Abstract
Background
Prodromal Huntington disease (prHD) is associated with a myriad of cognitive changes, but the domains that best predict time to clinical diagnosis have not been studied. This is a notable gap because some domains may be more sensitive to cognitive decline, which would inform clinical trials.
Objectives
The present study sought to characterize cognitive domains underlying a large test battery and for the first time, evaluate their ability to predict to time to diagnosis.
Methods
Participants included gene-negative and gene-positive prHD participants who were enrolled in the PREDICT-HD study. The CAG/Age Product (CAP) score was the measure of an individual’s genetic signature. A factor analysis of 18 tests was performed to identify sets of measures or latent factors that elucidated core constructs of tests. Factor scores were then fit to a survival model to evaluate their ability to predict time to diagnosis.
Results
Six factors were identified: 1) speed/inhibition, 2) verbal working memory, 3) motor planning/speed, 4) attention-information integration, 5) sensory-perceptual processing, and 6) verbal learning/memory. Factor scores were sensitive to a worsening of cognitive functioning in prHD, typically more so than performances on individual tests comprising the factors. Only the motor planning/speed and sensory-perceptual processing factors predicted time to diagnosis, after controlling for CAP scores and motor symptoms.
Conclusions
The results suggest that motor planning/speed and sensory-perceptual processing are important markers of disease prognosis. The findings also have implications for using composite indices of cognition in preventive HD trials where they may be more sensitive than individual tests.
Keywords: prodromal Huntington Disease, time to diagnosis, cognition, survival analysis
INTRODUCTION
A formal diagnosis of Huntington disease (HD) is made at the appearance of unequivocal motor signs. However, changes in the brain decades before a diagnosis not only produce subtle motor and psychiatric symptoms, but also cognitive changes. With the development of treatments that delay the onset or slow the progression of symptoms, international studies (PREDICT-HD, TRACK-HD, and REGISTRY/COHERT) have made a concerted effort to characterize cognition in prodromal HD (prHD) and determine when changes can be detected to evaluate the potential of cognitive measures as outcomes in clinical trials. Cognitive decline in prHD has been reported on tests of attention, working memory, processing speed, learning and memory, executive functions, sensory-perceptual functions, and emotion perception.[1–13] Most studies focus on a sole task or a few tests so that the relative sensitivity of different cognitive domains cannot be ascertained, which would inform selection of measures in clinical trials. Only one study has evaluated the prognostic significance of two cognitive tests in predicting time to diagnosis using a survival model.[14] However, genetic mutation information that determines whether a subject was at risk for developing HD was unavailable on the majority of cases and therefore, not used in the analysis. This may reduce the prognostic importance of cognitive measures. In addition, the value of multiple cognitive domains in predicting time to diagnosis has not been investigated, which is vital for identifying behavioral markers of disease prognosis.
With these issues in mind, the present study sought to characterize cognitive domains underlying a large test battery and evaluate their sensitivity to time to diagnosis in prHD participants enrolled in the PREDICT-HD study. We first performed a factor analysis of prHD participants’ data from cognitive and sensorimotor tests to identify sets of tests or latent factors that would help conceptualize core constructs of tests and potentially serve as more sensitive indices of functioning than single measures.[15] Then we investigated the cognitive domain(s) that best predicted time to diagnosis. This topic has been difficult to tackle due to the dearth of studies that track prHD individuals until they receive a clinical diagnosis. PREDICT-HD is the first study to prospectively follow genetically-tested prHD participants, evaluating them yearly on a large cognitive battery until a manifest diagnosis is made. Thus, the present study contains a large cohort of newly diagnosed HD subjects with baseline assessments, enabling an investigation of this issue for the first time.
METHODS
Participants
Study participants were enrolled in PREDICT-HD,[6] and included gene-positive prHD individuals and gene-negative controls with a family history of HD. Data were collected at 32 sites in the United States, Canada, Australia, Germany, Spain, and the United Kingdom from 2001 to 2008. Consent was obtained according to the Declaration of Helsinki. The study protocol was approved by the institutional review boards at the University of Iowa and participating sites.
Participants were 18 years of age or older and completed independent genetic testing for the HD CAG expansion prior to study entry. Confirmatory DNA testing was conducted on blood drawn at the baseline PREDICT visit. A polymerase chain-reaction method determined CAG-repeat length.[16] Gene-positive prHD participants had the expansion (≥ 36 CAG repeats) and gene-negative controls did not (< 36 CAG repeats). Exclusion criteria included alcohol or substance abuse within the previous year, learning disability or mental retardation requiring special education, a history of seizures, head trauma, or other neurological disease, a pacemaker or metallic implants, use of antipsychotic medications within the past 6 months, and use of phenothiazine-derivative antiemetic medications more than 3 times per month. Other prescribed and over-the-counter medicines were not restricted.
Another exclusion criterion concerned ratings on the Unified Huntington’s Disease Rating Scale (UHDRS) motor scale,[17] which contains 31 items that assess chorea, bradykinesia, rigidity, dystonia and oculomotor function. Item scores range from 0 (normal) to 4 (motor abnormalities, impairment) and are summed for a total motor score. Trained movement disorders specialists performed the motor exam and rated their level of confidence that any observed motor sign was a manifestation of HD (question 17). Confidence ratings ranged from 0 (normal, no abnormalities) to 4 (motor abnormalities, unequivocal signs, or ≥ 99% chance of HD). Participants with a diagnostic confidence level rating of 4 were excluded when they entered PREDICT-HD.
An prHD individual’s genetic signature at the time of study entry was based on the CAG-Age Product (CAP) score.[18] The CAP is computed by multiplying age at study entry (Age0) by a scaling of the CAG repeat length [CAP = Age0 × (CAG – 33.66)/432.3326]. Scaled CAP scores less than 1, equal to 1, and greater than 1 respectively indicate a 5-year diagnosis probability of less than 0.5, equal to 0.5, and greater than 0.5. The scaled CAP score is a proxy variable for time to diagnosis in a simple survival model containing only CAG and Age0. Hence, the CAP score is an index of the scaled cumulative mutation toxicity, with the scaling in reference to a 50–50 chance of diagnosis by 5 years. For one analysis, we stratified the prHD sample into low, medium, and high CAP groups. The CAP for the control group was assigned as 0, the low group was > 0 ≤ 0.67, the medium group ≥ 0.67 and < 0.85, and the high group > 0.85.[18]
Data from three samples of participants were analyzed. For the factor analysis, the sample included 580 prHD participants with complete data; 157, 197, and 226 had low, medium, and high CAP scores, respectively. In a second, intermediate analysis that examined the internal validity of factor scores, prHD participants were stratified into low, medium, and high CAP groups. Table 1 shows the characteristics of the 233 gene-negative and 821 prHD participants in these analyses. The groups were balanced in gender and years of education, but differed in age and CAG-repeat length as these variables are part of the CAP score. UHDRS motor scores increased with CAP group, since they correlate with CAG length. The sample for the main analysis that tested the ability of factor scores to predict time to diagnosis included 730 prHD participants that had at least one follow-up assessment; 137 (16.7%) were judged as having converted to HD based on a score of 4 on question 17 of the UHDRS during a follow-up exam. Follow-up periods ranged between .85 to 6.59 years. Table 2 details characteristics of the two groups at the baseline assessment.
Table 1.
Characteristics of participants included in ANCOVAs that compared the control and prodromal Huntington's disease groups on factor scores
| Controls (n=233) |
Low (n=222) |
Medium (n=281) |
High (n=318) |
p Value | |
|---|---|---|---|---|---|
| Estimated years to diagnosis | >12.8 | 7.6–12.8 | <7.6 | ||
| % Women | 63.5 | 68.0 | 65.1 | 58.8 | 0.151 |
| Age (years) | 43.5 (11.6) | 35.0 (7.7) | 41.2 (9.5) | 44.2 (9.8) | <0.0001 |
| Education (years) | 14.7 (2.7) | 14.5 (2.5) | 14.3 (2.7) | 14.2 (2.8) | 0.160 |
| CAG repeat length | 20.0 (3.5) | 40.9 (1.6) | 42.2 (2.1) | 43.8 (2.8) | <0.0001 |
| UHDRS motor score | 2.6 (3.3) | 3.0 (3.4) | 4.0 (4.3) | 6.8 (6.3) | <0.0001 |
Note, stratification of the prodromal Huntington's disease participants into low, medium, and high groups was based on the CAG–age product (CAP) score.18
UHDRS, Unified Huntington's Disease Rating Scale.
Table 2.
Characteristics of prodromal Huntington's disease participants at baseline who did and did not receive a clinical diagnosis at a follow-up examination
| Not diagnosed (n=593) | Diagnosed (n=137) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Low (n=179) | Medium (n=226) | High (n=188) | Low (n=9) | Medium (n=25) | High (n=103) | ||
| % Women | 65.9 | 65.0 | 55.9 | 88.9 | 68.0 | 65.0 | 0.069 |
| Age (years) | 35.6 (7.6) | 41.7 (9.2) | 43.9 (9.6) | 37.4 (5.5) | 42.8 (10.2) | 45.1 (10.1) | 0.205 |
| Education (years) | 14.6 (2.5) | 14.6 (2.7) | 14.2 (2.9) | 13.8 (2.0) | 12.8 (2.4) | 14.3 (2.6) | 0.113 |
| CAG repeat length | 40.8 (1.6) | 42.0 (1.9) | 43.5 (2.6) | 40.8 (1.3) | 42.2 (2.5) | 44.1 (3.0) | 0.072 |
| UHDRS motor score | 2.8 (3.1) | 3.6 (3.7) | 5.0 (5.2) | 7.8 (5.1) | 10.4 (5.4) | 10.1 (7.0) | 0.0001 |
| Follow-up period (years) | 3.6 (1.5) | 3.6 (1.4) | 3.6 (1.5) | 4.2 (1.1) | 4.2 (1.3) | 4.4 (1.2) | 0.0001 |
Note, stratification of the prodromal Huntington's disease participants into low, medium, and high groups was based on the CAG–age product (CAP) score.18 The p values are from comparisons between prodromal Huntington's disease participants who did and did not receive a diagnosis, adjusting for CAP group. The significant group difference in the follow-up period further stressed the importance of using survival analysis, for which the follow-up time is taken into consideration.
UHDRS, Unified Huntington's Disease Rating Scale.
Assessment Battery
The battery contained 18 measures from standardized clinical tests and computerized cognitive tests more commonly used in research. A detailed description of the tasks is provided by Stout and colleagues.[4] We selected tests and dependent variables that were the most sensitive in distinguishing gene-negative control and the prHD groups in a recent study[4] and in our latest unpublished, internal analysis of the PREDICT-HD database. Three clinical tests contained more than one subtest that was conceptually distinct (i.e., Stroop Test, Trail Making Test, and Hopkins Verbal Learning Test). For these tests, multiple measures were included, because they distinguished between the control and prHD groups. The present study reports baseline data from the first task administration for all analyses.
Eleven well-known clinical tests/subtests and their measures included the Stroop Test (color naming, word reading and interference; total correct in 45 sec);[19] the Trail Making Test (Parts A and B; time to complete);[20] the Wechsler Adult Intelligence Scale-III Letter-Number Sequencing (total correct); Phonemic Verbal Fluency (letters; total correct over three trials);[21] the Symbol Digit Modalities Test (SDMT; total correct in 90 sec);[22] the Hopkins Verbal Learning Test-Revised (HVLT-R; immediate (total learning) and delayed recall; total correct);[23] and the University of Pennsylvania Smell Identification Task (UPSIT; percent correct).[24]
There were 7 computerized tests. In the Dual Verbal Working Memory task,[25;26] participants name the color of digits presented serially every 1500 ms, and then recall the serial order of the digits. The set size begins at two and increases by one after 2 trials. The task is discontinued when recall fails for both trials of a set size; the measure is total correct recall. In the Emotion Recognition task, participants match the facial expression of faces with a verbal description of the emotion.[5] The measure is total correct negative emotions, which is sensitive in prHD. In the N-Back Working Memory task,[27] participants match the current letter with a letter presented two back in the series. Letters are presented every 3 sec and lures occur in the 1-back and 3-back positions. The measure is the discrimination score in the condition with lures. In the Maximum Tapping Speed task, participants tap at their maximum speed for 10 sec using the non-dominant hand. Five trials are administered and the measure is the mean of the inter-tap intervals. In the Paced Timing task,[2] participants begin tapping in synchrony with a 550 ms isochronous tone and then continue tapping without the tone at the same pace (continuation phase). The measure is the reciprocal of the within-subject inter-tap interval during the continuation phase, which reflects timing proficiency. In the Two-Choice Reaction Time task, one of two adjacent circles on a touch screen turns green and participants immediately press the circle. The measure is mean movement time, which reflects response selection processes. In the Cued Movement Sequence task,[28] filled circles are displayed in 12 vertical pairs along the bottom of a touch screen. Participants press sequentially illuminated circles. The next circle is illuminated when the finger is lifted from the previous circle. Eight error-free trials are administered; the measure is movement time, which reflects motor planning and sequencing skills.
Statistical Analyses
We first conducted an exploratory factor analysis (FA) with varimax rotation to elucidate the dimensional structure underlying the 18 tests. The FA used data from 580 prHD participants with complete observations. Measures that comprised a factor were those with factor loadings of ± 0.40 or greater, indicating that at least 16% of the variation in a variable was explained by an individual factor. Factors were not retained if a minimum of two variables failed to load on a factor. For each retained factor, factor scores were computed by 1) standardizing each cognitive variable, 2) multiplying the standardized variable by the factor coefficient for variables that loaded 0.40 or greater on the factor, and 3) summing over the weighed standardized variables.
In an intermediate analysis, an ANCOVA evaluated the internal validity of the factor scores by comparing the control, low CAP, medium CAP, and high CAP groups (Table 1) on each factor score, covarying age. Follow-up ANCOVAs were performed on each individual test comprising a factor score to qualitatively compare their sensitivity with the factor scores, as indexed by the adjusted R2 for the main effect of group.
A log-logistic accelerated failure time (AFT) survival model was used to predict time to diagnosis using data from 730 prHD participants with at least one follow-up assessment (Table 2). This model is an improvement over others because it directly models time to diagnosis in years since study entry to a HD diagnosis or to the last follow-up assessment for individuals who did not receive a diagnosis. A detailed description of the model is given by Zhang and colleagues.[18] The present study extended the model by including the UHDRS motor score and the factor scores as predictor variables, in addition to the CAP score. The ability of cognitive factor scores to predict time to diagnosis was assessed adjusting for the CAP and motor symptoms, which potentially confound associations between cognitive functioning and the primary endpoint. A stepwise variable-selection procedure was used wherein each factor score was sequentially added to the AFT model that included the CAP and the UHDRS motor score to identify the combination of factors that were the strongest unique predictors of time to diagnosis. Age at study entry did not add significantly to the model, so it was excluded in the final model.
RESULTS
Factor analysis
Seven factors were identified that accounted for 51% of the total variance. Factor 7 was discarded as all variable loadings were lower than ± 0.40 (i.e., ± 0.01 to 0.23). The first 6 factors were retained for further analyses. Table 3 summarizes variables that loaded on each factor, which accounted for 9% to 21.6% of the total common variance. Factor 1 was described as speed and inhibition, but consisted only of the three Stroop subtests. Though not a latent variable, subtests did not load with other factors, indicating they represent a different component of cognition that should be treated separately. This was also seen for factor 6, verbal learning and memory, which consisted of the two HVLT-R subtests. Latent variables were suggested by the remaining factors. Factor 2 was characterized by three tests of verbal working memory and phonemic verbal fluency, which involves tracking previously named words (i.e., monitoring contents of working memory). Factor 3 was characterized by tests of motor planning, but also motor speed (maximum tapping speed). Factor 4 was described by tests of attention and information integration. Factor 5 was characterized by tests of sensory and perceptual processing, including negative emotion recognition, smell perception, and timing.
Table 3.
Factor loadings for each test in the assessment battery
| Tests | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 |
|---|---|---|---|---|---|---|
| Speed and inhibition |
Verbal working memory |
Motor planning and speed |
Attention and information integration |
Sensory and perceptual processing |
Verbal learning and memory |
|
| Stroop: colour | 0.778 | |||||
| Stroop: word | 0.725 | |||||
| Stroop: interference | 0.575 | |||||
| Letter–number sequencing | 0.573 | |||||
| 2-back working memory | 0.569 | |||||
| Dual verbal working memory | 0.551 | |||||
| Phonemic verbal fluency | 0.418 | |||||
| Two choice reaction time | 0.694 | |||||
| Cued movement sequencing | 0.615 | |||||
| Maximum tapping speed | 0.594 | |||||
| Paced timing | −0.409 | 0.434 | ||||
| Trail Making Test: part A | 0.602 | |||||
| Trail Making Test: part B | 0.540 | |||||
| SDMT | −0.433 | |||||
| Emotion recognition | 0.526 | |||||
| UPSIT | 0.456 | |||||
| HVLT-R: delayed recall | 0.581 | |||||
| HVLT-R: immediate recall | 0.483 | |||||
| Per cent common variance | 21.6 | 21.0 | 19.8 | 13.6 | 13.0 | 9.0 |
HVLT-R, Hopkins Verbal Learning Test-revised; SDMT, Symbol Digit Modalities Test; UPSIT, University of Pennsylvania Smell Identification Task.
Internal validity of factor scores
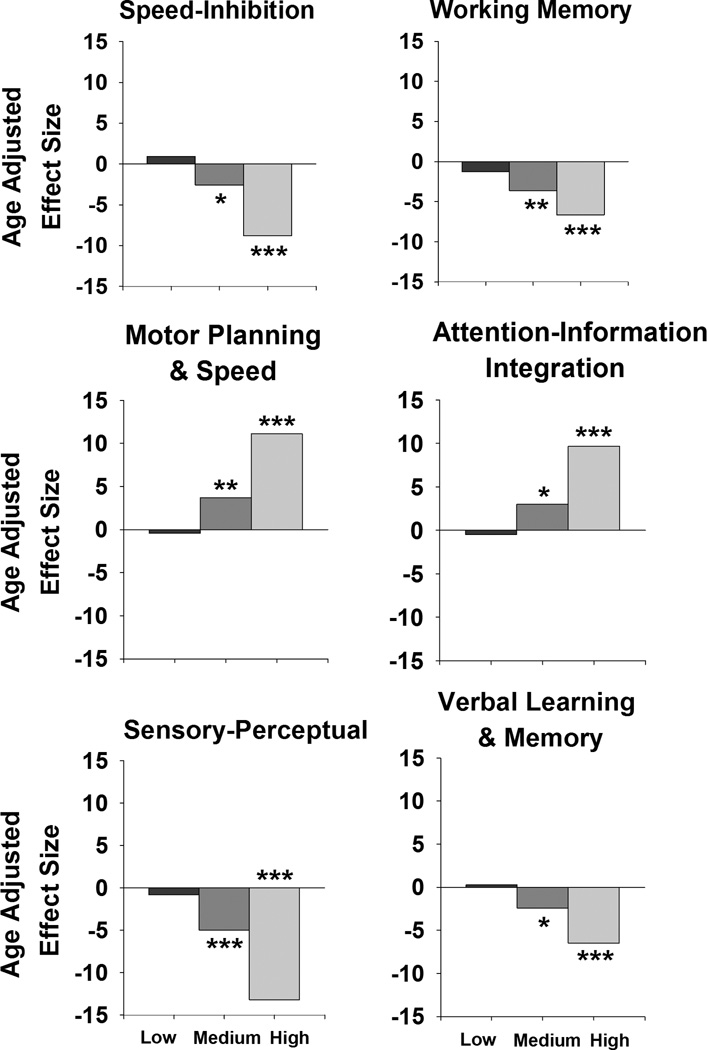
Intermediate ANCOVAs tested for group differences (control, low CAP, medium CAP, high CAP) in each factor score, covarying for age. All six factor scores differed significantly among the groups (factor 1: F(4,1041)=34.59, p<.0001; factor 2: F(4,812)=14.71, p<.0001; factor 3: F(4,948)=51.23, p<.0001; factor 4: F(4,1027)=45.20, p<.0001; factor 5: F(4,984)=58.21, p<.0001; and factor 6: F(4,1044)=16.16, p<.0001). Figure 1 plots the age-adjusted standardized effect sizes (i.e., effect size/standard error of the effect size) for each CAP group, which were obtained from pairwise comparisons with the control group. Greater negative effect sizes for factors 1, 2, 5 and 6 and greater positive effect sizes for factors 3 and 4 reflect worse performance. This figure shows the strong relationship between the CAP groups and factor scores. Though none of the factor scores discriminated the low CAP group from controls, all factors were significantly sensitive to worse performance in the medium and the high CAP groups, similar to findings for individual tests.[4] These results verified the internal validity of the factor scores. Qualitatively, the age-adjusted standardized effect sizes for the medium and high CAP groups were largest for sensory-perceptual processing and motor planning/speed, and the smallest for verbal learning and memory.
Figure 1.
Age adjusted effect sizes for the six factor scores in the low, medium, and high CAP groups. Standardized effect size = effect size/standard error of effect size. Asterisks denote the significance of pairwise comparisons between the control group and each CAP group where * = p<.02, ** = p<.001, and *** = p<.0001.
Follow-up ANCOVAs examining group differences on each individual test comprising a factor showed that factor scores typically discriminated the groups as well or better than any single test. Table 4 shows that the adjusted R2 for factor scores and the individual tests. Factors 2 (working memory), 3 (motor planning/speed) and 5 (sensory-perceptual processing) were more sensitive in discriminating among the groups than any single test comprising the factors. R2 values for factors 1 (speed-inhibition) and 4 (attention-integration) were similar to those for one test comprising each factor score (Stroop Interference and SDMT, respectively). Conversely, R2 values were lower for factor 6 (verbal learning and memory) than the HVLT-R immediate recall subtest (i.e., total recall).
Table 4.
Adjusted R2 from ANCOVA testing for group differences in factor scores and performances on individual tests comprising each factor
| Factor/test | Adjusted R2 |
Factor/Test | Adjusted R2 |
|---|---|---|---|
| Factor 1: speed–inhibition | 0.114** | Factor 4: attention–integration | 0.146** |
| Stroop: colour | 0.095** | Trail Making Test: part A | 0.074** |
| Stroop: word | 0.081** | Trail Making Test: part B | 0.099** |
| Stroop: interference | 0.113** | SDMT | 0.148** |
| Factor 2: working memory | 0.063** | Factor 5: sensory–perceptual | 0.188** |
| Letter–number sequencing | 0.035** | Paced timing | 0.132** |
| 2-back working memory | 0.049** | Emotion recognition | 0.121** |
| Dual verbal working memory | 0.039** | UPSIT | 0.064** |
| Phonemic verbal fluency | 0.030** | ||
| Factor 3: motor planning and speed | 0.174** | Factor 6: verbal learning and memory | 0.055** |
| Two choice reaction time | 0.097** | HVLT-R: immediate recall | 0.091** |
| Cued movement sequencing | 0.090** | HVLT-R: delayed recall | 0.010* |
| Maximum tapping speed | 0.126** | ||
| Paced timing | 0.134** |
The ANCOVAs adjusted for age. The main effect of group was significant for all factors and the measures comprising each factor at *p<0.006 or **p<0.0001.
HVLT-R, Hopkins Verbal Learning Test-revised; SDMT, Symbol Digit Modalities Test; UPSIT, University of Pennsylvania Smell Identification Task.
Cognitive domains and proximity to diagnosis
A stepwise variable-selection procedure determined the combination of factors that predicted time to diagnosis in the AFT model (Table 5). The final prediction equation was:
Y = exp[3.339 −1.217 × CAPs −0.039 × UHDRS Motor Score −0.099 × F3+0.172 × F5]
where Y is the predicted time to HD diagnosis since study entry given the CAP score, the UHDRS motor score, and scores on factors 3 (F3) and 5 (F5). The CAP and UHDRS motor scores were strongly related to time to diagnosis after adjusting for other variables in the equation. A half point increase in the CAP score decreases time to diagnosis by 46% (1 – exp(−1.217 * 0.5) and a one point increase in the UHDRS motor score decreases time to diagnosis by 4% (1-exp(−0.039). The main results showed that motor planning/seed (factor 3) and sensory-perceptual processing (factor 5) were the strongest unique predictors of time to diagnosis, after adjusting for the CAP and UHDRS motor scores. A one point increase in the motor planning/speed factor score (worse performance) would be expected to decrease time to diagnosis by 9% (i.e., 1 – exp(−0.099). A one point increase in the sensory-perceptual factor score (better performance) would be expected to increase time to diagnosis by 19% (i.e., exp(0.172) – 1). The remaining factors did not add to the prediction of time to diagnosis.
Table 5.
Log-logistic accelerated failure time model results
| Variable | Estimate | SD error | χ2 | p Value |
|---|---|---|---|---|
| CAP score | −1.217 | 0.299 | 16.565 | <0.0001 |
| UHDRS motor score | −0.039 | 0.008 | 21.437 | <0.0001 |
| Factor 3: motor planning and speed | −0.099 | 0.026 | 15.054 | <0.0001 |
| Factor 5: sensory–perceptual processing | 0.172 | 0.054 | 10.049 | <0.0015 |
CAP, CAG–age product; UHDRS, Unified Huntington's Disease Rating Scale.
DISCUSSION
The present study identified six conceptually meaningful cognitive domains that characterized functioning in prHD. Four latent factors (verbal working memory, motor planning/speed, attention-information integration and sensory-perceptual processing) were uncovered that elucidated the dimensional structure of 12 different tests. The two remaining factors (speed-inhibition and verbal learning/memory), were comprised of subtests from the same neuropsychological test, signifying that they should be treated separately. There was good definition between factors with all but one test clearly loading on one of the six factors. The paced timing task loaded on both the motor planning and the sensory-perceptual factors, which makes sense since timing proficiency is fundamental to planning and perception.
Our main results indicated that motor planning/speed and sensory-perceptual processing were the best indicators of time to diagnosis after controlling for CAP and UHDRS motor scores. This is notable given that the CAP score is by far the most robust predictor of time to diagnosis. A worse motor planning/speed score was a risk factor, with a one point increase expected to decrease time to diagnosis by 9%. This finding likely relates to changes in neural systems that support cognitive aspects of planning, since it was not confounded by motor symptoms. Conversely, a better sensory-perceptual score was a protective factor, with a one point increase expected to increase time to diagnosis by 19%. Most tests comprising these two factors are utilized in research rather than clinically, since many lack normative data. Hence, their use in clinical trials has been limited. Our results indicate that tests representative of these domains should be part of a clinical trials battery as they have more prognostic value than the other domains.
Factor scores are of limited utility for diagnosis. Changes in composite scores are also difficult to interpret for clinical purposes because they consist of linear combinations of variables. However, factor scores should be considered in clinical trials where the emphasis is on detecting treatment effects. Our results show that latent variables were more sensitive to cognitive decline than individual measures comprising the motor-planning/speed and sensory-perceptual factors (i.e., R2) or for that matter, any other factor. This is common when factors contain 3 or more measures from different tests.[15] Factor scores may also be more reliable since they are comprised of multiple measures, which would be advantageous for longitudinal designs. Sets of tests for the motor-planning and sensory-perceptual factors are also feasible for use in clinical trials as they each take 15–20 minutes to administer.
Although the neural bases of our findings are unknown, we speculate that motor planning/speed and sensory-perceptual processing may be mediated partly by different core networks that are especially weakened in prHD. For example, aspects of motor planning including timing and sequencing are mediated by the motor circuit (putamen, thalamus, supplementary motor area, and sensorimotor cortices) and parietal cortex.[29–31] Neuroimaging studies of timing and motor planning in prHD report abnormal activity in these systems, especially in individuals closer to diagnosis.[32;33] As for sensory-perceptual processing, the ventral striatum and the limbic system (orbitofrontal, cingulate, and anterior insular cortex) mediate timing, emotion, and odor recognition.[34–36] The insular cortex may be especially important as it integrates physiological states (e.g., emotionally charged or arousing events), which alter estimates of time.[34;37] Indeed, anterior insula activity is abnormal during timing in prHD individuals who are more than 12 years from diagnosis.[32;38] Insula atrophy also correlates with impaired recognition of fear in early HD.[39]
Other cognitive domains identified by the present study appeared less affected in prHD, possibly because different neural systems assume a more central role in mediating core functions. However, emerging neuroimaging studies suggest that functional markers can be more sensitive barometers of decline than behavioral indices. For example, connectivity in working memory networks (frontostriatal and frontoparietal) is weakened in prHD individuals near to diagnosis, despite normal working-memory performance.[40] Similarly, activation in a key inhibition hub (anterior cingulate) is abnormal in prHD despite normal performance on an interference task.[41] These findings may be due to the use of compensatory strategies, which can mask performance difficulties. More challenging cognitive tests might reveal behavioral impairments in these areas.
Limitations and future directions
The results provide insight into cognitive domains that are important markers of time to diagnosis. Whether these domains will be sensitive to longitudinal cognitive decline is unknown, although we plan to investigate this in the future. Our findings cannot be taken to mean that cognitive evaluations should only focus on motor planning/speed and sensory-perceptual processing. Rather, there are many facets to the constructs elucidated by the present study. More work is needed to address gaps in existing assessments. Domains that have been inadequately sampled by the PREDICT battery include inhibition and nonverbal working memory. The search for more sensitive indices of cognitive impairment is a vital endeavor as behavioral measures have considerable potential to serve as cost-effective and sensitive outcomes in clinical trials, and are important for diagnosis and tracking of disease progression.
Acknowledgments
We thank the PREDICT-HD sites, the study participants, and the National Research Roster for Huntington Disease Patients and Families. The full list of PREDICT-HD Contributors is shown below.
Funding
This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS40068) and CHDI Foundation, Inc.
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters
Active: September 2010–August 2011
Thomas Wassink, MD, Stephen Cross, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Jessica Wood, MD, PhD, Eric A. Epping, MD, PhD, and Leigh J. Beglinger, PhD (University of Iowa, Iowa City, Iowa, USA); Edmond Chiu, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, D.Psych, Chathushka Fonseka, Stephanie Antonopoulos and Samantha Loi (St. Vincent’s Hospital, The University of Melbourne, Kew, Victoria, Australia); Phyllis Chua, MD, and Angela Komiti, BS, MA (The University of Melbourne, Royal Melbourne Hospital, Melbourne, Australia); Lynn Raymond, MD, PhD, Rachelle Dar Santos, BSc, Kimberley Carter, BSc, and Joji Decolongon, MSC, CCRP (University of British Columbia, Vancouver, British Columbia, Canada); Adam Rosenblatt, MD, Christopher A. Ross, MD, PhD, Barnett Shpritz, BS, MA, OD, Nadine Yoritomo, RN and Claire Welsh (Johns Hopkins University, Baltimore, Maryland, USA); William M. Mallonee, MD, Greg Suter, BA, and Judy Addison (Hereditary Neurological Disease Centre, Wichita, Kansas, USA); Ali Samii, MD, and Alma Macaraeg, BS (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA); Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA); Roger A. Barker, BA, MBBS, MRCP, Sarah Mason, BSC, Anna Goodman, PhD, and Anna DiPietro (Cambridge Centre for Brain Repair, Cambridge, UK); Elizabeth McCusker, MD, Jane Griffith, RN, Clement Loy, MD, and David Gunn, BS (Westmead Hospital, Sydney, Australia); Bernhard G. Landwehrmeyer, MD, Michael Orth MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany); Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis, IN); Mark Guttman, MD, Alanna Sheinberg, BA, and Irita Karmalkar, BSc (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada); Susan Perlman, MD, Brian Clemente, and Arik Johnson, PsyD (University of California, Los Angeles Medical Center, Los Angeles, California, USA); Michael D. Geschwind, MD, PhD, Jon Gooblar, BA, and Gail Kang, MD (University of California San Francisco, California, USA); Tom Warner, MD, PhD, Maggie Burrows, RN, BA, Marianne Novak, MD, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, and Sarah Tabrizi, MD, PhD (National Hospital for Neurology and Neurosurgery, London, UK); Anne Rosser, MD, PhD, MRCP, Kathy Price, RN, and Sarah Hunt, BSc (Cardiff University, Cardiff, Wales, UK); Frederick Marshall, MD, Amy Chesire, LCSW-R, MSG, Mary Wodarski, BA, and Charlyne Hickey, RN, MS (University of Rochester, Rochester, New York, USA); Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, and Mary Lou Klimek, RN, BN, MA (University of Calgary, Calgary, Alberta, Canada); Peter Panegyres, MB, BS, PhD, Joseph Lee, and Steve Andrew (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia); Joel Perlmutter, MD, Stacey Barton, MSW, LCSW, and Amy Schmidt (Washington University, St. Louis, Missouri, USA); Zosia Miedzybrodzka, MD, PhD, Daniela Rae, RN, and Mariella D’Alessandro, PhD (Clinical Genetics Centre, Aberdeen, Scotland, UK); David Craufurd, MD, Ruth Fullam, BSC, Judith Bek, PhD, and Elizabeth Howard, MD (University of Manchester, Manchester, UK); Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA); Rajeev Kumar, MD and Diane Erickson, RN (Colorado Neurological Institute, Englewood, Colorado, USA); Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, Lisa Kjer, MSW, and Kathleen Baynes, PhD (University of California Davis, Sacramento, California, USA); Joseph Jankovic, MD, Christine Hunter, RN, CCRC, and William Ondo, MD (Baylor College of Medicine, Houston, Texas, USA); Wayne Martin, MD, Pamela King, BScN, RN, Marguerite Wieler and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada); Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Janice Zimbelman, PhD, PT, Alexandra Bea, BA, Emily Newman, BA, Alex Bura, BA, Lyla Mourany, and Juliet Schulz (Cleveland Clinic Foundation, Cleveland, Ohio, USA).
Steering Committee
Jane Paulsen, PhD, Principal Investigator, Eric A. Epping, MD, PhD, Hans Johnson, PhD, Megan Smith, PhD, Janet Williams, PhD, RN, FAAN, Leigh Beglinger, PhD, Jeffrey D. Long, PhD, James A. Mills, MS (University of Iowa, Iowa City, IA); Elizabeth Aylward, PhD (Seattle Children's Research Institute, WA); Kevin Biglan, MD (University of Rochester, Rochester, NY); Blair Leavitt, MD (University of British Columbia, Vancouver, BC, Canada); Marcy MacDonald, PhD (Massachusetts General Hospital); Martha Nance, MD (Hennepin County Medical Center, Minneapolis, MN); and Cheryl Erwin, JD, PhD (University of Texas Medical School at Houston).
Scientific Sections
Bio Markers: Blair Leavitt, MDCM, FRCPC (Chair) and Michael Hayden, PhD (University of British Columbia); Stefano DiDonato, MD (Neurological Institute “C. Besta,” Italy); Ken Evans, PhD (Ontario Cancer Biomarker Network); Wayne Matson, PhD (VA Medical Center, Bedford, MA); Asa Peterson, MD, PhD (Lund University, Sweden), Sarah Tabrizi, MD, PhD (National Hospital for Neurology and Neurology and Neurosurgery, London); Beth Borowsky, PhD (CHDI); Andrew Juhl, BS, James A. Mills, MS, Kai Wang, PhD (University of Iowa); and David Weir, BSc (University of British Columbia).
Brain: Jean Paul Vonsattell, PhD (Chair), and Carol Moskowitz, ANP, MS (Columbia University Medical Center); Anne Leserman, MSW, LISW, Lynn Schaul, BA, and Stacie Vik, BA (University of Iowa).
Cognitive: Deborah Harrington, PhD (Chair), Gabriel Castillo, BS, Jessica Morison, BS, and Jason Reed, BS (University of California, San Diego), Michael Diaz, PhD, Ian Dobbins, PhD, Tamara Hershey, PhD, Erin Foster, OTD, and Deborah Moore, BA (Washington University Cognitive Science Battery Development); Holly Westervelt, PhD (Chair, Quality Control and Training, Alpert Medical School of Brown University), Jennifer Davis, PhD, and Geoff Tremont, PhD, MS (Scientific Consultants, Alpert Medical School of Brown University); Megan Smith, PhD (Chair, Administration), David J. Moser, PhD, Leigh J. Beglinger, PhD, Kelly Rowe, Gloria Wenman, and Danielle Theriault, BS (University of Iowa); Carissa Gehl, PhD (VA Medical Center, Iowa City, IA); Kirsty Matheson (University of Aberdeen); Karen Siedlecki, PhD (Fordham University); Marleen Van Walsem (EHDN); Susan Bonner, BA, Greg Elias, BA, Mary Gover, Rachel Bernier, and Melanie Faust, BS (Rhode Island Hospital); Beth Borowski, PhD (CHDI); Noelle Carlozzi (University of Michigan); Kevin Duff, PhD (University of Utah); Nellie Georgiou-Karistianis (St. Vincent’s Hospital, The University of Melbourne, Australia); Julie Stout, PhD (Monash University, Melbourne, Australia); Herwig Lange (Air-Rahazentrum); and Kate Papp (University of Connecticut).
Functional: Janet Williams, PhD (Chair), Leigh J. Beglinger, PhD, Anne Leserman, MSW, LISW, Eunyoe Ro, MA, Lee Anna Clark, Nancy Downing, RN, PhD, Joan Laing, PhD, Kristine Rees, BA, Michelle Harreld, BS, and Stacie Vik, BA (University of Iowa); Rebecca Ready, PhD (University of Massachusetts); Anthony Vaccarino, PhD (Ontario Cancer Biomarker Network); Sarah Farias, PhD (University of California, Davis); Noelle Carlozzi, PhD (University of Michigan); and Carissa Gehl, PhD (VA Medical Center, Iowa City, IA).
Genetics: Marcy MacDonald, PhD (Co-Chair), Jim Gusella, PhD, and Rick Myers, PhD (Massachusetts General Hospital); Michael Hayden, PhD (University of British Columbia); Tom Wassink, MD (Co-Chair) Eric A. Epping, MD, PhD, Andrew Juhl, BA, James Mills, MS, and Kai Wang, PhD (University of Iowa); Zosia Miedzybrodzka, MD, PhD (University of Aberdeen); and Christopher Ross, MD, PhD (Johns Hopkins University).
Imaging: Administrative: Ron Pierson, PhD (Chair), Kathy Jones, BS, Jacquie Marietta, BS, William McDowell, AA, Greg Harris, BS, Eun Young Kim, MS, Hans Johnson, PhD, and Thomas Wassink, MD (University of Iowa); John Ashburner, PhD (Functional Imaging Lab, London); Steve Potkin, MD (University of California, Irvine); and Arthur Toga, PhD (University of California, Los Angeles). Striatal: Elizabeth Aylward, PhD (Chair, Seattle Children's Research Institute). Surface Analysis: Eric Axelson, BSE (University of Iowa). Shape Analysis: Christopher A. Ross (Chair), MD, PhD, Michael Miller, PhD, and Sarah Reading, MD (Johns Hopkins University); Mirza Faisal Beg, PhD (Simon Fraser University). DTI: Vincent A. Magnotta, PhD (Chair, University of Iowa); Karl Helmer, PhD (Massachusetts General Hospital); Kelvin Lim, MD (University of Ulm, Germany); Mark Lowe, PhD (Cleveland Clinic); Sasumu Mori, PhD (Johns Hopkins University); Allen Song, PhD (Duke University); and Jessica Turner, PhD (University of California, Irvine). fMRI: Steve Rao, PhD (Chair), Erik Beall, PhD, Katherine Koenig, PhD, Michael Phillips, MD, Christine Reece, BS, and Jan Zimbelman, PhD, PT (Cleveland Clinic); April Bryant, Andrew Juhl, BS, Kathy Jones, and Gloria Wenman (University of Iowa).
Motor: Kevin Biglan, MD (Chair) (University of Rochester); Karen Marder, MD (Columbia University); Jody Corey-Bloom, MD, PhD (University of California, San Diego); Michael Geschwind, MD, PhD (University of California, San Francisco); Ralf Reilmann, MD and Zerka Unds (Muenster, Germany); and Andrew Juhl, BS (University of Iowa).
Psychiatric: Eric A. Epping, MD, PhD (Chair), Nancy Downing, RN, PhD, Jess Fiedorowicz, MD, Robert Robinson, MD, Megan Smith, PhD, Leigh Beglinger, PhD, James Mills, MS, Kristine Rees, BA, Michelle Harreld, BS, Adam Ruggle, Stacie Vik, BA, Janet Williams, PhD, Dawei Liu, PhD, David Moser, PhD, and Kelly Rowe (University of Iowa); Karen Anderson, MD (University of Maryland); David Craufurd, MD (University of Manchester); Mark Groves, MD (Columbia University); Anthony Vaccarino, PhD and Ken Evans, PhD (Ontario Cancer Biomarker Network); Hugh Rickards, MD (Queen Elizabeth Psychiatric Hospital); Eric van Duijn, MD (Leiden University Medical Center, Netherlands); Phyllis Chua (The University of Melbourne, Royal Melbourne Hospital); and Kimberly Quaid, PhD (Indiana University School of Medicine).
Core Sections
Statistics: Jeffrey D. Long, PhD, Ji-In Kim, PhD, James A. Mills, MS, Blair Harrison, MPH, Ying Zhang, PhD, Dawei Liu, PhD, Wenjing Lu, and Spencer Lourens (University of Iowa).
Recruitment/Retention: Martha Nance, MD (Chair, University of Minnesota); Anne Leserman, MSW, LISW, Nicholas Doucette, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Kelli Thumma, BA, Elijah Waterman, BA, and Jeremy Hinkel, BA (University of Iowa).
Ethics: Cheryl Erwin, JD, PhD, (Chair, McGovern Center for Health, Humanities and the Human Spirit); Eric A. Epping, MD, PhD Janet Williams, PhD, Nicholas Doucette, BA, Anne Leserman, MSW, LISW, James Mills, MS, Lynn Schaul, BA, and Stacie Vik, BA (University of Iowa); Martha Nance, MD (University of Minnesota); and Lisa Hughes, MEd (University of Texas Medical School at Houston).
IT/Management: Hans Johnson, PhD (Chair), R.J. Connell, BS, Karen Pease, BS, Ben Rogers, BA, BSCS, Jim Smith, AS, Shuhua Wu, MCS, Roland Zschiegner, Erin Carney, Bill McKirgan, Mark Scully, and Ryan Wyse (University of Iowa); Jeremy Bockholt (AMBIGroup).
Program Management
Administrative: Chris Werling-Witkoske (Chair), Greg Ennis, MA, Stacie Vik, BA, Karla Anderson, BS, Kristine Bjork, BA, Sean Thompson, BA, Leann Davis, Machelle Henneberry, Ann Dudler, Jamy Schumacher, and Craig Stout (University of Iowa).
Financial: Steve Blanchard, MSHA, Kelsey Montross, BA, and Phil Danzer (University of Iowa).
Footnotes
Competing Interests
The authors report no competing interests.
References
- 1.Bechtel N, Scahill RI, Rosas HD, Acharya T, van den Bogaard SJ, Jauffret C, Say MJ, Sturrock A, Johnson H, Onorato CE, Salat DH, Durr A, Leavitt BR, Roos RA, Landwehrmeyer GB, Langbehn DR, Stout JC, Tabrizi SJ, Reilmann R. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology. 2010;75:2150–2160. doi: 10.1212/WNL.0b013e3182020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe KC, Paulsen JS, Langbehn DR, Duff K, Beglinger LJ, Wang C, O'Rourke JJ, Stout JC, Moser DJ. Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology. 2010;24:435–442. doi: 10.1037/a0018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duff K, Paulsen J, Mills J, Beglinger LJ, Moser DJ, Smith MM, Langbehn D, Stout J, Queller S, Harrington DL. Mild cognitive impairment in prediagnosed Huntington disease. Neurology. 2010 doi: 10.1212/WNL.0b013e3181eccfa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Carlozzi N, Duff K, Beglinger LJ, Langbehn DR, Johnson SA, Biglan KM, Aylward EH. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25:1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SA, Stout JC, Solomon AC, Langbehn DR, Aylward EH, Cruce CB, Ross CA, Nance M, Kayson E, Julian-Baros E, Hayden MR, Kieburtz K, Guttman M, Oakes D, Shoulson I, Beglinger L, Duff K, Penziner E, Paulsen JS. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain. 2007;130:1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Say MJ, Jones R, Scahill RI, Dumas EM, Coleman A, Santos RC, Justo D, Campbell JC, Queller S, Shores EA, Tabrizi SJ, Stout JC. Visuomotor integration deficits precede clinical onset in Huntington's disease. Neuropsychologia. 2011;49:264–270. doi: 10.1016/j.neuropsychologia.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Pirogovsky E, Gilbert PE, Jacobson M, Peavy G, Wetter S, Goldstein J, Corey-Bloom J, Murphy C. Impairments in source memory for olfactory and visual stimuli in preclinical and clinical stages of Huntington's disease. J Clin Exp Neuropsychol. 2007;29:395–404. doi: 10.1080/13803390600726829. [DOI] [PubMed] [Google Scholar]
- 9.Feigin A, Ghilardi MF, Huang C, Ma Y, Carbon M, Guttman M, Paulsen JS, Ghez CP, Eidelberg D. Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol. 2006;59:53–59. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon AC, Stout JC, Johnson SA, Langbehn DR, Aylward EH, Brandt J, Ross CA, Beglinger L, Hayden MR, Kieburtz K, Kayson E, Julian-Baros E, Duff K, Guttman M, Nance M, Oakes D, Shoulson I, Penziner E, Paulsen JS. Verbal episodic memory declines prior to diagnosis in Huntington's disease. Neuropsychologia. 2007;45:1767–1776. doi: 10.1016/j.neuropsychologia.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider SA, Wilkinson L, Bhatia KP, Henley SM, Rothwell JC, Tabrizi SJ, Jahanshahi M. Abnormal explicit but normal implicit sequence learning in premanifest and early Huntington's disease. Mov Disord. 2010;25:1343–1349. doi: 10.1002/mds.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009 doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsen JS, Zhao H, Stout JC, Brinkman RR, Guttman M, Ross CA, Como P, Manning C, Hayden MR, Shoulson I. Clinical markers of early disease in persons near onset of Huntington's disease. Neurology. 2001;57:658–662. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]
- 14.Langbehn DR, Paulsen JS. Predictors of diagnosis in Huntington disease. Neurology. 2007;68:1710–1717. doi: 10.1212/01.wnl.0000261918.90053.96. [DOI] [PubMed] [Google Scholar]
- 15.Gorsuch RL. Factor Analysis. Hillsdale: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 16.Warner JP, Barron LH, Brock DJ. A new polymerase chain reaction (PCR) assay for the trinucleotide repeat that is unstable and expanded on Huntington's disease chromosomes. Mol Cell Probes. 1993;7:235–239. doi: 10.1006/mcpr.1993.1034. [DOI] [PubMed] [Google Scholar]
- 17.Huntington Study Group. Unified Huntington's Disease Rating Scale: Reliability and consistency. Movement Disorders. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS. Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 20.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- 21.Benton AL, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment. Oxford, England: Oxford University Press; 1983. [Google Scholar]
- 22.Smith A. Symbol Digit Modalities Test (SDMT) manual (Revised) Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 23.Brandt J, Benedict R. Hopkins Verbal Learning Test-Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 24.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 25.Hale S, Bronik MD, Fry AF. Verbal and spatial working memory in school-age children: developmental differences in susceptibility to interference. Dev Psychol. 1997;33:364–371. doi: 10.1037//0012-1649.33.2.364. [DOI] [PubMed] [Google Scholar]
- 26.Turner MLERW. Is working memory capacity task dependent? Journal of Memory and Language. 1989;28:127–154. [Google Scholar]
- 27.Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- 28.Georgiou N, Bradshaw JL, Phillips JG, Chiu E, Bradshaw JA. Reliance on advance information and movement sequencing in Huntington's disease. Mov Disord. 1995;10:472–481. doi: 10.1002/mds.870100412. [DOI] [PubMed] [Google Scholar]
- 29.Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrington DL, Zimbelman JL, Hinton SC, Rao SM. Neural modulation of temporal encoding, maintenance, and decision processes. Cereb Cortex. 2010;20:1274–1285. doi: 10.1093/cercor/bhp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsinger CL, Harrington DL, Rao SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31:1177–1187. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Zimbelman JL, Paulsen JS, Mikos A, Reynolds NC, Hoffmann RG, Rao SM. fMRI detection of early neural dysfunction in preclinical Huntington's disease. J Int Neuropsychol Soc. 2007;13:758–769. doi: 10.1017/S1355617707071214. [DOI] [PubMed] [Google Scholar]
- 33.Kloppel S, Draganski B, Siebner HR, Tabrizi SJ, Weiller C, Frackowiak RS. Functional compensation of motor function in pre-symptomatic Huntington's disease. Brain. 2009;132:1624–1632. doi: 10.1093/brain/awp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington DL, Castillo GN, Fong CH, Reed JD. Neural underpinnings of distortions in the experience of time across senses. Frontiers in Integrative Neuroscience. 2011;5 doi: 10.3389/fnint.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walla P. Olfaction and its dynamic influence on word and face processing: cross-modal integration. Prog Neurobiol. 2008;84:192–209. doi: 10.1016/j.pneurobio.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Fan J, Gu X, Liu X, Guise KG, Park Y, Martin L, de MA, Tang CY, Minzenberg MJ, Hof PR. Involvement of the anterior cingulate and frontoinsular cortices in rapid processing of salient facial emotional information. Neuroimage. 2011;54:2539–2546. doi: 10.1016/j.neuroimage.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mella N, Conty L, Pouthas V. The role of physiological arousal in time perception: psychophysiological evidence from an emotion regulation paradigm. Brain and Cognition. 2011;75:182–187. doi: 10.1016/j.bandc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen JS, Zimbelman JL, Hinton SC, Langbehn DR, Leveroni CL, Benjamin ML, Reynolds NC, Rao SM. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington's Disease. AJNR Am J Neuroradiol. 2004;25:1715–1721. [PMC free article] [PubMed] [Google Scholar]
- 39.Henley SM, Wild EJ, Hobbs NZ, Warren JD, Frost C, Scahill RI, Ridgway GR, MacManus DG, Barker RA, Fox NC, Tabrizi SJ. Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia. 2008;46:2152–2160. doi: 10.1016/j.neuropsychologia.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Wolf RC, Sambataro F, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer B. Altered frontostriatal coupling in pre-manifest Huntington's disease: effects of increasing cognitive load. Eur J Neurol. 2008;15:1180–1190. doi: 10.1111/j.1468-1331.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- 41.Reading SA, Dziorny AC, Peroutka LA, Schreiber M, Gourley LM, Yallapragada V, Rosenblatt A, Margolis RL, Pekar JJ, Pearlson GD, Aylward E, Brandt J, Bassett SS, Ross CA. Functional brain changes in presymptomatic Huntington's disease. Ann Neurol. 2004;55:879–883. doi: 10.1002/ana.20121. [DOI] [PubMed] [Google Scholar]