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 39.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Di Lazzaro V, Pilato F, Dileone M, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 42.Lioumis P, Kicic D, Savolainen P, Makela JP, Kahkonen S. Reproducibility of TMS-evoked EEG responses. Hum Brain Mapp. 2009;30:1387–1396. doi: 10.1002/hbm.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kähkönen S, Komssi S, Wilenius J, Ilmoniemi RJ. Pre-frontal TMS produces smaller EEG responses than motor-cortex TMS: implications for rTMS treatment in depression. Psychopharmacology (Berl) 2005;181:16–20. doi: 10.1007/s00213-005-2197-3. [DOI] [PubMed] [Google Scholar]
- 44.Hampson M, Hoffman RE. Transcranial magnetic stimulation and connectivity mapping: tools for studying the neural bases of brain disorders. Front Syst Neurosci. 2010;4:40. doi: 10.3389/fnsys.2010.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrader LM, Stern JM, Koski L, Nuwer MR, Engel J., Jr Seizure incidence during single- and paired-pulse transcranial magnetic stimulation (TMS) in individuals with epilepsy. Clin Neurophysiol. 2004;115:2728–2737. doi: 10.1016/j.clinph.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 48.Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Helbig I, Scheffer IE, Mulley JC, Berkovic SF. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7:231–245. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- 50.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 51.Hattemer K, Knake S, Reis J, et al. Excitability of the motor cortex during ovulatory and anovulatory cycles: a transcranial magnetic stimulation study. Clin Endocrinol (Oxf) 2007;66:387–393. doi: 10.1111/j.1365-2265.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- 52.Badawy RA, Macdonell RA, Berkovic SF, Jackson GD. Cortical excitability and the menstrual cycle: reversal of normal patterns in new onset epilepsy. Clin Neurophysiol. 2010;121:S197. [abstract) [Google Scholar]
- 53.Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology. 2009;73:218–222. doi: 10.1212/WNL.0b013e3181ae7ca6. [DOI] [PubMed] [Google Scholar]
- 54.Salih F, Khatami R, Steinheimer S, Kretz R, Schmitz B, Grosse P. A hypothesis for how non-REM sleep might promote seizures in partial epilepsies: a transcranial magnetic stimulation study. Epilepsia. 2007;48:1538–1542. doi: 10.1111/j.1528-1167.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 55.Manganotti P, Bongiovanni LG, Fuggetta G, Zanette G, Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: a combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry. 2006;77:56–60. doi: 10.1136/jnnp.2004.041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology. 2006;67:1018–1022. doi: 10.1212/01.wnl.0000237392.64230.f7. [DOI] [PubMed] [Google Scholar]
- 57.Badawy R, Macdonell R, Jackson G, Berkovic S. The peri-ictal state: cortical excitability changes within 24 h of a seizure. Brain. 2009;132:1013–1021. doi: 10.1093/brain/awp017. [DOI] [PubMed] [Google Scholar]
- 58.Wright MA, Orth M, Patsalos PN, Smith SJ, Richardson MP. Cortical excitability predicts seizures in acutely drug-reduced temporal lobe epilepsy patients. Neurology. 2006;67:1646–1651. doi: 10.1212/01.wnl.0000242729.85335.a3. [DOI] [PubMed] [Google Scholar]
- 59.Delvaux V, Alagona G, Gerard P, De Pasqua V, Delwaide PJ, Maertens de Noordhout A. Reduced excitability of the motor cortex in untreated patients with de novo idiopathic “grand mal” seizures. J Neurol Neurosurg Psychiatry. 2001;71:772–776. doi: 10.1136/jnnp.71.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turazzini M, Manganotti P, Del Colle R, Silvestri M, Fiaschi A. Serum levels of carbamazepine and cortical excitability by magnetic brain stimulation. Neurol Sci. 2004;25:83–90. doi: 10.1007/s10072-004-0234-3. [DOI] [PubMed] [Google Scholar]
- 61.Manganotti P, Bongiovanni LG, Zanette G, Turazzini M, Fiaschi A. Cortical excitability in patients after loading doses of lamotrigine: a study with magnetic brain stimulation. Epilepsia. 1999;40:316–321. doi: 10.1111/j.1528-1157.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 62.Kazis DA, Kimiskidis VK, Papagiannopoulos S, et al. The effect of valproate on silent period and corticomotor excitability. Epileptic Disord. 2006;8:136–142. [PubMed] [Google Scholar]
- 63.Reutens DC, Berkovic SF, Macdonell RA, Bladin PF. Magnetic stimulation of the brain in generalized epilepsy: reversal of cortical hyperexcitability by anticonvulsants. Ann Neurol. 1993;34:351–355. doi: 10.1002/ana.410340308. [DOI] [PubMed] [Google Scholar]
- 64.Nezu A, Kimura S, Ohtsuki N, Tanaka M. Transcranial magnetic stimulation in benign childhood epilepsy with centro-temporal spikes. Brain Dev. 1997;19:134–137. doi: 10.1016/s0387-7604(96)00497-4. [DOI] [PubMed] [Google Scholar]
- 65.Cantello R, Civardi C, Varrasi C, et al. Excitability of the human epileptic cortex after chronic valproate: a reappraisal. Brain Res. 2006;1099:160–166. doi: 10.1016/j.brainres.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 66.Badawy RA, Macdonell RA, Berkovic SF, Newton MR, Jackson GD. Predicting seizure control: cortical excitability and antiepileptic medication. Ann Neurol. 2010;67:64–73. doi: 10.1002/ana.21806. [DOI] [PubMed] [Google Scholar]
- 67.Badawy RAB, Jackson GD, Berkovic SF, Macdonell RAL. Cortical excitability and refractory epilepsy; a three year longitudinal transcranial magnetic stimulation study. IJNS. 2013. (in press) [DOI] [PubMed]
- 68.Läppchen CH, Feil B, Fauser S, Wuwer Y, Glocker FX, Schulze-Bonhage A. Changes in intracortical excitability after successful epilepsy surgery. Epilepsy Res. 2008;79:55–62. doi: 10.1016/j.eplepsyres.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Kamida T, Fujiki M, Baba H, Ono T, Abe T, Kobayashi H. The relationship between paired pulse magnetic MEP and surgical prognosis in patients with intractable epilepsy. Seizure. 2007;16:113–119. doi: 10.1016/j.seizure.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Badawy RA, Macdonell RA, Berkovic SF, Jackson GD. Post-operative reduction in cortical excitability correlates with seizure freedom. Clin Neurophysiol. 2010;121:S197–S8. [abstract] [Google Scholar]
- 71.Di Lazzaro V, Oliviero A, Pilato F, et al. Effects of vagus nerve stimulation on cortical excitability in epileptic patients. Neurology. 2004;62:2310–2312. doi: 10.1212/01.wnl.0000131743.45131.ae. [DOI] [PubMed] [Google Scholar]
- 72.Molnar GF, Sailer A, Gunraj CA, et al. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology. 2006;66:566–571. doi: 10.1212/01.wnl.0000198254.08581.6b. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu T, Maehara T, Hino T, et al. Effect of multiple subpial transection on motor cortical excitability in cortical dysgenesis. Brain. 2001;124:1336–1349. doi: 10.1093/brain/124.7.1336. [DOI] [PubMed] [Google Scholar]
- 74.Reutens DC, Berkovic SF. Increased cortical excitability in generalised epilepsy demonstrated with transcranial magnetic stimulation. Lancet. 1992;339:362–363. doi: 10.1016/0140-6736(92)91679-3. [DOI] [PubMed] [Google Scholar]
- 75.Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol. 2007;61:324–331. doi: 10.1002/ana.21087. [DOI] [PubMed] [Google Scholar]
- 76.Brodtmann A, Macdonell RA, Gilligan AK, Curatolo J, Berkovic SF. Cortical excitability and recovery curve analysis in generalized epilepsy. Neurology. 1999;53:1347–9. doi: 10.1212/wnl.53.6.1347. [DOI] [PubMed] [Google Scholar]
- 77.Macdonell RA, King MA, Newton MR, Curatolo JM, Reutens DC, Berkovic SF. Prolonged cortical silent period after transcranial magnetic stimulation in generalized epilepsy. Neurology. 2001;57:706–708. doi: 10.1212/wnl.57.4.706. [DOI] [PubMed] [Google Scholar]
- 78.Klimpe S, Behrang-Nia M, Bott MC, Werhahn KJ. Recruitment of motor cortex inhibition differentiates between generalized and focal epilepsy. Epilepsy Res. 2009;84:210–216. doi: 10.1016/j.eplepsyres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Can changes in cortical excitability distinguish progressive from juvenile myoclonic epilepsy? Epilepsia. 2010;51:2084–2088. doi: 10.1111/j.1528-1167.2010.02557.x. [DOI] [PubMed] [Google Scholar]
- 80.Manganotti P, Bongiovanni LG, Zanette G, Fiaschi A. Early and late intracortical inhibition in juvenile myoclonic epilepsy. Epilepsia. 2000;41:1129–1138. doi: 10.1111/j.1528-1157.2000.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 81.Valzania F, Strafella AP, Tropeani A, Rubboli G, Nassetti SA, Tassinari CA. Facilitation of rhythmic events in progressive myoclonus epilepsy: a transcranial magnetic stimulation study. Clin Neurophysiol. 1999;110:152–157. doi: 10.1016/s0013-4694(98)00115-1. [DOI] [PubMed] [Google Scholar]
- 82.Inghilleri M, Mattia D, Berardelli A, Manfredi M. Asymmetry of cortical excitability revealed by transcranial stimulation in a patient with focal motor epilepsy and cortical myoclonus. Electroencephalogr Clin Neurophysiol. 1998;109:70–72. doi: 10.1016/s0924-980x(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 83.Varrasi C, Civardi C, Boccagni C, et al. Cortical excitability in drug-naive patients with partial epilepsy: a cross-sectional study. Neurology. 2004;63:2051–2055. doi: 10.1212/01.wnl.0000145770.95990.82. [DOI] [PubMed] [Google Scholar]
- 84.Werhahn KJ, Lieber J, Classen J, Noachtar S. Motor cortex excitability in patients with focal epilepsy. Epilepsy Res. 2000;41:179–189. doi: 10.1016/s0920-1211(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 85.Hamer HM, Reis J, Mueller HH, et al. Motor cortex excitability in focal epilepsies not including the primary motor area—a TMS study. Brain. 2005;128:811–818. doi: 10.1093/brain/awh398. [DOI] [PubMed] [Google Scholar]
- 86.Cantello R, Civardi C, Cavalli A, et al. Cortical excitability in cryptogenic localization-related epilepsy: interictal transcranial magnetic stimulation studies. Epilepsia. 2000;41:694–704. doi: 10.1111/j.1528-1157.2000.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 87.Cincotta M, Borgheresi A, Lori S, Fabbri M, Zaccara G. Interictal inhibitory mechanisms in patients with cryptogenic motor cortex epilepsy: a study of the silent period following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1998;107:1–7. doi: 10.1016/s0013-4694(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 88.Cicinelli P, Mattia D, Spanedda F, et al. Transcranial magnetic stimulation reveals an interhemispheric asymmetry of cortical inhibition in focal epilepsy. Neuroreport. 2000;11:701–707. doi: 10.1097/00001756-200003200-00010. [DOI] [PubMed] [Google Scholar]
- 89.Ertas NK, Gul G, Altunhalka A, Kirbas D. Cortical silent period following transcranial magnetic stimulation in epileptic patients. Epileptic Disord. 2000;2:137–140. [PubMed] [Google Scholar]
- 90.Cincotta M, Borgheresi A, Guidi L, et al. Remote effects of cortical dysgenesis on the primary motor cortex: evidence from the silent period following transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1340–1345. doi: 10.1016/s1388-2457(00)00330-8. [DOI] [PubMed] [Google Scholar]
- 91.von Giesen HJ, Roick H, Benecke R. Inhibitory actions of motor cortex following unilateral brain lesions as studied by magnetic brain stimulation. Exp Brain Res. 1994;99:84–96. doi: 10.1007/BF00241414. [DOI] [PubMed] [Google Scholar]
- 92.Manganotti P, Tamburin S, Zanette G, Fiaschi A. Hyperexcitable cortical responses in progressive myoclonic epilepsy: a TMS study. Neurology. 2001;57:1793–1799. doi: 10.1212/wnl.57.10.1793. [DOI] [PubMed] [Google Scholar]
- 93.Caramia MD, Gigli G, Iani C, et al. Distinguishing forms of generalized epilepsy using magnetic brain stimulation. Electroencephalogr Clin Neurophysiol. 1996;98:14–19. doi: 10.1016/0013-4694(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 94.Valentin A, Arunachalam R, Mesquita-Rodrigues A, et al. Late EEG responses triggered by transcranial magnetic stimulation (TMS) in the evaluation of focal epilepsy. Epilepsia. 2008;49:470–480. doi: 10.1111/j.1528-1167.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- 95.Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- 96.Trompetto C, Assini A, Buccolieri A, Marchese R, Abbruzzese G. Motor recovery following stroke: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1860–1867. doi: 10.1016/s1388-2457(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 97.Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 98.Netz J, Lammers T, Hömberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 99.Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 100.Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 101.Delvaux V, Alagona G, Gerard P, De Pasqua V, Pennisi G, de Noordhout AM. Post-stroke reorganization of hand motor area: a 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin Neurophysiol. 2003;114:1217–1225. doi: 10.1016/s1388-2457(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 102.Ahonen JP, Jehkonen M, Dastidar P, Molnar G, Hakkinen V. Cortical silent period evoked by transcranial magnetic stimulation in ischemic stroke. Electroencephalogr Clin Neurophysiol. 1998;109:224–229. doi: 10.1016/s0924-980x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 103.Liepert J, Restemeyer C, Kucinski T, Zittel S, Weiller C. Motor strokes: the lesion location determines motor excitability changes. Stroke. 2005;36:2648–2653. doi: 10.1161/01.STR.0000189629.10603.02. [DOI] [PubMed] [Google Scholar]
- 104.Kessler KR, Schnitzler A, Classen J, Benecke R. Reduced inhibition within primary motor cortex in patients with poststroke focal motor seizures. Neurology. 2002;59:1028–1033. doi: 10.1212/wnl.59.7.1028. [DOI] [PubMed] [Google Scholar]
- 105.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 106.Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- 107.Niehaus L, Bajbouj M, Meyer BU. Impact of interhemispheric inhibition on excitability of the non-lesioned motor cortex after acute stroke. Suppl Clin Neurophysiol. 2003;56:181–186. doi: 10.1016/s1567-424x(09)70220-x. [DOI] [PubMed] [Google Scholar]
- 108.Koch G, Oliveri M, Cheeran B, et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131:3147–1355. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 110.Bütefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- 111.Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34:2653–2658. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- 112.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 113.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 114.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–6. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 115.Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 117.Bruijn LI, Beal MF, Becher MW, et al. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc Natl Acad Sci U S A. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eisen A, Kim S, Pant B. Amyotrophic lateral sclerosis (ALS): a phylogenetic disease of the corticomotoneuron? Muscle Nerve. 1992;15:219–224. doi: 10.1002/mus.880150215. [DOI] [PubMed] [Google Scholar]
- 119.Schriefer TN, Hess CW, Mills KR, Murray NM. Central motor conduction studies in motor neurone disease using magnetic brain stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:431–437. doi: 10.1016/0168-5597(89)90032-4. [DOI] [PubMed] [Google Scholar]
- 120.Eisen A, Shytbel W, Murphy K, Hoirch M. Cortical magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve. 1990;13:146–151. doi: 10.1002/mus.880130211. [DOI] [PubMed] [Google Scholar]
- 121.Triggs WJ, Macdonell RA, Cros D, Chiappa KH, Shahani BT, Day BJ. Motor inhibition and excitation are independent effects of magnetic cortical stimulation. Ann Neurol. 1992;32:345–51. doi: 10.1002/ana.410320307. [DOI] [PubMed] [Google Scholar]
- 122.Triggs WJ, Menkes D, Onorato J, et al. Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology. 1999;53:605–611. doi: 10.1212/wnl.53.3.605. [DOI] [PubMed] [Google Scholar]
- 123.Berardelli A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Electrical and magnetic transcranial stimulation in patients with corticospinal damage due to stroke or motor neurone disease. Electroencephalogr Clin Neurophysiol. 1991;81:389–396. doi: 10.1016/0168-5597(91)90028-v. [DOI] [PubMed] [Google Scholar]
- 124.Attarian S, Azulay JP, Lardillier D, Verschueren A, Pouget J. Transcranial magnetic stimulation in lower motor neuron diseases. Clin Neurophysiol. 2005;116:35–42. doi: 10.1016/j.clinph.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 125.de Carvalho M, Turkman A, Swash M. Motor responses evoked by transcranial magnetic stimulation and peripheral nerve stimulation in the ulnar innervation in amyotrophic lateral sclerosis: the effect of upper and lower motor neuron lesion. J Neurol Sci. 2003;210:83–90. doi: 10.1016/s0022-510x(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 126.Miscio G, Pisano F, Mora G, Mazzini L. Motor neuron disease: usefulness of transcranial magnetic stimulation in improving the diagnosis. Clin Neurophysiol. 1999;110:975–981. doi: 10.1016/s1388-2457(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 127.Schulte-Mattler WJ, Muller T, Zierz S. Transcranial magnetic stimulation compared with upper motor neuron signs in patients with amyotrophic lateral sclerosis. J Neurol Sci. 1999;170:51–56. doi: 10.1016/s0022-510x(99)00201-4. [DOI] [PubMed] [Google Scholar]
- 128.Urban PP, Wicht S, Hopf HC. Sensitivity of transcranial magnetic stimulation of corticobulbar vs. corticospinal tract involvement in Amyotrophic Lateral Sclerosis (ALS) J Neurol. 2001;248:850–855. doi: 10.1007/s004150170068. [DOI] [PubMed] [Google Scholar]
- 129.Caramia MD, Cicinelli P, Paradiso C, et al. Excitability changes of muscular responses to magnetic brain stimulation in patients with central motor disorders. Electroencephalogr Clin Neurophysiol. 1991;81:243–250. doi: 10.1016/0168-5597(91)90009-m. [DOI] [PubMed] [Google Scholar]
- 130.Mills KR, Nithi KA. Corticomotor threshold is reduced in early sporadic amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:1137–1141. doi: 10.1002/(sici)1097-4598(199709)20:9<1137::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 131.Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin Neurophysiol. 2002;113:1688–1697. doi: 10.1016/s1388-2457(02)00288-2. [DOI] [PubMed] [Google Scholar]
- 132.Kohara N, Kaji R, Kojima Y, et al. Abnormal excitability of the corticospinal pathway in patients with amyotrophic lateral sclerosis: a single motor unit study using transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1996;101:32–41. doi: 10.1016/0013-4694(95)00166-2. [DOI] [PubMed] [Google Scholar]
- 133.Eisen A, Pant B, Stewart H. Cortical excitability in amyotrophic lateral sclerosis: a clue to pathogenesis. Can J Neurol Sci. 1993;20:11–16. doi: 10.1017/s031716710004734x. [DOI] [PubMed] [Google Scholar]
- 134.de Carvalho M, Evangelista T, Sales-Luis ML. The corticomotor threshold is not dependent on disease duration in amyotrophic lateral sclerosis (ALS) Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:39–42. doi: 10.1080/146608202317576525. [DOI] [PubMed] [Google Scholar]
- 135.Pouget J, Trefouret S, Attarian S. Transcranial magnetic stimulation (TMS): compared sensitivity of different motor response parameters in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 2):S45–S49. doi: 10.1080/14660820052415817. [DOI] [PubMed] [Google Scholar]
- 136.Mills KR. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain. 2003;126:2558–2566. doi: 10.1093/brain/awg260. [DOI] [PubMed] [Google Scholar]
- 137.Prout AJ, Eisen AA. The cortical silent period and amyotrophic lateral sclerosis. Muscle Nerve. 1994;17:217–223. doi: 10.1002/mus.880170213. [DOI] [PubMed] [Google Scholar]
- 138.Uozumi T, Tsuji S, Murai Y. Motor potentials evoked by magnetic stimulation of the motor cortex in normal subjects and patients with motor disorders. Electroencephalogr Clin Neurophysiol. 1991;81:251–256. doi: 10.1016/0168-5597(91)90010-u. [DOI] [PubMed] [Google Scholar]
- 139.Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Changes in motor cortex inhibition over time in patients with amyotrophic lateral sclerosis. J Neurol. 2002;249:1723–1728. doi: 10.1007/s00415-002-0926-7. [DOI] [PubMed] [Google Scholar]
- 140.Yokota T, Yoshino A, Inaba A, Saito Y. Double cortical stimulation in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1996;61:596–600. doi: 10.1136/jnnp.61.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enterzari-Taher M, Eisen A, Stewart H, Nakajima M. Abnormalities of cortical inhibitory neurons in amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:65–71. doi: 10.1002/(sici)1097-4598(199701)20:1<65::aid-mus9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 142.Ziemann U, Winter M, Reimers CD, Reimers K, Tergau F, Paulus W. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology. 1997;49:1292–1298. doi: 10.1212/wnl.49.5.1292. [DOI] [PubMed] [Google Scholar]
- 143.Vucic S, Kiernan MC. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain. 2006;129:2436–2446. doi: 10.1093/brain/awl172. [DOI] [PubMed] [Google Scholar]
- 144.Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- 145.Vucic S, Kiernan MC. Cortical excitability testing distinguishes Kennedy’s disease from amyotrophic lateral sclerosis. Clin Neurophysiol. 2008;119:1088–1096. doi: 10.1016/j.clinph.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 146.Vucic S, Cheah BC, Kiernan MC. Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Exp Neurol. 2009;220:177–182. doi: 10.1016/j.expneurol.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 147.Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol Aging. 1998;19:173–189. doi: 10.1016/s0197-4580(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 148.Yankner BA. The pathogenesis of Alzheimer’s disease. Is amyloid beta-protein the beginning or the end? Ann N Y Acad Sci. 2000;924:26–28. doi: 10.1111/j.1749-6632.2000.tb05555.x. [DOI] [PubMed] [Google Scholar]
- 149.Di Lazzaro V, Oliviero A, Pilato F, et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:555–559. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Di Lazzaro V, Oliviero A, Tonali PA, et al. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- 151.Liepert J, Bar KJ, Meske U, Weiller C. Motor cortex disinhibition in Alzheimer’s disease. Clin Neurophysiol. 2001;112:1436–1441. doi: 10.1016/s1388-2457(01)00554-5. [DOI] [PubMed] [Google Scholar]
- 152.Pepin JL, Bogacz D, de Pasqua V, Delwaide PJ. Motor cortex inhibition is not impaired in patients with Alzheimer’s disease: evidence from paired transcranial magnetic stimulation. J Neurol Sci. 1999;170:119–123. doi: 10.1016/s0022-510x(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 153.Julkunen P, Jauhiainen AM, Westeren-Punnonen S, et al. Navigated TMS combined with EEG in mild cognitive impairment and Alzheimer’s disease: a pilot study. J Neurosci Methods. 2008;172:270–276. doi: 10.1016/j.jneumeth.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 154.Ferreri F, Pasqualetti P, Määttä S, et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2011;54:90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 155.Di Lazzaro V, Oliviero A, Pilato F, et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005;76:1064–1069. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Di Lazzaro V, Pilato F, Dileone M, et al. Functional evaluation of cerebral cortex in dementia with Lewy bodies. Neuroimage. 2007;37:422–429. doi: 10.1016/j.neuroimage.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 157.Di Lazzaro V, Pilato F, Dileone M, et al. In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology. 2006;66:1111–1113. doi: 10.1212/01.wnl.0000204183.26231.23. [DOI] [PubMed] [Google Scholar]
- 158.Sanchez-Del-Rio M, Reuter U, Moskowitz MA. New insights into migraine pathophysiology. Curr Opin Neurol. 2006;19:294–298. doi: 10.1097/01.wco.0000227041.23694.5c. [DOI] [PubMed] [Google Scholar]
- 159.Sanchez del Rio M, Reuter U. Pathophysiology of headache. Curr Neurol Neurosci Rep. 2003;3:109–114. doi: 10.1007/s11910-003-0061-6. [DOI] [PubMed] [Google Scholar]
- 160.Ogilvie AD, Russell MB, Dhall P, et al. Altered allelic distributions of the serotonin transporter gene in migraine without aura and migraine with aura. Cephalalgia. 1998;18:23–26. doi: 10.1046/j.1468-2982.1998.1801023.x. [DOI] [PubMed] [Google Scholar]
- 161.Ophoff RA, Terwindt GM, Frants RR, Ferrari MD. P/Q-type Ca2+ channel defects in migraine, ataxia and epilepsy. Trends Pharmacol Sci. 1998;19:121–127. doi: 10.1016/s0165-6147(98)01182-1. [DOI] [PubMed] [Google Scholar]
- 162.Pietrobon D, Striessnig J. Neurobiology of migraine. Nature Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 163.Afra J, Mascia A, Gerard P, Maertens de Noordhout A, Schoenen J. Interictal cortical excitability in migraine: a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol. 1998;44:209–215. doi: 10.1002/ana.410440211. [DOI] [PubMed] [Google Scholar]
- 164.Bettucci D, Cantello R, Gianelli M, Naldi P, Mutani R. Menstrual migraine without aura: cortical excitability to magnetic stimulation. Headache. 1992;32:345–347. doi: 10.1111/j.1526-4610.1992.hed3207345.x. [DOI] [PubMed] [Google Scholar]
- 165.Siniatchkin M, Kroner-Herwig B, Kocabiyik E, Rothenberger A. Intracortical inhibition and facilitation in migraine—a transcranial magnetic stimulation study. Headache. 2007;47:364–370. doi: 10.1111/j.1526-4610.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- 166.van der Kamp W, MaassenVanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical excitability to magnetic stimulation in familial hemiplegic migraine. Neurology. 1997;48:1462–1464. doi: 10.1212/wnl.48.5.1462. [DOI] [PubMed] [Google Scholar]
- 167.Werhahn KJ, Wiseman K, Herzog J, Forderreuther S, Dichgans M, Straube A. Motor cortex excitability in patients with migraine with aura and hemiplegic migraine. Cephalalgia. 2000;20:45–50. doi: 10.1046/j.1468-2982.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 168.Gunaydin S, Soysal A, Atay T, Arpaci B. Motor and occipital cortex excitability in migraine patients. Can J Neurol Sci. 2006;33:63–67. doi: 10.1017/s0317167100004716. [DOI] [PubMed] [Google Scholar]
- 169.Bohotin V, Fumal A, Vandenheede M, Bohotin C, Schoenen J. Excitability of visual V1–V2 and motor cortices to single transcranial magnetic stimuli in migraine: a reappraisal using a figure-of-eight coil. Cephalalgia. 2003;23:264–270. doi: 10.1046/j.1468-2982.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 170.Aurora SK, al-Sayeed F, Welch KM. The cortical silent period is shortened in migraine with aura. Cephalalgia. 1999;19:708–712. doi: 10.1046/j.1468-2982.1999.019008708.x. [DOI] [PubMed] [Google Scholar]
- 171.Curra A, Pierelli F, Coppola G, et al. Shortened cortical silent period in facial muscles of patients with migraine. Pain. 2007;132:124–131. doi: 10.1016/j.pain.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 172.Ozturk V, Cakmur R, Donmez B, Yener GG, Kursad F, Idiman F. Comparison of cortical excitability in chronic migraine (transformed migraine) and migraine without aura. A transcranial magnetic stimulation study. J Neurol. 2002;249:1268–1271. doi: 10.1007/s00415-002-0834-x. [DOI] [PubMed] [Google Scholar]
- 173.Brighina F, Giglia G, Scalia S, Francolini M, Palermo A, Fierro B. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. Exp Brain Res. 2005;161:34–38. doi: 10.1007/s00221-004-2042-7. [DOI] [PubMed] [Google Scholar]
- 174.Badawy RA, Jackson GD. Cortical excitability in migraine and epilepsy: a common feature? J Clin Neurophysiol. 2012;29:244–249. doi: 10.1097/WNP.0b013e3182570fee. [DOI] [PubMed] [Google Scholar]
- 175.Battelli L, Black KR, Wray SH. Transcranial magnetic stimulation of visual area V5 in migraine. Neurology. 2002;58:1066–1069. doi: 10.1212/wnl.58.7.1066. [DOI] [PubMed] [Google Scholar]
- 176.Aurora SK, Ahmad BK, Welch KM, Bhardhwaj P, Ramadan NM. Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology. 1998;50:1111–1114. doi: 10.1212/wnl.50.4.1111. [DOI] [PubMed] [Google Scholar]
- 177.Gerwig M, Niehaus L, Kastrup O, Stude P, Diener HC. Visual cortex excitability in migraine evaluated by single and paired magnetic stimuli. Headache. 2005;45:1394–1399. doi: 10.1111/j.1526-4610.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 178.Young WB, Oshinsky ML, Shechter AL, Gebeline-Myers C, Bradley KC, Wassermann EM. Consecutive transcranial magnetic stimulation: phosphene thresholds in migraineurs and controls. Headache. 2004;44:131–135. doi: 10.1111/j.1526-4610.2004.04028.x. [DOI] [PubMed] [Google Scholar]
- 179.Antal A, Arlt S, Nitsche MA, Chadaide Z, Paulus W. Higher variability of phosphene thresholds in migraineurs than in controls: a consecutive transcranial magnetic stimulation study. Cephalalgia. 2006;26:865–870. doi: 10.1111/j.1468-2982.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 180.Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- 181.Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 182.Filipović SR, Ljubisavljević M, Svetel M, Milanović S, Kacar A, Kostić VS. Impairment of cortical inhibition in writer’s cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett. 1997;222:167–170. doi: 10.1016/s0304-3940(97)13370-5. [DOI] [PubMed] [Google Scholar]
- 183.Currà A, Romaniello A, Berardelli A, Cruccu G, Manfredi M. Shortened cortical silent period in facial muscles of patients with cranial dystonia. Neurology. 2000;54:130–135. doi: 10.1212/wnl.54.1.130. [DOI] [PubMed] [Google Scholar]
- 184.Allam N, Fonte-Boa PM, Tomaz CA, Brasil-Neto JP. Lack of effect of botulinum toxin on cortical excitability in patients with cranial dystonia. Clin Neuropharmacol. 2005;28:1–5. doi: 10.1097/01.wnf.0000152044.43822.42. [DOI] [PubMed] [Google Scholar]
- 185.Bütefisch CM, Boroojerdi B, Chen R, Battaglia F, Hallett M. Task-dependent intracortical inhibition is impaired in focal hand dystonia. Mov Disord. 2005;20:545–551. doi: 10.1002/mds.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gilio F, Curra A, Inghilleri M, et al. Abnormalities of motor cortex excitability preceding movement in patients with dystonia. Brain. 2003;126:1745–1754. doi: 10.1093/brain/awg188. [DOI] [PubMed] [Google Scholar]
- 188.Huang YZ, Trender-Gerhard I, Edwards MJ, Mir P, Roth-well JC, Bhatia KP. Motor system inhibition in dopa-responsive dystonia and its modulation by treatment. Neurology. 2006;66:1088–1090. doi: 10.1212/01.wnl.0000214304.03105.f4. [DOI] [PubMed] [Google Scholar]
- 189.Edwards MJ, Huang YZ, Wood NW, Rothwell JC, Bhatia KP. Different patterns of electrophysiological deficits in manifesting and non-manifesting carriers of the DYT1 gene mutation. Brain. 2003;126:2074–2080. doi: 10.1093/brain/awg209. [DOI] [PubMed] [Google Scholar]
- 190.Chen R, Wassermann EM, Canos M, Hallett M. Impaired inhibition in writer’s cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- 191.Rona S, Berardelli A, Vacca L, Inghilleri M, Manfredi M. Alterations of motor cortical inhibition in patients with dystonia. Mov Disord. 1998;13:118–124. doi: 10.1002/mds.870130123. [DOI] [PubMed] [Google Scholar]
- 192.Sommer M, Ruge D, Tergau F, Beuche W, Altenmuller E, Paulus W. Intracortical excitability in the hand motor representation in hand dystonia and blepharospasm. Mov Disord. 2002;17:1017–1025. doi: 10.1002/mds.10205. [DOI] [PubMed] [Google Scholar]
- 193.Kanovský P, Bares M, Streitová H, Klajblová H, Daniel P, Rektor I. Abnormalities of cortical excitability and cortical inhibition in cervical dystonia Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J Neurol. 2003;250:42–50. doi: 10.1007/s00415-003-0942-2. [DOI] [PubMed] [Google Scholar]
- 194.Weise D, Schramm A, Beck M, Reiners K, Classen J. Loss of topographic specificity of LTD-like plasticity is a trait marker in focal dystonia. Neurobiol Dis. 2011;42:171–176. doi: 10.1016/j.nbd.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 195.Quartarone A, Morgante F, Sant’angelo A, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- 196.Huang YZ, Rothwell JC, Lu CS, Wang J, Chen RS. Restoration of motor inhibition through an abnormal pre-motor-motor connection in dystonia. Mov Disord. 2010;25:689–696. doi: 10.1002/mds.22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Boroojerdi B, Cohen LG, Hallett M. Effects of botulinum toxin on motor system excitability in patients with writer’s cramp. Neurology. 2003;61:1546–1550. doi: 10.1212/01.wnl.0000095965.36574.0f. [DOI] [PubMed] [Google Scholar]
- 198.Tuite PJ, Krawczewski K. Parkinsonism: a review-of-systems approach to diagnosis. Semin Neurol. 2007;27:113–122. doi: 10.1055/s-2007-971174. [DOI] [PubMed] [Google Scholar]
- 199.Christine CW, Aminoff MJ. Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance. Am J Med. 2004;117:412–419. doi: 10.1016/j.amjmed.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 200.Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord. 2008;23(Suppl 3):S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 201.Litvan I, Campbell G, Mangone CA, et al. Which clinical features differentiate progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) from related disorders? A clinicopathological study. Brain. 1997;120:65–74. doi: 10.1093/brain/120.1.65. [DOI] [PubMed] [Google Scholar]
- 202.Swan L, Dupont J. Multiple system atrophy. Phys Ther. 1999;79:488–494. [PubMed] [Google Scholar]
- 203.Soysal A, Sobe I, Atay T, Sen A, Arpaci B. Effect of therapy on motor cortical excitability in Parkinson’s disease. Can J Neurol Sci. 2008;35:166–172. doi: 10.1017/s0317167100008581. [DOI] [PubMed] [Google Scholar]
- 204.Cantello R, Gianelli M, Bettucci D, Civardi C, De Angelis MS, Mutani R. Parkinson’s disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology. 1991;41:1449–1456. doi: 10.1212/wnl.41.9.1449. [DOI] [PubMed] [Google Scholar]
- 205.Valls-Solé J, Pascual-Leone A, Brasil-Neto JP, Cammarota A, McShane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson’s disease. Neurology. 1994;44:735–741. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- 206.Siebner HR, Mentschel C, Auer C, Lehner C, Conrad B. Repetitive transcranial magnetic stimulation causes a short-term increase in the duration of the cortical silent period in patients with Parkinson’s disease. Neurosci Lett. 2000;284:147–150. doi: 10.1016/s0304-3940(00)00990-3. [DOI] [PubMed] [Google Scholar]
- 207.Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain. 1994;117:317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- 208.Strafella A, Ashby P, Munz M, Dostrovsky JO, Lozano AM, Lang AE. Inhibition of voluntary activity by thalamic stimulation in humans: relevance for the control of tremor. Mov Disord. 1997;12:727–737. doi: 10.1002/mds.870120517. [DOI] [PubMed] [Google Scholar]
- 209.Young MS, Triggs WJ, Bowers D, Greer M, Friedman WA. Stereotactic pallidotomy lengthens the transcranial magnetic cortical stimulation silent period in Parkinson’s disease. Neurology. 1997;49:1278–1283. doi: 10.1212/wnl.49.5.1278. [DOI] [PubMed] [Google Scholar]
- 210.Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- 211.Chen R, Garg RR, Lozano AM, Lang AE. Effects of internal globus pallidus stimulation on motor cortex excitability. Neurology. 2001;56:716–723. doi: 10.1212/wnl.56.6.716. [DOI] [PubMed] [Google Scholar]
- 212.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 213.Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology. 2002;58:1665–1672. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- 214.Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115:2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 215.Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson’s disease. A study with paired magnetic stimulation. Brain. 1996;119:71–77. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- 216.Pierantozzi M, Palmieri MG, Marciani MG, Bernardi G, Giacomini P, Stanzione P. Effect of apomorphine on cortical inhibition in Parkinson’s disease patients: a transcranial magnetic stimulation study. Exp Brain Res. 2001;141:52–62. doi: 10.1007/s002210100839. [DOI] [PubMed] [Google Scholar]
- 217.Dauper J, Peschel T, Schrader C, et al. Effects of subthalamic nucleus (STN) stimulation on motor cortex excitability. Neurology. 2002;59:700–706. doi: 10.1212/wnl.59.5.700. [DOI] [PubMed] [Google Scholar]
- 218.Bares M, Kanovsky P, Klajblova H, Rektor I. Intracortical inhibition and facilitation are impaired in patients with early Parkinson’s disease: a paired TMS study. Eur J Neurol. 2003;10:385–389. doi: 10.1046/j.1468-1331.2003.00610.x. [DOI] [PubMed] [Google Scholar]
- 219.Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson’s disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- 220.Nardone R, Florio I, Lochner P, Tezzon F. Cholinergic cortical circuits in Parkinson’s disease and in progressive supranuclear palsy: a transcranial magnetic stimulation study. Exp Brain Res. 2005;163:128–131. doi: 10.1007/s00221-005-2228-7. [DOI] [PubMed] [Google Scholar]
- 221.Mascia MM, Valls-Sole J, Marti MJ, Salazar G. Sensorimotor integration in patients with parkinsonian type multisystem atrophy. J Neurol. 2005;252:473–481. doi: 10.1007/s00415-005-0678-2. [DOI] [PubMed] [Google Scholar]
- 222.Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 223.Eisen A, Bohlega S, Bloch M, Hayden M. Silent periods, long-latency reflexes and cortical MEPs in Huntington’s disease and at-risk relatives. Electroencephalogr Clin Neurophysiol. 1989;74:444–449. doi: 10.1016/0168-5597(89)90034-8. [DOI] [PubMed] [Google Scholar]
- 224.Tegenthoff M, Vorgerd M, Juskowiak F, Roos V, Malin JP. Postexcitatory inhibition after transcranial magnetic single and double brain stimulation in Huntington’s disease. Electroencephalogr Clin Neurophysiol. 1996;101:298–303. doi: 10.1016/0924-980x(96)94645-7. [DOI] [PubMed] [Google Scholar]
- 225.Modugno N, Curra A, Giovannelli M, et al. The prolonged cortical silent period in patients with Huntington’s disease. Clin Neurophysiol. 2001;112:1470–1474. doi: 10.1016/s1388-2457(01)00599-5. [DOI] [PubMed] [Google Scholar]
- 226.Abbruzzese G, Buccolieri A, Marchese R, Trompetto C, Mandich P, Schieppati M. Intracortical inhibition and facilitation are abnormal in Huntington’s disease: a paired magnetic stimulation study. Neurosci Lett. 1997;228:87–90. doi: 10.1016/s0304-3940(97)00363-7. [DOI] [PubMed] [Google Scholar]
- 227.Priori A, Berardelli A, Inghilleri M, Polidori L, Manfredi M. Electromyographic silent period after transcranial brain stimulation in Huntington’s disease. Mov Disord. 1994;9:178–182. doi: 10.1002/mds.870090209. [DOI] [PubMed] [Google Scholar]
- 228.Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral corticocortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci. 1996;140:109–116. doi: 10.1016/0022-510x(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 229.Lombroso PJ, Scahill L. Tourette syndrome and obsessive-compulsive disorder. Brain Dev. 2008;30:231–237. doi: 10.1016/j.braindev.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Orth M. Transcranial magnetic stimulation in Gilles de la Tourette syndrome. J Psychosom Res. 2009;67:591–598. doi: 10.1016/j.jpsychores.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 231.Heise KF, Steven B, Liuzzi G, et al. Altered modulation of intracortical excitability during movement preparation in Gilles de la Tourette syndrome. Brain. 2010;133:580–590. doi: 10.1093/brain/awp299. [DOI] [PubMed] [Google Scholar]
- 232.Orth M, Amann B, Robertson MM, Rothwell JC. Excitability of motor cortex inhibitory circuits in Tourette syndrome before and after single dose nicotine. Brain. 2005;128:1292–1300. doi: 10.1093/brain/awh473. [DOI] [PubMed] [Google Scholar]
- 233.Benito-Léon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. 2007;369:1152–1154. doi: 10.1016/S0140-6736(07)60544-3. [DOI] [PubMed] [Google Scholar]
- 234.Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord. 2008;23:174–182. doi: 10.1002/mds.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Romeo S, Berardelli A, Pedace F, Inghilleri M, Giovannelli M, Manfredi M. Cortical excitability in patients with essential tremor. Muscle Nerve. 1998;21:1304–1308. doi: 10.1002/(sici)1097-4598(199810)21:10<1304::aid-mus9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 236.Modugno N, Nakamura Y, Bestmann S, Curra A, Berardelli A, Rothwell J. Neurophysiological investigations in patients with primary writing tremor. Mov Disord. 2002;17:1336–1340. doi: 10.1002/mds.10292. [DOI] [PubMed] [Google Scholar]
- 237.Brown P, Ridding MC, Werhahn KJ, Rothwell JC, Marsden CD. Abnormalities of the balance between inhibition and excitation in the motor cortex of patients with cortical myoclonus. Brain. 1996;119:309–317. doi: 10.1093/brain/119.1.309. [DOI] [PubMed] [Google Scholar]
- 238.Thompson PD, Day BL. The anatomy and physiology of cerebellar disease. Adv Neurol. 1993;61:15–31. [PubMed] [Google Scholar]
- 239.Di Lazzaro V, Restuccia D, Molinari M, et al. Excitability of the motor cortex to magnetic stimulation in patients with cerebellar lesions. J Neurol Neurosurg Psychiatry. 1994;57:108–110. doi: 10.1136/jnnp.57.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Di Lazzaro V, Restuccia D, Nardone R, et al. Motor cortex changes in a patient with hemicerebellectomy. Electroencephalogr Clin Neurophysiol. 1995;97:259–263. doi: 10.1016/0013-4694(95)00110-k. [DOI] [PubMed] [Google Scholar]
- 241.Cruz-Martinez A, Arpa J. Transcranial magnetic stimulation in patients with cerebellar stroke. Eur Neurol. 1997;38:82–87. doi: 10.1159/000113165. [DOI] [PubMed] [Google Scholar]
- 242.Ugawa Y, Hanajima R, Kanazawa I. Motor cortex inhibition in patients with ataxia. Electroencephalogr Clin Neurophysiol. 1994;93:225–229. doi: 10.1016/0168-5597(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 243.Liepert J, Wessel K, Schwenkreis P, et al. Reduced intra-cortical facilitation in patients with cerebellar degeneration. Acta Neurol Scand. 1998;98:318–323. doi: 10.1111/j.1600-0404.1998.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 244.Schwenkreis P, Tegenthoff M, Witscher K, et al. Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect. Brain. 2002;125:301–309. doi: 10.1093/brain/awf023. [DOI] [PubMed] [Google Scholar]
- 245.Restivo DA, Lanza S, Saponara R, Rapisarda G, Giuffrida S, Palmeri A. Changes of cortical excitability of human motor cortex in spinocerebellar ataxia type 2. A study with paired transcranial magnetic stimulation. J Neurol Sci. 2002;198:87–92. doi: 10.1016/s0022-510x(02)00086-2. [DOI] [PubMed] [Google Scholar]
- 246.Tamburin S, Fiaschi A, Marani S, Andreoli A, Manganotti P, Zanette G. Enhanced intracortical inhibition in cerebellar patients. J Neurol Sci. 2004;217:205–210. doi: 10.1016/j.jns.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 247.Liepert J, Kucinski T, Tuscher O, Pawlas F, Baumer T, Weiller C. Motor cortex excitability after cerebellar infarction. Stroke. 2004;35:2484–2488. doi: 10.1161/01.STR.0000143152.45801.ca. [DOI] [PubMed] [Google Scholar]
- 248.Restivo DA, Lanza S, Giuffrida S, et al. Cortical silent period prolongation in spinocerebellar ataxia type 2 (SCA2) Funct Neurol. 2004;19:37–41. [PubMed] [Google Scholar]
- 249.Wessel K, Tegenthoff M, Vorgerd M, Otto V, Nitschke MF, Malin JP. Enhancement of inhibitory mechanisms in the motor cortex of patients with cerebellar degeneration: a study with transcranial magnetic brain stimulation. Electroencephalogr Clin Neurophysiol. 1996;101:273–280. doi: 10.1016/0924-980x(96)95531-9. [DOI] [PubMed] [Google Scholar]
- 250.Oechsner M, Zangemeister WH. Prolonged postexcitatory inhibition after transcranial magnetic stimulation of the motor cortex in patients with cerebellar ataxia. J Neurol Sci. 1999;168:107–111. doi: 10.1016/s0022-510x(99)00164-1. [DOI] [PubMed] [Google Scholar]