Abstract
Glutathionylspermidine synthetase/amidase (Gss) and the encoding gene (gss) have only been studied in Escherichia coli and several members of the Kinetoplastida phyla. In the present paper we have studied the phylogenetic distribution of Gss, and have found that Gss sequences are largely limited to certain bacteria and Kinetoplastids, and are absent in a variety of invertebrate and vertebrate species, Archea, plants and some Eubacteria. It is striking that almost all of the 75 Enterobacteria species that have been sequenced contain sequences with very high degree of homology to the E. coli Gss protein. In order to find out the physiological significance of glutathionylspermidine in E. coli, we have performed global transcriptome analyses. The microarray studies comparing gss+ and Δgss strains of E. coli show that a large number of genes are either upregulated (76 genes more than 3-fold) or downregulated (35 genes more than 3-fold) by the loss of the gss gene. Most significant categories of up-regulated genes include sulfur utilization, glutamine and succinate metabolism, polyamine and arginine metabolism, and purine and pyrimidine metabolism.
Keywords: Bacteria, Enterobacteria, microarray, polyamines, spermidine
INTRODUCTION
Earlier work from this laboratory showed that 95 percent of the intracellular spermidine and a large fraction of the intracellular glutathione are converted to monoglutathionylspermidine in Escherichia coli at the end of logarithmic growth (Dubin, 1959; Tabor and Tabor, 1970). Bollinger and his colleague (Bollinger et al., 1995) and Kwon and his group (Kwon et al., 1997) reported the purification of glutathionylspermidine synthetase/amidase of E. coli and showed that the bifunctional enzyme had a separate amidase and synthetase domains. Later Pai et al reported crystal structures of E. coli Gss in complex with substrate, product and inhibitor (Pai et al., 2006).
In 1985 Fairlamb and his group (Fairlamb et al., 1985) reported that glutathionylspermidine and diglutathionylspermidine (trypanothione) are present in trypanosomes, and that diglutathionylspermidine disulfide, rather than glutathione disulfide, is the substrate for a glutathionyl-like reductase in trypanosomes. These findings probably account for the therapeutic efficacy of difluoromethylornithine, an inhibitor of polyamine biosynthesis, in African trypanosomiasis (Fairlamb, 1988; Wyllie et al., 2009).
Trypanothione is not present in E. coli. In contrast to the large amount of glutathionylspermidine found in stationary and near stationary E. coli cultures, the earlier studies indicated that logarithmically-growing cultures of E. coli contain very little (Smith et al., 1995) or no detectable (Tabor and Tabor, 1976) glutathionylspermidine. Since the formation of glutathionylspermidine affects the intracellular levels of both spermidine and glutathione, we felt that it is important to test whether the Gss is only present in certain bacteria and Kinetoplastids. Therefore, we have carried out BLAST searches of the NCBI databases, and have found that the distribution of the Gss is indeed very limited. The small amount of glutathionylspermidine present in logarithmically growing cultures poses the question of whether glutathionylspermidine synthase has any physiological function in logarithmically growing E. coli. Therefore we have carried out microarray studies of E. coli, comparing a strain with a deletion in the gene coding for glutathionylspermidine synthetase (Δgss) with a gss+ strain, and have found that a large number of genes are upregulated or downregulated in the Δgss strain compared to the gss+ strain.
MATERIALS AND METHODS
Strains and culture medium
Strains used in this study are listed in Table 1. Cultures were grown in M9 medium (Miller, 1992) containing 0.4% glucose; incubation was at 37 ° C with shaking.
Table 1.
Strains used.
| Relevant genotype* | Source% | |
|---|---|---|
| HT761 | Δgss:kan | CGSC8299; JW29561 |
| HT779 | gss+ | CGSC7636; BW25113 |
| HT784 | Δgss | Transduction P1(HT761) into HT779 # |
All strains also had the following genotype: 7F−, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ−, rph-1, Δ(rhaD-rhaB)568, hsdR514
The kanamycin insert was excised from the transductant by the Flippase recombination enzyme as described by Baba et al (Baba et al., 2006) and by Datasenko and Wanner(Datsenko and Wanner, 2000).
CGSC: The Coli Genetic Stock Center. E. coli Genetic Resources at Yale. These strains were from their Keio collection. CGSC7636 (HT779) is the parent strain of the Keio collection.
Phylogenetic analyses
For a comparison of the different phyla BLAST searches were carried out comparing the E. coli Gss amino acid sequences (accession number AAC76024.1) with the non-redundant protein databases of the National Center for Biotechnology Information (NCBI). The cutoff level for significant homology, as defined by Hall (Hall, 2011) is e <10−3 and query coverage >55%.
Spermidine analysis
The cultures were incubated with shaking in air until the OD600 was 0.7–0.8 (log phase culture) or 2.8–3.0 (stationary phase culture). The cells were collected by centrifugation, extracted with perchloric acid and 5 µl of the 10% perchloric acid extract, representing 1 mg of cells (wet weight) were then analyzed by ion exchange chromatography essentially as described earlier (Murakami et al., 1989; Chattopadhyay et al., 2009b) using a Shim-pack column (Shimadzu, ISC-05/S0504); the eluting buffer was 1.6 M NaCl, 0.2 M sodium citrate.
Radioactive glutathionylspermidine analysis
Four ml of the M9 medium was inoculated with a single colony of each strain and grown at 37° C overnight. The overnight cultures were diluted into 50 ml of medium to an OD600 of 0.05 and grown for several hours to an OD600 of 0.2. To determine the relative amounts of glutathionylspermidine and of spermidine in each strain, cells were pre-labeled with 1.25 µCi of [14C]-spermidine trihydrochloride (12.5 nmoles), and the incubation was continued for either 2 hours ("log phase culture” OD600=0.7) or 20 hours ("overgrown culture"). The cultures were rapidly centrifuged at room temperature. The pellets were washed twice with medium and re-suspended in 10 % perchloric acid (1:5 wt/vol); the supernatants were subjected to HPLC chromatography on a Shim-pack cation exchange column with the elution system described in the previous section but with 1.0 M NaCl-0.2 M sodium citrate as the elution buffer. The elutes were collected at 2 minute intervals (0.7 ml/min), and a 100 µl aliquot from each fraction was counted in a Beckman scintillation counter (LS6500).
RNA isolation, Microarray analysis
Three independent cultures (109–1010 cells) from the E. coli gss+ and Δgss cells (OD600 of 0.7–0.8) were harvested, and resuspended in Tris-EDTA buffer (100 mM Tris, 10 mM EDTA, pH 8.0) containing 2 mg/ml lysozyme (Sigma). The cell suspensions were incubated for 5 min at room temperature to digest the cell wall. Total RNA was isolated according to the protocol described in the RNeasy mini kit (Qiagen, Germantown, MD). The mRNAs were enriched from total RNA by removing the 16S and 23S ribosomal RNAs using the MICROBExpress method and kit (Ambion, part # AM1905). The quantity and quality of RNA were evaluated by OD260/OD280 assays and by RNA capillary electrophoresis (Agilent Biotechnologies). Enriched mRNAs were reverse transcribed by Superscript II and random hexanucleotide primer (Invitrogen) and used for microarrays as described earlier (Chattopadhyay et al., 2009a) using Affymetrix (Santa Clara, CA) E. coli GeneChip arrays (Genome 2.0 array; n= 3 each for gss+ and Δgss). ANOVA (analysis of variance) was performed and p-values were calculated by using Partek Pro-software (Partek, St. Louis, MO) and plotted in negative log scale on y-axis against the Affimetrix signal ratios for each probe set on x-axis. Up- and down-regulated genes were selected based on p-values of <0.05 and fold change >+2 or −2. The complete microarray data can be obtained from GEO (accession number GSE30679).
RESULTS
Glutathionylspermidine synthetase/amidase is only found in two distinct groups: Eubacteria and Kinetoplastida
Most striking is that sequences homologous to E. coli Gss are only found in Eubacteria and the very distantly related Kinetoplastids (plus two fungal species with relatively low homology) (Table 2). No homologous sequences (as defined by the BLAST-P program) were found when the E. coli Gss sequence was compared with the human, rat, mouse, Arabidopsis, rice, worm, and Drosophila sequence databases (Table 2).
Table 2.
Distribution of Gss in different taxonomic groups*
| Taxonomic group | Number of sequences analyzed |
Number of species showing significant homology |
Identity# | e-value |
|---|---|---|---|---|
| Prokaryota | ||||
| Archaea | 91 | None | ||
| Eubacteria | ||||
| Actinobacteria | 170 | 42 | 20–30% | 3e−16−4e−28 |
| Bacteroidetes/Chlorobi | 113 | 7 | 25–28% | 7e−4−2e−31 |
| Chlamydiae | 11 | None | ||
| Cyanobacteria | 25 | 4 | 19–27% | 2e−23−4e−29 |
| Firmicutes | ||||
| Bacillles | 61 | 12 | 18–22% | 1e−4−4e−7 |
| Proteobacteria | ||||
| α-subdivision | 175 | 42 | 22–48% | 3e−04−1e−167 |
| β-subdivision | 105 | 5 | 24–26% | 4e−12−2e−21 |
| δ-subdivision | 46 | 1 | 27% | 1e−23 |
| ε-subdivision | 35 | 22 | 24–26% | 1e−12−3e−20 |
| γ-subdivision | ||||
| Enterobacteriales | 75 | 75 | 27–100% | 4e−32−0 |
| Pasteurellales | 18 | 9 | 28–66% | 7e−19−0 |
| Pseudomonadales | 14 | 7 | 25–28% | 3e−22−2e−30 |
| Vibrionales | 24 | 8 | 26–70% | 1e−10−0 |
| Xanthomonadales | 13 | 8 | 23–27% | 4e−18−2e−27 |
| Spirochaetes | 28 | None | ||
| Eukaryota | ||||
| Archaeoplastida (Plants &Algae) | 6 | None | ||
| Opisthokonta | ||||
| Fungi | 132 | 2 | 25–27% | 2e−14−4e−17 |
| Invertebrate | 51 | None | ||
| Vertebrate** | 23 | None | ||
| Excavata | ||||
| Kinetoplastida | 5 | 5 | 26–33% | 6e−31−5e−83 |
| Other Microbial Eukaryotes |
35 | 1 | 26% | 1e−19 |
The taxonomic classifications are based on the taxonomic groupings in NCBI database and BLAST P using NCBI database. The distribution of sequences homologous to the Gss amino acid sequences is based on BlastP analyses of the various microbial sequences in the NCBI database, omitting any redundant sequence for specific species.
Calculated for those species showing significant homology to the E. coli Gss amino acid sequence.
No significant homology was found when the E. coli Gss amino acid sequence was used to BLAST search in different vertebrate (mammals, zebra fish, chicken, western clawed frog, etc) and invertebrate (arthropode, nematode, echinoderm, chidaria, etc) protein sequence databases.
Among the bacterial species the homology was highest in the Proteobacteria and was particularly high in almost all of the 75 Enterobacteriales species that have been sequenced (27–100% homology in all of the Enterobacteria species, and >65% identity in 50 % of the species). A comparison of different species (Table 2) shows that outside of the Proteobacteria homologous sequences are only found in a few other bacterial species, and these have much less homology.
Glutathionylspermidine and spermidine levels in gss+ and Δgss strains
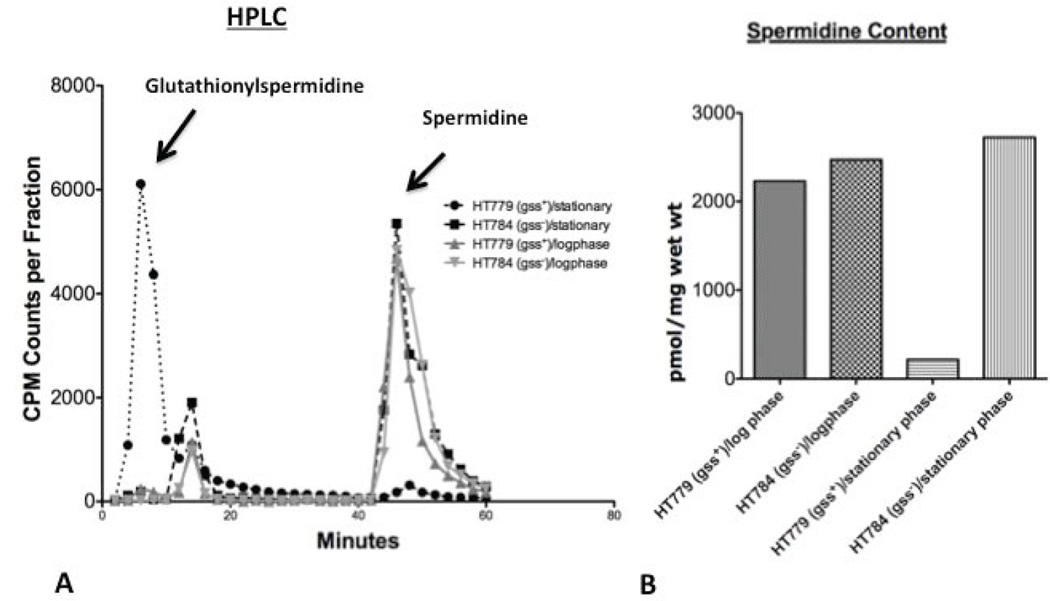
In order to measure the conversion of intracellular spermidine to glutathionylspermidine in stationary versus log phase cultures of gss+ and Δgss strains of E. coli, [14C]-spermidine labeled cells were analyzed on a cation exchange column as described in Materials and Methods. As shown in Figure 1A, confirming previous results from this and other laboratories, most of the spermidine in stationary cultures of a gss+ (wild type) strain was converted to glutathionylspermidine and a much smaller amount of conversion was found in log phase cultures (Dubin, 1959; Tabor and Tabor, 1970; Tabor and Tabor, 1976; Tabor and Tabor, 1971; Bollinger et al., 1995; Smith et al., 1995). No conversion of spermidine to glutathionylspermidine was found in cells containing a deletion in the gss gene (gss−) (Fig. 1A).
Figure 1. Comparison of spermidine and glutathionylspermidine levels in wild type (gss+) and Δgss mutant (gss−) in log phase and stationary phase cultures.
In both parts A and B cultures were grown in M9 medium to OD600 0.7 (log phase) or OD600 2.5 (stationary phase), harvested and washed. The cell pellets were extracted with 10% perchloric acid and analyzed by HPLC. In part A the cells were pre-labeled with [14C]-spermidine as described in the Materials and Methods and HPLC elutes were assayed for radioactivity. The peak near 12–14 minutes represents monoacetylspermidine. In part B the extracts were assayed for spermidine content by reaction with o-phthaldehyde as described in the text.
As shown in Fig. 1B, there was a very large decrease (85–90%) in the spermidine content of gss+ cells observed in a stationary phase culture (compared to a gss− control), but only a small decrease (10–15%) in the spermidine content of a log phase culture.
Comparison of global gene expression in gss+ and Δgss E. coli
We have applied microarray analysis to study the global gene expression profile of logarithmically growing E. coli cultures (OD600 of 0.7–0.8). We used logarithmically-growing cultures since, as shown in Figure 1, in stationary phase wild type E. coli converts most of the spermidine into glutathionylspermidine and global gene expression might be affected by a decrease in the glutathione and spermidine levels; in contrast only 10–15% of spermidine is converted to glutathionylspermidine in logarithmically growing cells.
The effects of the gss deletion on gene expression are shown in Fig. 2 and Tables 3 –4. There was no expression of the gss gene in Δgss cells, as compared to a high level of expression of gss in gss+ cells (Fig. 2, Table 3B). It is evident from the volcanic graph (Fig. 2), that the gss deletion has a pronounced effect on gene expression. To facilitate data analysis, the genes were grouped into functional categories based on Ecogene database, Affymetrix gene ID and gene ontology (GO). Top GO categories of up and down-regulated genes are presented in Table 3–4, and supplementary figure S3.
Figure 2. Microarray studies showing the differences in gene expression in Δgss and gss+ cultures.
Volcano plot demonstrates the relationship between significance in y-axis (expressed in p-values obtained from ANOVA) and Affimetrix signal ratios in x-axis (fold change) in gene relative expression in Δgss vs gss+ cells. The two boxes highlight genes that are more than four-fold up or down-regulated.
Table 3.
| Table 3A. Genes upregulated (4-fold or more) in gss mutant (Δgss) over wild type (gss+). In these tables data are the average of three independent experiments. "Annoted functions" are based on Ecogene and Affymetrix databases. | |||
|---|---|---|---|
| Pathways | Gene names |
Annoted functions | Fold change |
| Arginine /polyamine metabolism | |||
| carA | carbamoyl phosphate synthase small subunit | 7.5 | |
| carB | carbamoyl phosphate synthase large subunit | 5.5 | |
| puuD | gamma-glutamyl-gamma-aminobutyrate hydrolase | 4.5 | |
| Purine metabolism | |||
| purM | phosphoribosylaminoimidazolesynthetase | 7.7 | |
| purD | phosphoribosylglycinamidesynthetasephosphoribosylamine-glycine ligase | 6.9 | |
| purH | bifunctionalphosphoribosylaminoimidazolecarboxamideformyltransferase | 5.8 | |
| purL | phosphoribosylformyl-glycineamidesynthetase | 5.6 | |
| purK | phosphoribosylaminoimidazole carboxylase ATPase subunit | 5.5 | |
| purF | Amidophosphoribosyltransferase | 4.3 | |
| purE | phosphoribosylaminoimidazole carboxylase catalytic subunit | 4.1 | |
| Sulfur utilization/succinate metabolism | |||
| cysD | sulfateadenylyltransferase, subunit | 8.2 | |
| cysJ | sulfitereductase, subunit alpha | 7.4 | |
| cysP | Thiosulfate transporter subunit | 6.4 | |
| metF | 5,10-methylenetetrahydrofolate reductase | 5.1 | |
| cysI | sulfitereductase, beta subunit | 4.5 | |
| astD | succinylglutamicsemialdehyde dehydrogenase | 4.0 | |
| sdhD | succinate dehydrogenase cytochrome b556, small membrane subunit | 10.1 | |
| sdhC | succinate dehydrogenase cytochrome b556, large membrane subunit | 8.7 | |
| sdhA | succinate dehydrogenase, flavoprotein subunit | 6.2 | |
| Table 3B. Genes downregulated (−3 fold or more) in Δgss cells as compared to wild type (gss+) cells. | ||
|---|---|---|
| Gene names |
Annoted functions | Fold- change |
| gss | bifunctional glutathionylspermidine amidase/glutathionylspermidine synthetase | −75.8 |
| yghW | hypothetical protein | −10.8 |
| modA | molybdate transporter periplasmic protein | −6.6 |
| modB | molybdate ABC transporter permease protein | −6.0 |
| nirC | nitrite transporter | −5.1 |
| nirD | nitritereductase small subunit /// nitrite reductase, NAD(P)H-binding | −5.0 |
| yjjW | predicted pyruvate formatelyase activating enzyme | −4.6 |
| frdC | fumaratereductase subunit C (anaerobic) | −4.4 |
| cusF | periplasmic copper-binding protein | −4.4 |
| dcuC | C4-dicarboxylate transporter, (anaerobic) | −4.2 |
| cusB | copper/silver efflux system membrane fusion protein | −3.7 |
| modC | molybdate transporter ATP-binding protein | −3.7 |
| adiY | putative ARAC-type regulatory protein | −3.6 |
| hcr | HCP oxidoreductase, NADH-dependent | −3.6 |
| hypC | hydrogenase assembly chaperone | −3.4 |
| frdD | fumaratereductase subunit D (anaerobic) | −3.3 |
| gldA | glycerol dehydrogenase, NAD | −3.2 |
| frdB | fumaratereductase iron-sulfur subunit | −3.2 |
| dcuB | anaerobic C4-dicarboxylate transporter, antiporter | −3.1 |
| fumB | fumarase B /// fumaratehydratase class I, anaerobic | −3.0 |
| narI | nitratereductase 1 gamma subunit | −3.0 |
Table 4.
Effect of gss deletion on transcriptional regulators- up-regulated and down-regulated in Δgss cells over gss+ cells.
| Gene names | Annoted Functions | Fold- change |
|---|---|---|
| Up-regulated | ||
| mhpR | DNA-binding transcriptional activator of m-Hydroxyphenylpropionic acid operon | 4.4 |
| ydcI | Putative transcriptional regulator LYSR-type, function unknown | 3.9 |
| pdhR | Pyruvate dehydrogenase operon (pdhR-aceEF-lpd) repressor | 3.6 |
| betI | Repressor for the betIBAbetTdivergon (a pair of divergently transcribed operons that work together in the same biological system); choline-sensing | 3.4 |
| hcaR | Transcriptional activator of the hca operon; inducd by 3-phenylpropionate and cinnamic acid | 3.1 |
| glcC | Transcriptional activator for glc operon, glycolate-binding | 3.0 |
| puuR | Transcriptional repressor for the puudivergon; putrescine utilization pathway | 2.8 |
| putA | Trifunctional transcriptional regulator, Proline dehydrogenase and repressor for the putAPdivergon | 2.8 |
| lldR | Dual role activator/repressor for lldPRD operon | 2.4 |
| uhpA | Response regulator of two component system required for uhpT transcription | 2.0 |
| Down-regulated | ||
| adiY | Transcriptional activator for adiA, AraC family | −3.7 |
| ttdR | Transcriptional activator of ttdABT, tartrate-inducible; required for tartrate utilization; anaerobiosis nucleoid protein | −2.9 |
| cadC | Transcriptional activator for cadBA, low external pH-, low oxygen-, excess lysine-responsive | −2.8 |
| appY | Global transcription regulator, AraC family | −2.3 |
Effect of gss gene deletion on up-regulation of gene expression
When compared with the levels in the gss+ cells, in the Δgss cells transcripts of 183 genes were up-regulated more than 2-fold. 76 genes were up-regulated >3-fold, 24 genes were up-regulated >5-fold. Most significant categories of up-regulated genes include sulfur utilization, glutamine and succinate metabolism, polyamine and arginine metabolism, and purine and pyrimidine metabolism. As shown in Table 3 and supplementary figures S3A–S3D, many genes involved in polyamine and arginine metabolism, purine and pyrimidine biosynthesis, sulfate and succinate metabolism show 2–10-fold higher induction of gene expression in Δgss cells as compared to gss+ cells.
Effect of gss gene deletion on repression of gene expression
Deletion of gss gene resulted in down-regulation of 134 genes 2-fold as compared to wild type cells. 35 genes were down-regulated more than 3-fold, and 12 genes were down-regulated more than 4-fold. Several genes related to molybdate transporters (Table 3B, heat-map in supplementary figure S3E), nitrate transporters, copper transport/efflux (Table 3B and heat-map in supplementary figure S3F) and C4-dicarboxylate transporters were repressed in Δgss cells. Several oxidoreductases such as fumarate HCP oxidoreductase and glutaredoxin were also repressed.
Effect of deletion of gss gene on transcriptional regulators
Increased or decreased transcription of the large number of genes presented above may not be due to a direct effect of gss gene deletion; rather expression of twelve transcriptional regulators are increased in Δgss cells and four transcriptional activators are repressed in Δgss cells as compared to gss+ cells during log phase (Table 4).
DISCUSSION
It is striking that the Gss sequences have been conserved with a high degree of homology throughout the Enterobacteria (including E. coli, Salmonella enteric, and Klebsiella pneumoniae), where both the glutathionylspermidine synthetase and amidase domains have been conserved in most of the species. It seems possible that within the Enterobacteriales Gss have extensive inheritance, and thus they show more than 60% identity in many species. In addition, based on BLAST-P analysis among the closely related bacterial groups in the gamma-proteobacteria, Gss sequences are present in some species of the Pasteurellalel, Pseudomonadales, Vibrionales and Xanthomonadales groups, but absent in others. Many other bacteria either do not have Gss homologs (Table 2) or only possess lower homology with the synthetase domain (i.e. the C-terminal part).
As opposed to these results in various bacterial species, there are no homologs in nearly all other organisms (including S. cerevisiae, mammals, and plants) (Table 2). In contrast however, there is a high degree of homology between the E. coli Gss sequences and both the synthetase and amidase domains of both glutathionylspermidine synthetase (Gss) and trypanothione synthetase (Trs) of Kinetoplastids (Tetaud et al., 1998). The close relationship between Kinetoplastids and bacterial Gss sequences and the absence of such sequences in almost all other organisms suggest that either these organisms lost their respective ancestral sequences early in their lineage or Kinetoplastids have acquired the ability to synthesize both glutathionylspermidine from bacteria followed by gene duplication and modification to synthesize trypanothione. Large-scale phylogenetic analyses on genomic data have demonstrated that several distantly related microbial eukaryotes have acquired mostly metabolic genes from prokaryotic organisms (Opperdoes and Michels, 2007; Andersson, 2009). For instance, many protists encode genes not found universally among other eukaryotes and these are patchily distributed (such as tyrosyl t-RNA, genes for de novo pyrimidine biosynthesis and others) (For references see Opperdoes and Michels (Opperdoes &Michels, 2007), and Andersson (Andersson, 2009)).
Most organisms contain high concentrations of at least one low-molecular weight thiol for maintenance of an intracellular reducing environment, such as glutathione (most organisms including E. coli), homoglutathione (mung bean), glutathionylspermidine (E. coli, C. fasiculata), trypanothione (trypanosomatids) and L-γ-glutamyl-cystine (halobacteria) (Fairlamb and Cerami, 1992). Two important functions of these thiols are well documented-thiol modification of proteins and protection of DNA from ionizing radiation or oxidative damages. The most important function of these compounds is the modification of protein thiols either by the formation of mixed disulfides or by the formation of intramolecular disulfides. These post-translational modifications protect proteins from oxidative stress and can regulate their functions (Fairlamb and Cerami, 1992) oxidative stress, at least in part due to presence of trypanothione (Krieger et al., 2000). Thus, when the genes for trypanothione synthetase and reductase from T. cruzi were introduced into E. coli, the cells were protected from radiation induced DNA damage (Fitzgerald et al., 2010).
Although the high homology for the Gss sequences in the Enterobacteria suggests an important physiologic function for glutathionylspermidine in these organisms, no specific function has been described for this system in bacteria. One possible function of the enzyme glutathionylspermidine synthetase in E. coli could be a regulation of metabolites (both spermidine and glutathione) because of the presence of bifunctional activity of the enzyme Gss (Tabor and Tabor, 1975). It is also clear from our studies and from others that glutathionylspermidine and glutathione are not essential, as mutants of GSH or spermidine grow normally on minimal medium during normal aerobic growth (Greenberg and Demple, 1986; Chattopadhyay et al., 2009b). However, both glutathione and polyamines are absolutely required for protection against oxidative stress (Masip et al., 2006; Chattopadhyay et al., 2003) and polyamines are involved in other cellular functions (such as swarming, (Kurihara et al., 2009). Thus, it could be possible that glutathionylspermidine is essential during environmental stresses. Despite these changes in gene expression, we have not found any difference in the two strains (gss+ vs gss−) in their growth rate, their sensitivity to oxygen, the toxicity of copper sulfate or cadmium sulfate, or survival after long-time storage (data not shown). Since one of the older speculations suggested a function in protecting DNA (Krieger et al., 2000; Fitzgerald et al., 2010), we also tested their sensitivity to UV radiation, but found no significant difference in either survival or in development of fluorouracil resistant mutations (data not shown). Although we could not find any difference in oxygen sensitivity, Chiang et al have reported slight increase in the sensitivity of a combined grxΔ and gssΔ double mutant to hydrogen peroxide, but no difference between gss+ and gssΔcells (Chiang et al., 2010). They have also reported that glutathionyspermidine could form mixed disulfides with proteins, but their results do not exclude the possibility that comparable binding occurs with intracellular glutathione. In our C14-spermidine incorporation assays, we found more than 98% of the counts are in the TCA supernatant, and only less than 2% counts in TCA precipitate with the macromolecules. In this experiment the gss+ cells showed twice more counts than gss− cells in the TCA precipitate (data not shown).
Although we have not been able to define a specific function for the gss gene we feel that the microarray results clearly show that this gene has a considerable effect on the physiology and gene expression of the bacteria. Comparison of the gss+ and gss− strains in the microarray studies showed marked differences in the regulation of different mRNAs. These differences have been listed in Tables 3 and 4. Some of the gene expression changes in gss+ vs gss− cells are in the polyamine metabolisms and arginine metabolisms pathways, as expected. We felt that it was important to show that glutathionylspermidine is not just an inactive end-product, but is metabolically active. Our isotope exchange experiments show that glutathionylspermidine is metabolically active in both logarithmically- growing (data not shown) and stationary cultures (Tabor and Tabor, 1975). Thus it could be possible that even in the log-phase cells, where glutathionylspermidine content is less than 10%, there is always some change in spermidine and glutathione pools due to activities of both the synthetase and amidase domains of gss+ as compared to gss− cells. For further understanding of regulatory pathways involved in the gene expression pattern of up or down-regulated genes in gss+ vs gss- cells, we performed bioinformatics analyses. The microarray results show an up-regulation of succinate metabolism (sdhD, sdhC, sdhA), which increases fumarate synthesis in the cells and on the other hand down-regulation of fumarate metabolism (frdC, frdD and frdB), which could increase fumarate level in the gss− cells. The transcription of sdhCABD is enhanced during a switch from aerobic to anarerobic growth by ArcA transcriptional regulators (Maklashina et al., 1998). The carAB regulon is regulated by arginine, pyrimidine and purine levels (Devroede et al., 2004). The genes for purine metabolisms (purM, purD, purH, etc) are regulated by PurRP (Meng et al., 1990). The precise mechanism of how these genes are regulated by gss gene deletion is not known. However, as shown in Table 4, fourteen transcriptional regulators are either up or down regulated in gss culture. These transcriptional regulators may be directly or indirectly affected by the presence or absence of glutathionylspermidine in these two strains. Some of these transcriptional factors are related to growth in low oxygen or low pH. For example, pdhR a repressor involved in respiratory control of pyruvate dehydrogenase complex (Ogasawara et al., 2007) is induced in the gss cells; ttdR, a transcriptional activator required for tartrate utilization (Kim et al., 2009) and cadC, a transcriptional activator for cadBA induced during low oxygen and low pH (Haneburger et al., 2011) are repressed in gss cells. Apart from these, the puuR transcriptional repressor of the putrescine utilization pathway (Kurihara et al., 2008) is also induced in gss cells.
The phylogenetic data showing that the full Gss sequences are mainly found in two phyla, Enterobacteria and Kinetoplastida, and not in most other species, indicate that glutathionylspermidine and diglutathionylspermidine are not necessary for most species, but have specialized functions in Enterobacteria and Kinetoplastids. We do not know the function of glutathionylspermidine in Enterobacteria, but it seems likely that it is important for survival of these organisms (such as E. coli) in the crowded, anaerobic environment in the intestinal lumen.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health (National Institute of Diabetes, Digestive and Kidney Diseases). The authors declare no conflict of interest in this study.
Abbreviations
- Gss
monoglutathionylspermidine synthetase, encoded by the gss gene (formerly called "gsp")
- Trs
diglutathionylspermidine (trypanothione) synthetase
REFERENCES
- Andersson JO. Horizontal gene transfer between microbial eukaryotes. Methods Mol Biol. 2009;532:473–487. doi: 10.1007/978-1-60327-853-9_27. [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular systems biology. 2006;2:1–11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JMJ, Kwon DS, Huisman GW, Kolter R, Walsh CT. Glutathionylspermidine metabolism in Escherichia coli. Purification, cloning, overproduction, and characterization of a bifunctional glutathionylspermidine synthetase/amidase. J Biol Chem. 1995;270:14031–14041. doi: 10.1074/jbc.270.23.14031. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay MK, Chen W, Poy G, Cam M, Stiles D, Tabor H. Microarray studies on the genes responsive to the addition of spermidine or spermine to a Saccharomyces cerevisiae spermidine synthase mutant. Yeast. 2009a;26:531–544. doi: 10.1002/yea.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay MK, Tabor CW, Tabor H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci U S A. 2003;100:2261–2265. doi: 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay MK, Tabor CW, Tabor H. Polyamines are not required for aerobic growth of Escherichia coli: preparation of a strain with deletions in all of the genes for polyamine biosynthesis. J Bacteriol. 2009b;191:5549–5552. doi: 10.1128/JB.00381-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang BY, Chen TC, Pai CH, Chou CC, Chen HH, Ko TP, Hsu WH, Chang CY, Wu WF, Wang AH, Lin CH. Protein S-thiolation by Glutathionylspermidine (Gsp): the role of Escherichia coli Gsp synthetase/amidase in redox regulation. J. Biol. Chem. 2010;285:25345–25353. doi: 10.1074/jbc.M110.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devroede N, Thia-Toong TL, Gigot D, Maes D, Charlier D. Purine and Pyrimidine-specific Repression of the Escherichia coli carAB Operon are Functionally and Structurally Coupled. Journal of molecular biology. 2004;336:25–42. doi: 10.1016/j.jmb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Dubin DT. Evidence for a conjugate between polyamines and glutathione in E. coli. Biochem. Biophys. Res. Commun. 1959;1:262–265. [Google Scholar]
- Fairlamb AH. The role of glutathionylspermidine and trypanothione in regulation of intracellular spermidine levels during growth of Crithidia fasciculata. Adv Exp Med Biol. 1988;250:667–674. doi: 10.1007/978-1-4684-5637-0_59. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985;227:1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MP, Madsen JM, Coleman MC, Teoh MLT, Westphal SG, Spitz DR, Radi R, Domann FE. Transgenic biosynthesis of trypanothione protects Escherichia coli from radiation-induced toxicity. Radiation research. 2010;174:290–296. doi: 10.1667/RR2235.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986;168:1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BG. Phylogetic Trees Made Easy -A how to mannual. 4th Edition. Sunderland, MA: Sinauer Associates Inc; 2011. [Google Scholar]
- Haneburger I, Eichinger A, Skerra A, Jung K. New insights into the signaling mechanism of the pH-responsive, membrane-integrated transcriptional activator CadC of Escherichia coli. J Biol Chem. 2011;286:10681–10689. doi: 10.1074/jbc.M110.196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim O, Reimann J, Lukas H, Schumacher U, Grimpo J, Dunnwald P, Unden G. Regulation of tartrate metabolism by TtdR and relation to the DcuS-DcuR-regulated C4-dicarboxylate metabolism of Escherichia coli. Microbiology. 2009;155:3632–3640. doi: 10.1099/mic.0.031401-0. [DOI] [PubMed] [Google Scholar]
- Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Molecular microbiology. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- Kurihara S, Oda S, Tsuboi Y, Kim HG, Oshida M, Kumagai H, Suzuki H. gamma-Glutamylputrescine synthetase in the putrescine utilization pathway of Escherichia coli K-12. J Biol Chem. 2008;283:19981–19990. doi: 10.1074/jbc.M800133200. [DOI] [PubMed] [Google Scholar]
- Kurihara S, Suzuki H, Tsuboi Y, Benno Y. Dependence of swarming in Escherichia coli K-12 on spermidine and the spermidine importer. FEMS Microbiol Lett. 2009;294:97–101. doi: 10.1111/j.1574-6968.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- Kwon DS, Lin CH, Chen S, Coward JK, Walsh CT, Bollinger JMJ. Dissection of glutathionylspermidine synthetase/amidase from Escherichia coli into autonomously folding and functional synthetase and amidase domains. J Biol Chem. 1997;272:2429–2436. doi: 10.1074/jbc.272.4.2429. [DOI] [PubMed] [Google Scholar]
- Maklashina E, Berthold DA, Cecchini G. Anaerobic expression of Escherichia coli succinate dehydrogenase: functional replacement of fumarate reductase in the respiratory chain during anaerobic growth. Journal of bacteriology. 1998;180:5989–5996. doi: 10.1128/jb.180.22.5989-5996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masip L, Veeravalli K, Georgiou G. The many faces of glutathione in bacteria. Antioxidants & redox signaling. 2006;8:753–762. doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- Meng LM, Kilstrup M, Nygaard P. Autoregulation of PurR repressor synthesis and involvement of purR in the regulation of pur B, purC, purL, purMN and guaBA expression in Escherichia coli. European Journal of Biochemistry. 1990;187:373–379. doi: 10.1111/j.1432-1033.1990.tb15314.x. [DOI] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia Coli and Related Bacteria. NY: Cold Spring Harbor Laboratory Pr; 1992. [Google Scholar]
- Murakami Y, Nishiyama M, Hayashi S. Involvement of antizyme in stabilization of ornithine decarboxylase caused by inhibitors of polyamine synthesis. Eur J Biochem. 1989;180:181–184. doi: 10.1111/j.1432-1033.1989.tb14630.x. [DOI] [PubMed] [Google Scholar]
- Ogasawara H, Ishida Y, Yamada K, Yamamoto K, Ishihama A. PdhR (pyruvate dehydrogenase complex regulator) controls the respiratory electron transport system in Escherichia coli. J Bacteriol. 2007;189:5534–5541. doi: 10.1128/JB.00229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes FR, Michels PA. Horizontal gene transfer in trypanosomatids. Trends Parasitol. 2007;23:470–476. doi: 10.1016/j.pt.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Pai CH, Chiang BY, Ko TP, Chou CC, Chong CM, Yen FJ, Chen S, Coward JK, Wang AH, Lin CH. Dual binding sites for translocation catalysis by Escherichia coli glutathionylspermidine synthetase. EMBO J. 2006;25:5970–5982. doi: 10.1038/sj.emboj.7601440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Borges A, Ariyanayagam MR, Fairlamb AH. Glutathionylspermidine metabolism in Escherichia coli. Biochem J. 1995;312:465–469. doi: 10.1042/bj3120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C, Tabor H. The complete conversion of spermidine to a peptide derivative in Escherichia coli. Biochem. Biophys. Res. Commun. 1970;41:232–238. doi: 10.1016/0006-291x(70)90493-6. [DOI] [PubMed] [Google Scholar]
- Tabor H, Tabor CW. Glutathionylspermidine synthetase (Escherichia coli) Methods Enzymol. 1971;17B:815–817. [Google Scholar]
- Tabor H, Tabor C. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J. Biol. Chem. 1975;250:2648–2654. [PubMed] [Google Scholar]
- Tabor H, Tabor C. Glutathionylspermidine in Escherichia coli. Ital J Biochem. 1976;25:70–76. [PubMed] [Google Scholar]
- Tetaud E, Manai F, Barrett MP, Nadeau K, Walsh CT, Fairlamb AH. Cloning and characterization of the two enzymes responsible for trypanothione biosynthesis in Crithidia fasciculata. Journal of Biological Chemistry. 1998;273:19383–19390. doi: 10.1074/jbc.273.31.19383. [DOI] [PubMed] [Google Scholar]
- Wyllie S, Oza S, Patterson S, Spinks D, Thompson S, Fairlamb A. Dissecting the essentiality of the bifunctional trypanothione synthetase-amidase in Trypanosoma brucei using chemical and genetic methods. Mol. Microbiol. 2009;74:529–540. doi: 10.1111/j.1365-2958.2009.06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.