Abstract
Children with prenatal exposure to cocaine are at higher risk for negative behavioral function and attention difficulties, and have demonstrated brain diffusion abnormalities in frontal white matter regions. However, brain regions beyond frontal and callosal areas have not been investigated using diffusion tensor imaging (DTI). DTI data were collected on 42 youth aged 14–16 years; subjects were divided into three groups based on detailed exposure histories: those with prenatal exposure to cocaine but not alcohol (PCE, n=12), prenatal exposure to cocaine and alcohol (CAE, n=17), and controls (n=13). Tractography was performed and along-tract diffusion parameters were examined for group differences and correlations with executive function measures. In the right arcuate fasciculus and cingulum, the CAE group had higher fractional anisotropy (FA) and/or lower mean diffusivity (MD) than the other two groups. The PCE group demonstrated lower FA in the right arcuate and higher MD in the splenium of the corpus callosum than controls. Diffusion parameters in tracts with group differences correlated with measures of executive function. In conclusion, these diffusion differences in adolescents with prenatal cocaine exposure suggest localized, long-term structural brain alterations that may underlie attention and response inhibition difficulties.
Keywords: diffusion tensor imaging, magnetic resonance imaging, white matter, tractography
1. Introduction
Although initially there was concern that prenatal cocaine exposure (PCE) would lead to pervasive cognitive, emotional, and behavioral problems, the literature describing the impact of PCE on early development have yielded relatively small effects. Young children (<6 years) prenatally exposed to cocaine tend to exhibit physical growth, developmental test scores and language abilities within the normal range (Frank et al., 2001), and most deficits observed can be explained by other environmental variables such as prenatal alcohol exposure. However, several studies have identified behavior problems (Kodituwakku and Kodituwakku, 2011; Schneider et al., 2011) and attention and response inhibition difficulties (Accornero et al., 2007; Ackerman et al., 2008; Savage et al., 2005; Schroder et al., 2004) in children with PCE compared to non-exposed controls. Less is known about the effects of PCE during adolescence, though this is a key period of cognitive, emotional, and brain development. There is evidence of memory impairment in adolescents with PCE (Sullivan et al., 2010), suggesting that further investigation is warranted.
Understanding the long-term effects of PCE on both executive function and the underlying brain structure is important to provide a full picture of the consequences of PCE. Studies of cocaine-exposed children and adolescents (up to age 15 years) describe structural brain abnormalities including smaller caudate volume (Avants et al., 2007; Rao et al., 2007), reduced cortical gray matter and total brain volume (Rivkin et al., 2008), larger amygdala (Rao et al., 2007) and increased water diffusion in frontal areas (Warner et al., 2006). However some studies have found no brain volume abnormalities (Smith et al., 2001), no diffusion abnormalities (McKee et al., 2007), or effects that do not survive correction for other drug exposures (Rivkin et al., 2008). Furthermore, many individuals with PCE also have prenatal exposure to alcohol, yet only a few of the above-mentioned studies considered prenatal alcohol exposure in their analyses (Rao et al., 2007; Rivkin et al., 2008; Warner et al., 2006). As heavy alcohol exposure in utero has been linked to structural brain damage (Lebel et al., 2011), some findings in cocaine studies may not be specifically related to the cocaine exposure.
The goal of this study was to use diffusion tensor imaging (DTI), an imaging technique that provides a measure of white matter microstructure, to examine structural differences throughout the brain in youth with PCE, with and without additional alcohol exposure. Previous investigations of frontal and callosal regions in PCE suggest that any differences observed would be small (Liu et al., 2011; Warner et al., 2006), and therefore we used a sensitive method to measure parameters at multiple points along white matter tracts, allowing for a more complete picture of the extent and magnitude of structural abnormalities. Additional investigations of relationships between cognitive ability and brain structure were conducted to help understand observed brain abnormalities in the context of potential behavioral and cognitive difficulties.
2. Materials and Methods
2.1 Recruitment
Participants were part of a prospective study on the developmental effects of prenatal cocaine exposure; details of recruitment for that study are provided elsewhere (Eyler et al., 1998). The University of Florida institutional review board approved this study, and a federal Certificate of Confidentiality was obtained. In brief, pregnant women 18 years or older were recruited when they first sought prenatal care; women with previously diagnosed chronic illness that might affect pregnancy outcome or fetal development (e.g., diabetes, sickle cell disease, mental retardation) were excluded. Cocaine use was verified by interview and unanticipated urine screens (at both study enrollment and delivery); women with positive tests or admission of use of illicit drugs other than cocaine and marijuana were excluded. Non-cocaine using women were matched to cocaine users based on race, socioeconomic status, parity and location of prenatal care. At each stage of the study, informed consent from a parent/guardian and child assent were provided. Of the 296 surviving children enrolled in the original study, 263 participated in the 10-year follow-up assessment and completed all outcome measures. From this group, a subset was selected to participate in neuroimaging during adolescence on the basis of proximity to the University of Florida (imaging location) and hair tests for cocaine use at age 10.5 and 12.5 years. Youth with positive hair tests were selected along with negative hair test subjects matched for PCE and gender.
2.2 Participants
Imaging data was collected from 42 youth aged 14–16 years. 16 subjects participated in a previous DTI study of cocaine exposure approximately 4 years earlier at age 10–12 years (Warner et al., 2006). Since prenatal alcohol exposure has significant effects on DTI parameters (Lebel et al., 2008; Sowell et al., 2008; Wozniak et al., 2006), subjects were separated into three groups: those with no prenatal alcohol or cocaine exposure (controls, n=13), those with prenatal exposure to cocaine but not alcohol (PCE, n=12), and those with prenatal exposure to both cocaine and alcohol (CAE, n=17); see Table 1 for demographic information by group. As cocaine use is difficult to quantify, prenatal cocaine exposure was calculated as the number of weeks of use during pregnancy and the three months prior to pregnancy, divided by the total number of weeks over this time period. Prenatal alcohol exposure for these purposes was defined as an average of more than one half of one standard drink per week during pregnancy (equivalent to ≥9 mL of absolute alcohol per week). After a complete description of the study to the subjects, written informed consent was obtained.
Table 1.
Demographic information for subjects by group. Mean values are given with standard deviation (for age) or range (for exposures).
| Control (n=13) |
Cocaine Exposure (PCE, n=12) |
Cocaine & Alcohol Exposure (CAE, n=17) |
ANOVA F- statistic/Kru skal-Wallis |
ANOVA p-value |
|
|---|---|---|---|---|---|
| Age (y) | 15.2 ± 0.7 | 15.5 ± 0.5 | 15.3 ± 0.5 | 0.54 | 0.38 |
| Gender | 8 female/5 male |
8 female/4 male |
9 female/8 male |
0.84 | 0.58 |
| Hollingshead socioeconomic status (12.5y) |
3: 2 subjects 4: 8 subjects 5: 2 subjects |
2: 1 subject 3: 3 subjects 4: 4 subjects 5: 4 subjects |
2: 1 subject 3: 2 subjects 4: 5 subjects 5: 8 subjects |
0.57 | 0.57 |
| Home observation for measurement of the environment (HOME) | 41.7 ± 10.3 | 46.3 ± 6.8 | 40.0 ± 7.8 | 2.03 | 0.15 |
| Cocaine exposure (fraction of weeks with exposure) | 0 | 0.36 (0.15– 0.89) | 0.48 (0.04– 1.00) | n/a1 | n/a1 |
| Alcohol exposure (average mL/ week) | 1.4 (0–8.3) | 1.1 (0–5.4) | 72.9 (9.5–320.9) | n/a1 | n/a1 |
| Marijuana exposure (average joints/week) | 0 (0–0.3) | 1.2 (0–10.4) | 0.6 (0–8.5) | 1.03 | 0.13 |
| Tobacco exposure (average cigarettes/ week) | 0.2 (0–1.7) | 49 (0–151)* | 59 (0–146)* | 6.41 | 0.001 |
| Hair positive for cocaine use at 10.5y | 12 negative/1 positive |
9 negative/2 positive |
14 negative/1 positive |
1.03 | 0.60 |
| Hair positive for cocaine use at 12.5y2 | 8 negative/ 5 positive |
7 negative/4 positive |
11 negative/4 positive |
0.49 | 0.78 |
| WCST Perseverative Errors (std score) | 99.9 ± 14.8 | 100.5 ± 15.7 | 94.6 ± 10.3 | 0.88 | 0.42 |
| WCST Total Errors (std score) | 101.9 ± 13.3 | 106.7 ± 16.5 | 94.5 ± 11.3 | 2.95 | 0.06 |
| Stroop Color-Word (raw score) | 45.0 ± 9.4 | 42.9 ± 11.3 | 37.2 ± 6.6 | 3.05 | 0.06 |
| Trail Making A (s to complete) | 12.5 ± 4.9 | 11.3 ± 3.0 | 13.6 ± 4.6 | 0.65 | 0.36 |
| Trail Making B (s to complete) | 22.9 ± 7.5 | 24.7 ± 7.6 | 33.6 ± 11.2*+ | 4.27 | 0.006 |
Indicates significant differences from the control (no exposure) group
indicates significant differences from the cocaine-exposed group. WCST = Wisconsin Card Sorting Task.
As groups were defined based on cocaine and alcohol exposure, statistical tests for group differences of these variables are not appropriate.
Only one subject (who was in the PCE group) had positive hair tests at both time points, suggesting that most positive test reflect experimentation rather than regular use.
2.3 Cognitive assessment
All subjects but one were given cognitive assessments of executive function on the same day as MRI scanning: Trail Making Tests A and B, the Stroop Color-Word Test, and the Wisconsin Card Sorting Task (WCST); the examiner was blind to subject status. The Trail Making Test A measures motor speed and visual attention by asking participants to connect 25 number-containing circles in numerical order as fast as possible. In Part B, circles contain a number or a letter and participants must connect them in order of 1-A–2-B-etc; this measures not only visual attention and motor speed, but also cognitive flexibility (Gaudino et al., 1995). The Stroop Color-Word test assesses directed attention by asking participants to ignore the letters of a word and say the color of its text instead (Stroop, 1935); raw scores indicate the number of correct answers given in 45 seconds. The WCST is a card-matching task based on color, quantity, and design of shapes on a set of cards that measures set-shifting ability (Monchi et al., 2001). Age and cognitive scores were compared across groups using one-way ANOVAs with post-hoc tests. Gender, additional drug exposures, and hair testing were compared across groups using the nonparametric Kruskal-Wallis Test with post-hoc comparisons using the nonparametric Mann-Whitney Test for two related samples. For demographic tests, p<0.05 was considered significant. See Table 1.
2.4 Image acquisition and processing
Imaging data was collected on a 3 T Philips Achieva (Best, Netherlands) scanner at the University of Florida. The protocol included T1-weighted and T2-weighted imaging, and DTI using a spin-echo EPI sequence with 60 2 mm slices, 15 diffusion encoding gradient directions, field of view 256×256 cm, matrix of 128×128, TR/TE=10,000/60 ms, and b=800 s/mm2; total DTI acquisition time was 6 minutes.
Diffusion images were processed using Diffusion Toolkit (version 0.6, Athinoula Martinos Center for Biomedical Imaging, Boston, MA) to fit a tensor model to the data and generate FA and MD maps. Whole brain brute force tractography was performed in TrackVis (version 0.5, Athinoula Martinos Center for Biomedical Imaging, Boston, MA) according to the FACT algorithm (Mori et al., 1999) using an FA threshold of 0.2 and a maximum turning angle of 35°. Ten major white matter fiber bundles that are possible to reconstruct reliably using streamline tractography (the genu, body, and splenium of the corpus callosum, the dorsal cingulum, the corticospinal tract, the anterior thalamic radiations, the inferior longitudinal and fronto-occipital fasciculi, the uncinate fasciculus and the arcuate fasciculus) were delineated manually by the same operator (L.S.), following a strict protocol (see Supplementary Figure S1), blind to subject age, gender, and exposure group. Color maps were used as a guide for tractography, and the tracking protocol was developed and refined according to a priori information on tract location (Wakana et al., 2004) by two DTI experts. Inclusion and exclusion regions, as well as length restrictions were developed for each tract to ensure consistent and unbiased tracking among individuals. Generally, two inclusion regions were used, one at either end of the tract, and exclusion regions and length restrictions were implemented as necessary to eliminate spurious streamlines. For all tracts except the corpus callosum, left and right sides were delineated and analyzed separately.
2.5 Tract-based statistics
Diffusion parameters were calculated along each tract and statistical analysis was carried out using along-tract statistics (Colby et al., 2012). Briefly, tracts were parameterized along their length, and FA and MD parameters were extracted at multiple points along each tract by averaging across all streamlines at that cross-section; the number of points used was constant for each tract and based on its average length across subjects. First, MD and FA values were tested using mixed models to examine group differences and group-point interactions, with a threshold of p<0.003 (corrected for 17 multiple comparisons). Any tracts with significant group-point interactions were then examined for group differences at each point along the tract using ANOVAs and p<0.05.
2.6 Relationships between diffusion parameters and cognitive measures
Tracts with significant group differences were tested for correlations between cognitive assessments (Trail Making Tests A and B, WCST total errors and perseverative errors standard scores, Stroop Color-Word raw score) and diffusion parameters (FA and MD) along their lengths. A mixed models approach including test score, group, and point along tract was used, such that both main effects and interaction terms were included for all three variables. Tracts with significant main effects or interaction terms involving test score were examined further to determine the location of differences. A threshold of p<0.01 was used, which corresponds to p<0.05 corrected for 5 comparisons.
2.7 Other prenatal drug exposures
To ensure that observed effects could be attributed to cocaine and/or alcohol exposure rather than other drugs, follow-up analysis was run with prenatal tobacco exposure (average number of cigarettes per week) and prenatal marijuana exposure (average number of joints per week) included as covariates.
3. Results
3.1 Group demographics
Groups did not differ significantly on age, gender, prenatal marijuana exposure, socioeconomic status, home environment, hair tests for cocaine use, or performance on cognitive tests other than Trail Making B (see Table 1). Only one subject, from the PCE group, had positive hair samples for cocaine at both the 10.5 year and 12.5 year time points. The CAE group had significantly higher scores (slower performance) on the Trail Making Test B than both of the other groups. Control subjects had significantly lower tobacco exposure than both cocaine-exposed groups.
3.2 Tracking results
Qualitatively, tracking results were similar among groups, and most tracts were easily delineated in most subjects. The arcuate fasciculus can be difficult to delineate due to its asymmetry, and the right side was not successfully tracked in 10 subjects (1 control, 2 PCE, 7 CAE); the left side was not tracked in three subjects (1 control, 2 PCE). The right uncinate, left and right anterior thalamic radiations, right corticospinal tracts, and right inferior fronto-occipital fasciculus each failed to be delineated in one subject (different subjects for each tract). Two PCE youth and one CAE individual had failed tracking in the left inferior fronto-occipital fasciculus, while three different subjects had failed tracking in the left inferior longitudinal fasciculus (1 PCE, 2 CAE), and those three plus one more from the CAE group had failed tracking in the right inferior longitudinal fasciculus. Subjects were excluded only from analysis of the tracts that were not successfully delineated, and included for all other analysis; numbers for each comparison are given in Table 2.
Table 2.
Group differences of diffusion parameters and correlations with executive function measures.
| Fractional Anisotropy | Mean Diffusivity | |||
|---|---|---|---|---|
| Tract (n for CON/PCE/CAE) |
Group Differences |
Correlations with EF measures |
Group Differences |
Correlations with EF measures |
|
Anterior thalamic radiations (13/12/16) |
N.S. | n/a | N.S. | n/a |
|
Arcuate fasciculus (L: 12/10/17; R: 12/10/10) |
F=1.52/p=0.0026 R central, anterior: CAE>PCE>CON |
F=1.63/p=0.008 TM A in R anterior (pos in CAE, CON) |
N.S. | n/a |
|
Cingulum (13/12/17) |
F=1.68/p=0.004 R central, posterior: CAE>CON>PCE |
F=1.94/p=0.007 Stroop in R central, anterior (pos in PCE, neg in CAE) |
F=2.26/p=1.6×1 0−8 R anterior: CAE>PCE R central: PCE>CAE |
N.S. |
|
Corticospinal tract (L: 13/12/17; R:13/12/16) |
N.S. | n/a | N.S. | n/a |
| Genu (13/12/17) | N.S. | n/a | N.S. | n/a |
| Body (13/12/17) | N.S. | n/a | N.S. | n/a |
|
Splenium (13/12/17) |
N.S. | n/a | F=1.47/p=0.008 L hemisphere: PCE>CON>CA E |
F=2.36/p=0.3 × 10−13 L side with TM B (pos in all groups) |
|
Inferior fronto- occipital fasciculus (L: 13/10/16; R: 13/12/16) |
N.S. | n/a | N.S. | n/a |
|
Inferior longitudinal fasciculus (L:13/11/15; R: 13/11/14) |
N.S. | n/a | F=1.69/p=1.7 × 10−4 L temporal: PCE>CAE L central: PCE>CAE |
F=2.11/p=0.8 × 10−7 L central, occipital with WCST perseverative errs (pos in CON, neg in CAE) |
|
Uncinate fasciculus (L:13/12/17; R: 13/12/16) |
N.S. | n/a | N.S. | n/a |
EF = executive function; N.S. = not significant; n/a = not assessed; TM A/TM B = Trail Making A/B, Stroop = Stroop color-word; WCST = Wisconsin card sorting task
3.3 FA values
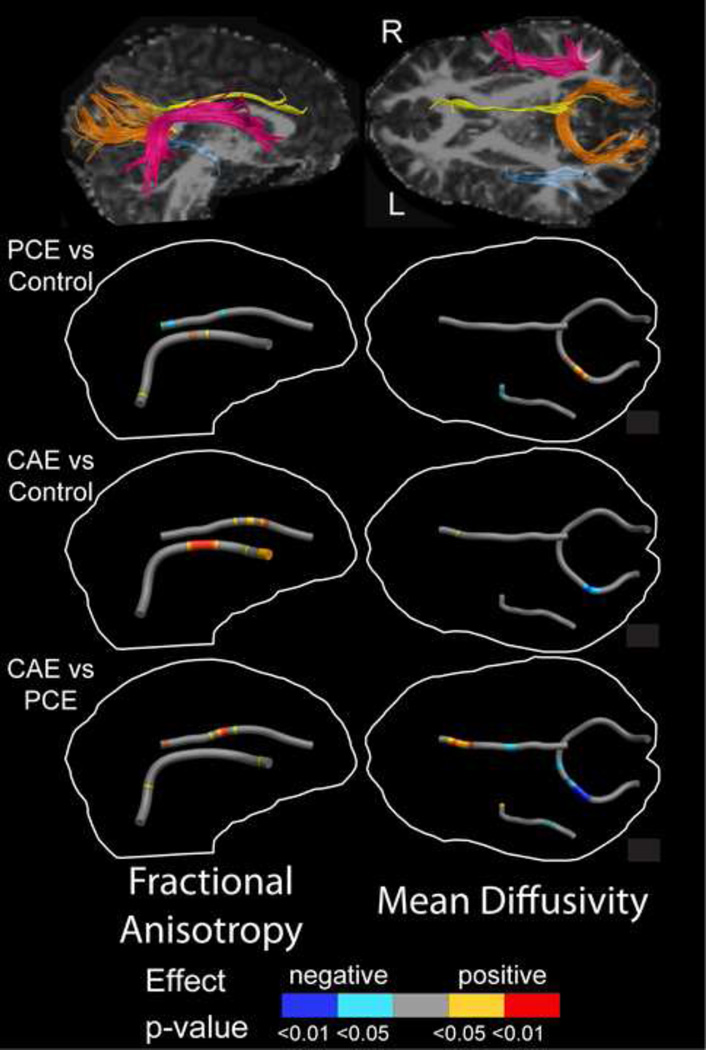
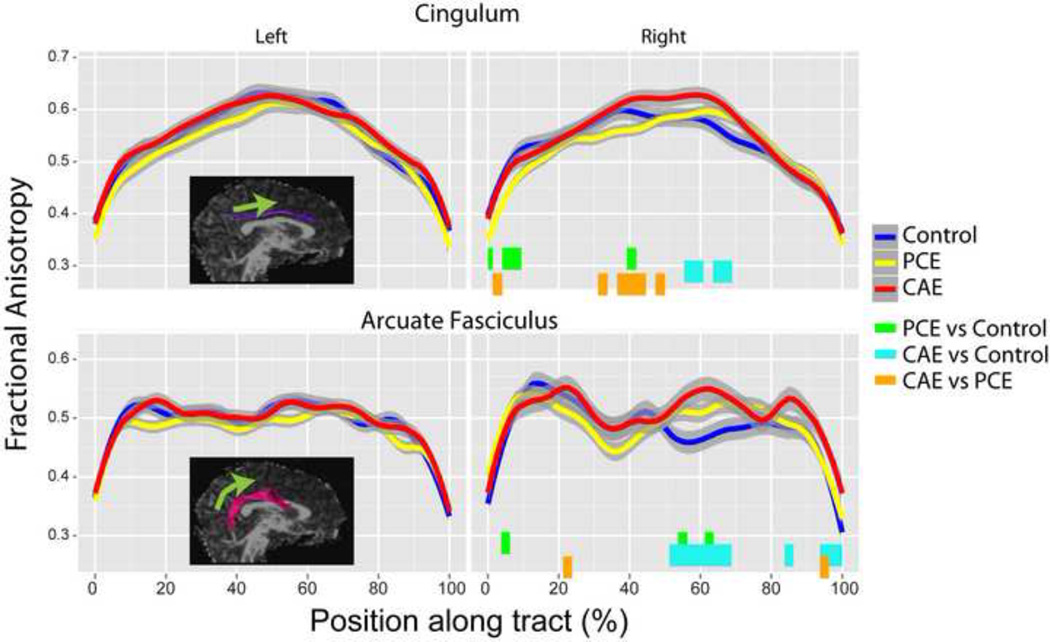
The right cingulum (df=74, 1443; F=1.68; p=0.004) and right arcuate fasciculus (df=80, 1160; F=1.53; p=0.0026) had significant point-group interactions (Table 2). Post hoc testing demonstrated that group differences in the right cingulum were localized to the central and posterior portions of the tract. In these regions, the PCE group had lower FA than both the control and CAE groups. In the central cingulum, the CAE group had higher FA than controls (Figures 1 and 2). For the arcuate fasciculus, the CAE group had higher FA than the PCE group and the PCE group had higher FA than controls at several small locations. In a larger section of the central and anterior arcuate, the CAE group had significantly higher FA than control subjects (Figures 1 and 2). For both of these tracts, FA differences ranged from approximately 0.04–0.09 FA units, or approximately 7–11%. No tracts had group differences when FA values were averaged across the entire tract.
Figure 1.
Tracts with significant group differences (right arcuate fasciculus - pink, right cingulum - yellow, splenium of the corpus callosum – orange, and left inferior longitudinal fasciculus – blue) are shown in the top row for a 14 year-old female in the cocaine and alcohol exposure (CAE) group. Group differences are overlaid onto a mean tract in the images below, indicating the direction and significance of the differences. The most prominent abnormalities were elevated FA in the right cingulum and arcuate fasciculus, and lower MD in the splenium of the corpus callosum in the CAE group compared to the other two. More localized effects were observed between the prenatal cocaine exposure (PCE) group and controls and the CAE and PCE groups for both FA and MD in the right cingulum, right arcuate fasciculus, left inferior longitudinal fasciculus and splenium of the corpus callosum.
Figure 2.
Fractional anisotropy (FA) values are shown along tracts for each group (± point-wise 95% confidence intervals) for tracts with significant point-group interaction terms. The locations of significant differences are indicated below FA values with colored bars. Points along each tract begin at the posterior end and continue toward the anterior end (indicated by green arrow). No significant differences were observed in the left cingulum or arcuate fasciculus, but these are shown for comparisons purposes. PCE=prenatal cocaine exposure; CAE=prenatal cocaine and alcohol exposure.
3.4 MD values
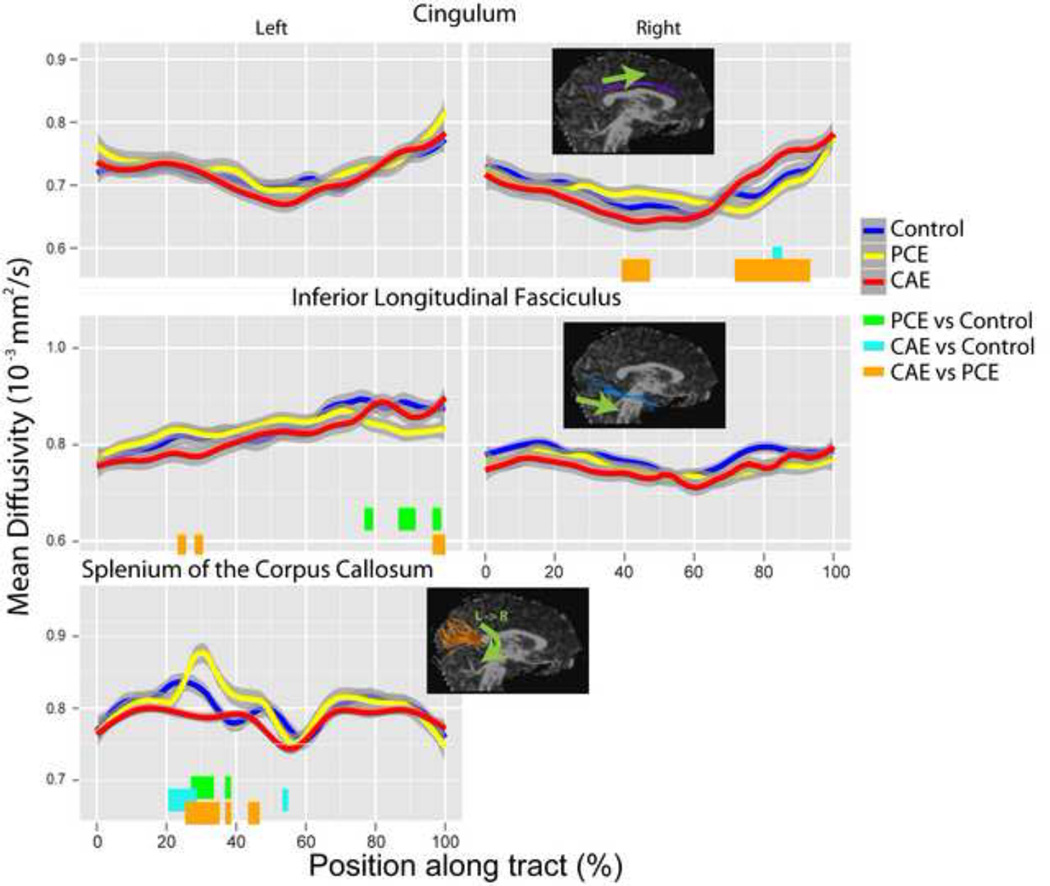
MD point-group interactions were significant for the right cingulum (df=74, 1443; F=2.26; p=1.6 × 10−8), left inferior longitudinal fasciculus (df=82, 1435; F=1.69; p=0.00017), and splenium of the corpus callosum (df=122, 2379; F=1.47; p=0.0008; Table 2). In the right cingulum, differences were localized to the central portion, where the PCE group had significantly higher MD than the CAE group, and the anterior cingulum, where the CAE group had significantly higher MD than the PCE group and controls (see Figures 1 and 3). In the left inferior longitudinal fasciculus, the PCE group had significantly lower MD than controls near the temporal end, higher MD than the CAE group in a small area near the occipital end and lower MD than the CAE group in a small region at the temporal end (see Figures 1 and 3). Splenium differences were located to the left of the midline, where the PCE group had significantly higher MD than controls and the CAE group, and controls had higher MD than the CAE group (Figures 1 and 3). MD differences ranged from 0.04–0.1 mm2/s (5–13%). No tract had group differences when MD values were averaged along the entire length of the tract.
Figure 3.
Mean diffusivity (MD) values are shown along tracts for each group for tracts with significant group-point interaction terms. Significant group differences are indicated with colored bars at the bottom of each plot. No group differences were observed in the left cingulum or right inferior longitudinal fasciculus, but these are shown for comparison purposes. PCE=prenatal cocaine exposure; CAE=prenatal cocaine and alcohol exposure.
3.5 Correlations with executive function measures
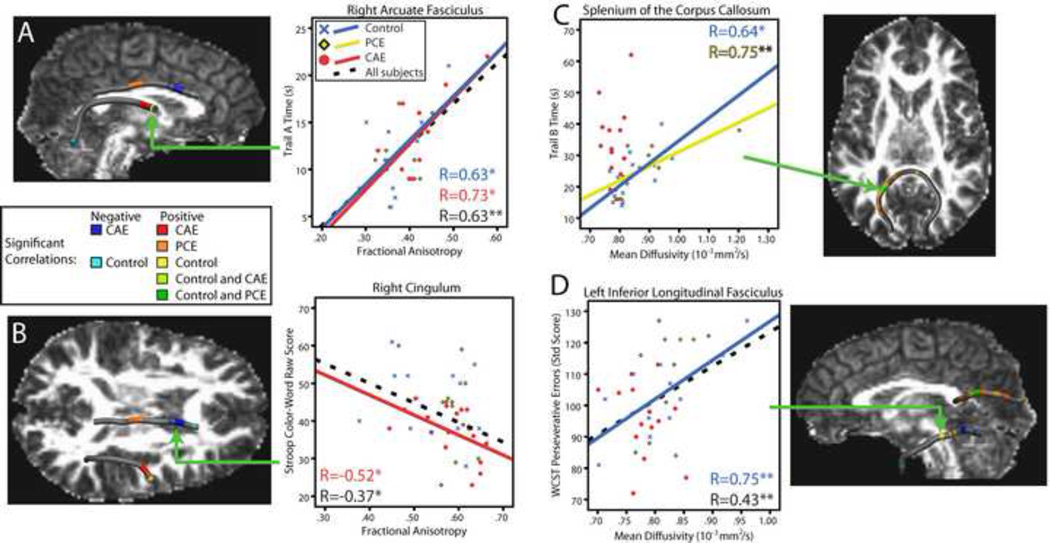
Structure-function relationships were significant for FA in the right arcuate fasciculus and right cingulum; in both cases, significant correlations overlapped the locations of group differences (Table 2). In the right arcuate, point-score interactions were significant for Trail Making Test A times (df=40, 1040; F=1.63; p=0.008). In the anterior portion, both the CAE and control groups had significant positive correlations of FA with Trail Making A times; one point at the temporal end of the arcuate had negative FA-Trail Making A correlations in control subjects (see Figure 4A).
Figure 4.
Significant correlations between diffusion parameters and cognitive assessments are shown. FA values in the right cingulum and arcuate fasciculus correlated with Stroop Color-Word test scores and Trail Making Test A times, respectively (A, B). In the splenium of the corpus callosum and the left inferior longitudinal fasciculus, correlations between MD and Trail Making B times and perseverative errors on the WCST, respectively, were significant (C, D). Note that higher Trail Making times and WCST scores indicate worse performance, whereas higher Stroop scores indicate better performance. Where significant, trend lines for each group (solid, colored), and across all subjects (black, dotted) are shown. * p<0.05; ** p<0.01.
In the right cingulum, point-score interactions were significant for Stroop Color-Word scores (df=37, 1332; F=1.94; p=0.0007). In the anterior right cingulum, correlations were negative in both the control and CAE groups; positive FA-Stroop correlations were observed in the central cingulum in the PCE group (Fig. 4B).
MD values correlated with executive function measures in both the splenium of the corpus callosum and the left inferior longitudinal fasciculus (Table 2). In the splenium of the corpus callosum, the point-group-score interaction was significant for Trail Making B (df=122, 2196; F=2.36; p=0.2 × 10−13). Positive correlations were observed between MD values and Trail Making B times for all three groups, located exclusively in the left hemisphere (see Figure 4C).
In the left inferior longitudinal fasciculus, the point-group-score interaction was significant for perseverative errors on the WCST (df=82, 1312; F=2.11; p=0.8 × 10−7 ).MD values correlated negatively with perseverative errors for the CAE group in the occipital region; similar correlations were negative in controls at the temporal end of this tract. In the central inferior longitudinal fasciculus, correlations were positive in controls (Fig. 4D).
In most cases mentioned above, the correlations were also significant across the entire group of subjects. No other correlations or interactions terms met the multiple comparison-corrected significance of p<0.01.
3.6 Other prenatal and postnatal drug exposures
When prenatal marijuana and tobacco exposure were included as covariates in the analysis, the original group differences remained significant for both FA and MD (p<0.003). Correlations between FA or MD and prenatal tobacco and marijuana exposure levels were not significant for any tract.
Analysis for FA and MD differences along tracts was conducted again after removal of the one subject with positive hair samples for cocaine use at both 10.5 year and 12.5 year time points (a PCE subject). Differences in the cingulum, inferior longitudinal fasciculus, and splenium of the corpus callosum remained significant. In the right arcuate fasciculus, the significance value for the point-group interaction was 0.01, which was below the Bonferonni-corrected threshold, though the uncorrected value remains significant. Investigation of the group differences along the right arcuate, however, indicated that all original differences remained significant despite removal of this one subject.
4. Discussion
DTI tractography has revealed localized differences in white matter microstructure that are related to PCE and do not appear to be attributable to concomitant exposure to other drugs. Diffusion abnormalities were localized to small regions of the right cingulum, right arcuate fasciculus, left inferior longitudinal fasciculus and splenium of the corpus callosum (Figure 1). Furthermore, these abnormalities were related to measures of executive function, indicating structure-function relationships within cocaine-exposed individuals and control subjects. Overall, this suggests measureable long-term structural brain differences associated with in utero exposure to cocaine.
The most robust effect of PCE (without concomitant alcohol exposure) was observed to the left of the midline in the splenium of the corpus callosum, where PCE subjects had higher MD values than controls. The only other published DTI study of PCE in adolescents found no significant differences in the corpus callosum (McKee et al., 2007), although they did not examine areas outside the midline. Some youth in the current study (16 of 42) participated in a previous imaging study when they were aged 10–12 years, which found elevated MD in left frontal callosal and right frontal projection regions in cocaine-exposed children compared to controls (Warner et al., 2006). No significant MD or FA differences were observed in these areas in the present study, which may be related to methodological differences (regions-of-interest versus tractography), differing populations, or age-related brain changes. Correlations in the splenium between MD values and Trail Making B Test scores were significant for both controls and PCE subjects, indicating that higher MD is associated with worse performance. In good agreement with these results, a previous study reported positive correlations between splenial MD and the Delis-Kaplan Executive Function System Trail Making Test in healthy adolescents (Fryer et al., 2008). The splenium is associated with visuomotor integration and even subtle degradation can affect cognitive processes (Schulte and Muller-Oehring, 2010). Thus, these findings are logical in that they indicate worse tissue microstructure in the splenium (associated with higher MD) of those with PCE, and that reduced tissue integrity there is associated with poorer cognitive flexibility and motor speed.
Diffusion abnormalities in the CAE group compared to controls occurred in the splenium, right arcuate and right cingulum, and may be due to alcohol and/or cocaine exposure, or an interaction effect between the two drugs. Most differences indicated increased FA and reduced MD in the CAE group, in contrast to previous studies of prenatal alcohol exposure that demonstrate reduced FA and increased MD relative to controls (Fryer et al., 2009; Lebel et al., 2008; Wozniak et al., 2006). However, increased FA in the right parietal lobe (Fryer et al., 2009) and decreased MD in the corpus callosum (Lebel et al., 2008) have been observed in prenatally alcohol-exposed children and adolescents. Furthermore, higher FA has been noted in the corpus callosum in children and adolescents exposed prenatally to tobacco (Jacobsen et al., 2007), and in frontal and parietal lobes of those with prenatal methamphetamine exposure (Cloak et al., 2009). Although somewhat unexpected, these results are informative and may highlight abnormalities due to the interaction effects of the two drug exposures rather than to the prenatal alcohol exposure specifically.
Children with PCE are more likely to have difficulties with sustained attention and impulse control than typically-developing children (Accornero et al., 2007; Ackerman et al., 2008; Savage et al., 2005; Schroder et al., 2004), and these abilities have been linked with activation of fronto-parietal regions, particularly in the right hemisphere (Lawrence et al., 2003). Cocaine-exposed children demonstrate abnormal activation patterns in right frontal and left occipital regions during response inhibition (Sheinkopf et al., 2009), supporting the notion that the diffusion differences observed in right parietal and left occipital regions may underlie attention and inhibition difficulties. Further support for this structure-function relationship in cocaine-exposed subjects was provided by correlations between diffusion parameters in the right frontal regions and the Stroop and Trail Making A tasks, both of which require sustained attention (Figure 4). In both of these cases, worse performance was associated with poorer white matter integrity in the PCE group, as indicated by lower FA values. In many areas, similar relationships were also observed in the CAE and/or control groups.
Cocaine blocks monoamine transporters, and through an accumulation of serotonin and norepinephrine, results in decreased blood flow to the fetus and intrauterine growth restriction (Ganapathy, 2011; Volpe, 1992). Alcohol, on the other hand, is a central nervous system depressant and can impact fetal development by altering neuronal proliferation and migration (Miller, 1986), or causing cell death (Ikonomidou et al., 2001). It is unclear why certain regions of the brain may be more susceptible to the effects of prenatal exposures, but the right parietal and callosal regions appear to be particularly vulnerable areas that are commonly found to be abnormal in studies of prenatal alcohol exposure (Lebel et al., 2012b; Lebel et al., 2011); differences have also been noted in prenatal methamphetamine exposure (Cloak et al., 2009). We observe similar findings related to prenatal cocaine exposure, suggesting that these regions are vulnerable to the teratogenic effects of multiple substances.
Brain structure is influenced by a large number of factors, including experiences such as learning a new skill or improving an existing one (Keller and Just, 2009; Scholz et al., 2009). Furthermore, brain development trajectories differ in children with different IQs (Shaw et al., 2006) and in children with prenatal alcohol exposure versus those without (Lebel et al., 2012c). Therefore, the structural brain abnormalities observed here may be due specifically to prenatal exposure or may be influenced by differing factors in the participants’ postnatal environments. Socioeconomic status and measures of home environment were collected several times during this longitudinal study, most recently when these subjects were aged 12.5 years. At no time did the three groups used in this study differ on these measures, demonstrating that subjects were well-matched demographically to minimize possible environmental influences. However, to properly separate the effects of prenatal and postnatal environments, longitudinal studies with large sample sizes and extensive environment data are necessary; future studies of this type may provide valuable information about the role of pre- and post-natal influences on brain structure.
The main strength of this study is its ability to attribute the observed effects more specifically to cocaine exposure. This study was prospective, excluded women using illegal drugs other than cocaine and marijuana, and obtained detailed exposure information about cocaine, alcohol, marijuana and tobacco during pregnancy. The group of subjects exposed to cocaine and not alcohol provides a unique study population and avoids the problems associated with examining subjects with multiple drug exposures that could confound interpretation. However, this study also has several limitations. The current preferred method for identifying prenatal cocaine exposure is meconium sampling, while cocaine use among pregnant women in this study was assessed via structured interview and urine samples. These methods were common at the time of study enrollment and have been shown to identify most, though not all, cocaine users (Eyler et al., 2005). Simulation studies recommend the use of at least 30 diffusion gradient encoding directions (Jones, 2004), and this study only used 15. However, studies on human subjects demonstrate tractography results with similar reliability between 6 and 30 directions, although absolute values varied slightly between protocols (Lebel et al., 2012a). Prenatal tobacco exposure was higher in the cocaine-exposed groups than in controls and has been linked to altered FA values in the corpus callosum (Jacobsen et al., 2007; McKee et al., 2007); however, a follow-up analysis including prenatal tobacco and marijuana exposure as covariates did not change the significance of FA or MD differences. Although well characterized and well-matched between groups, the sample size is relatively small with 12–17 subjects per group, and future studies with larger sample sizes and longitudinal data may further elucidate the effects of prenatal cocaine exposure on brain structure.
Like additional prenatal exposures, current drug use can be a potential confound in studies such as this one. The hair samples obtained at two time points prior to imaging indicate cocaine use by some of the youth, with even distributions across groups. Only one participant (a PCE subject) had positive hair samples at both time points, suggesting that the vast majority of positive hair samples were due to experimentation by these youth, rather than regular cocaine use. Although cocaine use has been shown to have effects on brain microstructure in adults (Lane et al., 2010; Lim et al., 2008), subjects in previous studies were heavy cocaine users for an average of 16–18 years prior to the study, whereas use in this study was mostly experimental. To ensure validity of these results, however, we reanalyzed the data excluding this individual. Results remained the same, though the significance of FA differences was slightly diminished in the arcuate fasciculus. Future studies will require larger samples to disentangle potential contributions of prenatal exposure and current drug use.
In conclusion, localized structural brain differences were observed in the right parietal lobe and the splenium of the corpus callosum in adolescents with PCE, indicating worse tissue microstructure than the control group. These areas are related to attention and response inhibition, and diffusion parameters there were correlated with performance on tests of executive function, further illustrating the structure-function relationships in this population as well as in control subjects. This study reveals measurable long-term effects of prenatal cocaine and alcohol exposure that may underlie cognitive and behavioral difficulties experienced by these youth, demonstrating the importance of appropriate prenatal counseling and early intervention and treatment to minimize deficits in affected children.
Supplementary Material
Acknowledgement
Funding for this study was provided by National Institute of Drug Abuse grants R21DA027561, R01DA05854, and R90DA023422; National Institute of General Medical Sciences grant T32GM008042, and National Institute on Alcohol Abuse and Alcoholism grant F30AA020431. Salary support was provided to CL by the Canadian Institutes for Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. Journal of developmental and behavioral pediatrics. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman JP, Llorente AM, Black MM, Ackerman CS, Mayes LA, Nair P. The effect of prenatal drug exposure and caregiving context on children's performance on a task of sustained visual attention. Journal of developmental and behavioral pediatrics. 2008;29:467–474. doi: 10.1097/DBP.0b013e3181903168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, Wang J, Gee JC. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatric Neurology. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM, Sowell ER. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59:3227–3242. doi: 10.1016/j.neuroimage.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcome from a prospective, matched study of prenatal crack/cocaine use: I. Interactive and dose effects on health and growth. Pediatrics. 1998;101:229–237. doi: 10.1542/peds.101.2.229. [DOI] [PubMed] [Google Scholar]
- Eyler FD, Behnke M, Wobie K, Garvan CW, Tebbett I. Relative ability of biologic specimens and interviews to detect prenatal cocaine use. Neurotoxicology and teratology. 2005;27:677–687. doi: 10.1016/j.ntt.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and cognition. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP. Characterization of White Matter Microstructure in Fetal Alcohol Spectrum Disorders. Alcoholism Clinical and Experimental Research. 2009;33:514–521. doi: 10.1111/j.1530-0277.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V. Drugs of abuse and human placenta. Life sciences. 2011;88:926–930. doi: 10.1016/j.lfs.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudino EA, Geisler MW, Squires NK. Construct validity in the Trail Making Test: what makes Part B harder? Journal of clinical and experimental neuropsychology. 1995;17:529–535. doi: 10.1080/01688639508405143. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochemical Pharmacology. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. Journal of Neuroscience. 2007;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magnetic Resonance in Medicine. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW, Kodituwakku EL. From research to practice: an integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychology Review. 2011;21:204–223. doi: 10.1007/s11065-011-9170-1. [DOI] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. Journal of cognitive neuroscience. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lebel C, Benner T, Beaulieu C. Six is enough? Comparison of diffusion parameters measured using six or more diffusion-encoding gradient directions with deterministic tractography. Magnetic Resonance in Medicine. 2012a;68:474–483. doi: 10.1002/mrm.23254. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012b;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O'Connor MJ, Narr KL, Kan E, Abaryan Z, Sowell ER. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. The Journal of neuroscience. 2012c;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcoholism Clinical and Experimental Research. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychology Review. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug and alcohol dependence. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cohen RA, Gongvatana A, Sheinkopf SJ, Lester BM. Impact of prenatal exposure to cocaine and tobacco on diffusion tensor imaging and sensation seeking in adolescents. Journal of Pediatrics. 2011;159:771–775. doi: 10.1016/j.jpeds.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harris GT, Rice ME, Silk L. Effects of a Snoezelen room on the behavior of three autistic clients. Research in developmental disabilities. 2007;28:304–316. doi: 10.1016/j.ridd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986;233:1308–1311. doi: 10.1126/science.3749878. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. The Journal of neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, Robson CD, Rose-Jacobs R, Frank DA. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. Journal of developmental and behavioral pediatrics. 2005;26:42–47. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychology Review. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder MD, Snyder PJ, Sielski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain and cognition. 2004;55:409–412. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychology Review. 2010;20:174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Developmental Neuroscience. 2009;31:159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, Walot I, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O’Connor MJ, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. Journal of Neuroscience. 2008;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Effect of cocaine use on the fetus. N Engl J Med. 1992;327:399–407. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract- based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clinical and experimental research. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.