Abstract
Purpose
The benefit of radiation therapy in extremity soft tissue sarcomas remains controversial. The purpose of this study was determine the effect of radiation therapy on overall survival among patients with primary soft tissue sarcomas of the extremity who underwent limb sparing surgery.
Patients and Methods
A retrospective study from the Surveillance, Epidemiology, and End Results (SEER) database that included data from January 1, 1988, to December 31st, 2005. A total of 6,960 patients comprised the study population. Overall survival curves were constructed using Kaplan-Meir Method and for patients with low and high grade tumors. Hazard ratios were calculated based on multivariable Cox proportional hazards models.
Results
Of the cohort, 47% received radiation therapy. There was no significant difference in overall survival among patients with low grade tumors by radiation therapy. In high grade tumors, the 3 year overall survival was 73% in patients who received radiation therapy vs. 63% for those who did not receive radiation therapy (p < 0.001). On multivariate analysis, patients with high grade tumors who received radiation therapy had an improved overall survival (HR 0.67, 95% CI 0.57-0.79).
In patients receiving radiation therapy, 13.5% received it in a neo-adjuvant setting. The incidence of patients receiving neo-adjuvant radiation did not change significantly between 1988 and 2005.
Conclusions
This is the largest population based study reported in patients undergoing limb sparing surgery for soft tissue sarcomas of the extremities and reports that radiation was associated with improved survival in patients with high grade tumors.
Keywords: Sarcoma, Extremity, Radiation, SEER
Background
Soft tissue sarcomas are rare malignancies that occurred in approximately 9,220 patients in the United States in 2007(1). Approximately half of these present in an extremity (2). These malignancies represent a heterogenous group of tumors, with many of them posing a high risk of local recurrence and distant metastasis. (3)
The major therapeutic goals of treating soft tissue extremity sarcomas are to maximize local tumor control using minimal surgery with limb preservation and to improve survival (4). A number of studies show that wide local excision with with pre- or post-operative radiation therapy results in equivalent outcomes regarding local control and overall survival when compared to amputations or more radical excisions (5-9).
For those patients who undergo limb-sparing surgery with wide local excision alone, the addition of radiation provides a local control benefit, but no improvement in overall survival has been demonstrated yet (10). The purpose of this study was to determine whether radiation therapy is associated with improved outcomes among patients with primary soft tissue sarcomas of the extremity who undergo limb sparing surgery.
Methods and Materials
Data and Study population
The Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute covers 26% of the US population and collects incidence and survival data from 17 population based cancer registries. The database contains information on primary tumor site, histology, stage at diagnosis, first course of treatment, follow-up, and cause of death.
Eligible patients had histologically confirmed invasive extremity soft tissue sarcoma. We identified patients using identified site codes C49.1 and C49.2 of the International Classification of Disease for Oncology, Third Edition. We restricted the analysis to patients aged > 20 years who underwent a limb-sparing surgery between 1988 – 2005. Patients who presented with distant metastasis or underwent amputation were excluded. Detailed information regarding the tumor size and grade were only available in SEER following 1988. Patients with Kaposi's sarcoma were also excluded because the majority of these patients have AIDS which would confound survival outcomes. The final sample size included a total of 6,960 patients.
Variables
Overall survival was the primary study end point. Overall survival was defined as the time from diagnosis to death. Exposure variables included categorical variables for whether patients received radiation therapy and the timing of radiation therapy (neoadjuvant or adjuvant). Information regarding the use of adjuvant chemotherapy, and specific radiation therapy technique (dose, fractionation, beam energy) were not available in the SEER database.
Covariates included in the analysis were all categorical and included: age (20-44 years, 45-59, 60-74, and 75+), sex, SEER registry, year of diagnosis, T stage (T1 ≤ 5cm, T2 > 5cm), nodal stage, grade (low grade = 1-2, high grade = 3-4), location (upper vs. lower), laterality, and histology. Detailed information regarding margin status at time of resection, local control, and performance status was not available in the SEER database.
Statistical Analysis
All data was analyzed using SAS Version 9.1 statistical software package (SAS Institute, Cary, NC). The Pearson's chi-square test was used to determine if associations existed between the use of radiation therapy and the covariates of interest. Estimates of overall survival stratified by the use of radiation therapy were calculated using the Kaplan-Meier method for patients with low and high grade tumors and for the subset of patients with large (>5cm) high grade tumors. The log rank test was used to determine if differences in survival curves were statistically significant. Results were considered significant when p < 0.05.
To determine the importance of radiation therapy as an independent predictor of overall and mortality, hazard ratios (HR) and their 95% confidence intervals (CI) were calculated based on multivariable Cox proportional hazards models adjusted for all covariates of interest. Models were run for the full sample, and for subsamples of patients with low and high grade tumors. Additional results were obtained to determine if use and timing of radiation therapy was associated with mortality for patients with high grade, large (> 5cm) tumors.
Results
Among the 6,960 patients in this study, 47% received radiation therapy as a component of their treatment. The categorical variables of sex, year of diagnosis, nodal status, and laterality were not associated with administration of radiation therapy (Table 1). The categorical variables of age, T stage, grade, location, and histology were significant predictors of the administration of radiation therapy. High grade, T2, lower extremity located tumors and certain histologies (fibrohistiocytic, synovial, and rhabdomyosarcomas) were more likely to receive radiation. Osseous and peripheral nerve tumors were less likely to receive radiation.
Table 1. Predictors of Radiation Therapy Use.
| No. of Patients* (n = 6,960) | % Who Received Radiation Therapy† (n = 3,692) | % Who Did Not Receive Radiation Therapy† (n = 3,268) | P-value‡ | |
|---|---|---|---|---|
| Overall | 47.0 | 53.1 | ||
| Patient characteristics | ||||
| Age in years | <.001 | |||
| 20-44 years | 1,917 | 51.4 | 48.6 | |
| 45-59 years | 1,774 | 53.3 | 46.7 | |
| 60-74 years | 1,760 | 57.8 | 42.2 | |
| 75+ years | 1,509 | 49.2 | 50.8 | |
| Sex | .21 | |||
| Male | 3,680 | 53.8 | 46.3 | |
| Female | 3,280 | 52.3 | 47.7 | |
| Location of SEER registry | <.001 | |||
| San Francisco | 594 | 61.5 | 38.6 | |
| Connecticut | 489 | 49.9 | 50.1 | |
| Detroit | 654 | 51.2 | 48.8 | |
| Hawaii | 218 | 56.0 | 44.0 | |
| Iowa | 531 | 53.9 | 46.1 | |
| New Mexico | 192 | 55.7 | 44.3 | |
| Seattle | 520 | 60.4 | 39.6 | |
| Utah | 235 | 57.5 | 42.6 | |
| Atlanta | 356 | 50.8 | 49.2 | |
| San Jose | 234 | 60.7 | 39.3 | |
| Los Angeles | 1,011 | 46.0 | 54.0 | |
| Alaska | 4 | 25.0 | 75.0 | |
| Georgia | 10 | 30.0 | 70.0 | |
| California (other than SF/SJ/LA) | 1,011 | 52.9 | 47.1 | |
| Kentucky | 230 | 51.3 | 48.7 | |
| Louisiana | 232 | 52.6 | 47.4 | |
| New Jersey | 439 | 49.4 | 50.6 | |
| Year of diagnosis | .055 | |||
| 1988 | 156 | 52.6 | 47.4 | |
| 1989 | 155 | 51.0 | 49.0 | |
| 1990 | 146 | 57.5 | 42.5 | |
| 1991 | 187 | 57.8 | 42.3 | |
| 1992 | 244 | 52.1 | 48.0 | |
| 1993 | 266 | 51.9 | 48.1 | |
| 1994 | 278 | 60.1 | 39.9 | |
| 1995 | 278 | 47.5 | 52.5 | |
| 1996 | 324 | 51.5 | 48.5 | |
| 1997 | 330 | 48.5 | 51.5 | |
| 1998 | 257 | 56.8 | 43.2 | |
| 1999 | 264 | 54.6 | 45.5 | |
| 2000 | 543 | 58.0 | 42.0 | |
| 2001 | 617 | 54.0 | 46.0 | |
| 2002 | 559 | 53.5 | 46.5 | |
| 2003 | 769 | 50.5 | 49.5 | |
| 2004 | 810 | 50.4 | 49.6 | |
| 2005 | 777 | 53.4 | 46.6 | |
| Tumor characteristics | ||||
| T stage | <.001 | |||
| T1 | 2,437 | 46.7 | 53.3 | |
| T2 | 3,438 | 63.6 | 36.4 | |
| N status | .87 | |||
| N0 | 6,845 | 53.1 | 46.9 | |
| N1 | 61 | 54.1 | 45.9 | |
| Grade | <.001 | |||
| Low grade (G1/2) | 2,317 | 41.3 | 58.7 | |
| High grade (G3/4) | 2,689 | 71.2 | 28.8 | |
| Location | <.001 | |||
| Upper | 1,998 | 49.1 | 51.0 | |
| Lower | 4,962 | 54.7 | 45.3 | |
| Laterality | .68 | |||
| Right | 3,402 | 52.9 | 47.2 | |
| Left | 3,549 | 53.3 | 46.7 | |
| Histology | <.001 | |||
| Fibrohistiocytic tumors | 2,350 | 57.2 | 42.8 | |
| Liposarcoma | 1,758 | 50.9 | 49.2 | |
| Leiomyosarcomas | 817 | 38.7 | 61.3 | |
| Synovial sarcomas | 438 | 67.8 | 32.2 | |
| Fibrosarcoma | 395 | 50.1 | 49.9 | |
| Osseous and chondromatous neoplasms of soft tissue | 154 | 40.3 | 59.7 | |
| Peripheral nerve sheath tumors | 142 | 43.0 | 57.0 | |
| Blood vessel tumors | 73 | 49.3 | 50.7 | |
| Rhabdomyosarcoma | 64 | 71.9 | 28.1 | |
| Other | 769 | 56.8 | 43.2 |
SEER: Surveillance, Epidemiology, and End Result. SF: San Francisco, SJ: San Jose, LA: Los Angeles.
Due to missing data, n for each variable ranges from 5,006 to 6,960.
Some percentages do not add to 100 due to rounding
P-values from Chi-square test.
Univariate Analysis
There was no significant difference in overall survival among patients with low grade tumors by radiation therapy. Among patients with high grade tumors, the three year overall survival was 73% for who received radiation vs. 63% for those who did not receive radiation therapy (p < 0.001; Figure 1). Among patients with large (>5cm) high grade tumors, the three year overall survival for those who received radiation was 66% vs. 53% for those who did not receive radiation therapy (p < 0.001; Figure 2).
Figure 1.
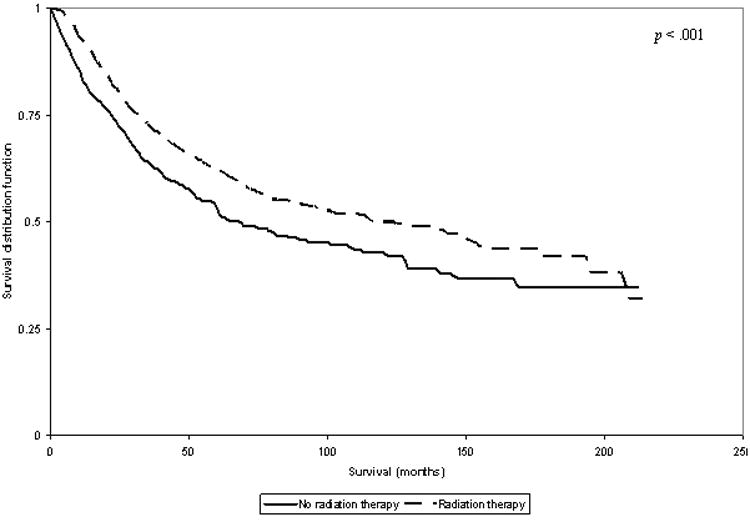
Plot of Overall Survival in Patients with High Grade Tumors, Stratified by Radiation Therapy Use.
Figure 2.
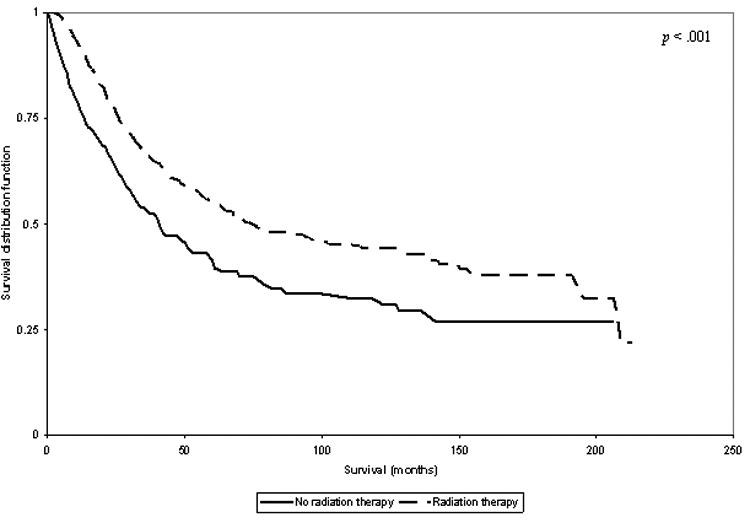
Plot of Overall Survival in Patients with Large (>5cm) High Grade Tumors, Stratified by Radiation Therapy Use.
Multivariate Analysis
Among all patients, radiation therapy was associated with significantly improved overall survival, even after adjustment for patient and tumor characteristics. Variables adversely affecting survival on multivariable analysis were increasing age, male sex, higher T stage, higher N stage, and higher grade tumors.
Additional analysis was performed on sub-samples of patients based on tumor grade. Patients with high grade tumors who received radiation therapy had an improved overall survival (HR 0.67, 95% CI 0.57-0.79) and cause specific survival (HR 0.76, 95% CI 0.63-0.91), even after adjustment for all available patient and tumor characteristics (Table 2). Patients with large (>5cm) high grade tumors had significantly improved overall survival (HR 0.63, 95% CI 0.53-0.77) associated with the administration of radiation therapy (Table 3).
Table 2.
Hazard Ratios and 95% Confidence Intervals for Multivariable Models of Overall Mortality, Stratified by Tumor Grade.
| All Patients (n=4,322) | Low grade (n=1,942) | High grade (n=2,380) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Radiation therapy* | ||||||
| No radiation | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| Radiation therapy | 0.78 | (0.69, 0.89) | 1.00 | (0.79, 1.27) | 0.67 | (0.57, 0.79) |
| Patient characteristics | ||||||
| Age in years | ||||||
| 20-44 years | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| 45-59 years | 1.31 | (1.05, 1.64) | 1.41 | (0.91, 2.19) | 1.29 | (0.99, 1.68) |
| 60-74 years | 2.39 | (1.94, 2.94) | 4.25 | (2.87, 6.30) | 1.90 | (1.49, 2.43) |
| 75+ years | 5.30 | (4.32, 6.51) | 12.65 | (8.57, 18.68) | 3.82 | (2.99, 4.87) |
| Sex | ||||||
| Male | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| Female | 0.75 | (0.66, 0.85) | 0.81 | (0.64, 1.02) | 0.72 | (0.63, 0.84) |
| Tumor characteristics | ||||||
| T stage | ||||||
| T1 | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| T2 | 1.70 | (1.48, 1.96) | 1.20 | (0.91, 1.58) | 1.97 | (1.67, 2.33) |
| N status | ||||||
| N0 | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| N1 | 2.40 | (1.39, 4.14) | 1.06 | (0.14, 8.21) | 3.00 | (1.68, 5.38) |
| Grade | ||||||
| G1/2 | 1.0 | Reference | NA | NA | NA | NA |
| G3/4 | 2.35 | (2.02, 2.73) | NA | NA | NA | NA |
| Location | ||||||
| Upper | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| Lower | 1.03 | (0.89, 1.18) | 1.11 | (0.84, 1.46) | 1.03 | (0.87, 1.22) |
| Laterality | ||||||
| Right | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| Left | 0.97 | (0.86, 1.09) | 1.09 | (0.87, 1.37) | 0.94 | (0.82, 1.09) |
| Histology | ||||||
| Fibrohistiocytic tumors | 1.0 | Reference | 1.0 | Reference | 1.0 | Reference |
| Liposarcoma | 0.70 | (0.59, 0.83) | 0.58 | (0.42, 0.80) | 0.78 | (0.63, 0.98) |
| Leiomyosarcomas | 1.11 | (0.90, 1.37) | 0.88 | (0.58, 1.34) | 1.16 | (0.91, 1.48) |
| Synovial sarcomas | 1.38 | (0.97, 1.95) | 2.06 | (1.09, 3.90) | 1.01 | (0.65, 1.55) |
| Fibrosarcoma | 0.80 | (0.56, 1.14) | 0.73 | (0.43, 1.23) | 0.76 | (0.46, 1.25) |
| Osseous and chondromatous | 1.09 | (0.73, 1.64) | 0.51 | (0.23, 1.10) | 1.56 | (0.96, 2.52) |
| neoplasms of soft tissue | ||||||
| Peripheral nerve sheath tumors | 1.49 | (0.99, 2.23) | 0.71 | (0.30, 1.67) | 1.86 | (1.17, 2.97) |
| Blood vessel tumors | 1.65 | (1.01, 2.71) | 0.67 | (0.16, 2.84) | 2.05 | (1.21, 3.48) |
| Rhabdomyosarcoma | 1.31 | (0.75, 2.30) | -- | -- | 1.43 | (0.81, 2.52) |
| Other | 1.13 | (0.93, 1.37) | 0.68 | (0.42, 1.11) | 1.20 | (0.97, 1.48) |
HR: Hazard Ratio. CI: Confidence Interval. NA: not applicable. ---: too few events to calculate results. Values in bold are significant at the p=.05 level.
Table 3. Hazard ratios in Univariate and Multivariable Models of Overall Mortality, for Patients with High Grade T2 Tumors.
| Overal l Survival | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| HR | 95% CI | HR | 95% CI | |
| Radiation therapy | ||||
| Did not receive radiation therapy | 1.0 | Reference | 1.0 | Reference |
| Received neo-adjvant radiation therapy | 0.59 | (0.44, 0.78) | 0.68 | (0.50, 0.94) |
| Received postoperative radiation therapy | 0.59 | (0.49, 0.72) | 0.61 | (0.49, 0.75) |
HR: Hazard Ratio. CI: Confidence Interval. Values in bold are significant at the p=.05 level.
Neo-adjuvant Radiation
Of the patients who received radiation therapy, 13.5% received it prior to surgical resection. Of this neo-adjuvant cohort, 75% were high grade lesions, and 78% were T2 lesions. The incidence of patients receiving neo-adjuvant radiation therapy did not change significantly between 1988 and 2005 (Figure 3). There was no difference in overall survival in patients with large (>5cm) high grade lesions who received neo-adjuvant radiation versus post-operative radiation therapy.
Figure 3.
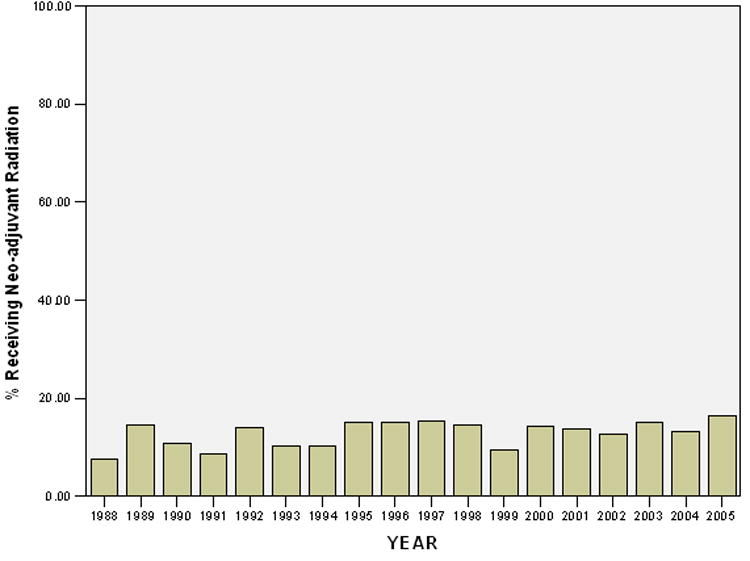
Proportion of Patients who received Neo-adjuvant Radiation Therapy from 1988-2005.
Discussion
This large population based cohort study examined the impact of radiation therapy on patients who had undergone a limb sparing surgical resection. This study is the first to report that radiation resulted in improved survival outcomes, with the greatest benefit seen in patients with large (>5cm) high grade tumors.
Wide local excision (WLE) has a high local failure rate up to 30%, and so adjuvant radiation has been added to bring failure rates <10% (11). Local recurrence was found to be an adverse risk factor by 4.4 times for distant metastasis in a large Scandinavian registry study of 559 patients (12). Intuitively, decreasing local failures and thus distant metastases would improve overall survival, but this has not been proven yet. In the largest prospective study completed at the NCI, 91 patients with soft tissue sarcomas of the extremity were randomized to receive or not receive post-operative radiation therapy after WLE (10). In high grade tumors, there were no local recurrences (0%) in the radiation arm compared to 22% in the no radiation arm. In low grade tumors that received radiation, there was a 4% local recurrence rate compared to 31% in patients who did not receive radiation therapy. Thus, it was found that that radiation decreased recurrences, however there was no survival difference between the two arms in both the high and low grade tumors. With so few patients in the study, this negative finding may have been due to a lack of power.
Our study did not find an overall survival benefit in the low grade tumors. This seems consistent with a prospective study that suggests that wide local excision alone for low grade, T1 tumors with complete resection margins is acceptable (13). This phase III adjuvant brachytherapy study of 164 patients demonstrated a 5 year local control benefit with radiation for high grade tumors (91% vs 70%), but not of any additional benefit for the low grade tumors. Despite the better local control for high grade tumors with radiation, this study, like the NCI study, also did not show any difference in distant metastasis or disease free survival. Our results confirm that radiotherapy is primarily effective in large high grade lesions and with a cohort of over 6,000 patients, our study also shows an overall survival benefit. (10, 13, 14) Treatment of sarcoma may be analogous to the breast cancer paradigm, where wide local excision and radiation is equivalent to more radical amputation / mastectomy, and the local control benefit of radiation was found to have an overall survival benefit for patients in a larger pooled analysis (15).
This study was limited primarily based on information availability in the SEER database. First, no information on radiotherapy technique (total dose, fraction size, beam energy) was available, so results could not determine if survival varies based on these factors. Second, although some patient characteristics that are potential confounding factors (such as margin or performance status) were not available in this data set, this study adjusted for all available patient and tumor characteristics. It is possible that the use of adjuvant radiation for low grade sarcomas may be justified if there are known, adverse risk factors (eg, incomplete resection, positive margins) not captured by the SEER database. It is possible that adjuvant radiation for these patients improved results to the level of the more favorable, low grade subgroup that would not need radiation according to the prospective wide local excision alone trial (13).
We also cannot comment on the influence of chemotherapy in this study because it was not recorded in SEER. Per NCCN guidelines, chemotherapy is not a standard treatment regardless of histology. We attempted to exclude pediatric tumors with an age eligibility >20 years old. Adjuvant chemotherapy was given to all high grade tumors in the NCI prospective study, but no change in overall survival was seen. A Lancet meta-analysis of 1568 patients from 14 trials using doxorubicine-based regimens failed to find an overall survival advantage (16). Thus, chemotherapy's role for adult sarcoma tissue is controversial and remains uncertain (16-19). Regardless, even if one assumes that adjuvant chemotherapy was preferentially to the radiation arm, the Lancet meta-analysis suggests a small absolute benefit of 5% at 5-10 years. This study suggests a benefit beyond any chemotherapy effect with an absolute overall survival difference of 10% in high grade lesions.
The majority of patients who received neo-adjuvant radiation in this study were in the large (>5cm) high grade subset but there was no difference in overall survival between patients with neo-adjuvant or post-operative radiation for this group. In regards to neo-adjuvant vs. post-operative radiation treatment preference, a large randomized trial from the NCI Canada Clinical Trial Group initiated in 1994, closed in 1997, and reported in 2002 showed that local recurrence and progression-free survival were similar between either arm (20). However, neo-adjuvant radiotherapy was associated with improved long-term functional outcomes when compared to post-operative radiotherapy (21). Despite these results, we found that the incidence of patients receiving neo-adjuvant radiotherapy nationwide has not changed significantly over the past twenty years.
Conclusions
This is the largest population based study in patients undergoing limb sparing surgery for soft tissue sarcomas of the extremities and our results indicate that radiation therapy was associated with improved survival outcomes in patients with high grade tumors. Furthermore, neo-adjuvant radiation appears equivalent to post-operative radiation therapy in terms of survival outcomes in patients with large high grade tumors. Finally, the rates of neo-adjuvant radiation therapy have not increased significantly over the past several years.
Acknowledgments
Shayna Rich's work on this study was supported by training grant T32 AG000262 from the National Institute on Aging, Bethesda, MD.
Footnotes
Conflict of Interest: There is no conflict of interest with any of the authors.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Mann GB, Lewis JJ, Brennan MF. Adult soft tissue sarcoma. Aust N Z J Surg. 1999;69:336–43. doi: 10.1046/j.1440-1622.1999.01568.x. [DOI] [PubMed] [Google Scholar]
- 3.Storm HH. Survival of adult patients with cancer of soft tissues or bone in europe. EUROCARE working group. Eur J Cancer. 1998;34:2212–7. doi: 10.1016/s0959-8049(98)00335-9. [DOI] [PubMed] [Google Scholar]
- 4.Muhic A, Hovgaard D, Mork Petersen M, et al. Local control and survival in patients with soft tissue sarcomas treated with limb sparing surgery in combination with interstitial brachytherapy and external radiation. Radiother Oncol. 2008;88:382–7. doi: 10.1016/j.radonc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Leibel SA, Tranbaugh RF, Wara WM, et al. Soft tissue sarcomas of the extremities: Survival and patterns of failure with conservative surgery and postoperative irradiation compared to surgery alone. Cancer. 1982;50:1076–83. doi: 10.1002/1097-0142(19820915)50:6<1076::aid-cncr2820500610>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg RD, Martin RG, Romsdahl MM, et al. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47:2391–7. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Fein DA, Lee WR, Lanciano RM, et al. Management of extremity soft tissue sarcomas with limb-sparing surgery and postoperative irradiation: Do total dose, overall treatment time, and the surgery-radiotherapy interval impact on local control? Int J Radiat Oncol Biol Phys. 1995;32:969–76. doi: 10.1016/0360-3016(95)00105-8. [DOI] [PubMed] [Google Scholar]
- 8.Abbatucci JS, Boulier N, de Ranieri J, et al. Local control and survival in soft tissue sarcomas of the limbs, trunk walls and head and neck: A study of 113 cases. Int J Radiat Oncol Biol Phys. 1986;12:579–86. doi: 10.1016/0360-3016(86)90066-0. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trovik CS, Scanadinavian Sarcoma Group Project Local recurrence of soft tissue sarcoma. A scandinavian sarcoma group project. Acta Orthop Scand Suppl. 2001;72:1–31. [PubMed] [Google Scholar]
- 13.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–68. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 14.Jebsen NL, Trovik CS, Bauer HC, et al. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: A scandinavian sarcoma group study. Int J Radiat Oncol Biol Phys. 2008;71:1196–203. doi: 10.1016/j.ijrobp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 16.Sarcoma Meta-analysis Collaboration (SMAC) Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev. 2000;(4):CD001419. doi: 10.1002/14651858.CD001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueredo A, Bramwell VH, Bell R, et al. Adjuvant chemotherapy following complete resection of soft tissue sarcoma in adults: A clinical practice guideline. Sarcoma. 2002;6:5–18. doi: 10.1080/13577140220127512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–47. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 19.Tierney JF, Mosseri V, Stewart LA, et al. Adjuvant chemotherapy for soft-tissue sarcoma: Review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72:469–75. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 21.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]


