Abstract
Background
The significance of tumor markers in patients with appendiceal carcinomatosis is poorly defined. We determined preoperative and postoperative tumor marker levels in patients undergoing cytoreductive surgery (CRS) and heated intraperitoneal chemoperfusion (HIPEC) and examined their association with clinicopathologic features and survival.
Methods
A total of 176 patients undergoing attempted CRS/HIPEC for appendiceal carcinomatosis had at least 1 tumor marker measured. Marker levels were correlated with tumor characteristics and oncologic outcomes. Kaplan–Meier curves and multivariate Cox regression models were used to identify prognostic factors affecting progression and survival.
Results
At least 1 marker was elevated prior to CRS/ HIPEC in 70 % of patients (CEA, 54.1 %; CA19-9, 47.7 %; CA-125, 47.2 %). Among patients with elevated preoperative marker levels, normalization occurred postoperatively in 79.4 % for CEA, 92.3 % for CA19-9, and 60 % for CA-125. Absolute preoperative tumor marker levels correlated with peritoneal carcinomatosis index (PCI) (p < .0002), and the number of elevated markers was associated with PCI and progression-free survival (PFS). Elevated postoperative CEA level was associated with decreased PFS (median, 13 vs 36 months, p = .0008). On multivariate Cox regression analysis, elevated preoperative CA19-9 was associated with shorter PFS (hazard ratio [HR] 2.9, 95 % confidence interval [95 % CI] 1.5–5.3, p = .0008), whereas elevated CA-125 was associated with shorter overall survival (HR 2.6, 95 % CI 1.3–5.4, p = .01).
Conclusions
Most patients with appendiceal carcinomatosis will have at least 1 elevated tumor marker and will normalize following CRS/HIPEC, allowing for ongoing surveillance. CA19-9 is a promising biomarker for early progression following CRS/HIPEC, whereas CA-125 is associated with shorter survival.
Appendiceal neoplasms with peritoneal dissemination comprise a spectrum of aggressiveness with varying potential for disease progression and death.1–4 In many patients, the natural history is marked by regional progression without extra-abdominal metastasis, and it is in these individuals that aggressive regional treatment is associated with disease control and the potential for long-term survival.5–11 The risks inherent in aggressive regional therapy for carcinomatosis have spurred efforts to improve prognostication and selection of appropriate patients for this treatment approach. Since serum tumor antigen levels are frequently elevated in patients with appendiceal neoplasms, these markers hold the potential to improve our ability to select suitable candidates for aggressive regional therapy, as well as to signal the opportunity for early reintervention in the setting of recurrent disease. The clinical usefulness of measuring the tumor markers carcinoembryonic antigen (CEA), CA19-9, and CA-125 has been well documented, as these biomarkers are widely used in a variety of tumor types to help confirm the presence of malignancy and serve as reliable indicators of response to chemotherapy and as harbingers of recurrent disease following curative surgery.12–14
To date, there is a paucity of information on the use of serum tumor markers in appendiceal cancer, relative to more common gastrointestinal malignancies, likely related to the rarity of this condition. The utility of measuring serum tumor marker levels in disseminated appendiceal neoplasms has recently been investigated, although consensus guidelines for their use have yet to emerge. Use of these markers has been advocated on the basis of their high sensitivity for this disease in the appropriate clinical setting, their wide availability, their correlation with extent of disease [peritoneal cancer index (PCI)] and completeness of cytoreduction (CC) score, and their prognostic significance.15–18 In addition, when used during posttreatment surveillance, elevated tumor markers have been shown to predate the radiographic appearance of recurrent disease by up to 9 months.17 Thus, a strong rationale for investigating the use of serum tumor markers in patients with disseminated appendiceal neoplasms has emerged on the basis of early reports.
To explore the utility of serum tumor marker levels in patients with disseminated appendiceal tumors, we adopted the practice of routinely measuring CEA, CA19-9, and CA125 in patients being considered for aggressive regional therapy, including cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC). In this paper, we describe our experience with serum tumor markers in a high-volume peritoneal surface malignancy program and correlate these levels with clinicopathologic features and oncologic outcomes in our patient population.
PATIENTS AND METHODS
Between May 2001 and July 2010, 282 patients with peritoneal carcinomatosis of appendiceal origin underwent attempted CRS with heated intraperitoneal chemotherapy (HIPEC). This cohort of patients has been described elsewhere.5 Among these patients, 176 had at least 1 tumor marker (i.e., CEA, CA19-9, or CA-125) measured either preoperatively or postoperatively, and this subset of patients constitutes the current study group. As previously described, patients were evaluated in a dedicated multi-disciplinary peritoneal surface malignancy clinic prior to being selected for CRS-HIPEC. Characteristics of patients undergoing this treatment regimen, including outcome variables, were collected as part of a University of Pitts-burgh Medical Center (UPMC) Institutional Review Board-approved protocol. CRS-HIPEC was carried out according to established and previously described methods.19,20
Briefly, after exploratory laparotomy and estimation of disease burden according to the Dutch simplified peritoneal carcinomatosis index (SPCI), CRS including peritonectomy and visceral resection were carried out with the aim of achieving complete cytoreduction (i.e., CC-0 or CC-1).21,22 HIPEC was administered via a closed technique with a roller-pump heat-exchanger perfusion machine (Thermo-Chem HT-100, ThermaSolutions, Melbourne, FL), using mitomycin C at a tissue temperature of 42 °C for 100 min as previously described.5
Serum levels of CEA, CA19-9, and CA-125 were recorded and were deemed elevated at levels of >5 ng/mL, >37 U/mL, and >35 kU/mL, respectively. Preoperative tumor antigen levels were recorded at the time of presentation to the UPMC multidisciplinary peritoneal surface malignancy clinic. Postoperative levels were obtained at the first postoperative office visit, typically within 2 weeks of discharge and within 30 days following CRS/HIPEC.
To investigate the association of clinicopathologic variables with tumor marker levels, patients were stratified on the basis of normal versus elevated serum concentrations of CEA, CA 19-9, and CA-125. Categorical variables were compared using chi-square or Fisher exact tests as appropriate. Continuous variables were compared using the t test. Absolute serum concentrations of each tumor marker were tested for correlation with volume of disease (SPCI) using the Spearman rank order correlation method, and the number of elevated markers was tested for correlation with SPCI using the Kruskal–Wallis test. Survival differences between subgroups were assessed using log-rank tests. Statistical significance for all tests was defined as a 2-tailed p value less than .05. Median follow-up was 28 months overall and 38 months in patients alive at the end of the study period. Multivariable analysis was carried out using stepwise backward Cox regression method as previously described.
RESULTS
Patient Characteristics, Operative Details, and Oncologic Outcomes
A total of 282 patients underwent attempted CRS/HI-PEC for disseminated appendiceal neoplasms between May 2001 and July 2010. Operative variables and outcomes have been presented elsewhere, but in summary, the patient group had a mean age of 54 years and the gender distribution was 50 % female and male.5 Disease was recurrent in 47.5 %, primary presentation in 48.6 %, and unknown in 3.6 %. Prior CRS had been performed in 34.1 % of patients, whereas 38.7 % of patients had received previous systemic chemotherapy. Most patients (~60 %) who received prior chemotherapy did so as adjuvant therapy after a previous operation, while ~30 % of these patients were treated with dedicated neoadjuvant systemic chemotherapy prior to CRS/HIPEC; the remainder had an unknown rationale for prior therapy. A small minority (7.6 %) had undergone previous HIPEC. Complete cyto-reduction (CCR0 or CCR1) was achieved in 81.6 % of patients, with 89 % receiving HIPEC. Tumor histologic classification was DPAM in 25.2 % and PMCA in 74.5 %; most patients (63.8 %) had low-grade tumors and 13.1 % showed signet ring cells. Median SPCI was 14. Lymph node metastases were present in 23.4 %. Major morbidity occurred in 24.8 %, with a 60-day mortality rate of 1.1 %. Updated median and 5 year overall survival were 73.6 months and 55.8 %; updated median and 5 year progression-free survival were 36.3 months and 37.3 %.5
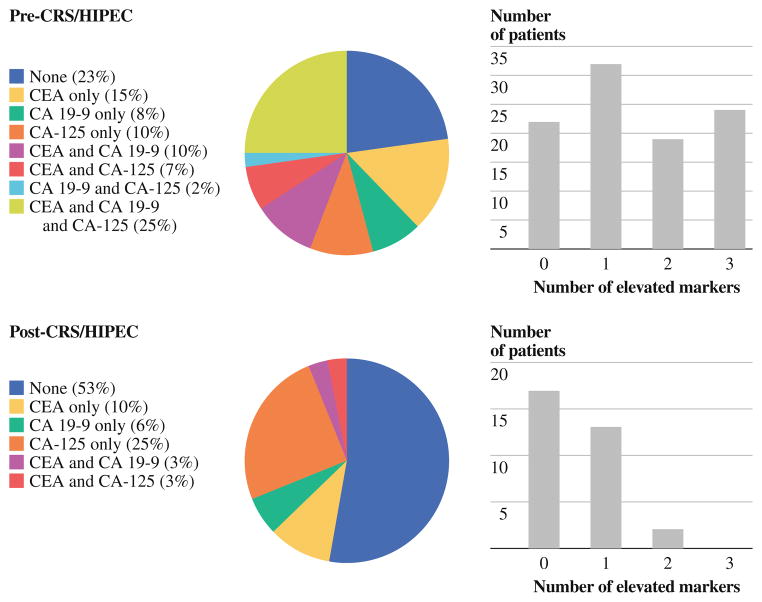
At least 1 tumor marker was measured preoperatively or postoperatively in 176 patients. Preoperative CEA, CA 19-9, and CA 125 levels were determined in 157, 111, and 106 patients, respectively. In 97 patients, all 3 tumor markers were determined preoperatively. The distribution and number of positive markers per patient are shown in Fig. 1. Univariate analysis of associations between clinicopathologic features and each tumor marker are shown in Table 1. Postoperatively, CEA, CA19-9, and CA125 levels were measured in 87, 45, and 38 patients, respectively.
FIG. 1.
Distribution of elevated tumor markers, pre-CRS/HIPEC and post-CRS/HIPEC
TABLE 1.
Univariate analysis of preoperative tumor marker levels
| CEA (ng/mL)
|
CA 19-9 (U/mL)
|
CA-125 (kU/mL)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤5, n (%) | > 5, n (%) | p value | ≤37, n (%) | > 37, n (%) | p value | ≤35, n (%) | > 3, n (%) | p value | |
| Overall | 72 (46) | 85 (54) | 58 (52) | 53 (48) | 56 (53) | 50 (47) | |||
| Age, mean | 53.9 | 57 | .2 | 56.1 | 53.9 | .3 | 55.9 | 55.3 | .8 |
| Gender | .2 | .3 | .4 | ||||||
| Male | 39 (54) | 37 (44) | 29 (50) | 21 (40) | 20 (36) | 22 (44) | |||
| Female | 33 (46) | 48 (56) | 29 (50) | 32 (60) | 36 (64) | 28 (56) | |||
| ASA | .1 | .4 | .5 | ||||||
| 1 | 1 (2) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 0 (0) | |||
| 2 | 15 (25) | 8 (12) | 8 (16) | 7 (16) | 9 (19) | 6 (14) | |||
| 3 | 36 (61) | 42 (66) | 36 (74) | 27 (61) | 31 (66) | 27 (64) | |||
| 4 | 7 (12) | 14 (22) | 5 (10) | 9 (21) | 6 (13) | 9 (22) | |||
| Recurrent disease | 27 (38) | 50 (60) | .01 | 22 (38) | 33 (64) | .01 | 32 (57) | 19 (39) | .08 |
| Symptomatic | 51 (71) | 50 (59) | .1 | 36 (62) | 35 (66) | .7 | 31 (55) | 33 (66) | .3 |
| CT evident disease | 47 (65) | 55 (66) | 1 | 37 (65) | 32 (60) | .7 | 27 (49) | 36 (72) | .02 |
| Prior chemotherapy | 28 (39) | 34 (40) | 1 | 19 (33) | 23 (43) | .3 | 22 (39) | 17 (34) | .7 |
| Prior HIPEC | 1 (1) | 8 (10) | .04 | 1 (2) | 4 (8) | .2 | 3 (5) | 1 (2) | .6 |
| Prior CRS | 21 (45) | 29 (59) | .2 | 15 (40) | 19 (63) | .09 | 16 (43) | 13 (50) | .6 |
| SPCI, median | 12 | 14 | .0002 | 13 | 16 | .0001 | 12 | 15 | .000007 |
| Complete cytoreduction | 57 (80) | 67 (80) | 1.0 | 48 (83) | 40 (77) | .5 | 46 (82) | 37 (76) | .5 |
| Diagnosis | .2 | .7 | .7 | ||||||
| DPAM | 14 (19) | 24 (29) | 13 (22) | 14 (27) | 16 (29) | 12 (24) | |||
| PMCA | 58 (81) | 60 (71) | 45 (78) | 38 (73) | 40 (71) | 37 (76) | |||
| High grade | 37 (51) | 22 (26) | .002 | 24 (41) | 18 (34) | .4 | 18 (32) | 21 (42) | .3 |
| Signet cells | 21 (29) | 9 (11) | .004 | 15 (26) | 9 (17) | .4 | 13 (23) | 8 (16) | .5 |
| Lymph node metastases | 16 (30) | 9 (16) | .1 | 10 (22) | 9 (23) | 1 | 10 (24) | 6 (15) | .4 |
CEA
Preoperative CEA levels were elevated in 85 of 157 patients (54.1 %). CEA was more commonly elevated in patients with recurrent appendiceal carcinomatosis compared with patients in their initial presentation (64.9 vs 42.9 %, p = .01) and was elevated in 8 of 9 patients who had previously undergone HIPEC (88.9 %, p = .04, Table 1). Elevated CEA levels were strongly associated with higher SPCI (Fig. 2a), although more than a third of patients with low PCI (0–7) had an elevated preoperative CEA level. CEA levels were more frequently elevated in patients with low-grade histology (63.9 vs 37.3 %, p = .002) and patients without signet cells (59.8 vs 30.0 %, p = .004). Patients in whom KRAS mutations were identified were more likely to have elevated preoperative CEA levels (61.0 vs 33.3 %, p = .03).
FIG. 2.
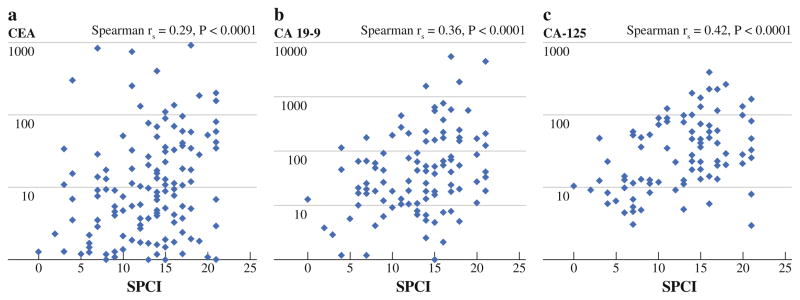
Correlation of absolute tumor marker levels with volume of disease (SPCI)
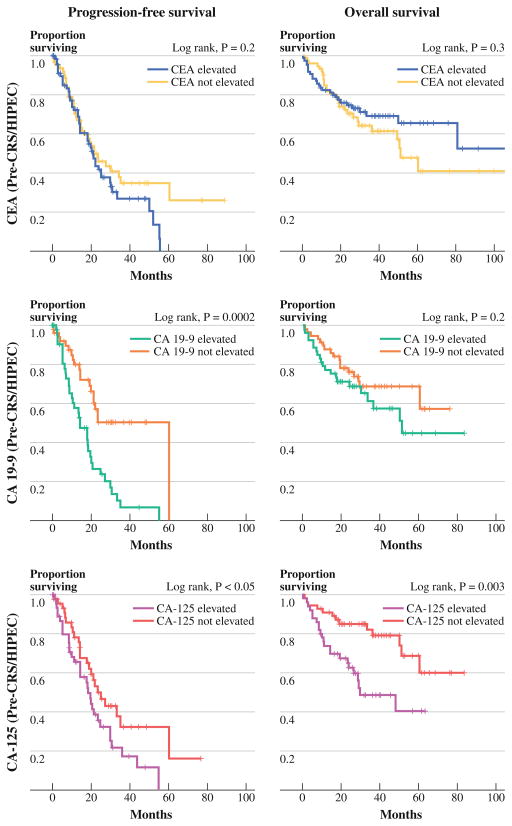
Preoperative CEA levels demonstrated no significant correlation with patient age or gender, presence of symptoms, presence of extraperitoneal disease, ability to achieve complete cytoreduction, histologic classification (DPAM vs. PMCA), or node positivity. There was no evidence of correlation between elevated preoperative CEA levels and levels of CA19-9 or CA125. Preoperative CEA was not associated with overall or progression-free survival on univariate analysis (Fig. 3).
FIG. 3.
Survival stratified by preoperative tumor marker elevation
Among 87 patients in whom postoperative CEA level was measured, only 20 (23 %) had an elevated level. Among 85 patients with an elevated preoperative CEA level, 34 had a postoperative CEA level measured, and 7 patients (20.6 %) were elevated, whereas 79.4 % experienced normalization of their elevated CEA level after CRS/ HIPEC. Among the 7 patients with a persistently elevated CEA, 2 had undergone incomplete cytoreduction, and 1 had extraperitoneal disease. Elevated postoperative CEA level was significantly associated with shorter progression-free survival on univariate analysis (Fig. 4), with a median progression-free survival of 13.1 months versus 36.2 months (p = .0008). This difference persisted among patients undergoing complete cytoreduction (not shown). No significant difference in overall survival on the basis of postoperative CEA level was observed (Fig. 4).
FIG. 4.
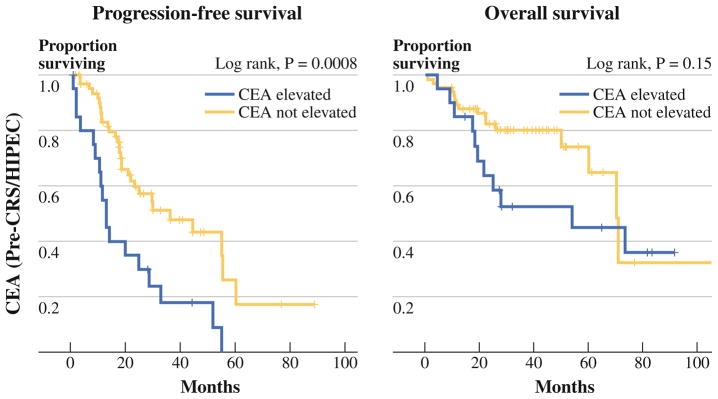
Survival stratified by postoperative CEA elevation
CA19-9
Preoperative CA19-9 level was elevated in 53 of 111 patients (47.7 %). Elevated preoperative CA19-9 level was associated with higher SPCI (Fig. 2b) and recurrent versus initial presentation of disease (63.5 vs 37.9 %, p = .01; Table 1). No association was found between elevated CA19-9 and patient age, gender, symptoms, presence of extraperitoneal disease, ability to achieve a complete cytoreduction, histologic diagnosis, grade, presence of signet cells, presence of positive lymph nodes, or KRAS mutation status. A positive association between CA19-9 and CA-125 was noted, in that 59.1 % of patients with elevated CA19-9 also had elevated CA-125, whereas 66.7 % of patients with normal CA19-9 also had normal CA-125 (p = .01). Patients with elevated preoperative CA19-9 levels exhibited a shorter progression-free survival relative to those with normal CA 19-9 levels (median, 14.4 vs. 60.3 months, p = .0002), whereas no association with overall survival was seen. The observed difference in progression-free survival was also seen when restricting the analysis to patients undergoing treatment during their initial presentation with disease and excluding those being treated for recurrent carcinomatosis (p = .03).
Postoperative CA19-9 level was measured in 45 patients and was normal in 37 (82 %). Among patients with an elevated preoperative CA19-9 level and a known postoperative CA19-9 level, 12 of 13 (92 %) had normalized following surgery; the 1 patient in whom CA19-9 failed to normalize had a complete cytoreduction and no known extraperitoneal disease. Postoperative CA19-9 level was not significantly associated with overall or progression-free survival.
CA125
Preoperative CA-125 level was elevated in 50 of 106 patients (47.2 %). Elevated preoperative CA-125 was significantly associated with higher SPCI (Fig. 2c), but was not associated with patient age, gender, histologic diagnosis, grade, presence of signet cells, presence of nodal metastasis, presence of symptoms, presence of extraperitoneal disease, or ability to achieve complete cytoreduction (Table 1). As noted previously, elevation of CA-125 was correlated with elevation of CA19-9. On univariate analysis, elevated preoperative CA-125 was significantly associated with shorter progression-free survival (median, 18.6 vs. 24.9 months, p < .05), as well as overall survival (median, 29.9 vs. not reached, p = .003) (Fig. 3). When restricting the analysis to patients treated during their initial presentation with carcinomatosis, the differences in progression-free and overall survival were no longer statistically significant.
Postoperative CA-125 level was measured in 38 patients and was elevated in 10 patients. A total of 21 patients had both preoperative and postoperative CA-125 levels measured. Among 10 patients with an elevated preoperative CA-125 level, 6 had normal postoperative CA-125 levels and 4 failed to normalize postoperatively in spite of having undergone complete cytoreduction and having no extraperitoneal disease. Postoperative CA-125 was not associated with overall or progression-free survival.
Analysis of Number of Elevated Tumor Markers
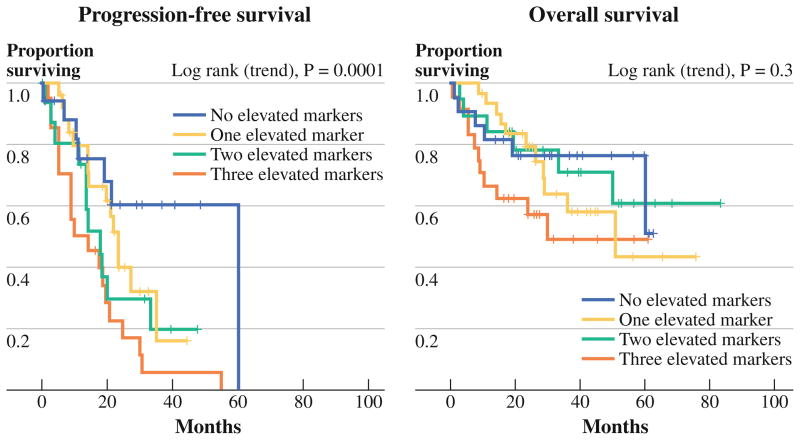
We noted a correlation between SPCI and the number of elevated preoperative tumor markers, in that patients with no elevated markers had a mean SPCI of 10.2, compared with 12.5 for patients with a single elevated marker, 13.4 for 2 elevated markers, and 15.5 for patients with 3 elevated markers (p = .006). Patients were stratified on the basis of number of elevated preoperative tumor marker levels, in order to assess for an impact on survival (Fig. 5). Decreased progression-free survival was found to be associated with increasing number of preoperatively elevated tumor markers (p = .004), and overall survival of patients with elevated preoperative levels of all 3 markers was shorter relative to patients with 0–2 elevated markers (p = .003).
FIG. 5.
Survival stratified by number of elevated preoperative tumor markers
Postoperatively, 17 patients had normal levels of all 3 tumor markers. Comparing these patients against 34 who had at least 1 elevated postoperative marker, we found no significant differences in clinicopathologic features or survival.
Multivariate Analysis of Progression-Free and Overall Survival
To examine whether serum tumor markers hold independent prognostic significance, we performed Cox regression analysis incorporating well-characterized risk factors for progression and death in patients with appendiceal carcinomatosis (Table 2). We found that elevated preoperative serum CA 19–9 level was independently predictive of progression (hazard ratio [HR] 2.9, p = .0008), along with patient age, SPCI, high-grade histology, and incomplete cytoreduction. Due to the association of CA19-9 with recurrent disease on univariate analysis, we performed a bivariate analysis incorporating CA19-9 elevation and recurrent disease status to assess whether the observed association between CA19-9 and progression is independent of recurrent disease status. In this model, CA19-9 was confirmed to be independent of recurrent disease status, with a hazard ratio of 2.4 for progression (95 % confidence interval [95 % CI], 1.3–4.4, p = .004). Elevated preoperative CA-125 was independently associated with poor overall survival (HR 2.6, p = .01), along with patient age, prior chemotherapy, SPCI, and high-grade histology.
TABLE 2.
Cox regression analysis of progression-free and overall survival
| Factor | Progression-free survival HR (95 % CI) | Overall survival HR (95 % CI) |
|---|---|---|
| Age | 1.04 (1.01–1.06), p = .003 | 1.06 (1.02–1.1), p = .003 |
| Prior chemotherapy | NS | 3.2 (1.5–6.7), p = .003 |
| SPCI | 1.1 (1.02–1.2), p = .01 | 1.1 (1.05–1.2), p = .001 |
| High grade | 3.2 (1.8–5.9), p = .0002 | 4.6 (2.1–10.1), p = .0001 |
| Incomplete cytoreduction | 2.5 (1.3–4.9), p = .009 | NS |
| Elevated preoperative CEA | NS | NS |
| Elevated preoperative CA19-9 | 2.9 (1.5–5.3), p = .0008 | NS |
| Elevated preoperative CA-125 | NS | 2.6 (1.3–5.4), p = .01 |
HR hazard ratio, CI confidence interval, SPCI simplified peritoneal carcinomatosis index, NS not statistically significant
DISCUSSION
Serum tumor marker levels play an important role in the management of a number of gastrointestinal malignancies. Tumor markers can aid in securing a diagnosis of malignancy, in determining the primary site of a malignancy, in assessing response to therapy, in estimating prognosis, and in detecting recurrence.12–14 In the treatment of patients with peritoneal dissemination from primary appendiceal tumors, tumor markers have been used to judge response to systemic chemotherapy administered prior to CRS, and in some cases may be used to help decide whether a patient is a good candidate for CRS/HI-PEC. Moreover, since recurrence rates are high following CRS/HIPEC, serum tumor marker levels may aid in detecting recurrence earlier than cross-sectional imaging, thereby identifying candidates for retreatment with systemic and/or regional therapy at a time of minimal disease burden. In order to refine our understanding of the utility of serum tumor markers in patients with carcinomatosis of appendiceal origin, we examined tumor markers in a large series of patients undergoing CRS/HIPEC.
The reported prevalence of elevated preoperative serum CEA in patients with appendiceal carcinomatosis has ranged from 44 to 75 % and was 54 % in our population.15–17,23 We found that elevated preoperative CEA levels were associated with low-grade histology and with increased burden of disease, as measured by SPCI. Prior studies have examined CEA as a prognostic factor in appendiceal carcinomatosis. Groups in the Netherlands, the UK, and Italy have suggested a relationship between elevated CEA and shorter progression-free survival, although no independent association based on multivariate analysis has been shown.16,17,23 Carmignani et al., from the Washington Cancer Institute, demonstrated an association between CEA elevation and decreased overall survival, but, again, no multivariable analysis of this association was presented.15 In our series, we did not observe any relationship between preoperative CEA levels and survival, possibly due to differences in selection criteria for CRS/ HIPEC or other disparities in patient characteristics being treated at different centers. However, we demonstrated worse progression-free survival among patients with elevated postoperative CEA levels.
The prevalence of elevated preoperative CA19-9 serum levels in our patients was 48 %, compared with a reported range of 38–67 %.15–17,23 We found a strong association of preoperative CA19-9 levels with SPCI and confirmed in our population a strong independent association of elevated preoperative CA19-9 with reduced progression-free survival, as has been previously reported by van Ruth et al. in the Netherlands, Baratti et al. in Italy, and Elias et al. in France.16,17,24
The prevalence of elevated preoperative CA-125 serum levels was reported to be 41 % by Alexander-Sefre et al. (UK) and 59 % by the Italian group, compared with our finding of 47 %.16,23 Preoperative CA-125, like CEA and CA19-9, was strongly correlated with SPCI in our population. In our series, preoperative CA-125 was a strong independent predictor of decreased overall survival with a hazard ratio of 2.6 (p < .01), a result that was not obtained in prior smaller studies examining CA-125 in patients with appendiceal carcinomatosis.16,23
In our population, the absolute preoperative serum concentration of each tumor marker strongly correlated with burden of disease, as measured by PCI. This relationship was also seen for CEA and CA19-9 in the Netherlands cohort, as well as for CA-125 in the Italian cohort.16,17 In spite of the relationship between tumor marker levels and volume of disease, we did not observe a relationship between elevation in any tumor marker and the ability to achieve a complete cytoreduction. Therefore, there is no suggestion of threshold serum tumor marker concentrations that could be used to exclude patients from an attempt at CRS/HIPEC.
We have adopted the practice of measuring all 3 tumor marker levels at the time of initial evaluation of patients with appendiceal carcinomatosis. This practice is supported by several of our findings. Firstly, one-third of patients will have only 1 preoperatively elevated tumor marker, with the distribution of elevated tumor markers split relatively evenly among CEA (15 %), CA19-9 (8 %), and CA-125 (10 %) (Fig. 1). Second, elevations in different markers carry different prognostic significance—preoperative CA 19-9 being a marker of increased risk for progression and preoperative CA-125 correlating with increased risk for progression as well as risk for death. Third, determining the number of elevated tumor markers is useful since incremental worsening of SPCI and survival were noted with each additional elevated marker in our series, similar to a previous report by Ross et al.18 It is important to measure tumor marker levels prior to CRS/HIPEC since a majority of patients will have normalization of serum levels postoperatively. This provides an opportunity for postoperative surveillance, early detection of disease recurrence, and timely intervention, with repeat CRS/HIPEC and/or systemic chemotherapy, in a disease where recurrence rates are high.
Further research will be necessary to clarify the role of tumor marker levels in the early postoperative period. Our data suggest that measuring CEA following CRS/HIPEC may identify a subset of patients with high risk for recurrence—namely those who fail to show normalization of CEA levels in spite of a complete cytoreduction. However, at this time we have insufficient numbers of patients with postoperative tumor marker levels and long-term follow-up to determine whether postoperative CEA elevation is an independent predictor of recurrence or death. Ongoing work will aim to determine whether CA 19-9 and CA-125 carry similar prognostic information when measured after CRS/HIPEC.
Other relevant areas for further investigation are suggested by our findings. It will be interesting to determine whether the prognostic utility of serum tumor markers differs among patients during their initial presentation with appendiceal carcinomatosis, as opposed to those experiencing recurrent disease. Also, the utility of serum tumor marker levels to assess response to treatment in patients receiving systemic chemotherapy prior to CRS/HIPEC should be explored. In clinical practice, both radiologic and serologic markers of response to systemic therapy are often incorporated into the decision of whether and when to perform CRS/HIPEC on patients with disseminated appendiceal cancer, although there is little data available on which to base this practice. Prospective studies examining the association between changes in serum tumor marker levels during neoadjuvant treatment and oncologic outcomes following subsequent CRS/HIPEC are underway and could improve our ability to select appropriate candidates for CRS/HIPEC in whom a complete cytoreduction is likely to be achieved.
Our insights into appropriate utilization of serum tumor markers in appendiceal carcinomatosis are likely to expand as our follow-up continues to mature. Nevertheless, based on current data, a strong rationale has emerged for measuring CEA, CA 19-9, and CA-125 in all patients with carcinomatosis of appendiceal origin being considered for CRS/HIPEC. Most patients will have an elevation of at least 1 marker, and the majority will normalize following CRS/HIPEC, allowing for postoperative serologic surveillance. CA 19-9 has now been shown in multiple series to be an important independent predictor of progression following CRS/HIPEC. CA-125 is a potentially important predictor of death following CRS/HIPEC, although this finding will need to be confirmed in other institutions. Elevated CEA following complete cytoreduction is associated with early disease progression, although additional research will be needed to clarify the utility of tumor marker measurement in the early post-CRS/HIPEC setting.
References
- 1.Sugarbaker PH, Chang D, Koslowe P. Prognostic features for peritoneal carcinomatosis in colorectal and appendiceal cancer patients when treated by cytoreductive surgery and intraperitoneal chemotherapy. Cancer Treat Res. 1996;81:89–104. doi: 10.1007/978-1-4613-1245-1_9. [DOI] [PubMed] [Google Scholar]
- 2.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089–103. doi: 10.1097/00000478-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bradley RF, Stewart JH, 4th, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinico-pathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–9. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 5.Austin F, Mavanur A, Sathaiah M, Steel J, Lenzner D, Ramalingam L, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19:1386–93. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484–92. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 7.Loungnarath R, Causeret S, Bossard N, Faheez M, Sayag-Beaujard A-C, Brigand C, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudo-myxoma peritonei: a prospective study. Dis Colon Rectum. 2005;48:1372–9. doi: 10.1007/s10350-005-0045-5. [DOI] [PubMed] [Google Scholar]
- 8.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FAN. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–9. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JH, 4th, Shen P, Russell GB, Bradley RF, Hundley JC, Loggie BL, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intra-peritoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 10.Baratti D, Kusamura S, Nonaka D, Langer M, Andreola S, Favaro M, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:526–34. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 11.Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93:1270–6. doi: 10.1002/bjs.5427. [DOI] [PubMed] [Google Scholar]
- 12.Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–60. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–70. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC. Accuracy of CA 125 in the diagnosis of ovarian tumors: a quantitative systematic review. Eur J Obstet Gynecol Reprod Biol. 2009;142:99–105. doi: 10.1016/j.ejogrb.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugar-baker PH. Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol. 2004;87:162–6. doi: 10.1002/jso.20107. [DOI] [PubMed] [Google Scholar]
- 16.Baratti D, Kusamura S, Martinetti A, Seregni E, Laterza B, Oliva DG, et al. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14:2300–8. doi: 10.1245/s10434-007-9393-9. [DOI] [PubMed] [Google Scholar]
- 17.van Ruth S, Hart AAM, Bonfrer JMG, Verwaal VJ, Zoetmulder FAN. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2002;9:961–7. doi: 10.1007/BF02574513. [DOI] [PubMed] [Google Scholar]
- 18.Ross A, Sardi A, Nieroda C, Merriman B, Gushchin V. Clinical utility of elevated tumor markers in patients with disseminated appendiceal malignancies treated by cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2010;36:772–6. doi: 10.1016/j.ejso.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15:204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 20.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15:754–63. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 21.Portilla AG, Shigeki K, Dario B, Marcello D. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol. 2008;98:228–31. doi: 10.1002/jso.21068. [DOI] [PubMed] [Google Scholar]
- 22.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 23.Alexander-Sefre F, Chandrakumaran K, Banerjee S, Sexton R, Thomas JM, Moran B. Elevated tumour markers prior to complete tumour removal in patients with pseudomyxoma peritonei predict early recurrence. Colorectal Dis. 2005;7:382–6. doi: 10.1111/j.1463-1318.2005.00773.x. [DOI] [PubMed] [Google Scholar]
- 24.Elias D, Honoré C, Ciuchendéa R, Billard V, Raynard B, Lo Dico R, et al. Peritoneal pseudomyxoma: results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2008;95:1164–71. doi: 10.1002/bjs.6235. [DOI] [PubMed] [Google Scholar]